Abstract
TiO2/Karaya composite was synthesized by the sol-gel method for the photoinactivation of pathogens. This is the first time that we have reported this composite for an antimicrobial approach. The structure, morphology, and optical properties were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-rays (EDS), Fourier transform infrared spectroscopy (FTIR), and diffuse reflectance, and the surface area was characterized by the BET method. The XRD and EDS results showed that the TiO2/Karaya composite was successfully stabilized by the crystal structure and pore diameter distribution, indicating a composite of mesoporous nature. Furthermore, antibacterial experiments showed that the TiO2/Karaya composite under light was able to photoinactivate bacteria. Therefore, the composite is a promising candidate for inhibiting the growth of bacteria.
1. Introduction
With advances in technology and the growing population without adequate planning, the limitless release of residual effluents is directly impacting the environment and represents an eminent concern in environmental management policy [1,2]. In addition, high concentrations of micropollutants, organic/synthetic chemicals, pesticides, heavy metals, and other emerging contaminants are significant concerns for human health and environmental safety [3,4].
Among the emerging pollutants, antibiotics are natural or synthesized substances that act to inhibit bacterial growth. They are formulated to destroy the growth of bacteria and treat infections by their antimicrobial potential [3,5]. However, they present different mechanisms of action and chemical structures that make it challenging to remove them from aquatic matrices, making them persistent, according to the World Health Organization (WHO) [3,6]. Although they have inhibitory functions, antimicrobial contamination favors the emergence of bacteria that are highly resistant to antibiotics [7]. Examples of bacteria include Escherichia coli and Staphylococcus aureus, which differ in terms of their cell walls and cause different infections. The investigation of photocatalysts to combat pathogenic microorganisms through photocatalytic processes has been conducted. This process guarantees a photochemical mechanism that can cause changes in the cell membrane that cause the death of bacteria [8,9,10,11].
Inorganic oxides with antibacterial performance have been essential in the current scenario. The presence of irradiation (visible light, fluorescent lamps, and LED) has the ability to inactivate several pathogenic microorganisms due to reactive oxygen species (ROS). They can promote oxidative stress and, consequently, disrupt the bacterial growth structure through electrostatic interactions, inactivating cellular functions [12,13,14]. Furthermore, the production of ROS (O2•−, HO2, •OH, and H2O2) in the reaction medium occurs through photochemical mechanisms based on electron transfer to form oxygen radicals and/or by direct energy transfer among the photosensitizing materials that produce singlet oxygen (1O2), which is considered toxic to bacterial cells [15,16]. According to Yao et al. [17], ROS generation contributes to optimizing and improving antibacterial performance in chemically modified TiO2 nanoparticles, reinforcing its essential role in cell survival, cell death, cell differentiation, and inflammations.
Due to its effectiveness and attractive properties, such as chemical stability, selectivity, biocompatibility, and non-toxicity, titanium dioxide (TiO2) is highly functional in the degradation of organic pollutants [18], as well as in the treatment of various bacterial pathogens [19,20,21]. TiO2 can be chemically modified through surface strategies (immobilization, deposition, doping, and stabilization) that optimize its deficiencies and facilitate its antibacterial performance [14,22,23]. Yao et al. [17] showed that the heterojunction between nanoparticles (SnSO4-modified TiO2) significantly promoted the inhibition of E. coli and S. aureus. In addition, the enhanced photoinactivation of sulfur-doped TiO2 nanoparticles under visible light to combat the spread of Vibrio cholerae was reported by Tariq et al. [24]. Studies of antibacterial activity showed that the doping of other synthesized membranes with silver nanoparticles significantly increased the inhibition of E. coli bacteria.
Stabilization using natural gums is considered an effective strategy to improve the mobility of charge carriers and light responses [25]. Furthermore, gums are examples of green eco-materials with high availability and low cost [26]. Therefore, they are more efficient in terms of structural changes, as reported by Araujo et al. [25,27,28], who combined oxides to degrade synthetic dyes under visible irradiation. This work aimed to synthesize a TiO2/Karaya composite, called GKT, for the photoinactivation of bacteria. This is the first time that we have reported this composite, TiO2/Karaya, for an antimicrobial approach. This study used Gram-positive Staphylococcus aureus ATCC 25923 (SA) and Gram-negative Escherichia coli bacteria ATCC 25922 (EC). The composite was synthesized by the sol-gel method and characterized by XRD, SEM, Diffuse Reflectance, and FTIR. Finally, the mechanistic responses of inactivation and growth against infection-causing pathogens were based on the action of reactive oxygen species (ROS).
2. Materials and Methods
2.1. Materials
The reagents used to synthesize the photocatalysts were Karaya Gum (KG)—Lot SLBP5629V (Aldrich, St. Louis, MO, USA), ethyl alcohol 99.8% (Aldrich), titanium isopropoxide 97% (Aldrich), methylene blue 97% (Dinâmica, Indaiatuba, SP, Brazil), and ultrapure water. For antibacterial tests, the reagents used were Brain Heart Infusion and Mueller Hinton media (HIMEDIA, Mumbai, MA, India) and Mueller Hinton with sodium chloride (IMPEX, Kolkata, WB, India).
