Effect of Oxide Metallurgy on Inclusions in 125 ksi Grade OCTG Steel with Sulfide Stress Corrosion Resistance
Abstract
:1. Introduction
2. Experimental Method
2.1. Preparation of the Experimental Steels
2.2. The Hot Rolling and Heat Treatment of the Experimental Steels
2.3. Microstructure Observation
2.4. Mechanical Properties Experiments
2.5. NACE-A Test
3. Results and Discussion
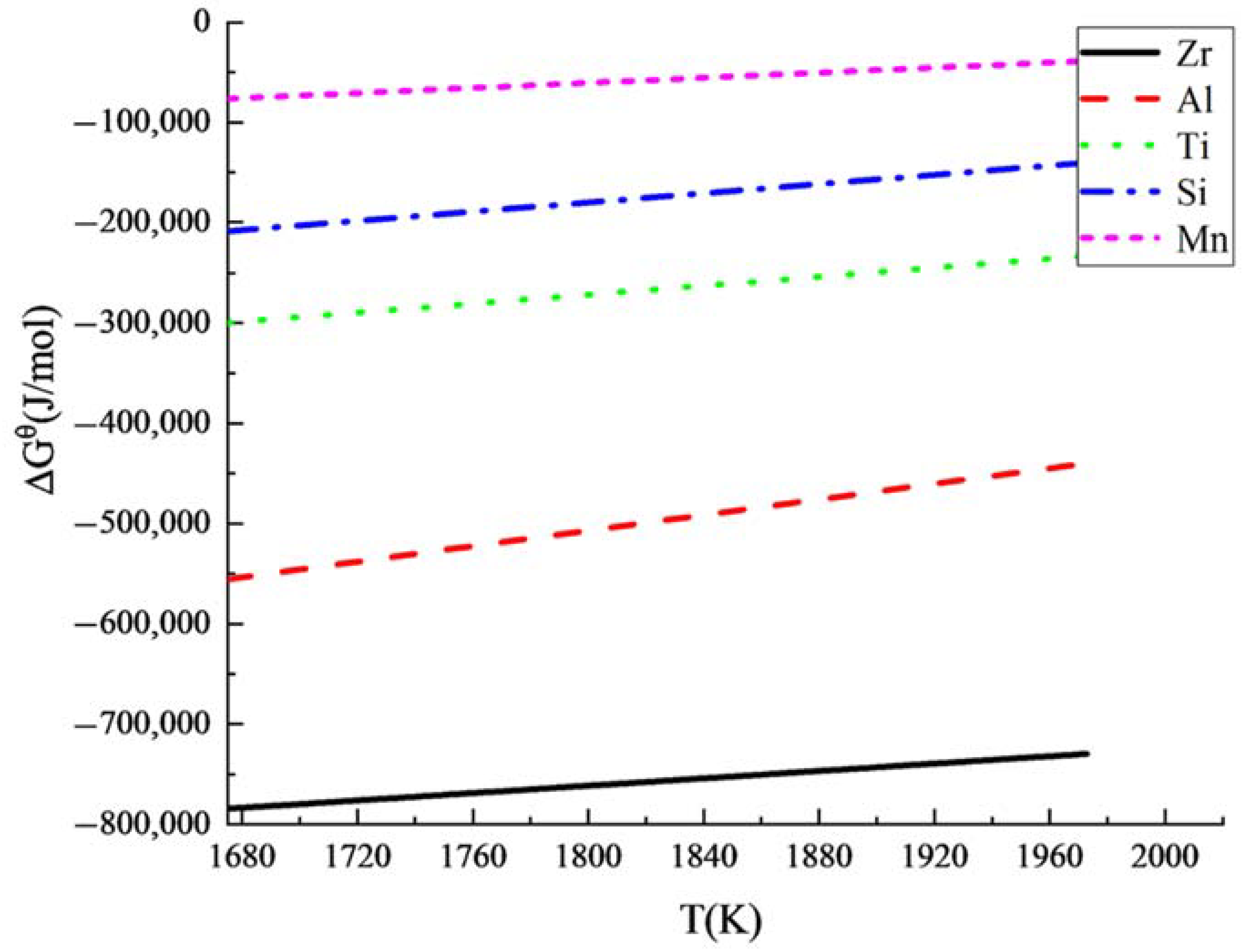
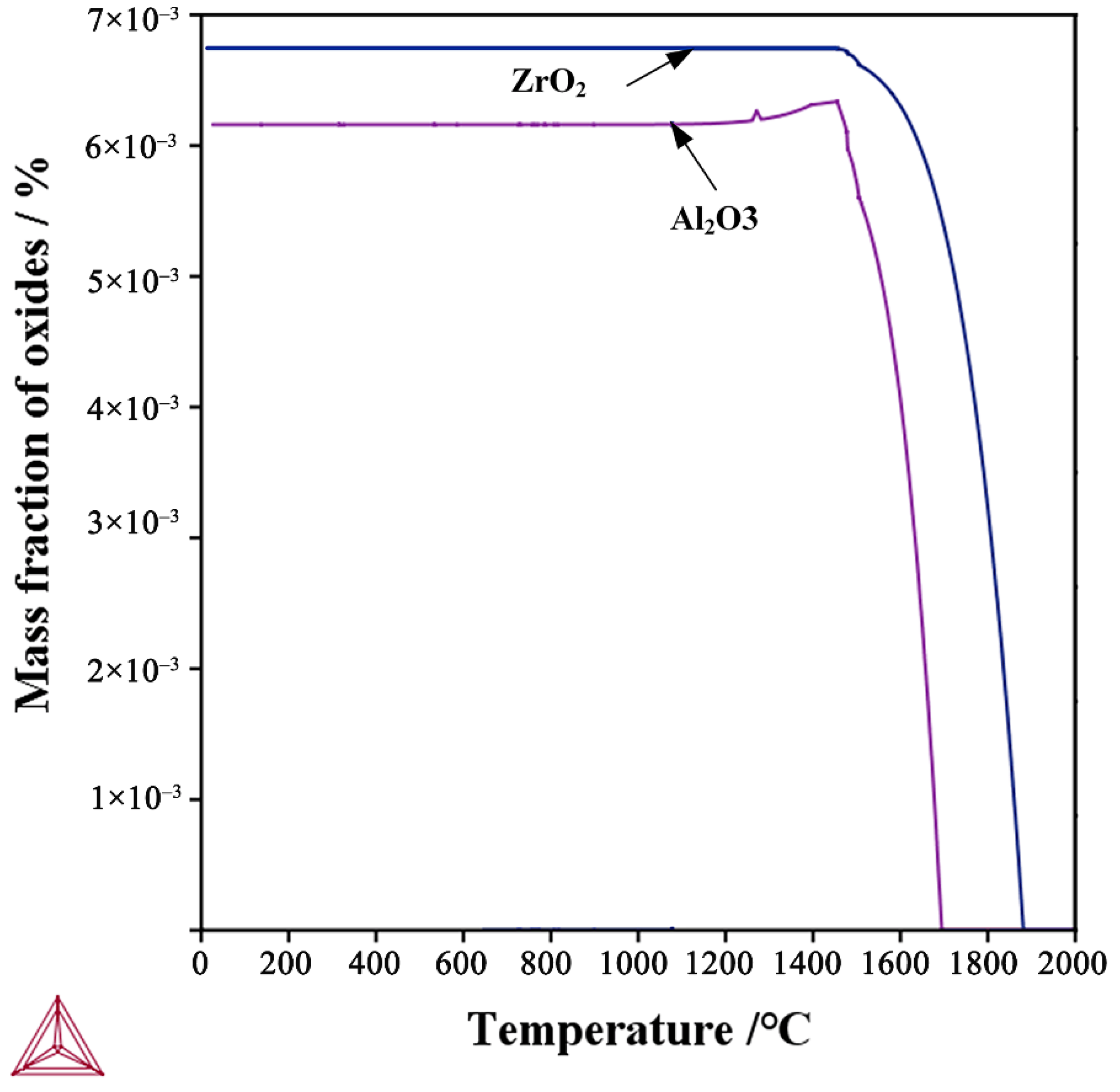
3.1. Thermodynamic Analysis of Deoxidation
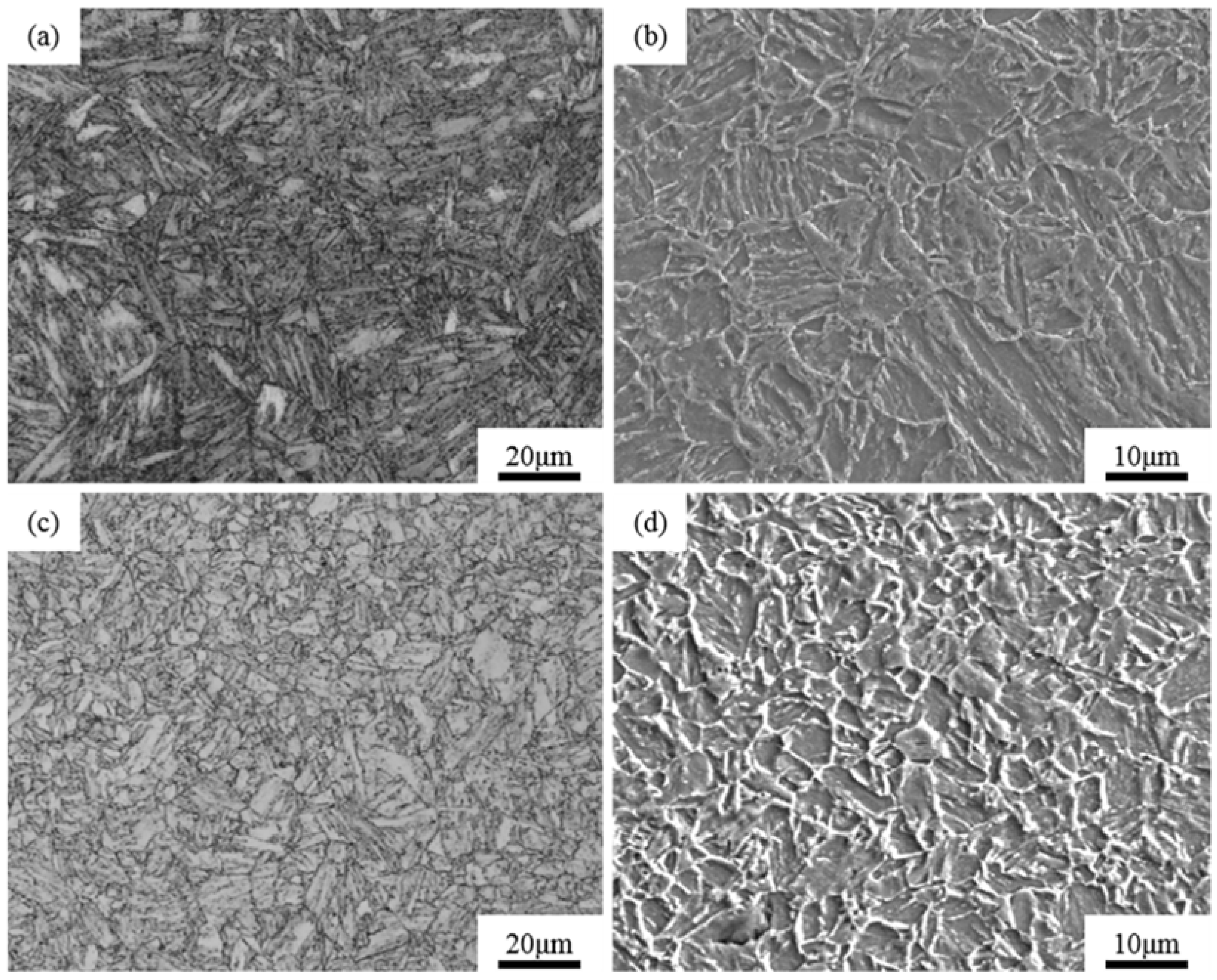
3.2. Microstructures
3.3. Mechanical Properties
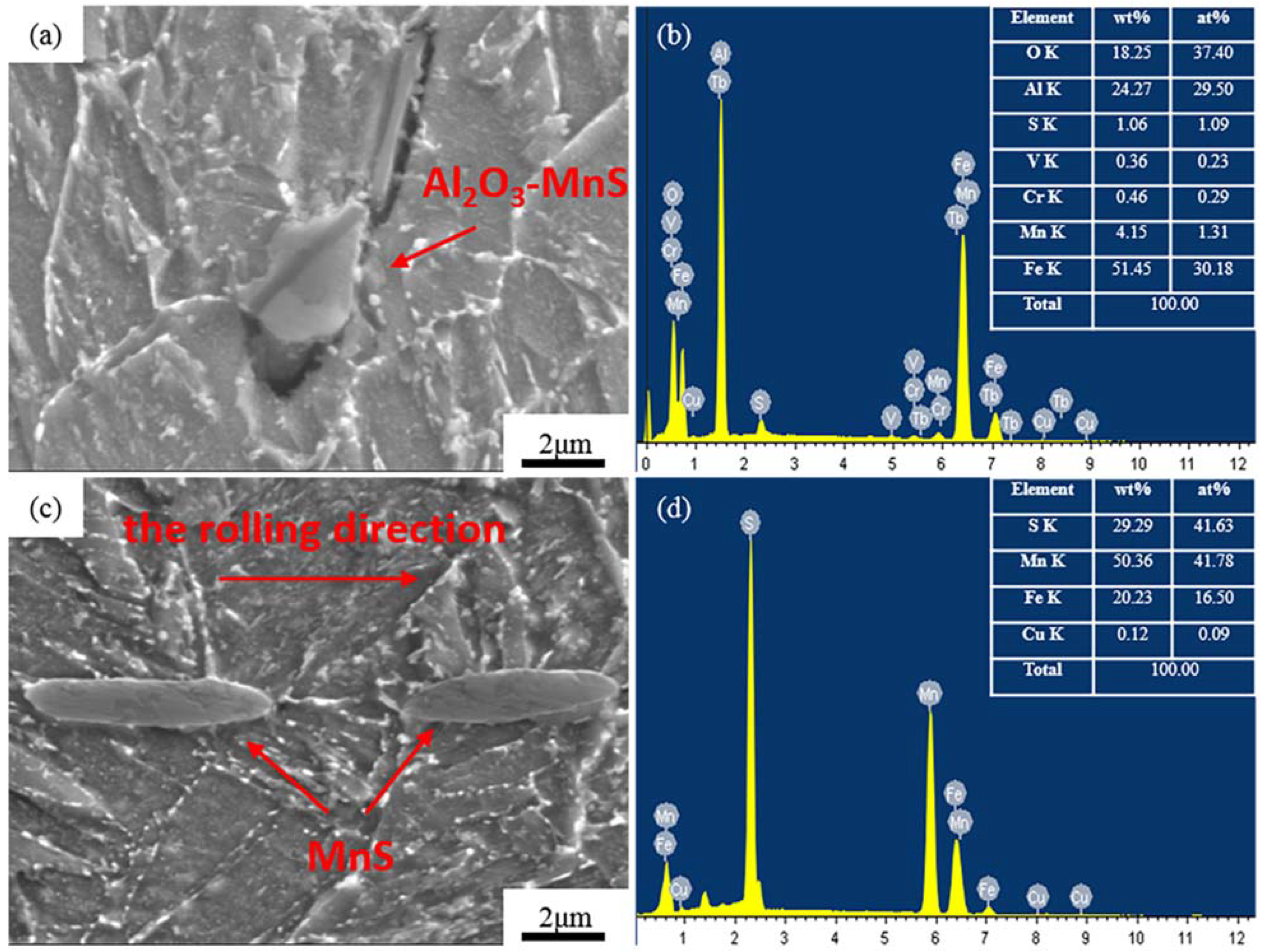
3.4. Inclusions
