Single-Step Process for Titanium Surface Micro- and Nano-Structuring and In Situ Silver Nanoparticles Formation by Ultra-Short Laser Patterning
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florian, C.; Kirner, S.V.; Krüger, J.; Bonse, J. Surface functionalization by laser-induced periodic surface structures. J. Laser Appl. 2020, 32, 022063. [Google Scholar] [CrossRef]
- Sugioka, K. Progress in ultrafast laser processing and future prospects. Nanophotonics 2017, 6, 393–413. [Google Scholar] [CrossRef] [Green Version]
- Vorobyev, A.Y.; Guo, C.L. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Dos Santos Courrol, D.; Borges Lopes, C.R.; da Silva Cordeiro, T.; Franzolin, M.R.; Dias Vieira Junior, N.; Samad, R.E.; Courrol, L.C. Optical properties and antimicrobial effects of silver nanoparticles synthesized by femtosecond laser photoreduction. Opt. Laser Technol. 2018, 103, 233–238. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, C.; Qiu, J.; Yang, Y.; Chen, G. Preparation and optical properties of silver nanoparticles induced by a femtosecond laser irradiation. J. Cryst. Growth 2007, 300, 519–522. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast laser processing of materials: From science to industry. Light Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.C. Wetting behaviour of femtosecond laser textured ti-6al-4v surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Murillo, D.; García-Girón, A.; Romano, J.M.; Cardoso, J.T.; Cordovilla, F.; Walker, M.; Dimov, S.S.; Ocaña, J.L. Wettability modification of laser-fabricated hierarchical surface structures in Ti-6Al-4V titanium alloy. Appl. Surf. Sci. 2019, 463, 838–846. [Google Scholar] [CrossRef]
- Bonse, J.; Koter, R.; Hartelt, M.; Spaltmann, D.; Pentzien, S.; Hohm, S.; Rosenfeld, A.; Kruger, J. Femtosecond laser-induced periodic surface structures on steel and titanium alloy for tribological applications. Appl. Phys. A Mater. Sci. Process. 2014, 117, 103–110. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Mei, X.; Pan, A.; Zhang, Y. Femtosecond laser-induced periodic oxidization of titanium film: Structural colors both in reflection and transmission mode. Opt. Mater. 