2. Experimental Procedures
The substrate used to prepare the solder joints was a Cu electroplated layer deposited on a 1 cm × 3 cm Cu foil (99.99 wt.%, UMAT Co., Hsinchu, Taiwan). The electrodeposition of Cu was performed in a Haring cell containing an electrolyte of high-purity CuSO4⋅5H2O, H2SO4, and specific additives. The specific additives were suppressor (polyethylene glycol, PEG, 2000 g/mole, 50 ppm), accelerator (bis(3-sulfopropyl) disulfide, SPS, 1 ppm), and chloride ions (NaCl, 60 ppm). They were added in the plating solutions to reduce the impurity concentrations in the Cu electroplated layer and to regulate the deposition behaviors of reduced Cu atoms. Furthermore, the Cu foil was placed on the cathode side to grow the Cu electroplated layer and a 0.04 wt.% phosphorus-containing Cu plate (UMAT Co., Hsinchu, Taiwan) was used as the anode. During the electroplating process for 1 h, the current density was kept as constant at 3.44 ASD, and the temperature of the Haring cell was controlled at 28 °C. After electroplating, the Cu substrate was rinsed using deionized water and dried by an air gun. To define the reaction area with the solder alloy, a heat-resistant tape (solder mask) with an aperture of 1.8 mm diameter was attached on the Cu electroplated layer.
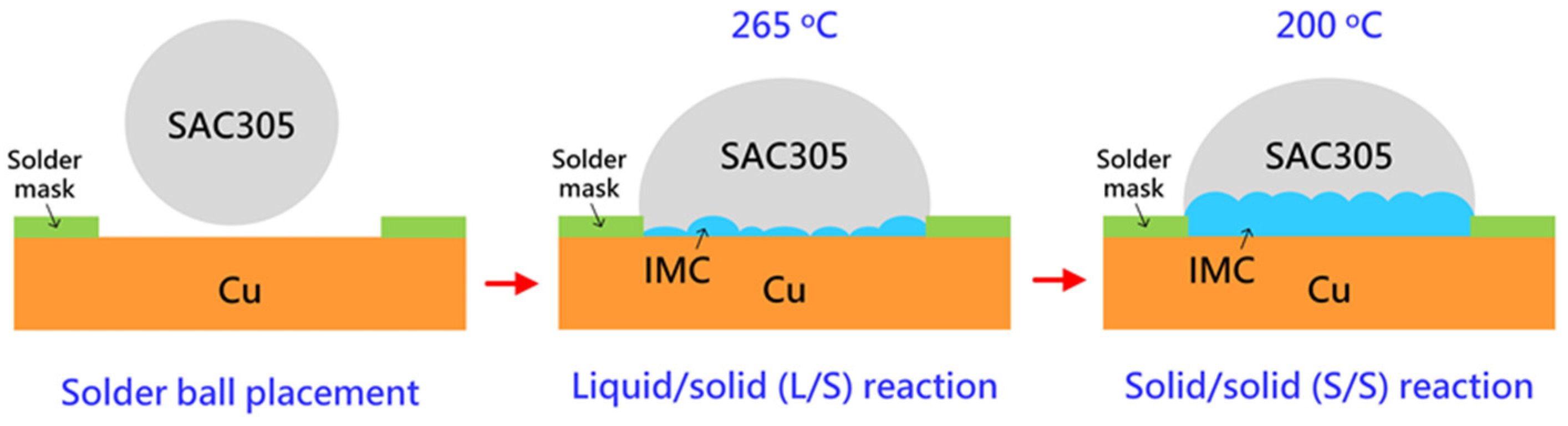
Commercially available SAC305 (Sn—3 wt.% Ag—0.5 wt.% Cu, Senju Metal Industry Co., Ltd., Tokyo, Janan) was used as the solder alloy. Proper amounts of alloy ingots of 22 mg were weighed and heated on a hot plate at 250 °C, during which the alloy ingots melted and transformed into a tiny ball. The tiny solder ball was coated with flux and placed on the exposed surface of Cu defined by the aperture of the heat-resistant tape. The liquid/solid (L/S) reaction between SAC305 and Cu was performed by placing the samples on a hot plate at 265 °C for 1, 10, 30, and 60 min. The as-joined SAC305/Cu samples for various L/S reaction times were denominated as L/S-1, L/S-10, L/S-30, and L/S60, where the number after L/S represents the reaction time. After the L/S reaction, the SAC305/Cu samples were placed in an oven at 200 °C to perform the solid/solid (S/S) reaction for 72, 168, and 360 h. The preparation and reaction of the SAC305/Cu samples are schematically shown in
Figure 1.