3.4.1. Inclusions in the Al-Steel
3.4.2. Inclusions of the Zr-Steel
4. Conclusions
- (1)
- The yield strength of the Al-Steel and Zr-Steel was 922 MPa and 939 MPa, respectively; the impact energy was 60 ± 6 J and 132 ± 6 J, respectively; the fracture time of the NACE-A experiment increased from 28 h (Al-Steel) to 720 h (Zr-Steel) without fracture. The oxide metallurgy process improved the sulfide stress corrosion resistance of the steel.
- (2)
- The oxide metallurgy process increased the number of inclusions and decreased the size of the inclusions. These fine particles can pin the austenite grain boundary, refining the prior austenite grain. The average diameter of the austenite grains of the Al-Steel and the Zr-Steel was 9.1 μm and 8 μm, respectively. After the heat treatment, both experimental steels consisted of a tempered martensite structure, and the average grain size of the martensite was 8.2 μm (Al-Steel) and 3.8 μm (Zr-Steel), respectively. The microstructure of the oxide metallurgy process was an ultra-fine grain, contributing to a fine grain strengthening and consumption of the fracture energy. This is the most ideal microstructure for sulfide stress corrosion resistant high-strength steel.
- (3)
- Based on the thermodynamic calculation, it is feasible to deoxidize using Zr instead of Al in steelmaking because of the strong affinity of Zr with oxygen. The number of fine composite inclusions with a high melting point increased due to the oxide metallurgy process by adding zirconium (Zr). The Al-Steel and Zr-Steel contained 22.38 and 68.77 inclusions per unit area, respectively; the fraction of the inclusions with a diameter less than 2μm was 73.46%, and 89.63%, respectively. The average diameter of the inclusions in the Al-Steel (2.45 μm) was larger than that of the Zr-Steel (1.65 μm). An inclusion refinement reduced the lattice defects, and the hydrogen atoms could not be concentrated in the irreversible hydrogen trap, so as to improve the SSC resistance of the steel.