2.2. Synthesis of TiO2/Karaya Composite (GKT)
GKT was synthesized by the sol-gel method as previously reported [25,27,29] with some modifications. More detail about this modification is provided in the supplementary materials. A mixture of 2% of Karaya Gum (KG) against the volume of the titanium with 100.0 mL of ethyl alcohol was kept under stirring for 30 min. Then, 6.0 mL of titanium isopropoxide was slowly added to the gum solution under magnetic stirring. After 30 min, 6.0 mL of ultrapure water was slowly added and allowed to stir for a further 30 min. Afterward, the solution was left to rest for 24 h and then dried inside an incubator at 75 °C. Finally, the GKT was calcined at 400 °C.
2.3. Physico-Chemical Characterization
The crystal structure was investigated by X-ray diffraction in a scanning range of 2θ = 3° to 75° using an X-ray diffractometer Shimadzu, model Labx-XDR 6000, with Cu-Kα radiation (λ = 1.5406 Å in the Bragg–Betano configuration. Scanning Electron Microscopy (SEM) using a scanning electron microscope with an electron source by field emission FEG (Field Emission Gun), Quanta FEI 250. Spectroscopy in the Fourier Transform Infrared Region (FTIR) was performed on a Perkin Elmer SPECTRUM 400 (FTIR/FT-NIR) spectrometer with a sweep from 4000 to 400 cm−1. The textural properties of the solids were investigated based on the nitrogen adsorption–desorption isotherms using Quantachrome (Autosorb-iQ Instruments) results. The surface area, pore volume, and diameter were calculated using the Brunauer–Emmett–Teller (BET) method based on N2 adsorption–desorption. The material’s bandgap (Eg) was determined using a Shimadzu spectrophotometer Model UV-3600 with a diffuse reflectance accessory monitoring the region of 200 to 800 nm and was calculated through a series of mathematical transformations proposed in the Kubelka–Munk method.
2.4. Photoinactivation of Bacteria
Standard strains of Gram-positive Staphylococcus aureus ATCC 25923 (SA) and Gram-negative Escherichia coli ATCC 25922 (EC) were used to perform the antibacterial activity test. Both were provided by the Microbiology Research Laboratory of the Federal University of Piauí. The bacterial cultures were obtained by transferring the inoculum from the bacterial growth stock to Brain Heart Infusion (BHI) medium and incubated at 37 °C for 24 h. After the incubation period, the inoculum suspension was standardized in saline solution at 1.5 × 108 colony-forming units per mL (CFU/mL). After preparing the standard inoculum, 1 mL of this suspension was added to Eppendorf containing 10 mg of the TiO2/Karaya composite [30]. Then, the Eppendorf containing the suspension with the material was separated into two groups, under darkness and under light. Irradiation occurred for 2 h under a UV-Vis light source–neutral white LED system (4000–4500 K) LK1230_M001. The irradiation process took place both for the solution containing the bacterial strains and TiO2/Karaya, and for the solution containing only the bacterial strains. The control and the samples were left in the dark to compare process efficiency. Finally, 100 μL of the solutions was transferred to Petri dishes containing Mueller Hinton agar medium and seeded with a loop of Drigalski. Bacterial viability was determined by a direct contact test in a solid medium [31]. Subsequently, the antimicrobial activity followed the same methodology of the experiments carried out under darkness with incubation at 37 °C for 24 h [11].
The inactivation of bacteria by each test solution was calculated by Equation (1).
where ƞ is defined as the inactivation of bacteria, N1 is the arithmetic average of the number of CFU of the control plates, and N2 is the arithmetic average of the number of CFU of each tested solution. The method described was carried out individually for each proposed strain. Figure 1 displays the scheme of the methodology for the photoinactivation tests.
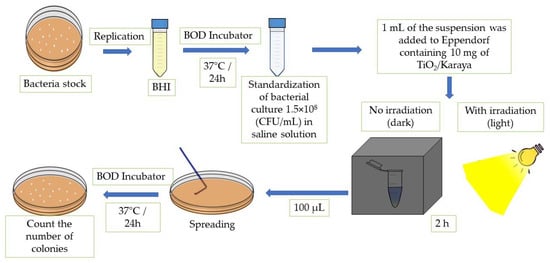
Figure 1.
Scheme of the methodology for the photoinactivation tests.