2020, 109, 110240. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; von Alvensleben, F.; Tunnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundaram, S.K. Ultrafast laser processing of materials: A review. Adv. Opt. Photonics 2015, 7, 684–712. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- Dahmen, C.; von Plessen, G. Optical Effects of Metallic Nanoparticles. Aust. J. Chem. 2007, 60, 447–456. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Hongwei, L.; Colleen, L.N.; Jason, H.H. Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine 2006, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Palermo, G.; Ritacco, T.; Aceti, D.M.; Pezzi, L.; Giocondo, M.; De Luca, A. Photo-thermal effects in 1D gratings of gold nanoparticles. Crystals 2017, 7, 14. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zang, Y.-L.; Wang, J.-N.; Wang, L.; Xia, H.; Chen, Q.-D.; Ding, H.; Sun, H.-B. Controllable assembly of silver nanoparticles induced by femtosecond laser direct writing. Sci. Technol. Adv. Mater. 2015, 16, 024805. [Google Scholar] [CrossRef]
- Barton, P.; Mukherjee, S.; Prabha, J.; Boudouris, B.W.; Pan, L.; Xu, X. Fabrication of silver nanostructures using femtosecond laser-induced photoreduction. Nanotechnology 2017, 28, 505302. [Google Scholar] [CrossRef] [Green Version]
- Toriyama, S.; Mizeikis, V.; Ono, A. Fabrication of silver nano-rings using photo-reduction induced by femtosecond pulses. Appl. Phys. Express 2019, 12, 015004. [Google Scholar] [CrossRef]
- Ritacco, T.; Pagliusi, P.; Giocondo, M. Insight into diffusive and convective processes affecting gold nanoparticles microclustering by multiphoton photoreduction. Colloids Surf. A Phys. Eng. Asp. 2021, 610, 125927. [Google Scholar] [CrossRef]
- Klos, A.; Sedao, X.; Itina, T.E.; Helfenstein-Didier, C.; Donnet, C.; Peyroche, S.; Vico, L.; Guignandon, A.; Dumas, V. Ultrafast Laser Processing of Nanostructured Patterns for the Control of Cell Adhesion and Migration on Titanium Alloy. Nanomaterials 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Zouani, O.F.; Plawinski, L. Human mesenchymal stem cell behavior