- (4)
- The MnS was obviously spheroidized in the steels treated by the oxide metallurgy process. The stress concentration caused by anisotropy was effectively avoided, and the low-temperature impact toughness and SSC resistance of the steels were improved. The critical stress of fracture increased with the decrease in the inclusion size according to the Griffith theory.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Case, R. The influence of H2S on hydrogen absorption and sulfide stress cracking resistance of high strength low alloy carbon steel C110. J. Nat. Gas Sci. Eng. 2022, 99, 104418. [Google Scholar] [CrossRef]
- Rihan, R.; Waka, A.B.; Tanoli, N.; Shalaby, H. The susceptibility of P110 downhole tubular steel to sulfide stress cracking in H2S and NaCl. J. Pet. Sci. Eng. 2019, 174, 1034–1041. [Google Scholar] [CrossRef]
- Chen, L.; Case, R.; Liu, L.L.; Xiang, S.S.; Castaneda, H. Assessment of sulfide corrosion cracking and hydrogen permeation behavior of ultrafine grain high strength steel. Corros. Sci. 2022, 198, 110142. [Google Scholar] [CrossRef]
- Wang, X.T.; Liu, M.; Zhou, G.Y.; Jiang, H.; Li, X.; Luo, M.; Liu, Y.H.; Zhang, Z.H.; Cao, G.H. Effects of Chromium and Tungsten on Sulfide Stress Cracking in high Strength Low Alloy 125ksi Grade Casing Steel. Corros. Sci. 2019, 160, 108163. [Google Scholar] [CrossRef]
- Luo, M.; Liu, M.; Wang, X.T.; Li, M.C.; Li, X.; Ren, Z.M.; Cao, G.H.; Zhang, Z.H. Effect of tempering temperature at high temperature zone on sulfide stress cracking behavior for casing steel. Eng. Fail. Anal. 2019, 105, 227–236. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, L.H.; Gu, H.X.; Lu, Y.S. A New Understanding of Deoxidation by Aluminum in Steels. J. Iron Steel Res. 1999, 11, 65–69. [Google Scholar]
- Cao, J.; Yang, W.; Wang, X.H. Changes of Non-Metallic Inclusions for Low-Carbon Aluminium-Killed Steel Sheet at the Start of Continuous Casting. Iron Steel 2011, 46, 37–39. [Google Scholar]
- André, L.V.C.S. The effects of non-metallic inclusions on properties relevant to the performance of steel in structural and mechanical applications. J. Mater. Res. Technol. 2019, 8, 2408–2422. [Google Scholar]
- Wang, Y.F.; Cheng, G.X.; Wu, W.; Li, Y. Role of inclusions in the pitting initiation of pipeline steel and the effect of electron irradiation in SEM. Corros. Sci. 2017, 130, 252–260. [Google Scholar] [CrossRef]
- Ciaraldi, S.W. Microstructural Observations on the Sulfide Stress Cracking of Low Alloy Steel Tubulars. Corrosion 1984, 40, 77–81. [Google Scholar] [CrossRef]
- Torkkeli, J.; Saukkonen, T.; Hanninen, H. Effect of MnS inclusion dissolution on carbon steel stress corrosion cracking in fuel-grade ethanol. Corros. Sci. 2015, 96, 14–22. [Google Scholar] [CrossRef]
- Wang, L.W.; Xin, J.C.; Cheng, L.J.; Zhao, K.; Sun, B.Z.; Li, J.R.; Wang, X.; Cui, Z.Y. Influence of inclusions on initiation of pitting corrosion and stress corrosion cracking of X70 steel in near-neutral pH environment. Corros. Sci. 2019, 147, 108–127. [Google Scholar] [CrossRef]
- Melander, A.; Gustavsson, A. An FEM study of driving forces of short cracks at inclusions in hard steels. Int. J. Fatigue 1996, 18, 389–399. [Google Scholar] [CrossRef]
- André, L.V.C.S. Non-metallic inclusions in steels—origin and control. J. Mater. Res. Technol. 2018, 7, 283–299. [Google Scholar]
- Raphael, M.S.; Marcia, S.O.; Jose, R.O.; Eduardo, J.; Victor, B.T.; Felipe, F.G. Analysis of predictors for modification of alumina inclusions in medium carbon steel. J. Mater. Res. Technol. 2021, 14, 2257–2266. [Google Scholar]
- Al-Mansour, M.; Alfantazi, A.M.; El-Boujdaini, M. Sulfide stress cracking resistance of API-X100 high strength low alloy steel. Mater. Des. 2009, 30, 4088–4094. [Google Scholar] [CrossRef]