3. Results and Discussion
3.1. Physico-Chemical Characterization
Figure 2 shows the XRD pattern for the GKT samples. The diffraction peaks at 25°, 38°, 48°, 54°, 55°, 63°, 69°, and 70°, indexed to the (101), (004), (200), (105), (211), (204), (116), and (220) planes, respectively, belonged to the anatase phase of TiO2 and were identified by the Crystallographic Open Database (reference code: 96-101-0943), while the emerging peak at 31° was associated with the brookite (121) phase (reference code: 96-900-4138). For the compound synthesized from natural reagents with a calcination temperature of 400°, the XRD pattern suggested the formation of a crystalline structure due to the narrow and well-defined peaks’ presence [32,33,34,35,36]. Using the Williamson and Hall equation, the average crystallite size (D) and lattice strain (ε) were calculated for the majority anatase phase. The D and ε values were 10 nm and 1.65 %, respectively, being close to the reported in the literature for TiO2 synthesized by the sol-gel method under other conditions [37].
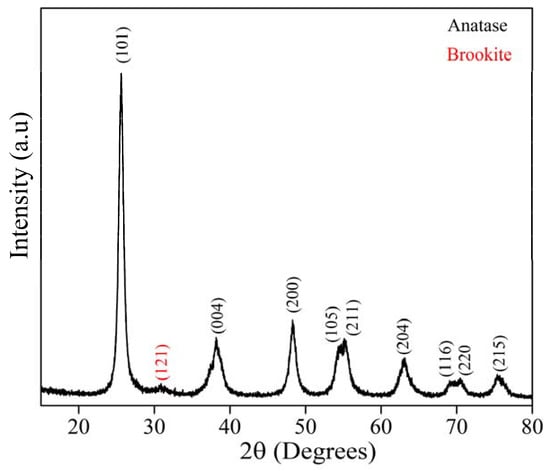
Figure 2.
XRD pattern of TiO2 synthesized in the presence of Karaya Gum calcinated at 400 °C.
The functional groups present in the GKT were investigated by FTIR, as shown in Figure 3a. The band between 3100 and 3500 cm−1 was attributed to the bond vibration of the stretching of the hydroxyl groups [36,38]. The deformation vibrations of the hydroxyl group are present around 1620 cm−1 [39,40,41]. Studies have reported that the bands between 900 and 400 cm−1 are the characteristic bands of TiO2 [40,42,43]. The UV–Vis reflectance value was used to determine the optical band gap energy (Eg) for the TiO2/Karaya composite. Eg was determined by extrapolating the linear part of the plot of (αhν)2 versus hν, as shown in Figure 3b. The reflectance spectrum is shown in the supplementary material (Figure S1).

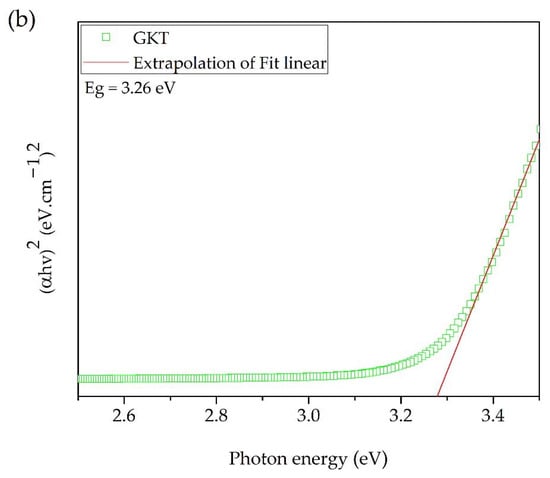
Figure 3.
(a) FTIR spectrum and (b) bandgap value (Eg) of GKT determined according to the Kubelka–Munk method.
The nitrogen adoption–desorption isotherms of the GKT are shown in Figure 4a,b. The isotherm was similar to the IUPAC type-IV classification, which indicated the existence of a mesoporous structure [44,45,46]. Table 1 shows the surface area, pore volume, and pore size of the GKT. The pore diameter had an approximate value of 5 nm. Figure 4b displays the distribution of pore diameters between 2–50 nm, confirming the classification of mesoporous materials. GKT had a surface area of 38 g m2 g−1. This information is shown in Table 1.

Figure 4.
(a) N2 adsorption–desorption isotherm curve; (b) pore size distribution of GKT.

Table 1.
BET parameters of TiO2/Karaya composite.
The morphological analysis results are shown in Figure 5a,b,d. Both images demonstrate the spherical shape of the nanoparticles, and the average diameter of the composite was 0.128 μm ± 0.05. In addition, it was observed that the sample exhibited small agglomerates. The EDS analysis results are shown in Figure 5c. The peaks referring to TiO2 demonstrate that the composite was synthesized with success and indicate the purity of the material. The peak indicating C refers to the double-sided tape used for the deposition and fixation of the material. Figure 5d shows the Ti and O particles present in the GKT, with Ti represented by the color red and O represented by cyan.
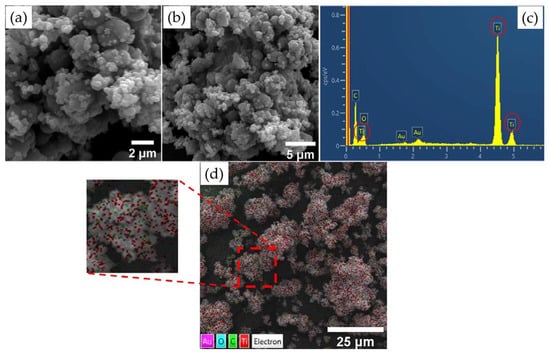
Figure 5.