After the S/S reactions, the SAC305/Cu samples were removed from the oven and cooled down to room temperature naturally. The aged SAC305/Cu samples were mounted in epoxy resin and were ground in a direction perpendicular to the SAC305/Cu interface using SiC sandpaper. The exposed SAC305/Cu interfacial zone was polished using fine Al2O3 suspensions to offer scratch-free microstructures for examinations using optical microscopy (OM, BX51, Olympus, Tokyo, Japan). Energy-dispersive X-ray spectroscopy (EDX) attached to scanning electron microscopy (SEM, UltraPlus, Zeiss, Oberkochen, Germany) was used to analyze the elemental compositions of the IMCs formed at the SAC305/Cu interface. The average thickness of the IMCs was determined by dividing the area of the IMC layers by the linear length of the SAC305/Cu interface using an image processing software.
3. Results and Discussion
There were four SAC305/Cu joint samples obtained from the L/S reaction, and they were denominated as L/S-1, L/S-10, L/S-30, and L/S-60, for various reaction times from 1 min to 60 min.
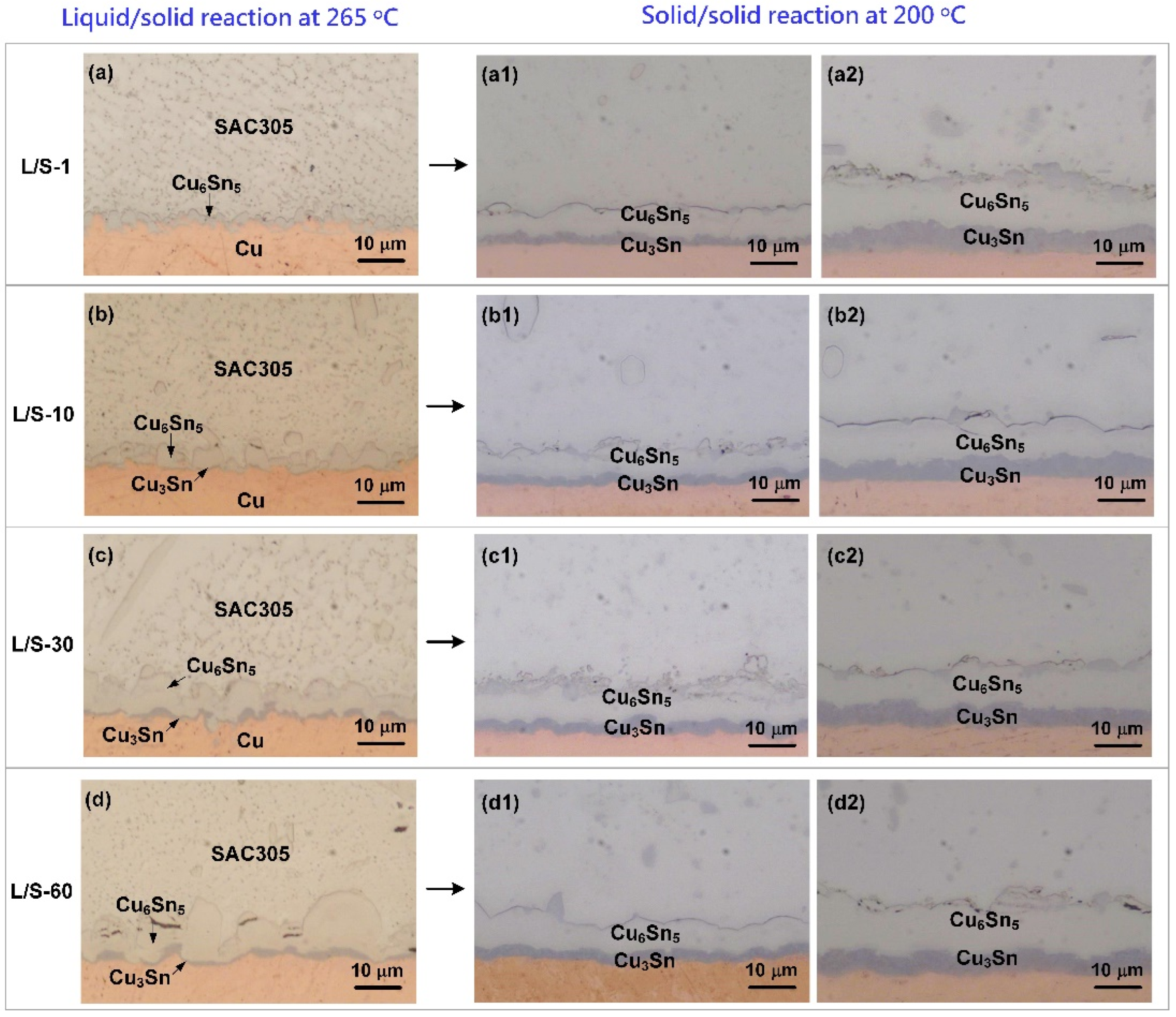
Figure 2a–d show the OM micrographs of the cross-sections of the four SAC305/Cu joints after the L/S reaction. Only one IMC was formed at the SAC305/Cu interface after L/S reaction for 1 min as seen in
Figure 2a, and it was identified as the Cu
6Sn
5 phase according to the EDX results (57.8 at.% Cu, 42.2 at.% Sn). A new IMC was formed at the Cu
6Sn
5/Cu interface after longer L/S reaction times (10, 30, and 60 min) as seen in
