The Kinetics of the Redox Reaction of Platinum(IV) Ions with Ascorbic Acid in the Presence of Oxygen
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods of Analysis
3. Results and Discussion
3.1. Experimental Conditions
3.2. Spectra of Reagents
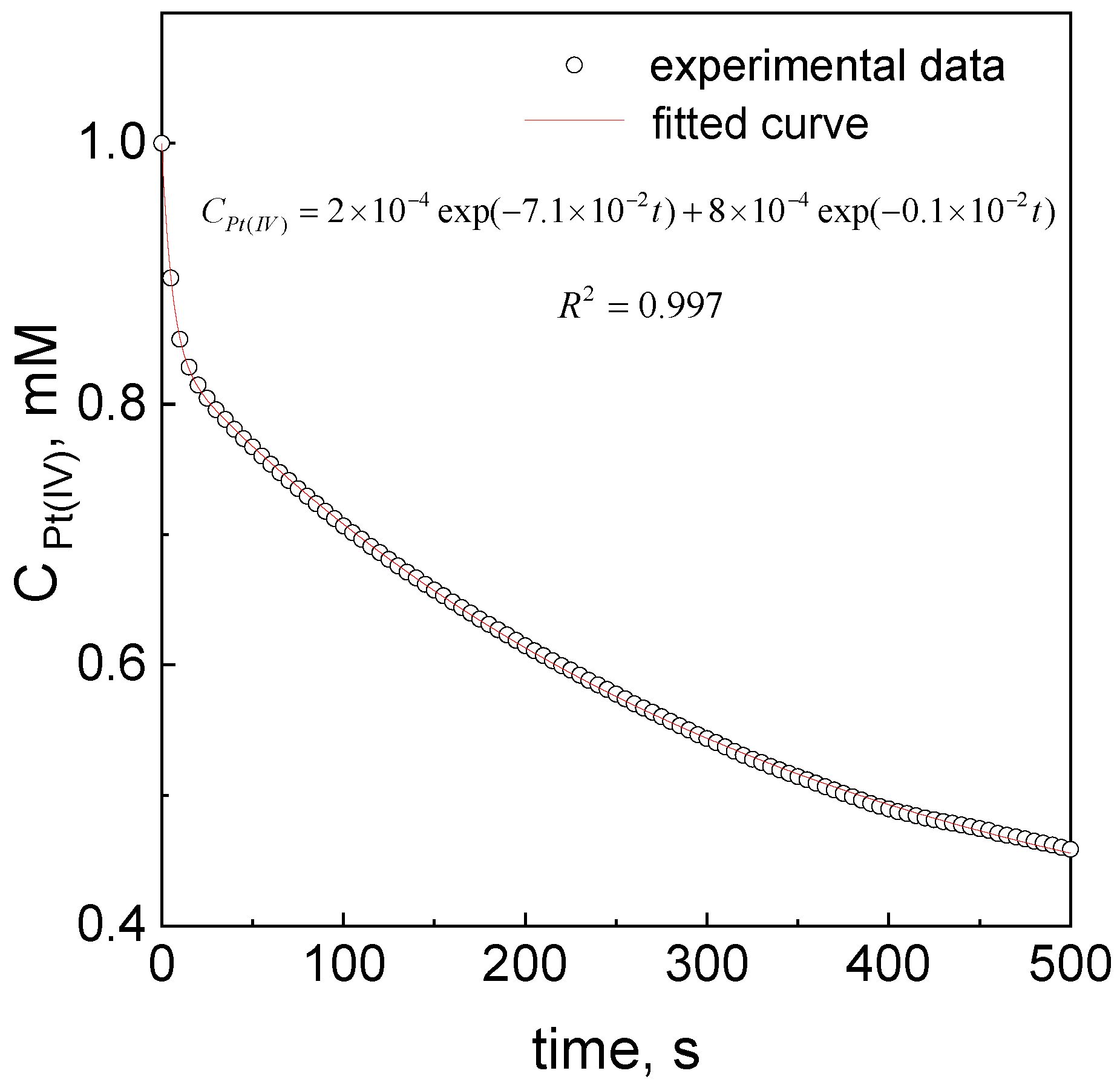
3.3. Kinetic Curves Determination and Proposed Mechanism
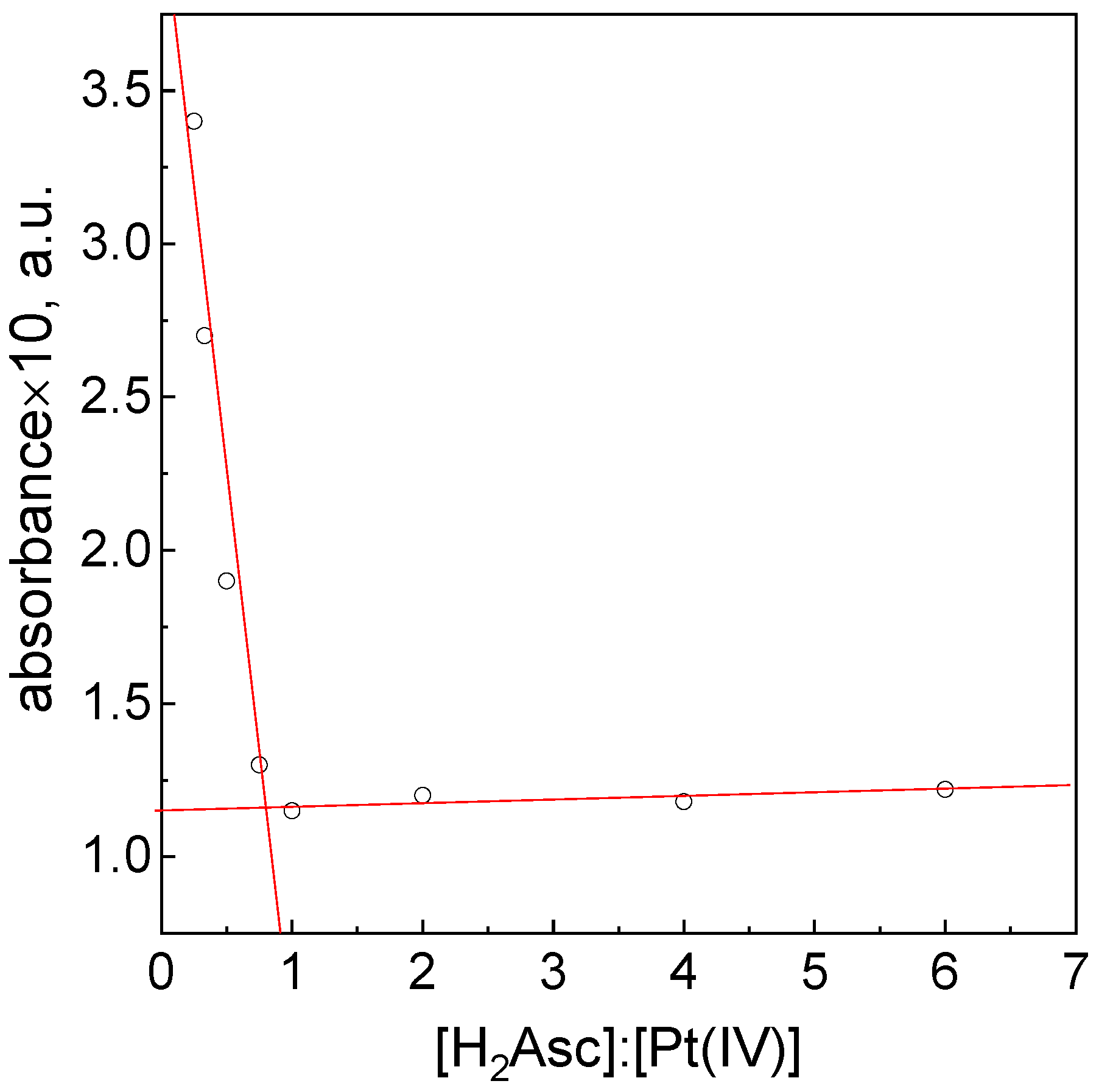
3.4. Stoichiometry
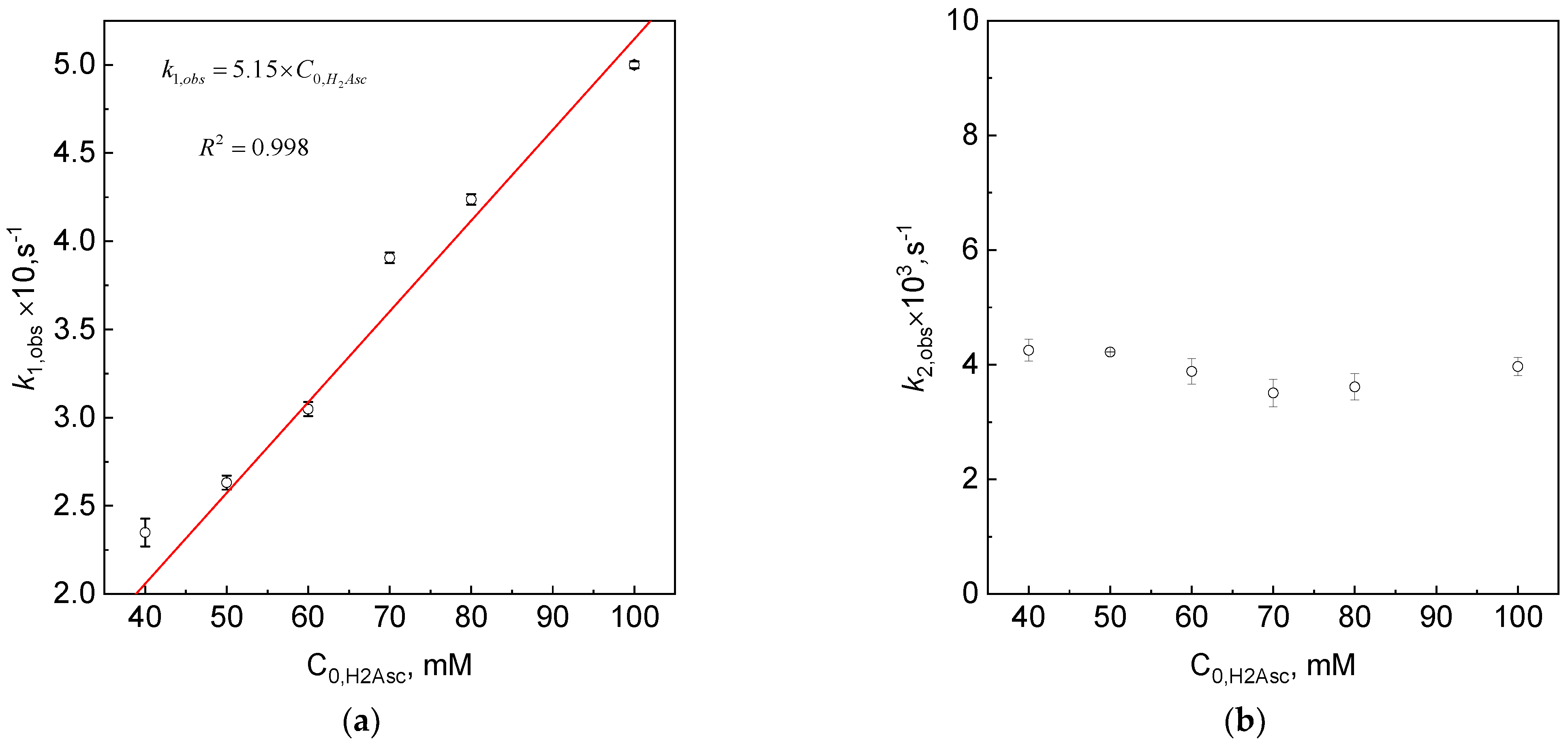
3.5. The Dependency of kobs vs. Initial Concentration of the Reductant
3.6. The Influence of Temperature on Second-Order Rate Constant
3.7. The Influence of pH
3.8. The Influence of Ionic Strength
3.9. The Influence of Chloride Concentration
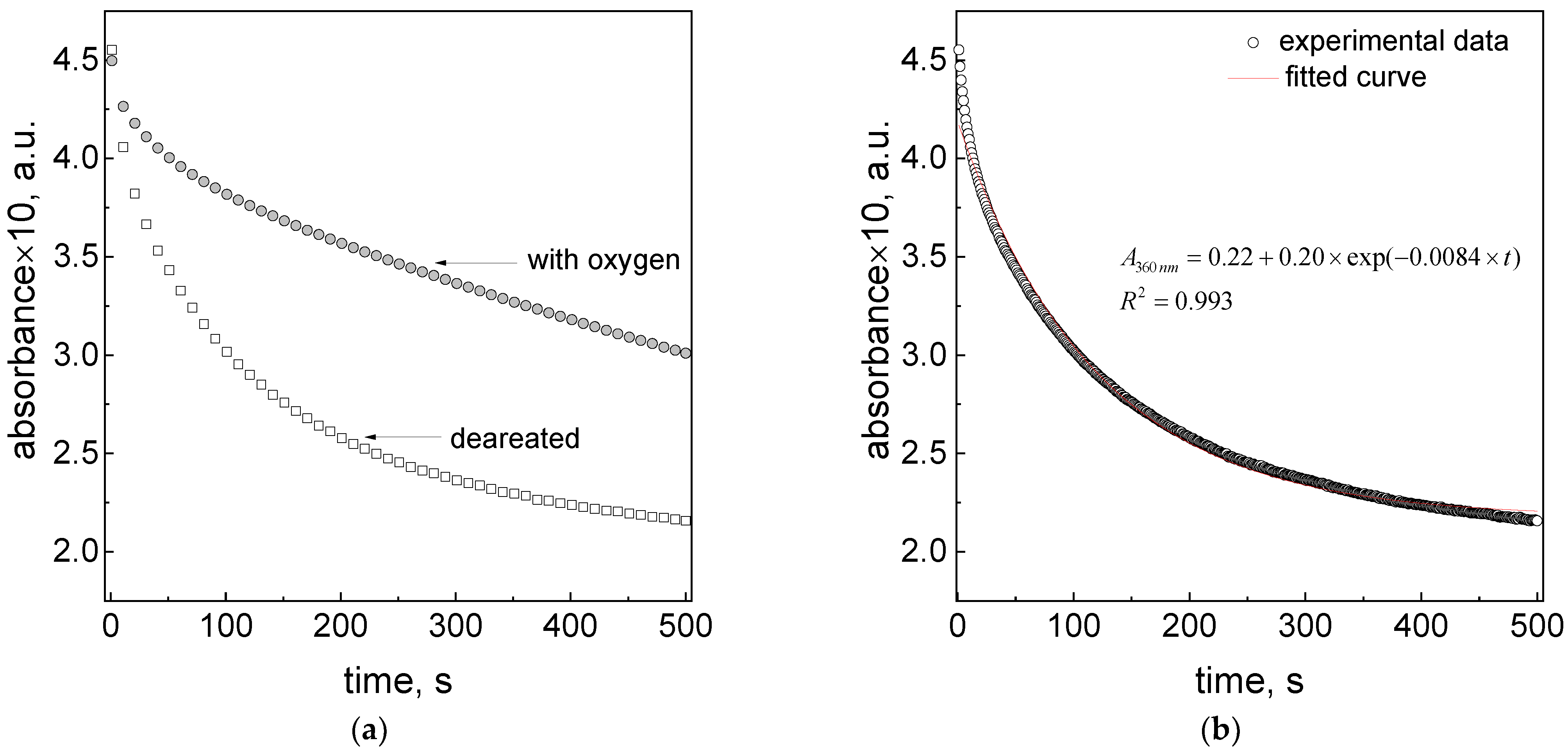
3.10. The Role of Oxygen Dissolved in Aqueous Solution in the Reduction Process
3.11. Mechanism Description
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemma, K.; Berglund, J.; Farrell, N.; Elding, L.I. Kinetics and mechanism for reduction of anticancer-active tetrachloroam(m)ine platinum(IV) compounds by glutathione. J. Biol. Inorg. Chem. 2000, 5, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wojnicki, M.; Żabiński, P.; Csapó, E. Kinetic Studies of Pt(IV) Chloride Complex Ions Reduction Reaction Using Potassium Formate. Arch. Metall. Mater. 2020, 65, 1135–1140. [Google Scholar]
- Jovanović, S.; Petrović, B.; Bugarčić, Ž.D.; van Eldik, R. Reduction of some Pt(iv) complexes with biologically important sulfur-donor ligands. Dalton Trans. 2013, 42, 8890–8896. [Google Scholar] [CrossRef] [PubMed]