GKT: (a) SEM and semi-quantitative analysis at a scale of 5 µm, (b) SEM and semi-quantitative analysis at a scale of 2 µm, (c) EDS mapping, (d) and elemental mapping.
3.2. Photoinactivation of Bacteria
Figure 6 shows the antibacterial activity of the TiO2/Karaya composite against Gram-positive and Gram-negative bacteria. GKT presented an antibacterial inhibitory effect of around 20% for S. aureus strains and 29% for E. coli strains. The antibacterial activity results demonstrate the inhibitory effect under light. The inactivation of S aureus strain bacteria could be explained by the oxidative action of the peptidoglycan layer and the E. coli strain by lipid peroxidation. Both produced reactive oxygen species when GKT was irradiated under light [47]. The TiO2/Karaya composite exhibited photoinactivation of around 40% against S. aureus and 70% against E. coli. The production of radicals is based on the photocatalytic process.

Figure 6.
Inactivation of TiO2/Karaya against Gram-positive and Gram-negative bacteria under darkness and under light.
Photocatalysis occurs when a semiconductor is irradiated with enough energy to promote the electron (e−) from the valence band (VB) to the conduction band (CB). This process promotes electrons (e−) to the conduction band and generates a hole (h+) in the valence band (Equation (2)). The hole produces very positive potentials that form hydroxyl radicals from the reaction with water (Equation (3)). The e− reacts with the O2 adsorbed in water to form O2− radicals (Equation (4)). H+, e−, and OH are the main ROS responsible for bacterial photoinactivation and the degradation of organic pollutants (Equation (5)) [3,48,49]. The degradation of methylene blue proved the formation of ROS, which were responsible for the photoinactivation of bacteria. The model molecule’s kinetic photodegradation is displayed in the supplementary material (Figure S2).
ROS are responsible for bacterial inactivation by oxidizing membranes, proteins, lipids, and genetic material [50]. However, Gram-negative and Gram-positive strains have structural differences in their cell walls. Gram-positive strains have multiple layers of peptidoglycan, resulting in a thicker layer populated with lipoic and teichoic acids. On the other hand, Gram-negative bacteria have a polysaccharide membrane above the peptidoglycan layer [47,51]. Upon semiconductor activation, the ROS oxidize the peptidoglycan layer and the polysaccharide layer, facilitating the reduction in cell viability in the system [52,53]. Cell membrane destruction can also occur through the interaction between microbes and particles from the deposition of bacteria on the semiconductor agglomerate [30].
The TiO2/Karaya composite exhibited the anatase phase of the titanium matrix, as shown in Figure 2. TiO2 has three types of crystal structure: rutile, anatase, and brookite. Among the polymorphic types of TiO2, anatase is the phase that has the best photocatalytic performance [36,54]. The calcination temperature during the synthesis process directly influences the material’s crystal structure. The anatase phase is formed at temperatures ranging between 400 and 700 °C. The FTIR presented in Figure 3 did not present any functional groups different from those of TiO2, indicating that the gum was eliminated in the calcination process. Figure 4a,b demonstrates the formation of a mesoporous material. Depending on the average diameter, pores are classified as micropores (<2 nm), mesopores (2–50 nm), and macropores (>50 nm) [55].
Figure 6 shows the results of the bacterial photoinactivation assay for S. Aureus and E. coli in the presence of light and under dark conditions. The bacteria irradiated by light did not undergo bacterial inactivation, demonstrating that the lamp used did not influence the process of destroying the cell membrane of the bacteria. The inhibitory effect was observed when there was a photocatalyst. Under dark conditions, the bacterial inactivation was around 20% for S. aureus strains and 29% for E. coli strains. The direct interaction between bacteria and GKT showed a better inhibitory effect on S. Aureus bacteria. The thicker peptidoglycan layer offered more excellent resistance to the TiO2/Karaya composite than the thin layer of peptidoglycan in E.coli around the lipopolysaccharide layer (LPS) [52].
