A Simple Replica Method as the Way to Obtain a Morphologically and Mechanically Bone-like Iron-Based Biodegradable Material
Abstract
:1. Introduction
2. Materials and Methods
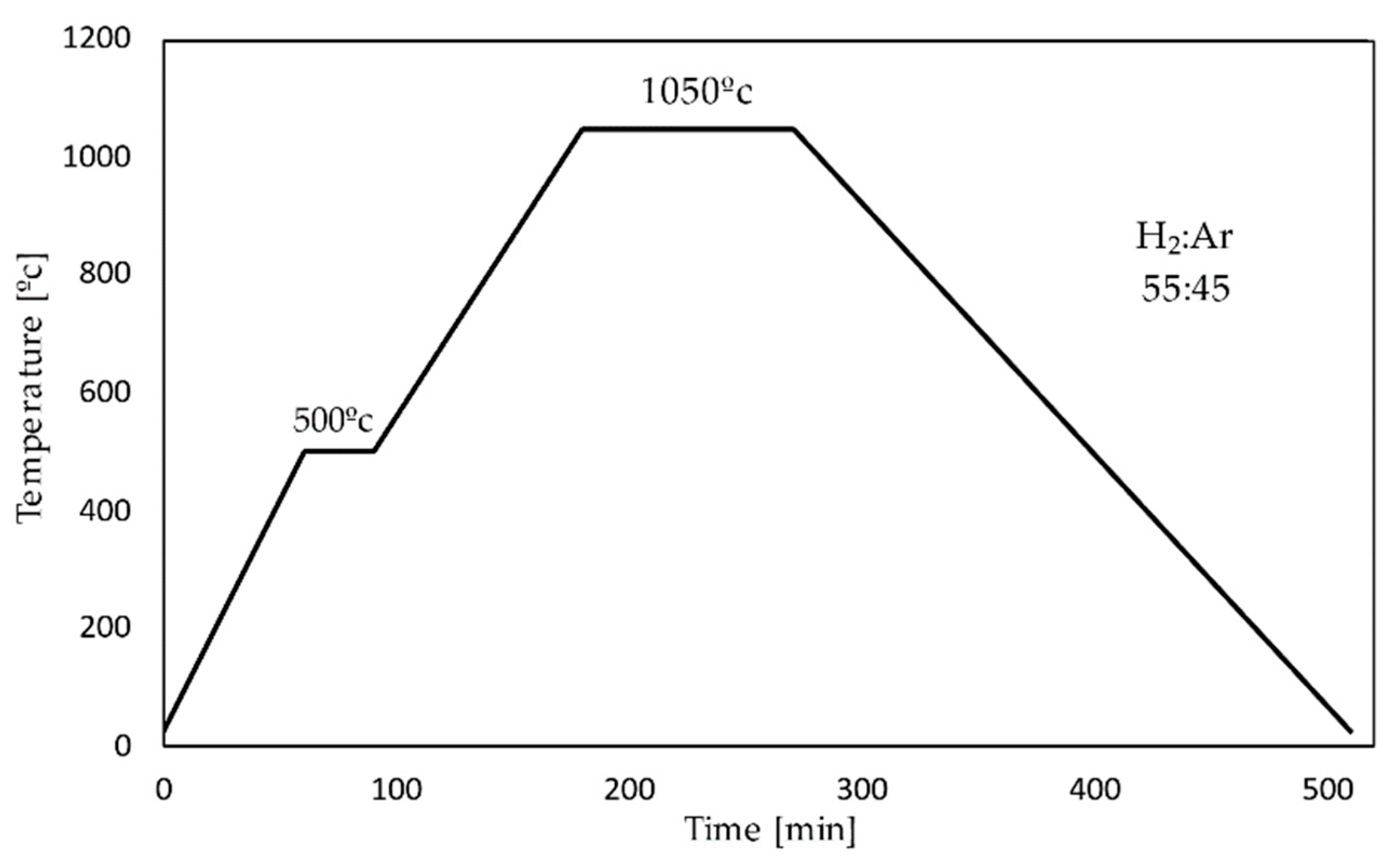
2.1. Fe Scaffolds Preparation
2.2. Fe Scaffolds Characterization
2.3. Immersion Enthalpy and Surface Energy Determination
2.4. Nanomechanical Properties of Fe Scaffolds
2.5. Degradation of Fe Scaffolds
2.6. Electrochemical Degradation of Fe Scaffolds
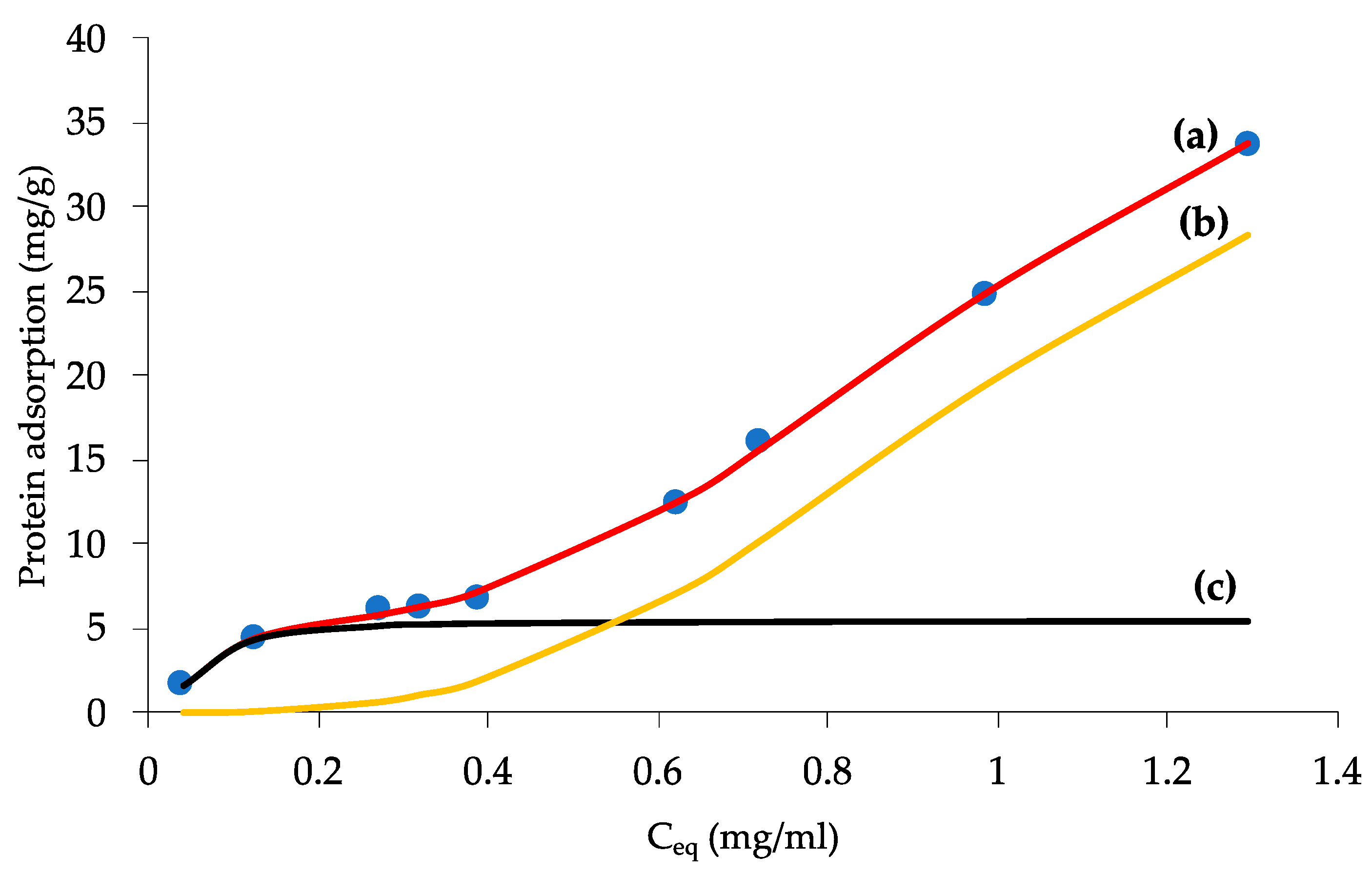
2.7. Albumin Adsorption
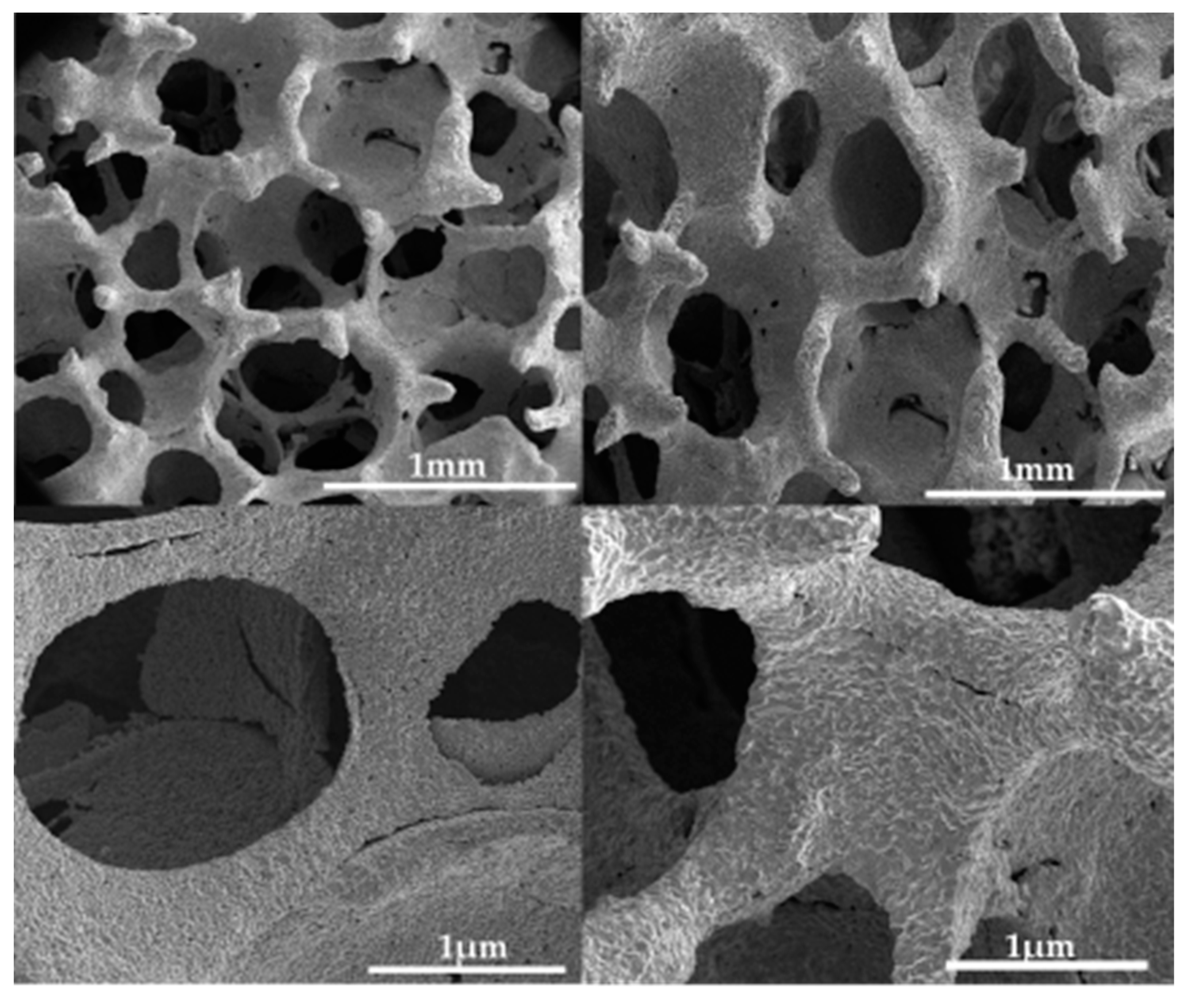
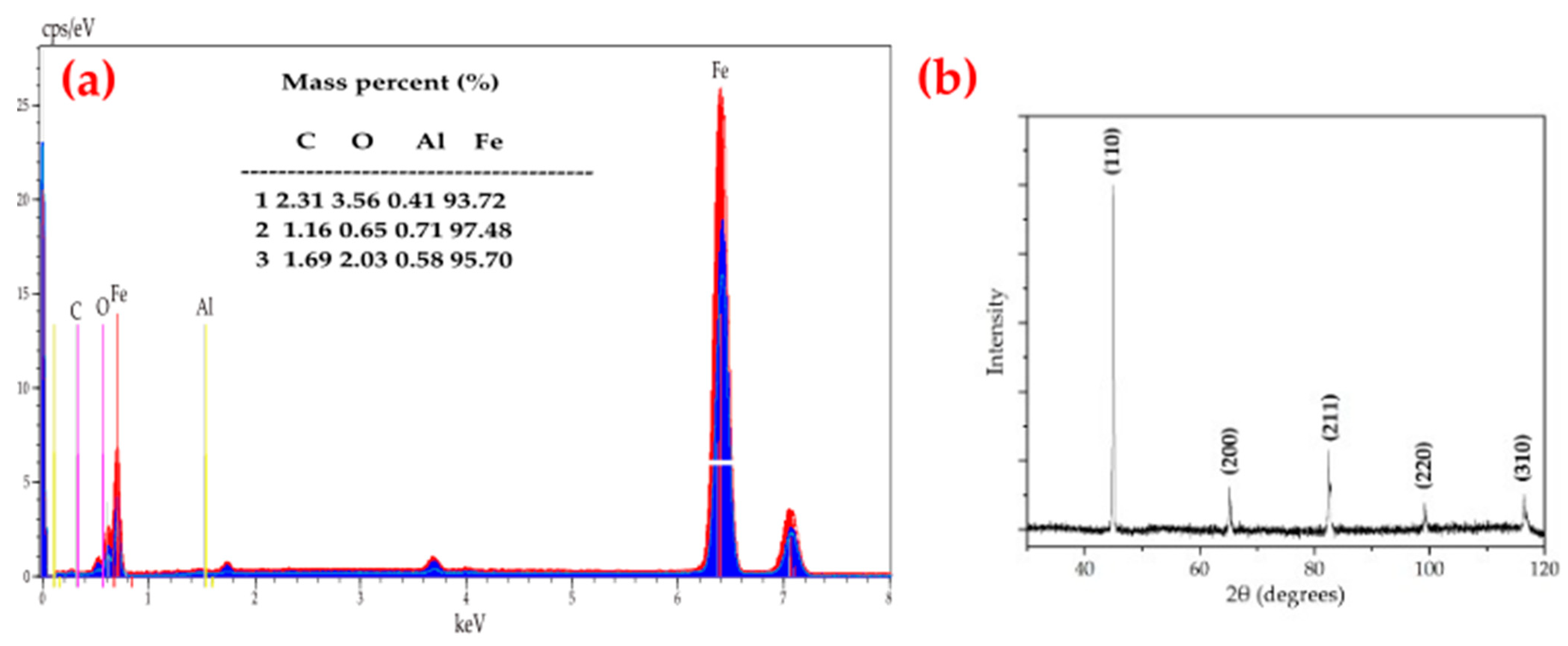
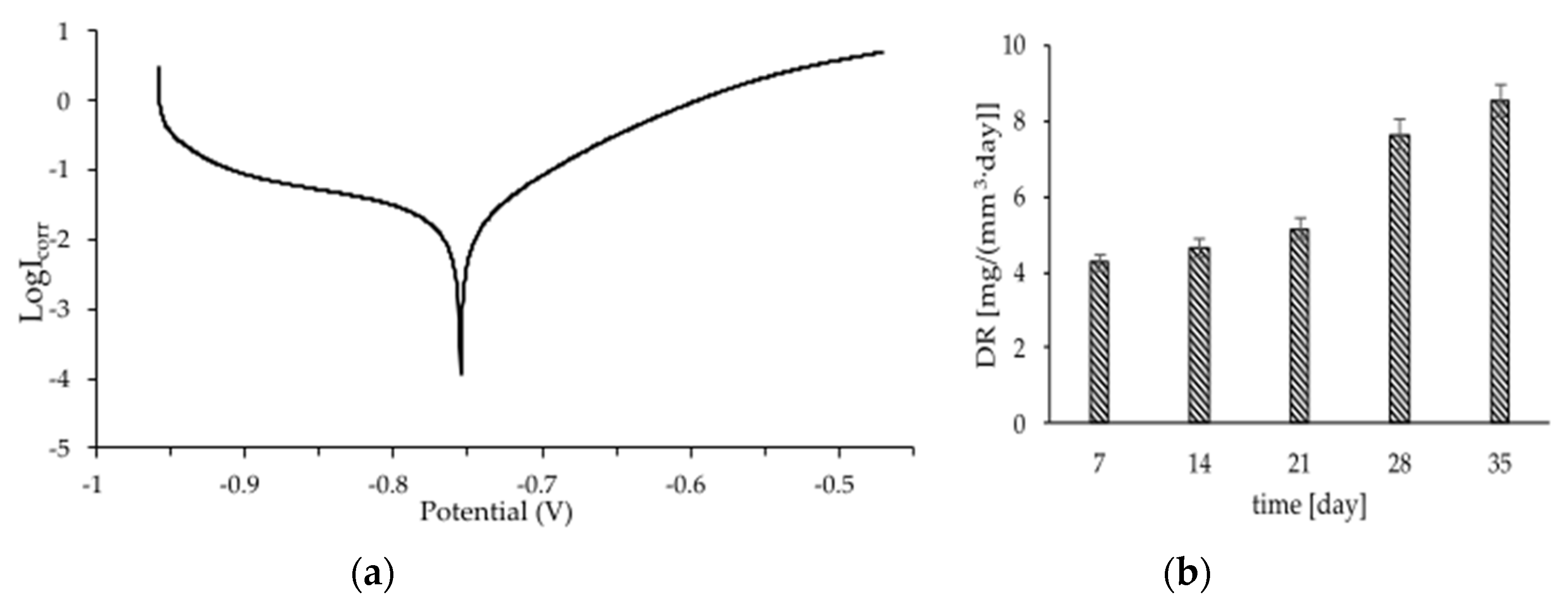
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Hussain, M.; Rizvi, S.A.; Abbas, N.; Sajjad, U.; Shad, M.; Badshah, M.; Malik, A. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Losic, D. Advancing of titanium medical implants by surface engineering: Recent progress and challenges. Expert Opin. Drug Deliv. 2021, 18, 1355–1378. [Google Scholar] [CrossRef]
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloy. 2021, 10, 627–669. [Google Scholar] [CrossRef]
- Albrektsson, T.; Becker, W.; Coli, P.; Jemt, T.; Mölne, J.; Sennerby, L. Bone loss around oral and orthopedic implants: An immunologically based condition. Clin. Implant Dent. Relat. Res. 2019, 21, 786–795. [Google Scholar] [CrossRef]
- Rahim, M.I.; Ullah, S.; Mueller, P.P. Advances and Challenges of Biodegradable Implant Materials with a Focus on Magnesium-Alloys and Bacterial Infections. Metals 2018, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Sarian, M.N.; Iqbal, N.; Sotoudehbagha, P.; Razavi, M.; Ahmed, Q.U.; Sukotjo, C.; Hermawan, H. Potential bioactive coating system for high-performance absorbable magnesium bone implants. Bioact. Mater. 2022, 12, 42–63. [Google Scholar] [CrossRef]