Figure 2b–d, and it was confirmed as the Cu
3Sn phase according to the EDX results (74.8 at.% Cu, 25.2 at.% Sn). The phase formation of Cu
6Sn
5 and Cu
3Sn at the SAC305/Cu interface subjected to L/S reaction was consistent with previous studies [
5,
24]. For the Cu
6Sn
5 phase in the early stage of L/S reaction (L/S-1 sample) in
Figure 2a, it grew in the form of small granules at the interface, and its average thickness was 2.65 µm. The Cu
6Sn
5 phase in the later stage of L/S reaction (L/S-60 sample) in
Figure 2d grew thicker (6.9 µm in the average thickness) and exhibited a scallop-shaped morphology. Due to the scallop-shaped morphology, the thickness of the Cu
6Sn
5 phase was uneven; some Cu
6Sn
5 scallops grew to be as thick as 15 µm in the direction perpendicular to the interface, while some were 10 µm in the thickness. The Cu
6Sn
5 phase formed at the valleys in between two neighboring scallops was less than 5 µm thick. In the other two samples (L/S-10 and L/S-30), as seen in
Figure 2b,c, the Cu
6Sn
5 phase also grew into a scallop-shaped structure, while the scallop size was smaller.
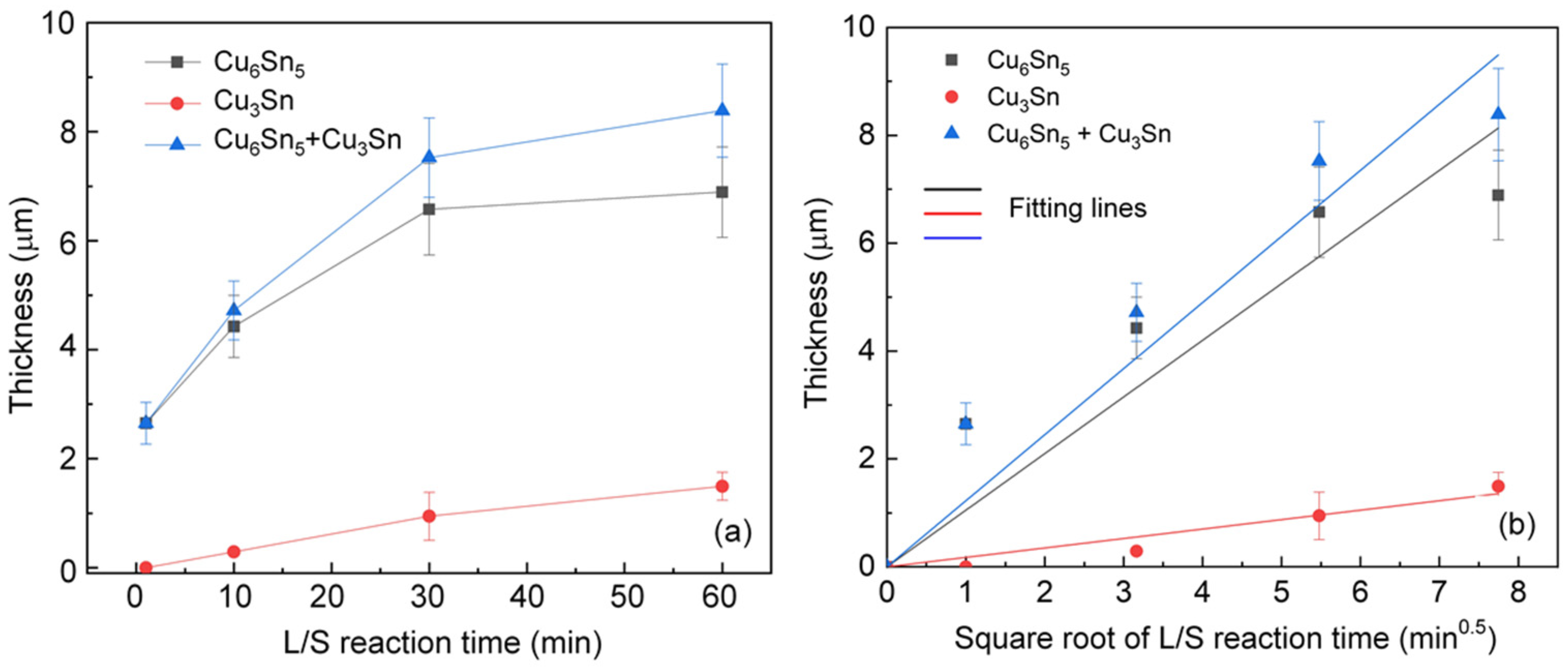
The average thickness of IMCs formed in the L/S joint samples was measured and plotted as a function of the reaction time as shown in
Figure 3a. Overall, the thicknesses of the IMCs were found to increase with increasing reaction time. In general, the thickness of IMC can be formulated as a function of the square root of reaction time by assuming a diffusion-controlled growth mechanism [
20].