- Tsay, L.W.; Chen, Y.C.; Chan, S. Sulfide stress corrosion cracking and fatigue crack growth of welded TMCP API 5L X65 pipe-line steel. Int. J. Fatigue 2001, 23, 103–113. [Google Scholar] [CrossRef]
- Ioffe, A.V.; Tetyueva, T.V.; Vyboishchik, M.A.; Knyaz’kin, S.A.; Zyryanov, A.O. Corrosion-mechanical fracture of tubing from carbon and alloy steels operating in environments containing hydrogen sulfide. Met. Sci. Heat Treat. 2013, 54, 492–497. [Google Scholar] [CrossRef]
- Koh, S.U.; Kim, J.S.; Yang, B.Y.; Kim, K.Y. Effect of line pipe steel microstructure on susceptibility to sulfide stress cracking. Corrosion 2004, 60, 244–253. [Google Scholar] [CrossRef]
- Jin, T.Y.; Liu, Z.Y.; Cheng, Y.F. Effect of non-metallic inclusions on hydrogen-induced cracking of API 5L X100 steel. Int. J. Hydrog. Energy 2010, 35, 8014–8021. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Characterization of inclusions of X80 pipeline steel and its correlation with hydrogen-induced cracking. Corros. Sci. 2011, 53, 1201–1208. [Google Scholar] [CrossRef]
- Omura, T.; Numata, M.; Takayama, T.; Aral, Y.; Souma, A.; Ohe, T.; Amaya, H.; Ueda, M. Super-high Strength Low Alloy Steel OCTG with Improved Sour Resistance. Nippon Steel Technol. News 2013, 397, 17–22. [Google Scholar]
- Liu, Z.Y.; Li, X.G.; Du, C.W.; Lu, L.; Zhang, Y.R.; Cheng, Y.F. Effect of inclusions on initiation of stress corrosion cracks in X70 pipeline steel in an acidic soil environment. Corros. Sci. 2009, 51, 895–900. [Google Scholar] [CrossRef]
- Guo, J.B. Process of Research on Morphology Control of Al2O3 Inclusions in Aluminum Dexidized Steel. Anhui Metall. 2014, 4, 19–23. [Google Scholar]
- Van, E.M.; Guo, M.; Zinngrebe, E.; Blanpain, B.; Jung, I.J. Evolution of non-metallic inclusions in secondary steelmaking: Learning from inclusion size distributions. ISIJ Int. 2013, 53, 1974–1982. [Google Scholar]
- Choudhary, S.K.; Ghosh, A. Thermodynamic evaluation of formation of oxide–sulfide duplex inclusions in steel. ISIJ Int. 2008, 48, 1552–1559. [Google Scholar] [CrossRef] [Green Version]
- Wael, A.A.; Ahmed, M.A.E.; Mohamed, A.S.; Asmaa, I.Z.; Huda, S.A.; Mohamed, A.M.; Adel, A.E. Influence of CeO2 loading on the catalytic performance of CoNiMoS/CeO2–Al2O3 toward vacuum gas oil hydrotreatme. Mater. Chem. Phys. 2022, 276, 125165. [Google Scholar]
- Ren, Q.; Zhang, L.F. Effect of cerium content on inclusions in an ultra-low-carbon aluminum-killed steel. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2020, 51, 589–600. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.G.; Li, S.J.; Dai, W.X. Effect of cerium on the behavior of inclusions in H13 steel. Steel Res. Int. 2018, 89, 1800371. [Google Scholar] [CrossRef]
- Wang, H.; Bao, Y.P.; Zhi, J.G.; Duan, C.Y.; Gao, S.; Wang, M. Effect of rare earth Ce on the morphology and distribution of Al2O3 inclusions in high strength IF steel containing phosphorus during continuous casting and rolling process. ISIJ Int. 2021, 61, 657–666. [Google Scholar] [CrossRef]
- Liu, X.J.; Yang, J.C.; Zhang, F.; Fu, X.Y.; Li, H.W.; Yang, C.Q. Experimental and DFT study on cerium inclusions in clean steels. Rare Earths. 2021, 39, 477–486. [Google Scholar] [CrossRef]
- Mehrabian, R.; Keane, M.; Flemings, M.C. Interdendritic fluid flow and macrosegregation; influence of gravity. Metall. Mater. Trans.B 1970, 1, 1209–1220. [Google Scholar] [CrossRef]
- Tehovnik, F.; Burja, J.; Arh, B.; Knap, M. Submerged entry nozzle clogging during continuous casting of Al-killed steel. Metalurgija 2015, 54, 371–374. [Google Scholar]
- Ghosh, A.; Chatterjee, A. Ironmaking and Steelmaking: Theory and Practice; PHI Learning Private Limited: New Delhi, India, 2010; Volume 30. [Google Scholar]