- Walker, M.H.E.; Heiden, E.W.A.L.S.M. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation—A Review. J. Biotechnol. Biomater. 2015, 5, 1. [Google Scholar] [CrossRef]
- Lane, J.M.; Mait, J.E.; Unnanuntana, A.; Hirsch, B.P.; Shaffer, A.D.; Shonuga, O.A. 6.616—Materials in Fracture Fixation. In Comprehensive Bi-omaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 219–235. ISBN 978-0-08-055294-1. [Google Scholar]
- Granke, M.; Does, M.D.; Nyman, J.S. The Role of Water Compartments in the Material Properties of Cortical Bone. Calcif. Tissue Res. 2015, 97, 292–307. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Kang, J.-H. Mechanical Properties of Compact Bone Defined by the Stress-Strain Curve Measured Using Uniaxial Tensile Test: A Concise Review and Practical Guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef]
- Amini, A.R.; Wallace, J.S.; Nukavarapu, S.P. Short-term and long-term effects of orthopedic biodegradable implants. J. Long-term Eff. Med. Implant. 2011, 21, 93–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Liu, B.; Wu, Y.; Zheng, Y. Comparative in vitro Study on Pure Metals (Fe, Mn, Mg, Zn and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Liu, Q.; Liao, H.; Coddet, C. Vacuum heat treatment of iron parts produced by selective laser melting: Microstructure, residual stress and tensile behavior. Mater. Des. 2014, 54, 727–733. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Frank, M.A.; Detsch, R.; Boccaccini, A.R.; Virtanen, S. Accelerated Degradation Behavior and Cytocompatibility of Pure Iron Treated with Sandblasting. ACS Appl. Mater. Interfaces 2016, 8, 26482–26492. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, G.; Szczepański, J.; Radtke, A. Biodegradable Iron-Based Materials—What Was Done and What More Can Be Done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef]
- Génin, J.-M.; Olowe, A.; Refait, P.; Simon, L. On the stoichiometry and pourbaix diagram of Fe(II)-Fe(III) hydroxy-sulphate or sulphate-containing green rust 2: An electrochemical and Mössbauer spectroscopy study. Corros. Sci. 1996, 38, 1751–1762. [Google Scholar] [CrossRef]
- Oriňáková, R.; Oriňák, A.; Bučková, L.M.; Giretová, M.; Medvecký, Ľ.; Labbanczová, E.; Kupková, M.; Hrubovčáková, M.; Kovaľ, K. Iron Based Degradable Foam Structures for Potential Orthopedic Applications. Int. J. Electrochem. Sci. 2013, 8, 15. [Google Scholar]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Kurokawa, H.; Matsui, H. Mitochondrial reactive oxygen species and heme, non-heme iron metabolism. Arch. Biochem. Biophys. 2020, 700, 108695. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Crossing the Iron Gate: Why and How Transferrin Receptors Mediate Viral Entry. Annu. Rev. Nutr. 2018, 38, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C.; Schmidt, P.J. Iron Homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Nauman, E.A.; Stanciu, L.A. Investigation of porosity on mechanical properties, degradation and in-vitro cytotoxicity limit of Fe30Mn using space holder technique. Mater. Sci. Eng. C 2019, 99, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Pandey, P.M. Mechanical behaviour of 3D printed ordered pore topological iron scaffold. Mater. Sci. Eng. A 2020, 783, 139293. [Google Scholar] [CrossRef]