where
x,
x0,
k, and
t are the IMC thickness after the reaction, the initial IMC thickness before the reaction, the growth rate constant, and the reaction time, respectively. By plotting the relationship between Δ
x and
t0.5 as shown in
Figure 3b, the growth rate constant (
k) can be determined from the slope of the fitting line according to Equation (1). The values of
k for the IMCs formed in the L/S reactions are listed in
Table 1. It was found that the growth rate constant of Cu
6Sn
5 was 1.05 µm/min
0.5, which was approximately six times larger than that of Cu
3Sn (0.175 µm/min
0.5). The growth of Cu
3Sn was generally governed by the phase transformation described below.
Because the phase transformation involved the solid-state reaction between Cu6Sn5 and Cu, the Cu3Sn phase was expected to have a smaller growth rate constant compared with the Cu6Sn5 phase, which was formed as a result of the liquid/solid reaction.
After the L/S reactions, the four SAC305/Cu joint samples were thermally aged at 200 °C to perform the S/S reaction for various lengths of time. As shown in
Figure 2(a1–d2), Cu
6Sn
5 and Cu
3Sn were observed at the interfaces of the four samples, and both displayed a uniform layer-type structure. The morphological change from the scallop type (L/S reaction) into layer type (S/S reaction) was accomplished by faster atomic diffusion at the scallop valleys, which accelerated the IMC growth there to catch up with the growth front at the scallop peaks, resulting in the planarization of the Cu
6Sn
5 scallops [
18].
Figure 4 depicts the thicknesses of the IMCs in the four samples after S/S reaction for various lengths of time. Both Cu
6Sn
5 and Cu
3Sn grew thicker with increasing reaction time as shown in
Figure 4a,b, respectively, was as did the total IMCs (Cu
6Sn
5 + Cu
3Sn), as shown in
Figure 4c. The thickness data of the IMCs were also plotted as a function of the square root of reaction time, as shown in
Figure 5. The fitting results based on Equation (1) indicated that the growth of all IMCs obeyed the parabolic law, and this growth was diffusion-controlled.
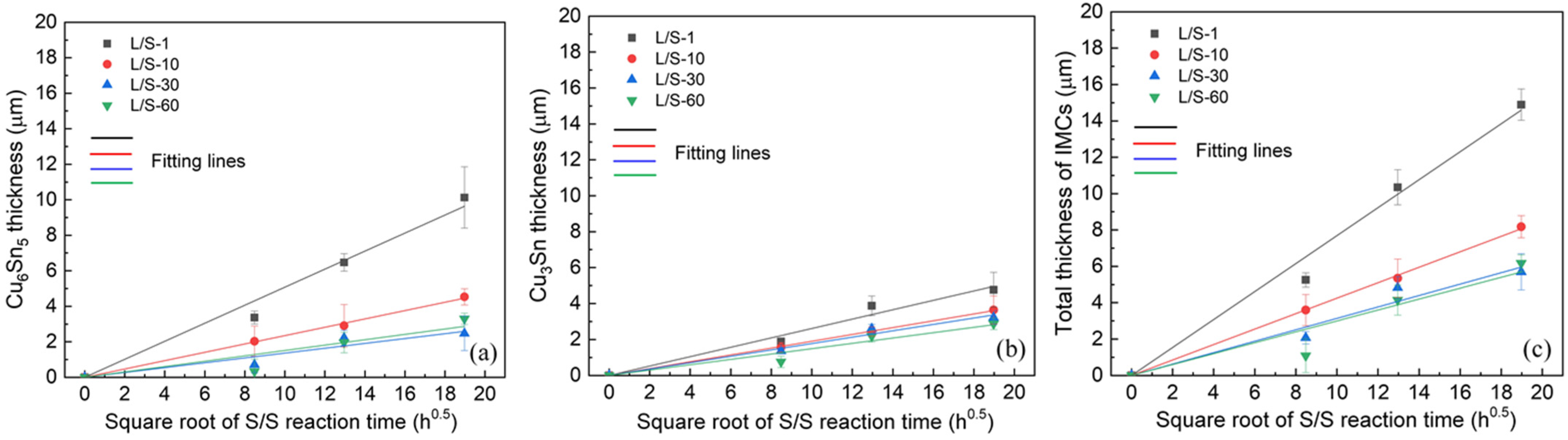
Table 2 lists the magnitudes of
k for the growth of IMCs in the four L/S samples during the S/S reactions. The magnitude of
k for Cu
6Sn