Several TiO2-based composites with antibacterial properties under dark conditions have been reported in the literature, as shown in Table 2. The morphology of the material is an essential factor for evaluating its antibacterial potential. TiO2-based nanoparticles (NPs) were previously investigated for the influence of size and their antibacterial properties [30]. Smaller nanoparticles have better antibacterial activity [30,56]. In this study, the average diameter of the composite was 0.128 μm ± 0.05, as illustrated in Figure 4. This indicates that the smaller aggregates influenced the antibacterial approach of the material. Anandgaonker et al. synthesized TIO2 nanoparticles by an electrochemical method varying the current density from 10 mA/cm2 to 14 mA/cm2, resulting in particles with average crystalline sizes of 25 nm and 20 nm, respectively. The samples synthesized with a current of 14 mA/cm2 showed a better antibacterial activity, indicating that smaller crystallite sizes achieve better results [57]. Manjunath et al. synthesized TiO2 nanoparticles with an average crystallite size and average nanoparticle size of 40 nm and 35 nm, respectively. The nanoparticles showed antibacterial activity against Gram-negative (Klebsiella aerogenes, Pseudomonas desmolyticum, and E. Coli) and Gram-positive strains (S. aureus) [42]. Li et al. synthesized TiO2/chitosan composites that exhibited growth reduction of 49.4% and 74.6% in an antibacterial assay with Xanthomonas oryzae pv. Oryzae strain with and without extracellular polysaccharides, respectively [58].

Table 2.
TiO2-based composite, synthesis method, and bacterial strain studied.
Therefore, the TiO2/Karaya composite (GKT) in the presence of light showed promising bacterial photoinactivation results. The results indicate that the TiO2 synthesized with Karaya gum presented antibacterial activity of around 40% for S. aures and 70% for E. coli. ROS oxidized E.coli LPS, quickly destroying the thin peptidoglycan layer. The destruction of the cell wall allowed bacterial inactivation, providing the intracellular oxidation of the bacteria. However, intracellular oxidation occurs by DNA denaturation due to an increased catalyst particle size. The reduction of cell viability will occur due to the leaching of K+ ions and the breakage of protons, directly influencing the cell osmotic activity and the synthesis of adenosine triphosphate (ATP), respectively. Figure 7 summarizes the processes of the photoinactivation of bacteria using GKT as a photocatalyst.
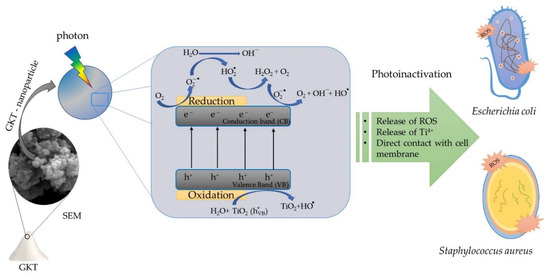
Figure 7.
Photoinactivation of GKT against Gram-positive and Gram-negative bacteria.
4. Conclusions
TiO2/Karaya composite was successfully synthesized by the sol-gel method. The XRD showed the formation of a highly crystalline material exhibiting the anatase phase of TiO2. The characteristic bands from the FTIR spectra indicated the presence of TiO2. SEM showed that the composite was nanoparticles and had agglomerates. The isotherms of nitrogen adsorption and desorption and the average pore size distribution result indicated mesoporous structures. The TiO2/Karaya composite (GKT) presented antibacterial activity under dark and light conditions. The better inactivation of bacteria occurred under light, and the production of ROS can explain why this behavior was able to photo-inactivate bacteria. Therefore, the TiO2/Karaya composite (GKT) is a promising material for antibacterial applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma15134559/s1, Figure S1: Reflectance spectrum of TiO2/Karaya composite (GKT), Figure S2: Photocatalytic degradation of methylene blue.
Author Contributions
A.C.B.L.: methodology and writing—original draft; F.P.A.: conception; L.M.C.H.: writing and editing; A.I.S.M.: conceptualization and visualization; I.S.d.L.: methodology; L.C.A.: formal analysis; R.P.G.: Methodology; E.C.S.-F.: writing—review and editing; M.B.F.: writing—review—and funding; J.A.O.: supervision, project administration, and writing—review. All authors have read and agreed to the published version of the manuscript.