- Murakami, T.; Akagi, T.; Kasai, E. Development of Porous Iron based Material by Slag Foaming and its Reduction. Procedia Mater. Sci. 2014, 4, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef]
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. 3D Printed Fe Scaffolds with HA Nanocoating for Bone Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 608–616. [Google Scholar] [CrossRef]
- Putra, N.; Leeflang, M.; Minneboo, M.; Taheri, P.; Fratila-Apachitei, L.; Mol, J.; Zhou, J.; Zadpoor, A. Extrusion-based 3D printed biodegradable porous iron. Acta Biomater. 2020, 121, 741–756. [Google Scholar] [CrossRef]
- Moravej, M.; Prima, F.; Fiset, M.; Mantovani, D. Electroformed iron as new biomaterial for degradable stents: Development process and structure–properties relationship. Acta Biomater. 2010, 6, 1726–1735. [Google Scholar] [CrossRef]
- Rabeeh, V.P.M.; Hanas, T. Progress in manufacturing and processing of degradable Fe-based implants: A review. Prog. Biomater. 2022, 11, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, S.C.; San-Miguel, V.; Wang, Y.; García-Peñas, A. Bioresorbable Metals for Cardiovascular and Fracture Repair Implants. Nanohybrids Future Mater. Biomed. Appl. 2021, 87, 134–155. [Google Scholar]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Alamdari, H.; Mantovani, D.; Dubé, D. Iron–manganese: New class of metallic degradable biomaterials prepared by powder metallurgy. Powder Met. 2008, 51, 38–45. [Google Scholar] [CrossRef]
- Hermawan, H.; Mantovani, D. Process of prototyping coronary stents from biodegradable Fe–Mn alloys. Acta Biomater. 2013, 9, 8585–8592. [Google Scholar] [CrossRef]