- Suito, H.; Inoue, R. Thermodynamics on Control of Inclusions Composition in Ultra-clean Steels. ISIJ Int. 1996, 36, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Waudby, P.E. Rare earth additions to steel. Int. Met. Rev. 1978, 23, 74–98. [Google Scholar] [CrossRef]
- Wang, L.M.; Lin, Q.; Ji, J.W.; Lan, D.N. New study concerning development of application of rare earth metals in steels. Alloy. Compd. 2006, 408–412, 384–386. [Google Scholar] [CrossRef]
- Tian, C.; Yu, J.K.; Jin, E.D.; Wen, T.P.; Jia, D.B.; Liu, Z.L.; Fu, P.X.; Yuan, L. Effect of interfacial reaction behaviour on the clogging of SEN in the continuous casting of bearing steel containing rare earth elements. J. Alloy. Compd. 2019, 792, 1–7. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; Lu, B.; Zhi, J.G.; Zhang, S.; Liu, Y. Analysis on clogging of submerged entry nozzle in continuous casting of high strength steel with rare earth. J. Iron Steel Res. Int. 2021, 29, 34–43. [Google Scholar] [CrossRef]
- Roos, E.; Karasev, A.; Jönsson, P.G. Effect of Si and Ce Contents on the Nozzle Clogging in a REM Alloyed Stainless Steel. Steel Res. Int. 2015, 86, 1279–1288. [Google Scholar] [CrossRef]
- Zhou, S.C. Study of the Clogging of the Submersible Nozzle in the Continuous Casting of Stainless Steel RE-253MA. Metallurgist 2013, 57, 510–515. [Google Scholar]
- Andrey, K.; Hideaki, S. Effect of Particle Size Distribution on Austenite Grain Growth in Fe–0.05mass%C Alloy Deoxidized with Mn–Si, Ti, Mg, Zr and Ce. ISIJ Int. 2006, 46, 718–727. [Google Scholar]
- Shi, M.H.; Kannan, R.; Zhang, J.; Yuan, X.G.; Li, L.J. Effect of Zr Microalloying on Austenite Grain Size of Low-Carbon Steels. Metall. Mater. Trans. B 2019, 50, 2574–2585. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, J.; Chen, H.L.; Sun, Y.H.; Kuo, C.L.; Su, Y.H.; Chen, S.H.; Lin, K.J.; Hsieh, P.H.; Hwang, W.S. Effects of Rare Earth Metals on Steel Microstructures. Materials 2016, 9, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Jin, L.Y.; Gong, Y.D.; Wen, X.L.; Yin, G.Q.; Wen, Q.; Tang, B.J. Experimental evaluation of surface generation and force time-varying characteristics of curvilinear grooved micro end mills fabricated by EDM. J. Manuf. Processes 2022, 73, 799–814. [Google Scholar] [CrossRef]
- NACE Standard TM 0177-2005; Standard Test Method Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments. NACE International: Houston, TX, USA, 2016; p. 21212.
- Zheng, S.W. Study on the Competitive Priority Precipitation Mechanism of Intra-Granular Ferrite in the Process of Oxide Metallurgy. Master’s Thesis, North China University of Science and Technology, Tangshan, China, 2019. [Google Scholar]
- Qiu, G.X.; Zhan, D.P.; Niu, B.; Zhou, Y.X.; Jiang, Z.H.; Zhang, H.S. Effect of deoxidization process on inclusions in steel. J. Iron Steel Res. 2016, 28, 25–30. [Google Scholar]
- Lenkovskiy, T.M.; Kulyk, V.V.; Duriagina, Z.A.; Kovalchuk, R.A.; Topilnytskyy, V.H.; Vira, V.V.; Tepla, T.L. Mode I and mode II fatigue crack growth resistance characteristics of high tempered 65G steel. Arch. Mater. Sci. Eng. 2017, 1, 34–41. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, M.; Liu, Y.H.; Luo, M.; Zhang, C.X.; Wang, C.H.; Cao, G.H. A systematical analysis with respect to multiple hydrogen traps influencing sulfide stress cracking behavior of API-5CT-C110 casing steel. Mater. Sci. Eng. A. 2018, 721, 81–88. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Dong, Y.; Meng, Q.W.; Misra, R.D.K. Effect of Ti variation on microstructure evolution and mechanical properties of low carbon medium Mn heavy plate steel. Mater. Charact. 2019, 152, 21–35. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Wang, J.J. Effect of V on intragranular ferrite nucleation of high Ti bearing steel. Scr. Mater. 2013, 68, 953–956. [Google Scholar] [CrossRef]