on femtosecond laser-textured Ti-6Al-4V surfaces. Nanomedicine 2015, 10, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Scharnweber, T.; Pfleging, W.; Besser, H.; Majumdar, J.D. Laser surface textured titanium alloy (Ti–6Al–4V)–Part II–Studies on bio-compatibility. Appl. Surf. Sci. 2015, 357, 750–758. [Google Scholar] [CrossRef]
- Schweitzer, L.; Cunha, A.; Pereira, T.; Mika, K.; Do Rego, A.M.B.; Ferraria, A.M.; Kieburg, H.; Geissler, S.; Uhlmann, E.; Schoon, J. Preclinical in vitro assessment of submicron-scale laser surface texturing on ti6al4v. Materials 2020, 13, 5342. [Google Scholar] [CrossRef]
- Orazi, L.; Maksym, P.; Deineka, V.; Husak, E.; Korniienko, V.; Mishchenko, O.; Reggiani, B. Osteoblast Cell Response to LIPSS-Modified Ti-Implants. Key Eng. Mater. 2019, 813, 322–327. [Google Scholar] [CrossRef]
- Martínez, E. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef]
- Watari, S.; Hayashi, K.; Wood, J.A.; Russell, P.; Nealey, P.F.; Murphy, C.J.; Genetos, D.C. Modulation of osteogenic differentiation in hMSCs cells by submicron topographically-patterned ridges and grooves. Biomaterials 2012, 33, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Helbig, R.; Günther, D.; Friedrichs, J.; Rößler, F.; Lasagni, A.; Werner, C. The impact of structure dimensions on initial bacterial adhesion. Biomater. Sci. 2016, 4, 1074–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowthu, S.; Hoffmann, P. Chapter 3—Versatile micro- and nanotexturing techniques for antibacterial applications. In Functional Nanostructured Interfaces for Environmental and Biomedical Applications, 1st ed.; Dinca, V., Suchea, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–62. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirdeshmukh, N.; Dongre, G. Laser micro & nano surface texturing for enhancing osseointegration and antimicrobial effect of biomaterials: A review. Mater. Today Proc. 2021, 44, 2348–2355. [Google Scholar] [CrossRef]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef]