- Wegener, B.; Sichler, A.; Milz, S.; Sprecher, C.; Pieper, K.; Hermanns, W.; Jansson, V.; Nies, B.; Kieback, B.; Müller, P.E.; et al. Development of a novel biodegradable porous iron-based implant for bone replacement. Sci. Rep. 2020, 10, 9141. [Google Scholar] [CrossRef]
- Wiśniewski, M.; Rychlicki, G.; Arcimowicz, A. Experimental and theoretical estimations of the polar force contributions to the heat of immersion of carbon nanotubes. Chem. Phys. Lett. 2010, 485, 331–334. [Google Scholar] [CrossRef]
- Douillard, J.-M.; Salles, F.; Henry, M.; Malandrini, H.; Clauss, F. Surface energy of talc and chlorite: Comparison between electronegativity calculation and immersion results. J. Colloid Interface Sci. 2007, 305, 352–360. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Overview of Poly(lactic Acid). In Handbook of Biopolymers and Biodegradable Plastics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 11–54. ISBN 9781455728343. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 629270. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-Ray Diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Taguta, J.; McFadzean, B.; O’Connor, C. The relationship between the flotation behaviour of a mineral and its surface energy properties using calorimetry. Miner. Eng. 2019, 143, 105954. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast–biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Hong, S. Surface energy anisotropy of iron surfaces by carbon adsorption. Curr. Appl. Phys. 2003, 3, 457–460. [Google Scholar] [CrossRef]
- Navrotsky, A.; Ma, C.; Lilova, K.; Birkner, N. Nanophase Transition Metal Oxides Show Large Thermodynamically Driven Shifts in Oxidation-Reduction Equilibria. Science 2010, 330, 199–201. [Google Scholar] [CrossRef]