- Lemma, K.; Shi, T.; Elding, L.I. Kinetics and Mechanism for Reduction of the Anticancer Prodrug trans,trans,trans-[PtCl2(OH)2(c-C6H11NH2)(NH3)] (JM335) by Thiols. Inorg. Chem. 2000, 39, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Huo, S.; Shen, S.; Xu, J.; Shi, T.; Elding, L.I. Reactivity of the glutathione species towards the reduction of ormaplatin (or tetraplatin). Bioorganic Med. Chem. Lett. 2016, 26, 4261–4266. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, H.; Xu, L.; Zhou, L.; Wang, J.; Xu, B.; Liu, C.; Elding, L.I.; Shi, T. Investigations of the Kinetics and Mechanism of Reduction of a Carboplatin Pt(IV) Prodrug by the Major Small-Molecule Reductants in Human Plasma. Int. J. Mol. Sci. 2019, 20, 5660. [Google Scholar] [CrossRef]
- Lemma, K.; House, D.A.; Retta, N.; Elding, L.I. Kinetics and mechanism for reduction of halo- and haloam(m)ine platinum(IV) complexes by l-ascorbate. Inorg. Chim. Acta 2002, 331, 98–108. [Google Scholar] [CrossRef]
- Whitehead, C.B.; Özkar, S.; Finke, R.G. LaMer’s 1950 Model for Particle Formation of Instantaneous Nucleation and Diffusion-Controlled Growth: A Historical Look at the Model’s Origins, Assumptions, Equations, and Underlying Sulfur Sol Formation Kinetics Data. Chem. Mater. 2019, 31, 7116–7132. [Google Scholar] [CrossRef]
- Wojnicki, M.; Fitzner, K.; Luty-Błocho, M. Kinetic studies of nucleation and growth of palladium nanoparticles. J. Colloid Interface Sci. 2016, 465, 190–199. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Grzonka, J.; Kurzydłowski, K.J.; Fitzner, K. Linking the Gold Nanoparticles Formation Kinetics with Their Morphology. Int. J. Chem. Kinet. 2018, 50, 204–214. [Google Scholar] [CrossRef]