Funding
Convênio UFPI-IFPI 45-2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the CAPES and CNPq agencies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Honorio, L.M.C.; Trigueiro, P.A.; Viana, B.C.; Ribeiro, A.B.; Osajima, J.A. Nanostructured Materials for the Photocatalytic Degradation of Organic Pollutants in Water. In Nanostructured Materials for Treating Aquatic Pollution; Springer: Cham, Switzerland, 2019; pp. 65–90. ISBN 9783030337445. [Google Scholar]
- Brillas, E.; Martínez-Huitle, C.A. Applied Catalysis B: Environmental Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods: An Updated Review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous Removal of Antibiotics and Inactivation of Antibiotic-Resistant Bacteria by Photocatalysis: A Review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Vashisht, D.; Kumar, A.; Mehta, S.K.; Ibhadon, A. Analysis of Emerging Contaminants: A Case Study of the Underground and Drinking Water Samples in Chandigarh, India. Environ. Adv. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic Pollution in Surface Fresh Waters: Occurrence and Effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Kaistha, S.; Das, A.J.; Kumar, R. Antibiotic Resistant Bacteria: A Challenge to Modern Medicine; Springer: Singapore, 2019; ISBN 978-981-13-9878-0. [Google Scholar]
- Liang, X.-L.; Liang, Z.-M.; Wang, S.; Chen, X.-H.; Ruan, Y.; Zhang, Q.-Y.; Zhang, H.-Y. An Analysis of the Mechanism Underlying Photocatalytic Disinfection Based on Integrated Metabolic Networks and Transcriptional Data. J. Environ. Sci. 2020, 92, 28–37. [Google Scholar] [CrossRef]
- Ateia, M.; Alalm, M.G.; Awfa, D.; Johnson, M.S.; Yoshimura, C. Modeling the Degradation and Disinfection of Water Pollutants by Photocatalysts and Composites: A Critical Review. Sci. Total Environ. 2020, 698, 134197. [Google Scholar] [CrossRef]
- Hwangbo, M.; Claycomb, E.C.; Liu, Y.; Alivio, T.E.G.; Banerjee, S.; Chu, K.-H. Effectiveness of Zinc Oxide-Assisted Photocatalysis for Concerned Constituents in Reclaimed Wastewater: 1,4-Dioxane, Trihalomethanes, Antibiotics, Antibiotic Resistant Bacteria (ARB), and Antibiotic Resistance Genes (ARGs). Sci. Total Environ. 2019, 649, 1189–1197. [Google Scholar] [CrossRef]
- Rosendo, F.R.G.V.; Pinto, L.I.F.; de Lima, I.S.; Trigueiro, P.; Honório, L.M.d.C.; Fonseca, M.G.; Silva-Filho, E.C.; Ribeiro, A.B.; Furtini, M.B.; Osajima, J.A. Antimicrobial Efficacy of Building Material Based on ZnO/Palygorskite against Gram-Negative and Gram-Positive Bacteria. Appl. Clay Sci. 2020, 188, 105499. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, S.; Fu, Y.; Wang, Y.; Mi, J.; Lu, T.; Wang, X.; Lü, C. Size-Controllable Preparation and Antibacterial Mechanism of Thermo-Responsive Copolymer-Stabilized Silver Nanoparticles with High Antimicrobial Activity. Mater. Sci. Eng. C 2020, 110, 110735. [Google Scholar] [CrossRef]
- Sharma, G.; Prema, D.; Venkataprasanna, K.S.; Prakash, J.; Sahabuddin, S.; Devanand Venkatasubbu, G. Photo Induced Antibacterial Activity of CeO2/GO against Wound Pathogens. Arab. J. Chem. 2020, 13, 7680–7694. [Google Scholar] [CrossRef]
- Chen, J.; Shan, M.; Shi, X.; Zhang, S.; Li, J.; Luan, J.; Duan, L.; Hou, H. BiSnSbO6–TiO2 Composites Enhance LED Light-Driven Photocatalytic Antibacterial Activity. Ceram. Int. 2022, 48, 19036–19046. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 70, pp. 343–394. ISBN 9780128128343. [Google Scholar]
- Sułek, A.; Pucelik, B.; Kobielusz, M.; Łabuz, P.; Dubin, G.; Dąbrowski, J.M. Surface Modification of Nanocrystalline TiO2 Materials with Sulfonated Porphyrins for Visible Light Antimicrobial Therapy. Catalysts 2019, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, B.; Cui, S.; Yang, S.; Tang, X. Fabrication of SnSO4-Modified TiO2 for Enhance Degradation Performance of Methyl Orange (MO) and Antibacterial Activity. Appl. Surf. Sci. 2021, 551, 149419. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Menazea, A.A.; Awwad, N.S. Antibacterial Activity of TiO2 Doped ZnO Composite Synthesized via Laser Ablation Route for Antimicrobial Application. J. Mater. Res. Technol. 2020, 9, 9434–9441. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Akpeji, B.; Azeez, S.O.; Ayipo, Y.O.; Abdulsalam, Z.A.; Adebayo, Z.F.; Ajao, A.T.; Zakariyah, A.T.; Elemike, E.E. Biosynthesis of Ag and TiO2 Nanoparticles and the Evaluation of Their Antibacterial Activities. Inorg. Chem. Commun. 2022, 141, 109503. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and Antibiofilm Efficacy of Self-Cleaning Surfaces Functionalized by TiO2 Photocatalytic Nanoparticles against Staphylococcus Aureus and Pseudomonas Putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef]