5 formed in the L/S-1 sample was 0.508 µm/h
0.5, but it decreased to 0.235 µm/h
0.5, 0.136 µm/h
0.5, and 0.151 µm/h
0.5 for the L/S-10, L/S-30, and L/S-60 samples, respectively. The decreasing trend of
k was also observed for the Cu
3Sn phase, as well as the total IMCs (Cu
6Sn
5 + Cu
3Sn). This phenomenon indicated that the initial characteristics of IMCs (mainly Cu
6Sn
5) played an important role in the growth behavior of IMCs in the thermal aging process. When the initial morphology of the Cu
6Sn
5 phase was thin and of the layer type (L/S-1), it grew at a faster rate with a higher
k of 0.508 µm/h
0.5. However, the growth of the Cu
6Sn
5 phase with an initial scallop-shaped morphology (L/S-60) was sluggish due to a lower
k of 0.151 µm/h
0.5.
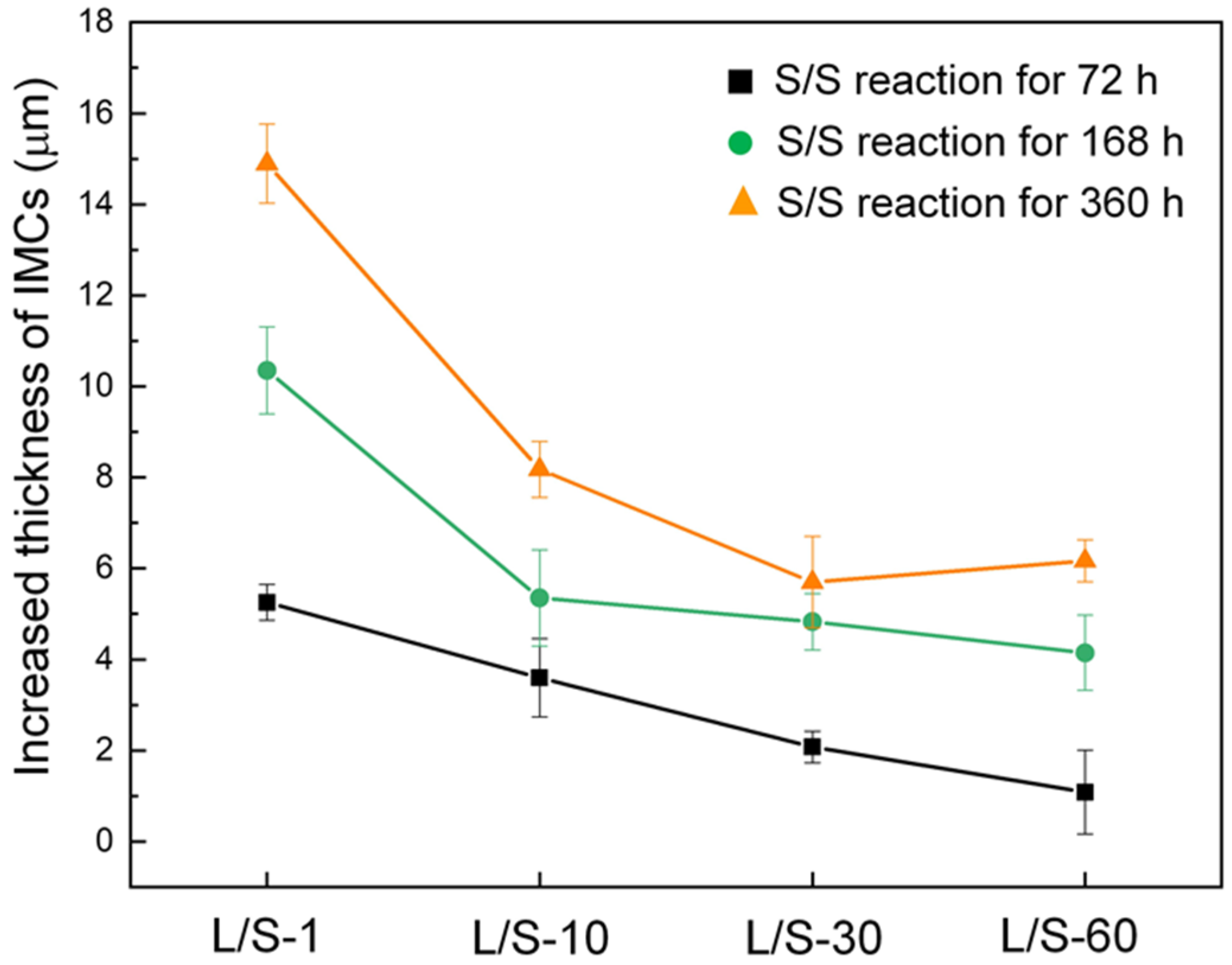
Figure 6 depicts the increment in total thickness of the IMCs for the four L/S samples during the S/S reaction. For the L/S-1 sample, the thickness of IMCs increased by 5 µm, 10 µm, and 15 µm after the S/S reaction for 72 h, 168 h, and 360 h, respectively. The increased thicknesses of IMCs were only 1 µm, 4 µm, and 6 µm for the L/S-60 sample after the same S/S reaction times, which were reduced by 80%, 60%, and 60%, respectively, compared to those in the L/S-1 sample. As mentioned above, the Cu
6Sn
5 phase predominated the growth of the IMCs at the SAC305/Cu interface due to its faster growth rate. For simplicity, only the Cu
6Sn
5 phase was taken into consideration in the discussion of the growth of IMCs. In addition, Sn was considered as the faster diffusion species that controlled the growth of Cu
6Sn
5 at 200 °C [
12]. Therefore, the growth of the IMCs (Cu
6Sn
5) was governed by the atomic diffusion flux, as described by Equation (3).
where
JSn,
DSn,
CSn, and
x are the diffusion flux of Sn, the diffusivity of Sn, the concentration of Sn, and the distance along the diffusion direction, respectively. According to Equation (3), the growth rate of Cu
6Sn
5 is influenced by two parameters; one is the diffusivity of Sn (
DSn), and the other is the concentration gradient (
dCSn/
dx). The latter parameter,
dCSn/
dx, can be calculated for quantitative comparison. Assuming a linear concentration gradient within the Cu
6Sn
5 phase, the magnitude of
dCSn/
dx is equal to Δ
CSn/Δ
x, where Δ