- Streszewski, B.; Jaworski, W.; Pacławski, K.; Csapó, E.; Dékány, I.; Fitzner, K. Gold nanoparticles formation in the aqueous system of gold(III) chloride complex ions and hydrazine sulfate—Kinetic studies. Colloids Surf. A Physicochem. Eng. Asp. 2012, 397, 63–72. [Google Scholar] [CrossRef]
- Pacławski, K.; Streszewski, B.; Jaworski, W.; Luty-Błocho, M.; Fitzner, K. Gold nanoparticles formation via gold(III) chloride complex ions reduction with glucose in the batch and in the flow microreactor systems. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 208–215. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Csapo, E.; Fitzner, K. On the Rate of Interaction of Sodium Borohydride with Platinum (IV) Chloride Complexes in Alkaline Media. Materials 2021, 14, 3137. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate oxidation by iron, copper and reactive oxygen species: Review, model development, and derivation of key rate constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Pacławski, K.; Wojnicki, M.; Fitzner, K. The kinetics of redox reaction of gold(III) chloride complex ions with l-ascorbic acid. Inorg. Chim. Acta 2013, 395, 189–196. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, X.; Jing, J.; Liu, H.; Wu, H.; Yang, W. Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 172–176. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Fitzner, K. Gold Nanoparticles Formation via Au(III) Complex Ions Reduction with l-Ascorbic Acid. Int. J. Chem. Kinet. 2017, 49, 789–797. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Grzonka, J.; Kurzydłowski, K.J. The Synthesis of Stable Platinum Nanoparticles in the Microreactor. Arch. Metall. Mater. 2014, 59, 509–512. [Google Scholar] [CrossRef]