- Schwibbert, K.; Menzel, F.; Epperlein, N.; Bonse, J.; Krüger, J. Bacterial adhesion on femtosecond laser-modified polyethylene. Materials 2019, 12, 3107. [Google Scholar] [CrossRef] [Green Version]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Mathew, S.; Nayana, A.R.; Mathews, J.; Radhakrishnan, E.K. Microbially and phytofabricated Ag NPs with different mode of bactericidal action were identified to have comparable potential for surface fabrication of central venous catheters to combat Staphylococcus aureus biofilm. J. Photochem. Photobiol. B 2017, 171, 96–103. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, M.; Lee, D.; Han, S.; Moon, J.-H.; Lim, H.-N.; Kwon, I.K. Preparation and characterization of antibacterial orthodontic resin containing silver nanoparticles. Appl. Surf. Sci. 2018, 432, 317–323. [Google Scholar] [CrossRef]
- Mirzaee, M.; Vaezi, M.; Palizdar, Y. Synthesis and characterization of silver doped hydroxyapatite nanocomposite coatings and evaluation of their antibacterial and corrosion resistance properties in simulated body fluid. Mater. Sci. Eng. C 2016, 69, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ghilini, F.; Fagali, N.; Pissinis, D.E.; Benítez, G.; Schilardi, P.L. Multifunctional titanium surfaces for orthopedic implants: Antimicrobial activity and enhanced osseointegration. ACS Appl. Bio Mater. 2021, 4, 6451–6461. [Google Scholar] [CrossRef] [PubMed]
- Arens, D.; Zeiter, S.; Nehrbass, D.; Ranjan, N.; Paulin, T.; Alt, V. Antimicrobial silver-coating for locking plates shows uneventful osteotomy healing and good biocompatibility results of an experimental study in rabbits. Injury 2020, 51, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, X.; Maclean, M.; Qin, Y.; Duxbury, M.; Ding, F. A single-step fabrication approach for development of antimicrobial surfaces. J. Mater. Process. Technol. 2019, 271, 249–260. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Liu, X.; Sun, J. Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface. Int. J. Nanomed. 2012, 7, 875. [Google Scholar] [CrossRef] [Green Version]
- Bui, V.D.; Mwangi, J.W.; Meinshausen, A.K.; Mueller, A.J.; Bertrand, J.; Schubert, A. Antibacterial coating of Ti-6Al-4V surfaces using silver nano-powder mixed electrical discharge machining. Surf. Coat. Technol. 2020, 383, 125254. [Google Scholar] [CrossRef]