- Yerli-Soylu, N.; Akturk, A.; Kabak, Ö.; Erol-Taygun, M.; Karbancioglu-Guler, F.; Küçükbayrak, S. TiO2 Nanocomposite Ceramics Doped with Silver Nanoparticles for the Photocatalytic Degradation of Methylene Blue and Antibacterial Activity against Escherichia Coli. Eng. Sci. Technol. Int. J. 2022, 101175. [Google Scholar] [CrossRef]
- Dhanalakshmi, R.; Pandikumar, A.; Sujatha, K.; Gunasekaran, P. Photocatalytic and Antimicrobial Activities of Functionalized Silicate Sol–Gel Embedded ZnO–TiO2 Nanocomposite Materials. Mater. Express 2013, 3, 291–300. [Google Scholar] [CrossRef]
- Tariq, F.; Hussain, R.; Noreen, Z.; Javed, A.; Shah, A.; Mahmood, A.; Sajjad, M.; Bokhari, H.; Rahman, S. ur Enhanced Antibacterial Activity of Visible Light Activated Sulfur-Doped TiO2 Nanoparticles against Vibrio Cholerae. Mater. Sci. Semicond. Process. 2022, 147, 106731. [Google Scholar] [CrossRef]
- Araujo, F.P.; Honorio, L.M.C.; Lima, I.S.; Trigueiro, P.; Almeida, L.C.; Fechine, P.B.A.; Santos, F.E.P.; Peña-Garcia, R.; Silva-Filho, E.C.; Osajima, J.A. New Composite TiO2/Naturals Gums for High Efficiency in Photodiscoloration Process. Ceram. Int. 2020, 46, 15534–15543. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Rhim, J.-W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and Characterization of Functional Sodium Caseinate/Guar Gum/TiO2/Cumin Essential Oil Composite Film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.P.; Trigueiro, P.; Honório, L.M.C.; Oliveira, D.M.; Almeida, L.C.; Garcia, R.P.; Lobo, A.O.; Cantanhêde, W.; Silva-Filho, E.C.; Osajima, J.A. Eco-Friendly Synthesis and Photocatalytic Application of Flowers-like ZnO Structures Using Arabic and Karaya Gums. Int. J. Biol. Macromol. 2020, 165, 2813–2822. [Google Scholar] [CrossRef]
- Araujo, F.P.; Trigueiro, P.; Honório, L.M.C.; Furtini, M.B.; Oliveira, D.M.; Almeida, L.C.; Garcia, R.R.P.; Viana, B.C.; Silva-Filho, E.C.; Osajima, J.A. A Novel Green Approach Based on ZnO Nanoparticles and Polysaccharides for Photocatalytic Performance. Dalton Trans. 2020, 49, 16394–16403. [Google Scholar] [CrossRef]
- Morais, A.Í.S.; Oliveira, W.V.; de Oliveira, V.V.; Honorio, L.M.C.; Araujo, F.P.; Bezerra, R.D.S.; Fechine, P.B.A.; Viana, B.C.; Furtini, M.B.; Silva-Filho, E.C.; et al. Semiconductor Supported by Palygorskite and Layered Double Hydroxides Clays to Dye Discoloration in Solution by a Photocatalytic Process. J. Environ. Chem. Eng. 2019, 7, 103431. [Google Scholar] [CrossRef]
- Khater, M.S.; Kulkarni, G.R.; Khater, S.S.; Gholap, H.; Patil, R. Study to Elucidate Effect of Titanium Dioxide Nanoparticles on Bacterial Membrane Potential and Membrane Permeability. Mater. Res. Express 2020, 7, 035005. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Zhu, J.-F. Study on Antimicrobial Activity of Chitosan with Different Molecular Weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Guo, X.-P.; Zang, P.; Li, Y.-M.; Bi, D.-S. TiO2-Powdered Activated Carbon (TiO2/PAC) for Removal and Photocatalytic Properties of 2-Methylisoborneol (2-MIB) in Water. Water 2021, 13, 1622. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Ceylan, A.; Yilmaz, E. Synthesis of Ag and TiO2 Modified Polycaprolactone Electrospun Nanofibers (PCL/TiO2-Ag NFs) as a Multifunctional Material for SERS, Photocatalysis and Antibacterial Applications. Ecotoxicol. Environ. Saf. 2020, 188, 109856. [Google Scholar] [CrossRef]
- Maheswari, P.; Ponnusamy, S.; Harish, S.; Ganesh, M.R.; Hayakawa, Y. Hydrothermal Synthesis of Pure and Bio Modified TiO2: Characterization, Evaluation of Antibacterial Activity against Gram Positive and Gram Negative Bacteria and Anticancer Activity against KB Oral Cancer Cell Line. Arab. J. Chem. 2020, 13, 3484–3497. [Google Scholar] [CrossRef]
- Abdul Razak, K.; Che Halin, D.S.; Abdullah, M.M.A.; Mohd Salleh, M.A.A.; Mahmed, N.; Azani, A.; Chobpattana, V. Factors of Controlling the Formation of Titanium Dioxide (TiO2 ) Synthesized Using Sol-Gel Method—A Short Review. J. Phys. Conf. Ser. 2022, 2169, 012018. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Facile Synthesis of TiO2 Nanoparticles of Different Crystalline Phases and Evaluation of Their Antibacterial Effect Under Dark Conditions Against E. Coli. J. Clust. Sci. 2019, 30, 379–391. [Google Scholar] [CrossRef]