- Zysset, P.K.; Guo, X.E.; Hoffler, C.E.; Moore, E.K.; Goldstein, A.S. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Asl, S.K.F.; Nemeth, S.; Tan, M. Electrophoretic deposition of hydroxyapatite coatings on AZ31 magnesium substrate for biodegradable implant applications. Prog. Cryst. Growth Charact. Mater. 2014, 60, 74–79. [Google Scholar] [CrossRef]
- Matsugi, K.; Endo, T.; Choi, Y.-B.; Sasaki, G. Alloy Design of Ti Alloys Using Ubiquitous Alloying Elements and Characteristics of Their Lev-itation-Melted Alloys. Mater. Trans. 2010, 51, 740–748. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Tan, Y.J.; Chow, C.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Malladi, L.; Mahapatro, A.; Gomes, A.S. Fabrication of magnesium-based metallic scaffolds for bone tissue engineering. Mater. Technol. 2017, 33, 173–182. [Google Scholar] [CrossRef]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.M.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pshyk, A.; Coy, L.; Yate, L.; Załęski, K.; Nowaczyk, G.; Pogrebnjak, A.; Jurga, S. Combined reactive/non-reactive DC magnetron sputtering of high temperature composite AlN–TiB2–TiSi2. Mater. Des. 2016, 94, 230–239. [Google Scholar] [CrossRef]
- Xie, Z.; Lugovy, M.; Orlovskaya, N.; Graule, T.; Kuebler, J.; Mueller, M.; Gao, H.; Radovic, M.; Cullen, D. Hexagonal OsB2: Sintering, microstructure and mechanical properties. J. Alloy. Compd. 2015, 634, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/Material Interfaces: Influence of Surface Chemistry and Surface Topography on Cell Adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 81B, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhou, X.; Xia, Q.; Peng, J.; Wang, H.; Li, Z. Preparation and Adsorption Performance of GrO@Cu-BTC for Separation of CO2/CH4. Ind. Eng. Chem. Res. 2014, 53, 11176–11184. [Google Scholar] [CrossRef]