- Lin, C.-H.; Jiang, L.; Chai, Y.-H.; Xiao, H.; Chen, S.-J.; Tsai, H.-L. One-Step Fabrication of Nanostructures by Femtosecond Laser for Surface-Enhanced Raman Scattering. Opt. Express 2009, 17, 21581–21589. [Google Scholar] [CrossRef]
- Yin, Z.; Geng, Y.; Xie, Q.; Hong, X.; Tan, X.; Chen, Y.; Wang, L.; Wang, W.; Li, X. Photoreduced silver nanoparticles grown on femtosecond laser ablated D-shaped fiber probe for surface-enhanced Raman scattering. Appl. Opt. 2016, 55, 5408. [Google Scholar] [CrossRef]
- Han, Y.; Liang, Z.; Sun, H.; Xiao, H.; Tsai, H.L. Nanostructured substrate with nanoparticles fabricated by femtosecond laser for surface-enhanced Raman scattering. Appl. Phys. A 2011, 102, 415–419. [Google Scholar] [CrossRef]
- Youfu, G.; Zhen, Y.; Xiaoling, T.; Yu, D.; Xueming, H.; Xuejin, L. Femtosecond Laser Ablated Polymer SERS Fiber Probe with Photoreduced Deposition of Silver Nanoparticles. IEEE Photonics J. 2016, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Hang, R.; Gao, A.; Zhang, X.; Huang, X.; Wang, Y.; Tang, B.; Zhao, L.; Chu, P.K. Nanostructured titanium–silver coatings with good antibacterial activity and cytocompatibility fabricated by one-step magnetron sputtering. Appl. Surf. Sci. 2015, 355, 32–44. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Sharonova, A.; Loza, K.; Surmeneva, M.; Surmenev, R.; Prymak, O.; Epple, M. Synthesis of positively and negatively charged silver nanoparticles and their deposition on the surface of titanium. IOP Conf. Ser. Mater. Sci. Eng. 2016, 116, 012009. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Zhang, H.; Xu, Z.; Zhao, L.; Wang, J.; Qiu, Y.; Liu, B. Improvements on biological and antimicrobial properties of titanium modified by AgNPs-loaded chitosan-heparin polyelectrolyte multilayers. J. Mater. Sci. Mater. Med. 2019, 30, 52. [Google Scholar] [CrossRef]