CSn is the concentration difference of Sn at the two boundaries of the Cu
6Sn
5 layer, and Δ
x is the thickness of Cu
6Sn
5. Δ
CSn can be calculated from the homogeneity range information of Cu
6Sn
5 in the Sn–Cu phase diagram with the assumption of a local equilibrium [
25]. At 200 °C, the homogeneity range of Cu
6Sn
5 ranged from 43.4 at.% Sn at the Cu
3Sn/Cu
6Sn
5 interface to 44.3 at.% Sn at the Cu
6Sn
5/Sn interface; thus, Δ
CSn was calculated to be −0.9 at.%, which is a constant independent of the IMC thickness. In the L/S-1 sample, the thickness of initial Cu
6Sn
5 was measured to be 2.65 µm, as shown in
Figure 4a. Then, Δ
CSn/Δ
x was determined to be −0.34 at.%/µm for the initial Cu
6Sn
5 phase prior to the S/S reaction. Following a similar calculation process, the magnitudes of |Δ
CSn/Δ
x| (absolute value) of the Cu
6Sn
5 phase at the different stages of S/S reaction for all L/S reaction samples were determined as listed in
Table 3. Their relationship with the reaction time is plotted in
Figure 7.
As shown in
Figure 7, all L/S samples displayed a decreasing trend of |Δ
CSn/Δ
x| with increasing reaction time due to an increasing Δ
x. As expected, the L/S-1 sample had a larger value of |Δ
CSn/Δ
x| at the initial stage of the S/S reaction due to a smaller Δ
x. A larger value of |Δ
CSn/Δ
x| gave rise to a larger
JSn, which accelerated the growth of Cu
6Sn
5 in the L/S-1 sample (
Figure 4). With increasing S/S reaction time, the thickness of Cu
6Sn
5 increased; hence, the magnitude of |Δ
CSn/Δ
x| decreased. When the reaction time reached 72 h, the |Δ
CSn/Δ
x| of the L/S-1 sample decreased to a level very close to those of the other three samples (L/S-10, L/S-30, and L/S-60) due to the high similarity in the IMC thickness. After the S/S reaction for 168 h and 360 h, the values of |Δ
CSn/Δ
x| for all four L/S samples were very similar to each other, as shown in
Figure 7. It was expected that the growth rates of the IMCs in the four L/S samples would differ very slightly after S/S reaction for 168 h due to the high similarity of |Δ
CSn/Δ
x| and
JSn. However, in the L/S-1 sample, the IMCs continued to grow, and the thickness exceeded that of the other samples after S/S reaction for 168 h, as shown in
Figure 4. The discrepancy can be explained from the perspective of grain microstructures of IMCs.