- Tu, Y.-J.; Njus, D.; Schlegel, H.B. A theoretical study of ascorbic acid oxidation and HOO˙/O2˙− radical scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef]
- Wojnicki, M.; Pacławski, K.; Socha, R.P.; Fitzner, K. Adsorption and reduction of platinum(IV) chloride complex ions on activated carbon. Trans. Nonferrous Met. Soc. China 2013, 23, 1147–1156. [Google Scholar] [CrossRef]
- Bao, H.; Bihr, T.; Smith, A.-S.; Klupp Taylor, R.N. Facile colloidal coating of polystyrene nanospheres with tunable gold dendritic patches. Nanoscale 2014, 6, 3954–3966. [Google Scholar] [CrossRef]
- Murray, P.; Koch, K.R.; van Eldik, R. Mechanism of tetrachloroplatinate(ii) oxidation by hydrogen peroxide in hydrochloric acid solution. Dalton Trans. 2014, 43, 6308–6314. [Google Scholar] [CrossRef]
- Granados-Fernández, R.; Montiel, M.A.; Díaz-Abad, S.; Rodrigo, M.A.; Lobato, J. Platinum Recovery Techniques for a Circular Economy. Catalysts 2021, 11, 937. [Google Scholar] [CrossRef]
- El-Mehdawi, R.; Bryan, S.A.; Roundhill, D.M. Axial ligand anation and aquation reactions in diplatinum(III) complexes. Comparison of aquation rates between hexachloroplatinate(IV) anion and diplatinum(III) chloro complexes having.mu.-phosphato or.mu.-pyrophosphito ligands. J. Am. Chem. Soc. 1985, 107, 6282–6286. [Google Scholar] [CrossRef]
- Archibald, E.H. CXXV.—The hydrolysis of platinum salts. Part I. Potassium platinichloride. J. Chem. Soc. Trans. 1920, 117, 1104–1120. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Berdyugin, S.; Komarov, V.; Sheven, D.; Kolesov, B.; Filatov, E.; Tkachev, S. Hydrolysis of [PtCl6]2– in Concentrated NaOH Solutions. Inorg. Chem. 2022, 61, 5926–5942. [Google Scholar] [CrossRef]