- MacKenzie, M.; Chi, H.; Varma, M.; Pal, P.; Kar, A.; Paterson, L. Femtosecond laser fabrication of silver nanostructures on glass for surface enhanced Raman spectroscopy. Sci. Rep. 2019, 9, 17058. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wan, Y.; Yu, M.; Wang, H.; Cai, Y.; Liu, C.; Zhang, D. Biocompability evaluation of micro textures coated with zinc oxide on Ti-6Al-4V treated by nanosecond laser. Surf. Coat. Technol. 2021, 422, 127453. [Google Scholar] [CrossRef]
- Flores, C.Y.; Diaz, C.; Rubert, A.; Benítez, G.A.; Moreno, M.S.; Fernández Lorenzo de Mele, M.A.; Salvarezza, R.C.; Schilardi, P.L.; Vericat, C. Spontaneous Adsorption of Silver Nanoparticles on Ti/TiO2 Surfaces. Antibacterial Effect on Pseudomonas Aeruginosa. J. Colloid Interface Sci. 2010, 350, 402–408. [Google Scholar] [CrossRef]
- Landis, E.C.; Phillips, K.C.; Mazur, E.; Friend, C.M. Formation of nanostructured TiO2 by femtosecond laser irradiation of titanium in O2. J. Appl. Phys. 2012, 112, 063108. [Google Scholar] [CrossRef]
- Kwon, Y.-H.; Ossig, R.; Hubenthal, F.; Kronfeldt, H.-D. Influence of surface plasmon resonance wavelength on SERS activity of naturally grown silver nanoparticle ensemble. J. Raman Spec. 2012, 43, 1385–1391. [Google Scholar] [CrossRef]
- Bonse, J.; Kirner, S.V.; Krüger, J. Laser-Induced Periodic Surface Structures (LIPSS). In Handbook of Laser Micro- and Nano-Engineering; Sugioka, K., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Rebollar, E.; Castillejo, M.; Ezquerra, T.A. Laser Induced Periodic Surface Structures on Polymer Films: From Fundamentals to Applications. Eur. Polym. J. 2015, 73, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Schnell, G.; Lund, H.; Bartling, S.; Polley, C.; Riaz, A.; Senz, V.; Springer, A.; Seitz, H. Heat accumulation during femtosecond laser treatment at high repetition rate–A morphological, chemical and crystallographic characterization of self-organized structures on Ti6Al4V. Appl. Surf. Sci. 2021, 570, 151115. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell meets Marangoni—A review of theories on laser-induced periodic surface structures. Laser Photonics Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Oliveira, V.; Ausset, S.; Vilar, R. Surface micro/nanostructuring of titanium under stationary and non-stationary femtosecond laser irradiation. Appl. Surf. Sci. 2009, 255, 7556–7560. [Google Scholar] [CrossRef]