- Bolibok, P.; Wiśniewski, M.; Roszek, K.; Terzyk, A.P. Controlling enzymatic activity by immobilization on graphene oxide. Sci. Nat. 2017, 104, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erwardt, P.; Roszek, K.; Wiśniewski, M. Determination of Graphene Oxide Adsorption Space by Lysozyme Uptake─Mechanistic Studies. J. Phys. Chem. B 2022, 126, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Jeppu, G.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, P. 4.406—Protein Interactions with Biomaterials. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 63–73. ISBN 978-0-08-055294-1. [Google Scholar]
- Scott, C.F. Mechanism of the participation of the contact system in the Vroman effect. Review and summary. J. Biomater. Sci. Polym. Ed. 1991, 2, 173–181. [Google Scholar] [CrossRef]
- Hirsh, S.L.; McKenzie, D.R.; Nosworthy, N.J.; Denman, J.A.; Sezerman, O.U.; Bilek, M. The Vroman effect: Competitive protein exchange with dynamic multilayer protein aggregates. Colloids Surf. B Biointerfaces 2012, 103, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Gilmore, C.P.; Cooper, E.; Leggett, G.J.; Downes, S. Protein adsorption and human osteoblast-like cell attachment and growth on alkylthiol on gold self-assembled monolayers. J. Biomed. Mater. Res. 2002, 59, 84–99. [Google Scholar] [CrossRef]

| hwater [J/m2] | hn-heptane [J/m2] | hformamide [J/m2] | [J/m2] | ||||
|---|---|---|---|---|---|---|---|
| −1.763 (0.21) | −0.352 (0.0201) | −1.951 (0.141) | 0.827 (0.17) | 10.67 (0.22) | 0.278 (0.03) | 2.550 (0.16) | 1.881 (0.09) |