- Wickramasinghe, L.A.; Sharp, P.R. Photoreduction of Pt(IV) Halo-Hydroxo Complexes: Possible Hypohalous Acid Elimination. Inorg. Chem. 2014, 53, 1430–1442. [Google Scholar] [CrossRef]
- Wickramasinghe, L.A.; Sharp, P.R. Dihydrogen Trioxide (HOOOH) Photoelimination from a Platinum(IV) Hydroperoxo-Hydroxo Complex. J. Am. Chem. Soc. 2014, 136, 13979–13982. [Google Scholar] [CrossRef]
- Wickramasinghe, L.A.; Sharp, P.R. Hydroxo Radicals, C–H Activation, and Pt–C Bond Formation from 77 K Photolysis of a Platinum(IV) Hydroxo Complex. Inorg. Chem. 2014, 53, 11812–11814. [Google Scholar] [CrossRef]
- Cox, L.E.; Peters, D.G.; Wehry, E.L. Photoaquation of hexachloroplatinate(IV). J. Inorg. Nucl. Chem. 1972, 34, 297–305. [Google Scholar] [CrossRef]
- Jameson, R.F.; Blackburn, N.J. Role of copper dimers and the participation of copper(III) in the copper-catalysed autoxidation of ascorbic acid. Part III. Kinetics and mechanism in 0.100 mol dm–3 potassium chloride. J. Chem. Soc. Dalton Trans. 1976, 16, 1596–1602. [Google Scholar] [CrossRef]
- Cabelli, D.E.; Bielski, B.H.J. Kinetics and mechanism for the oxidation of ascorbic acid/ascorbate by HO2/O2− (hydroperoxyl/superoxide) radicals. A pulse radiolysis and stopped-flow photolysis study. J. Phys. Chem. 1983, 87, 1809–1812. [Google Scholar] [CrossRef]