- Koch, J.; Taschner, S.; Suttmann, O.; Kaierle, S. Surface functionalization under water using picosecond and femtosecond laser pulses–first observations and novel effects. Procedia CIRP 2018, 74, 381–385. [Google Scholar] [CrossRef]
- Shen, M.; Carey, J.; Crouch, C.; Kandyla, M.; Stone, H.; Mazur, E. High-density regular arrays of nanometer-scale rods formed on silicon surfaces via femtosecond laser irradiation in water. Nano Lett. 2008, 8, 2087–2091. [Google Scholar] [CrossRef]
- Rivera, L.P.; Munoz-Martin, D.; Chávez-Chávez, A.; Morales, M.; Gómez-Rosas, G.; Molpeceres, C. Subwavelength LIPSS formation on SS304 by picosecond laser irradiation under water confinement. Mater. Sci. Eng. B 2021, 273, 115393. [Google Scholar] [CrossRef]
- Luo, X.; Liu, W.; Chen, C.; Jiang, G.; Hu, X.; Zhang, H.; Zhong, M. Femtosecond laser micro-nano structured Ag SERS substrates with unique sensitivity, uniformity and stability for food safety evaluation. Opt. Laser Technol. 2021, 139, 106969. [Google Scholar] [CrossRef]
- George, J.E.; Unnikrishnan, V.; Mathur, D.; Chidangil, S.; George, S.D. Flexible superhydrophobic SERS substrates fabricated by in situ reduction of Ag on femtosecond laser-written hierarchical surfaces. Sens. Actuators B Chem. 2018, 272, 485–493. [Google Scholar] [CrossRef]
- De Giacomo, A.; Dell’Aglio, M.; Gaudiuso, R.; Koral, C.; Valenza, G. Perspective on the use of nanoparticles to improve libs analytical performance: Nanoparticle enhanced laser induced breakdown spectroscopy (nelibs). J. Anal. Atom. Spectrom. 2016, 31, 1566–1573. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Alrifai, R.; De Giacomo, A. Nanoparticle enhanced laser induced breakdown spectroscopy (nelibs), a first review. Spectrochim. Acta B 2018, 148, 105–112. [Google Scholar] [CrossRef]
- Guglielmelli, A.; Pierini, F.; Tabiryan, N.; Umeton, C.; Bunning, T.J.; De Sio, L. Thermoplasmonics with gold nanoparticles: A new weapon in modern optics and biomedicine. Adv. Photonics Res. 2021, 2, 2000198. [Google Scholar] [CrossRef]