Figure 8 shows the top-view SEM micrographs of the SAC305/Cu interfaces in the L/S samples subjected to the S/S reaction for various lengths of time. The solder portion was entirely removed using an etching solution (10% HNO
3) to expose the Cu
6Sn
5 grains for three-dimensional observation. Before the S/S reaction, the Cu
6Sn
5 grains in the L/S samples displayed a granular (scallop) microstructure with a smaller grain size in the L/S-1 sample but a much larger grain size in the L/S-60 sample. After the S/S reaction, planarization of the Cu
6Sn
5 grains occurred by means of faster atomic diffusion in the scallop valleys. Grain ripening and growth also occurred, which was evidenced by the presence of some tiny grains surrounding giant grains in both samples. The average grain sizes in the L/S-1 and L/S-60 samples after S/S reaction for 168 h were measured to be 27.2 µm
2 and 54.7 µm
2, respectively, using an image processing software as shown in
Figure 9. The grain size in the L/S-1 sample was smaller than that in the L/S-60 sample, meaning a higher grain boundary quantity in the Cu
6Sn
5 layer of the L/S-1 sample, which might have influenced the atomic diffusion behavior.
The apparent diffusion coefficient (
Dapp) of Sn in the Cu
6Sn
5 layer can be formulated by Equation (4) [
26].
where
Dl and
Db are the volume and grain boundary diffusion coefficients, respectively, and
δ and
d are the grain boundary thickness and grain size of Cu
6Sn
5, respectively. The ratio of
δ/
d is an indicator that can reflect the contribution of grain boundary diffusion to the total atomic diffusion. Because the Cu
6Sn
5 phase in the L/S-1 sample had a smaller grain size (
d) compared to that in the L/S-60 sample, the grain boundary diffusion should have made a greater contribution in the L/S-1 sample than in the L/S-60 sample. As a result, the magnitude of
Dapp was enhanced in the L/S-1 sample, giving rise to an enhanced atomic flux (
JSn), as well as a faster growth rate of the Cu
6Sn
5 phase. In contrast, the grain size (
d) in the L/S-60 sample was larger, which resulted in a smaller size ratio (
δ/
d) and weakened the contribution of grain boundary diffusion.
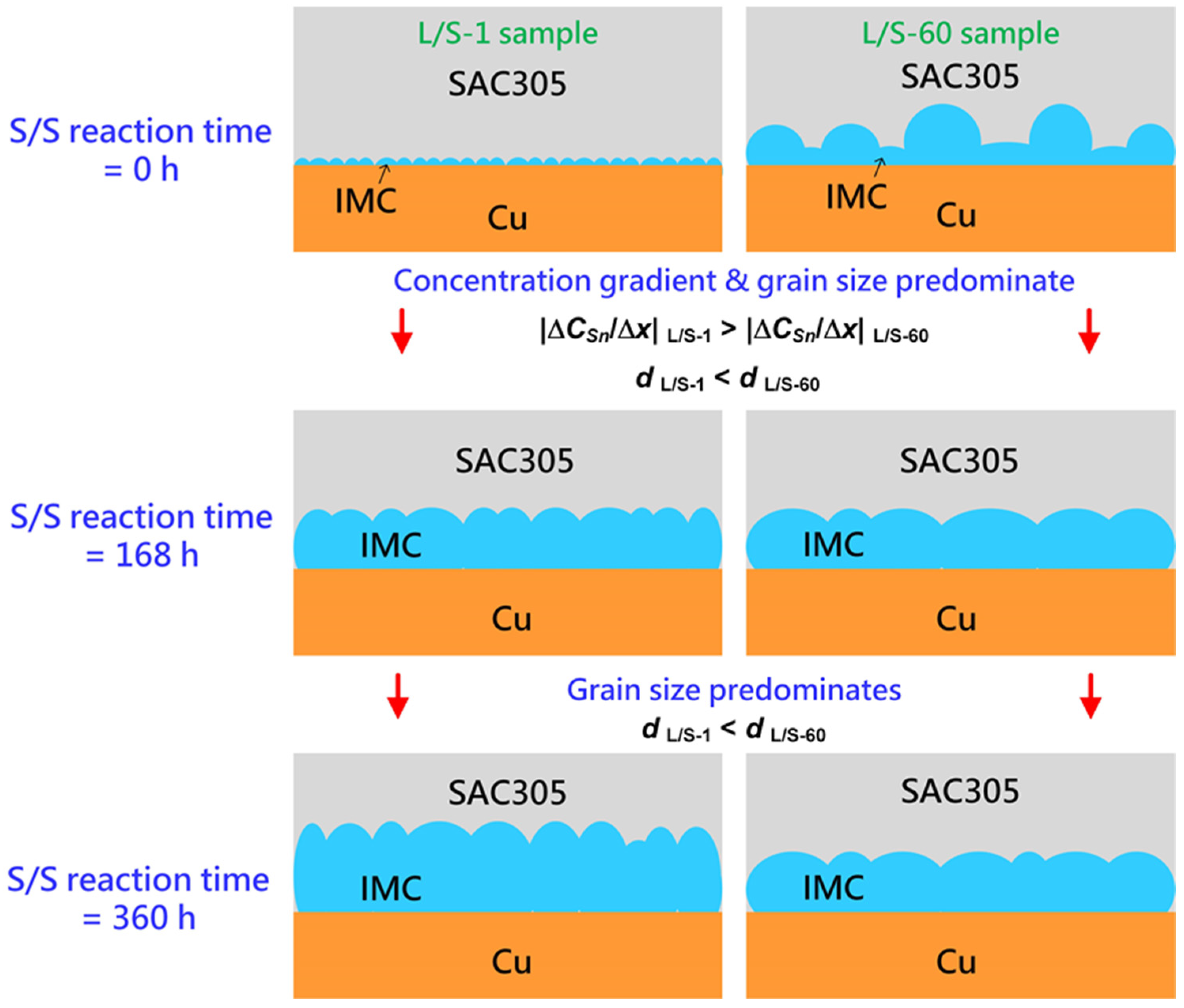