- Jiang, D.; Li, X.; Liu, L.; Yagnik, G.B.; Zhou, F. Reaction Rates and Mechanism of the Ascorbic Acid Oxidation by Molecular Oxygen Facilitated by Cu(II)-Containing Amyloid-β Complexes and Aggregates. J. Phys. Chem. B 2010, 114, 4896–4903. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J. Reevaluation of the Spectral and Kinetic Properties of HO2 and O2− free radicals. Photochem. Photobiol. 1978, 28, 645–649. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Allen, A.O.; Schwarz, H.A. Mechanism of the disproportionation of ascorbate radicals. J. Am. Chem. Soc. 1981, 103, 3516–3518. [Google Scholar] [CrossRef]
- Spieker, W.A.; Liu, J.; Miller, J.T.; Kropf, A.J.; Regalbuto, J.R. An EXAFS study of the co-ordination chemistry of hydrogen hexachloroplatinate(IV): 1. Speciation in aqueous solution. Appl. Catal. A Gen. 2002, 232, 219–235. [Google Scholar] [CrossRef]
- Senapati, S.; Das, S.P.; Patnaik, A.K. Kinetics and Mechanism of Oxidation of L-Ascorbic Acid by Pt(IV)(aq) in Aqueous Hydrochloric Acid Medium. Adv. Phys. Chem. 2012, 2012, 143734. [Google Scholar] [CrossRef]
- Znakovskaya, I.V.; Sosedova, Y.A.; Glebov, E.M.; Grivin, V.P.; Plyusnin, V.F. Intermediates formed by laser flash photolysis of [PtCl6]2− in aqueous solutions. Photochem. Photobiol. Sci. 2005, 4, 897–902. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Rimstidt, J.D.; Michel, F.M. Kinetic Effect of Surface Chemisorbed Oxygen on Platinum-Catalyzed Hydrogen Peroxide Decomposition. Catal. Lett. 2021, 151, 138–146. [Google Scholar] [CrossRef]
- McKee, D.W. Catalytic decomposition of hydrogen peroxide by metals and alloys of the platinum group. J. Catal. 1969, 14, 355–364. [Google Scholar] [CrossRef]



| Initial Concentration of Reagents | Ionic Strength | T | pH | |
|---|---|---|---|---|
| C0,Pt(IV), mM | C0,H2Asc, M | I, M | K | |
| The stoichiometry | ||||