| Material | Fe Foams |
|---|---|
| Hardness [GPa] | 2.584 ± 0.692 |
| Young’s Modulus [GPa] | 28.457 ± 5.601 |
| E/H ratio [−] | 0.093 ± 0.027 |
| Plastic energy [nJ] | 14.566 ± 3.448 |
| Total energy [nJ] | 25.095 ± 3.329 |
| D ratio [−] | 0.577 ± 0.085 |
| Material | ECORR [mV] | icorr [µA/cm2] |
|---|---|---|
| Fe foams | −755 | 68.6 |
| Qm,1 [mg/gFe] | K1 [L/g] | n1 | Qm,2 [mg/gFe] | K2 [L/g] | n2 | R2 |
|---|---|---|---|---|---|---|
| 5.44 (0.11) | 16.41 (1.77) | 0.51 (0.02) | 43.16 (0.81) | 0.95 (0.05) | 0.32 (0.02) | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grodzicka, M.; Gąsior, G.; Wiśniewski, M.; Bartmański, M.; Radtke, A. A Simple Replica Method as the Way to Obtain a Morphologically and Mechanically Bone-like Iron-Based Biodegradable Material. Materials 2022, 15, 4552. https://doi.org/10.3390/ma15134552
Grodzicka M, Gąsior G, Wiśniewski M, Bartmański M, Radtke A. A Simple Replica Method as the Way to Obtain a Morphologically and Mechanically Bone-like Iron-Based Biodegradable Material. Materials. 2022; 15(13):4552. https://doi.org/10.3390/ma15134552
Chicago/Turabian StyleGrodzicka, Marlena, Gabriela Gąsior, Marek Wiśniewski, Michał Bartmański, and Aleksandra Radtke. 2022. "A Simple Replica Method as the Way to Obtain a Morphologically and Mechanically Bone-like Iron-Based Biodegradable Material" Materials 15, no. 13: 4552. https://doi.org/10.3390/ma15134552
APA StyleGrodzicka, M., Gąsior, G., Wiśniewski, M., Bartmański, M., & Radtke, A. (2022). A Simple Replica Method as the Way to Obtain a Morphologically and Mechanically Bone-like Iron-Based Biodegradable Material. Materials, 15(13), 4552. https://doi.org/10.3390/ma15134552