Figure 10 shows a schematic drawing of the IMC growth in the two L/S samples (L/S-1 and L/S-60) which had a very different initial thickness and morphology of IMC (mainly Cu
6Sn
5) before performing the S/S reaction. At the initial stage of the S/S reaction (t = 0 h), the Cu
6Sn
5 phase was thin (2.65 µm) in the L/S-1 sample but was thicker (6.9 µm) in the L/S-60 sample. In addition, the grain size of Cu
6Sn
5 was extremely different in the two samples. The Cu
6Sn
5 grains were much smaller in the L/S-1 sample (4.5 µm
2) than in the L/S-60 sample (46.3 µm
2) as shown in
Figure 9. When the two samples were subjected to thermal aging at 200 °C (S/S reaction), the Cu
6Sn
5 phase continued to grow due to atomic interdiffusion occurring at the SAC305/Cu interface. During the early stage of S/S reaction (t = 0–168 h), the atomic interdiffusion was enhanced in the L/S-1 sample due to a synergy resulting from a higher concentration gradient (|Δ
CSn/Δ
x|) and a higher apparent diffusion coefficient (
Dapp). As a result, the growth rate of Cu
6Sn
5 was enhanced in the L/S-1 sample. Even though the initial Cu
6Sn
5 phase was thin in the L/S-1 sample, it could catch up that of the L/S-60 sample, which had a thicker initial Cu
6Sn
5 phase. By contrast, the growth rate of Cu
6Sn
5 was sluggish in the L/S-60 sample due to a lower concentration gradient (|Δ
CSn/Δ
x|) and a lower apparent diffusion coefficient (
Dapp). In the later stage of S/S reaction (t = 168–360 h), the influence of concentration gradient was similar in the two samples. However, the Cu
6Sn
5 phase still had a smaller grain size in the L/S-1 sample than in the L/S-60 sample, indicating that the apparent diffusion coefficient should have been higher in the L/S-1 sample. Therefore, the Cu
6Sn
5 phase in the L/S-1 sample still grew at a faster rate than that in the L/S-60 sample as seen in
Figure 4a. In summary, the initial characteristics of thickness and morphology significantly influenced the growth kinetics of Cu
6Sn
5 during the S/S reaction. According to the growth kinetics analysis (
Table 2), the growth rate constant of Cu
6Sn
5 was remarkably reduced to 0.151 µm/h
0.5 for the L/S-60 sample, which was a significant reduction of 70% compared to that of the L/S-1 sample (0.508 µm/h
0.5).