| C0,H2Asc:C0,Pt(IV) 0.25 0.33 0.5 0.75 1 2 4 6 | 0.10 | 313 | 2.50 | |
| Dependency of kobs vs. initial reductant concentration | ||||
| 1.00 | 0.04 | 0.40 | 313 | 2.50 |
| 0.05 | ||||
| 0.06 | ||||
| 0.07 | ||||
| 0.08 | ||||
| 0.10 | ||||
| The influence of temperature | ||||
| 1.00 | 0.06 | 0.06 | 287 | 2.50 |
| 298 | ||||
| 303 | ||||
| 308 | ||||
| 313 | ||||
| The influence of pH | ||||
| 1.00 | 0.06 | 0.06 | 313 | 2.15 2.50 |
| 3.65 | ||||
| 4.17 | ||||
| 5.04 | ||||
| The influence of ionic strength (addition of NaClO4) on the rate constants | ||||
| 1.00 | 0.06 | 0.00 0.05 0.10 0.20 0.30 0.40 | 313 | 2.50 |
| The influence of chloride ions on the rate constants (at constant value of [Na+] = 0.4 M) | ||||
| 1.00 | 0.06 | 0.4 | 313 | NaCl addition 0.00 0.05 0.10 0.20 0.30 0.40 |
| , mM | s−1 | k1 M−1s−1 | s−1 | M−1s−1 |
|---|---|---|---|---|
| 40 | 23.49 ± 0.79 | 3.91 ± 0.13 | 4.25 ± 0.19 | 7.08 ± 0.32 |
| 50 | 26.32 ± 0.40 | 4.38 ± 0.07 | 4.22 ± 0.04 | 7.04 ± 0.37 |
| 60 | 30.49 ± 0.40 | 5.08 ± 0.07 | 3.89 ± 0.22 | 6.48 ± 0.40 |
| 70 | 39.06 ± 0.32 | 5.51 ± 0.05 | 3.51 ± 0.24 | 5.85 ± 0.40 |
| 80 | 42.37 ± 0.30 | 7.06 ± 0.05 | 3.61 ± 0.23 | 6.02 ± 0.38 |
| 100 | 50.00 ± 0.20 | 8.33 ± 0.03 | 3.97 ± 0.16 | 6.61 ± 0.27 |
| ln A | [M−1s−1] | EA/R |
EA |
|---|---|---|---|
| 21.7 | 26 | 6587 | 54 |
| ΔH/(R) | ΔH± [kJ × mol−1] | (23.77 + Δs/R) | ∆S± [J × K−1 × mol−1] |
|---|---|---|---|
| 6279 | 52 | 15 | −73 |
| pH | s−1 | s−1 |
|---|---|---|
| 2.15 ± 0.05 | 10.86 ± 0.06 | 1.20 ± 0.06 |
| 2.50 ± 0.05 | 16.76 ± 0.22 | 0.66 ± 0.18 |
| 3.65 ± 0.05 | 62.89 ± 1.02 | 62.89± 0.18 |
| 4.17 ± 0.05 | 89.28 ± 0.03 | - |