- El-sheikh, S.M.; Zhang, G.; El-hosainy, H.M.; Ismail, A.A.; Shea, K.E.O.; Falaras, P.; Kontos, A.G.; Dionysiou, D.D. High Performance Sulfur, Nitrogen and Carbon Doped Mesoporous Anatase—Brookite TiO2 Photocatalyst for the Removal of Microcystin-LR under Visible Light Irradiation. J. Hazard. Mater. 2014, 280, 723–733. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.; Romero-Toledo, R.; Yahia, E.; Pérez-Larios, A. Synthesis and Characterization of TiO2-ZnO-MgO Mixed Oxide and Their Antibacterial Activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef] [Green Version]
- Aytekin Aydın, M.T.; Hoşgün, H.L.; Dede, A.; Güven, K. Synthesis, Characterization and Antibacterial Activity of Silver-Doped TiO2 Nanotubes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 503–507. [Google Scholar] [CrossRef]
- Jing, F.; Suo, H.; Cui, S.; Tang, X.; Zhang, M.; Shen, X.; Lin, B.; Jiang, G.; Wu, X. Facile Synthesis of TiO2/Ag Composite Aerogel with Excellent Antibacterial Properties. J. Sol-Gel Sci. Technol. 2018, 86, 590–598. [Google Scholar] [CrossRef]
- Iqbal, T.; Farman, S.; Afsheen, S.; Riaz, K.N. Novel Study to Correlate Efficient Photocatalytic Activity of WO3 and Cr Doped TiO2 Leading to Enhance the Shelf-Life of the Apple. Appl. Nanosci. 2022, 12, 87–99. [Google Scholar] [CrossRef]
- Manjunath, K.; Reddy Yadav, L.S.; Jayalakshmi, T.; Reddy, V.; Rajanaika, H.; Nagaraju, G. Ionic Liquid Assisted Hydrothermal Synthesis of TiO2 Nanoparticles: Photocatalytic and Antibacterial Activity. J. Mater. Res. Technol. 2018, 7, 7–13. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 Nanoparticles: Synthesis and Antibacterial Capacity. J. Non-Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Salehi, M.; Eshaghi, A.; Tajizadegan, H. Synthesis and Characterization of TiO2/ZnCr2O4 Core-Shell Structure and Its Photocatalytic and Antibacterial Activity. J. Alloys Compd. 2019, 778, 148–155. [Google Scholar] [CrossRef]
- Miao, G.; Chen, L.; Qi, Z. Facile Synthesis and Active Photocatalysis of Mesoporous and Microporous TiO2 Nanoparticles. Eur. J. Inorg. Chem. 2012, 2012, 5864–5871. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green Synthesis of TiO2 Nanoparticles Using Acorus Calamus Leaf Extract and Evaluating Its Photocatalytic and In Vitro Antimicrobial Activity. Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Gupta, R.; Modak, J. Bacterial Lysis via Photocatalysis—A Critical Mechanistic Review. ChemCatChem 2020, 12, 2148–2170. [Google Scholar] [CrossRef]
- Bodzek, M. Nanoparticles for Water Disinfection by Photocatalysis: A Review. Arch. Environ. Prot. 2022, 48, 3–17. [Google Scholar] [CrossRef]
- Iravani, S. Nanophotocatalysts against Viruses and Antibiotic-Resistant Bacteria: Recent Advances. Crit. Rev. Microbiol. 2022, 48, 67–82. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Evolution of Phthalocyanine Structures as Photodynamic Agents for Bacteria Inactivation. Chem. Rec. 2022, 22, e202100292. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef]
- Thomas-Moore, B.A.; del Valle, C.A.; Field, R.A.; Marín, M.J. Recent Advances in Nanoparticle-Based Targeting Tactics for Antibacterial Photodynamic Therapy. Photochem. Photobiol. Sci. 2022, 1–21. [Google Scholar] [CrossRef]
- Lebedeva, N.S.; Koifman, O.I. Supramolecular Systems Based on Macrocyclic Compounds with Proteins: Application Prospects. Russ. J. Bioorg. Chem. 2022, 48, 1–26. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent Development and Future Prospects of TiO2 Photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO Nanoparticles Immobilized on Clay in Wastewater Treatment: A Review; Springer International Publishing: Cham, Switzerland, 2020; Volume 10, ISBN 0123456789. [Google Scholar]
- Ijaz, M.; Zafar, M.; Islam, A.; Afsheen, S.; Iqbal, T. A Review on Antibacterial Properties of Biologically Synthesized Zinc Oxide Nanostructures. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2815–2826. [Google Scholar] [CrossRef]
- Anandgaonker, P.; Kulkarni, G.; Gaikwad, S.; Rajbhoj, A. Synthesis of TiO2 Nanoparticles by Electrochemical Method and Their Antibacterial Application. Arab. J. Chem. 2019, 12, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhang, Y.; Yang, Y.; Qiu, W.; Wang, X.; Liu, B.; Wang, Y.; Sun, G. Synthesis, Characterization, and Antibacterial Activity of Chitosan/TiO2 Nanocomposite against Xanthomonas Oryzae Pv. Oryzae. Carbohydr. Polym. 2016, 152, 825–831. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).