- Zhu, M.; Baffou, G.; Meyerbröker, N.; Polleux, J. Micropatterning thermoplasmonic gold nanoarrays to manipulate cell adhesion. ACS Nano 2012, 6, 7227–7233. [Google Scholar] [CrossRef] [PubMed] [Green Version]

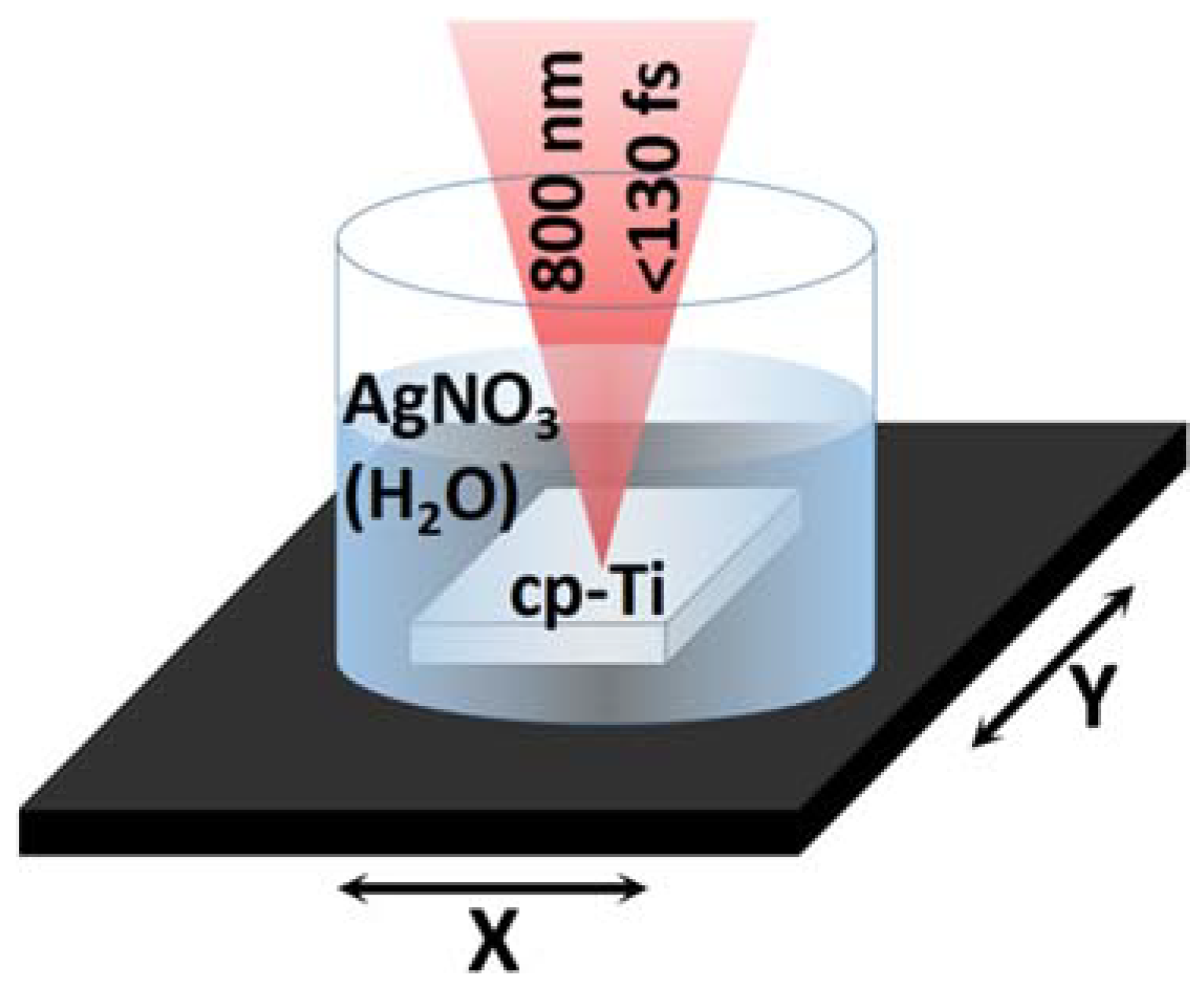
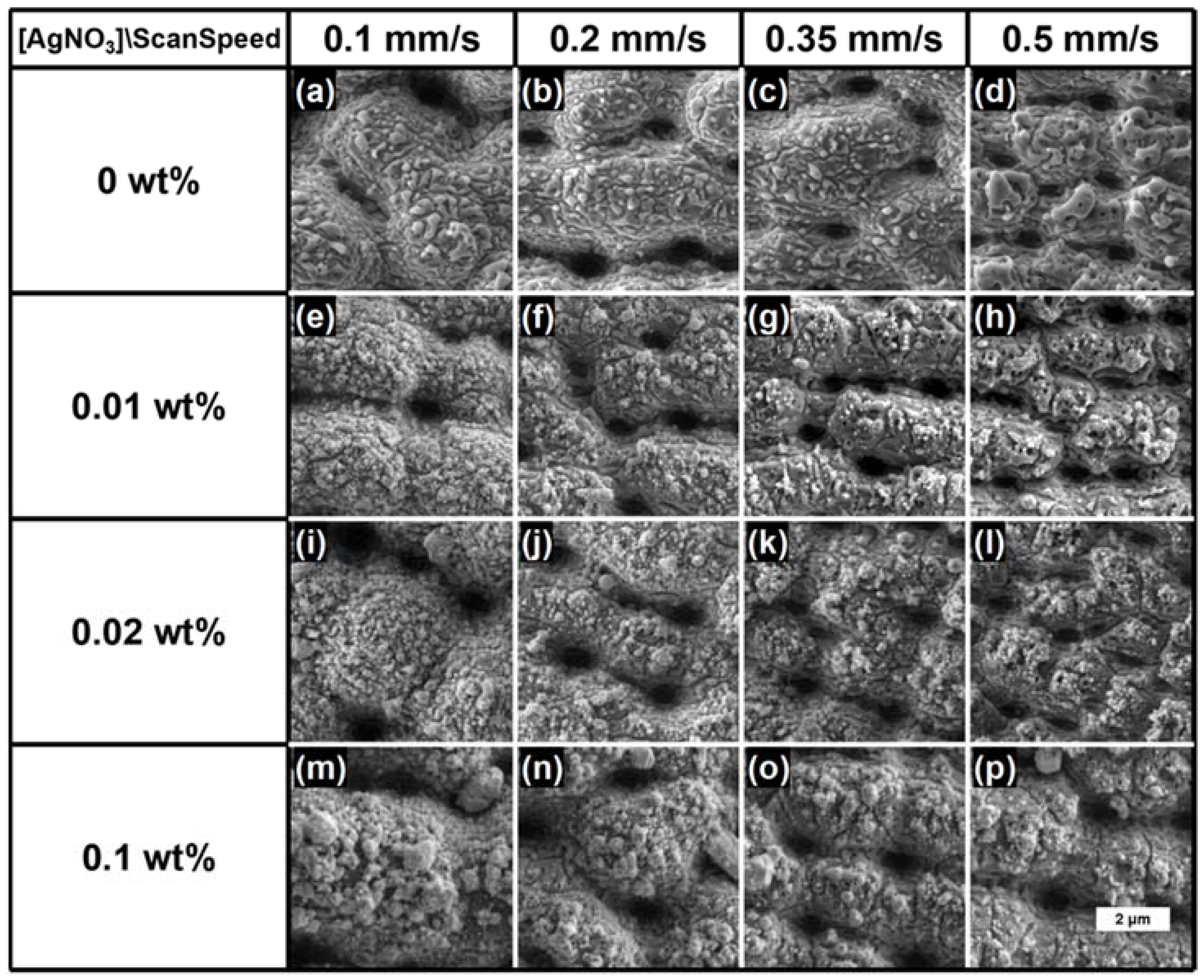



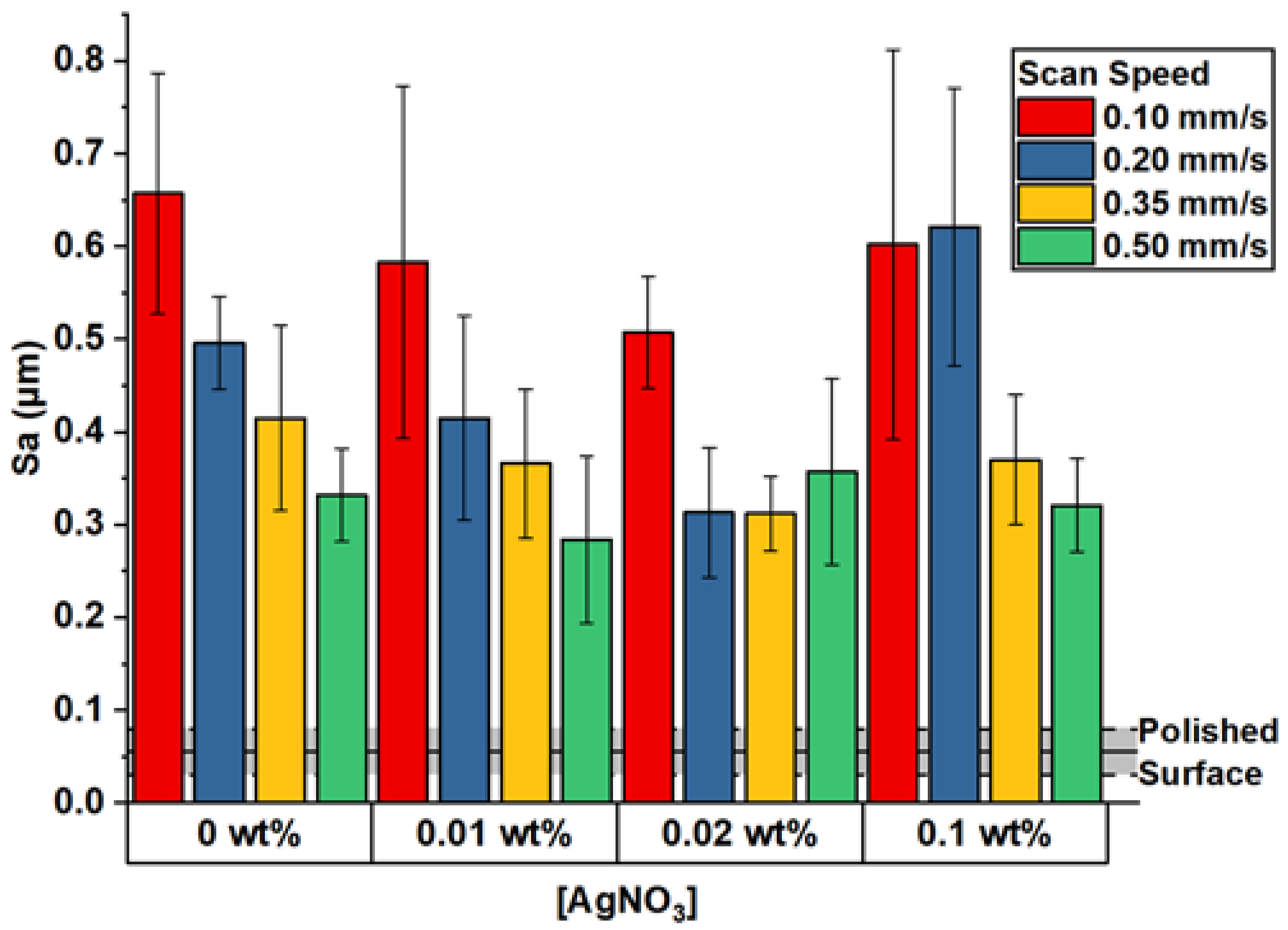
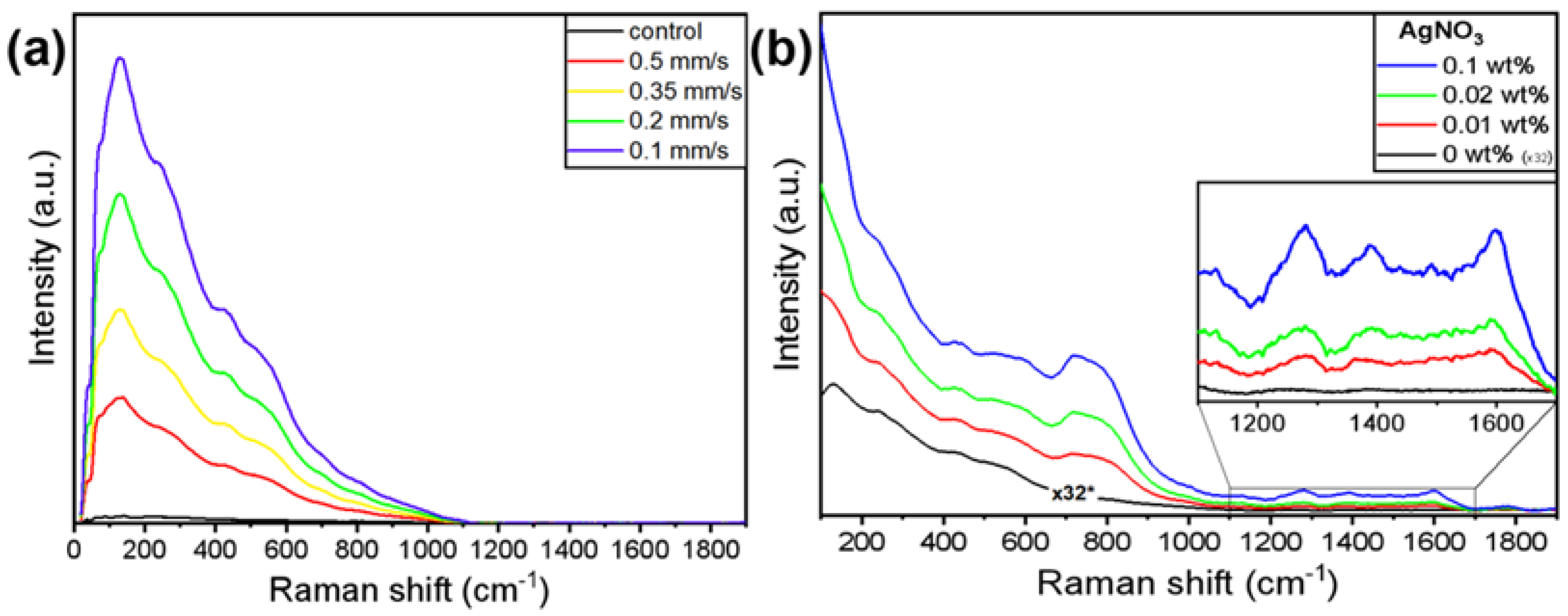
| Scan Speed | Ag_0.01 | Ag_0.02 | Ag_0.1 |
|---|---|---|---|
| (mm/s) | (μm) | (μm) | (μm) |
| 0.10 | 0.50 ± 0.01 | 1.10 ± 0.01 | 2.00 ± 0.01 |
| 0.20 | 0.40 ± 0.01 | 0.60 ± 0.01 | 1.50 ± 0.01 |
| 0.35 | 0.18 ± 0.01 | 0.50 ± 0.01 | 1.20 ± 0.01 |
| 0.50 | 0.12 ± 0.01 | 0.30 ± 0.01 | 1.00 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aceti, D.M.; Filipov, E.; Angelova, L.; Sotelo, L.; Fontanot, T.; Yousefi, P.; Christiansen, S.; Leuchs, G.; Stanimirov, S.; Trifonov, A.; et al. Single-Step Process for Titanium Surface Micro- and Nano-Structuring and In Situ Silver Nanoparticles Formation by Ultra-Short Laser Patterning. Materials 2022, 15, 4670. https://doi.org/10.3390/ma15134670
Aceti DM, Filipov E, Angelova L, Sotelo L, Fontanot T, Yousefi P, Christiansen S, Leuchs G, Stanimirov S, Trifonov A, et al. Single-Step Process for Titanium Surface Micro- and Nano-Structuring and In Situ Silver Nanoparticles Formation by Ultra-Short Laser Patterning. Materials. 2022; 15(13):4670. https://doi.org/10.3390/ma15134670
Chicago/Turabian StyleAceti, Dante Maria, Emil Filipov, Liliya Angelova, Lamborghini Sotelo, Tommaso Fontanot, Peyman Yousefi, Silke Christiansen, Gerd Leuchs, Stanislav Stanimirov, Anton Trifonov, and et al. 2022. "Single-Step Process for Titanium Surface Micro- and Nano-Structuring and In Situ Silver Nanoparticles Formation by Ultra-Short Laser Patterning" Materials 15, no. 13: 4670. https://doi.org/10.3390/ma15134670
APA StyleAceti, D. M., Filipov, E., Angelova, L., Sotelo, L., Fontanot, T., Yousefi, P., Christiansen, S., Leuchs, G., Stanimirov, S., Trifonov, A., Buchvarov, I., & Daskalova, A. (2022). Single-Step Process for Titanium Surface Micro- and Nano-Structuring and In Situ Silver Nanoparticles Formation by Ultra-Short Laser Patterning. Materials, 15(13), 4670. https://doi.org/10.3390/ma15134670