| 5.04 ± 0.05 | 105.2 ± 0.10 | - |
| I M | s−1 | s−1 |
|---|---|---|
| 0.00 | 17.45 ± 0.06 | 3.30 ± 0.02 |
| 0.05 | 25.00 ± 0.02 | 2.11 ± 0.05 |
| 0.10 | 27.78 ± 0.02 | 2.36 ± 0.03 |
| 0.20 | 29.76 ± 0.02 | 3.44 ± 0.05 |
| 0.30 | 29.82 ± 1.31 | 3.52 ± 0.19 |
| 0.40 | 30.49 ± 0.40 | 3.89 ± 0.22 |
| [Cl−] M | s−1 | k1 M−1s−1 | s−1 | M−1s−1 |
|---|---|---|---|---|
| 0.00 | 30.48 ± 0.40 | 5.08 | 3.89 ± 0.05 | 6.48 |
| 0.05 | 39.06 ± 0.30 | 6.51 | 4.41 ± 0.03 | 7.34 |
| 0.10 | 35.84 ± 0.39 | 5.97 | 4.52 ± 0.05 | 7.54 |
| 0.20 | 37.45 ± 0.10 | 6.24 | 5.21 ± 0.06 | 8.68 |
| 0.30 | 40.32 ± 0.20 | 6.72 | 5.57 ± 0.06 | 9.41 |
| 0.40 | 40.65 ± 0.20 | 6.77 | 5.41 ± 0.10 | 9.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luty-Błocho, M.; Szot, A.; Hessel, V.; Fitzner, K. The Kinetics of the Redox Reaction of Platinum(IV) Ions with Ascorbic Acid in the Presence of Oxygen. Materials 2023, 16, 4630. https://doi.org/10.3390/ma16134630
Luty-Błocho M, Szot A, Hessel V, Fitzner K. The Kinetics of the Redox Reaction of Platinum(IV) Ions with Ascorbic Acid in the Presence of Oxygen. Materials. 2023; 16(13):4630. https://doi.org/10.3390/ma16134630
Chicago/Turabian StyleLuty-Błocho, Magdalena, Aleksandra Szot, Volker Hessel, and Krzysztof Fitzner. 2023. "The Kinetics of the Redox Reaction of Platinum(IV) Ions with Ascorbic Acid in the Presence of Oxygen" Materials 16, no. 13: 4630. https://doi.org/10.3390/ma16134630
APA StyleLuty-Błocho, M., Szot, A., Hessel, V., & Fitzner, K. (2023). The Kinetics of the Redox Reaction of Platinum(IV) Ions with Ascorbic Acid in the Presence of Oxygen. Materials, 16(13), 4630. https://doi.org/10.3390/ma16134630