- Kusche, C.F.; James, S.K.; Gibson, L.; Wollenweber, M.A.; Kerzel, S.K. On the mechanical properties and deformation mechanisms of manganese sulphide inclusions. Mater. Design. 2020, 193, 108801. [Google Scholar] [CrossRef]
- Shimahashi, N.; Muto, I.; Sugawara, Y.; Hara, N. Effects of Corrosion and Cracking of Sulfide Inclusions on Pit Initiation in Stainless Steel. J. Electrochem. Soc. 2014, 161, 494–500. [Google Scholar] [CrossRef]
- Huang, F.; Li, X.G.; Liu, J.; Qu, Y.M.; Jia, J.; Du, C.W. Hydrogen-induced cracking susceptibility and hydrogen trapping efficiency of different microstructure X80 pipeline steel. J. Mater. Sci. 2011, 46, 715–722. [Google Scholar] [CrossRef]
- Lv, Z.A.; Ni, H.W.; Zhang, H.; Liu, C.S. Evolution of MnS inclusions in Ti-bearing X80 pipeline steel. J. Iron Steel Res. Int. 2017, 24, 654–660. [Google Scholar] [CrossRef]
- Guo, A.M.; Li, S.R.; Guo, J.; Li, P.H.; Ding, Q.F.; Wu, K.M.; He, X.L. Effect of zirconium addition on the impact toughness of the heat affected zone in a high strength low alloy pipeline steel. Mater. Charact. 2008, 59, 134–139. [Google Scholar] [CrossRef]
- Ma, Y.; Song, L.R.; Liu, G.S.; Li, J.F.; Yan, G.L.; Lai, X.F. Zirconium Dioxide: A Versatile High-performance New Material. Mod. Chem. Res. 2021, 1, 134–138. [Google Scholar]
- Xia, S.Y.; Hu, C.B. Review of Physical Property Calculations of Liquid Aluminum and Alumina. J. Propuls. Technol. 2019, 40, 961–969. [Google Scholar]
- Anderson, T.L. Fracture Mechanics—Fundamentals and Applications; Taylor & Francis Group: Abingdon, UK, 2005; pp. 27–28. [Google Scholar]
- Peng, Z.X. Interaction between Inclusions and Hydrogen in Pipeline Steel and Its Effect on HIC Sensitivity; Wuhan University of Science and Technology: Wuhan, China, 2021; p. 12. [Google Scholar]
- Curry, D.A.; Knott, J.F. Effect of Microstructure on Cleavage Fracture Toughness of Quenched and Tempered Steels. Mater. Sci. Technol. 2013, 13, 341–345. [Google Scholar] [CrossRef]
- Lenkovskiy, T.M.; Kulyk, V.V.; Duriagina, Z.A.; Dzyubyk, L.V.; Vira, V.V.; Dzyubyk, A.R.; Tepla, T.L. Finite elements analysis of the side grooved I-beam specimen for mode II fatigue crack growth rates determination. J. Achiev. Mater. Manuf. Eng. 2018, 2, 70–77. [Google Scholar] [CrossRef]





| No. | C | Si | Mn | P | S | Cr | Ti | V, Mo, Ni, Cu | Al | Zr | O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Steel | 0.28 | 0.29 | 0.69 | 0.010 | 0.009 | 0.98 | 0.013 | <1.7 | 0.01 | 0 | 0.0029 |
| Zr-Steel | 0.27 | 0.31 | 0.70 | 0.011 | 0.005 | 0.97 | 0.011 | <1.7 | - | 0.009 | 0.0020 |
| Deoxidation Reaction | Standard Gibbs Free Energy (J·mol−1) | ΔGθ (T = 1873 K) (J·mol−1) | Reference |
|---|---|---|---|
| [Zr] + 2[O] = ZrO2 | ΔGθ = −1,092,000 + 183.7 T | −0.747930 × 10−6 | [47] |
| 2[Al] + 3[O] = Al2O3 | ΔGθ = −1,205,090 + 387.73 T | −0.478872 × 10−6 | [48] |
| [Ti] + 2[O] = TiO2 | ΔGθ = −675,720 + 224.6 T | −0.255044 × 10−6 | [47] |
| [Si] + 2[O] = SiO2 | ΔGθ = −594,128 + 230 T | −0.163338 × 10−6 | [48] |
| [Mn] + [O] = MnO | ΔGθ = −288,773 + 126.82 T | −0.051239 × 10−6 | [48] |
| No. | Rσ (MPa) | R (MPa) | Rσ/R | A (%) | Akv/0 °C (J) | NACE-A [SMYS-85%] (h) | |
|---|---|---|---|---|---|---|---|
| Al-Steel | 922 | 964 | 0.96 | 15 | 65 | 28 | |
| 54 | 60 ± 6 | ||||||
| 61 | |||||||
| Zr-Steel | 939 | 978 | 0.96 | 14 | 129 | >720 | |
| 135 | 132 ± 3 | ||||||
| 132 | |||||||
| Compound | Crystal Structure | Planes (hkl) | Interplanar Distance (Å) | Crystal Plane Angle, β | Relative Intensity | Lattice Parameters (Å) |
|---|---|---|---|---|---|---|
| ZrO2 | Monoclinic | 002 | 2.621 | 20 | a = 5.145 | |
| 022 | 1.847 | 99.2 | 14 | b = 5.207 | ||
| 113 | 1.509 | 3 | 4 | c = 5.311 | ||
| MnS | Fcc | 111 | 2.612 | 100 | a = 5.224 | |
| 220 | 1.847 | 90 | 50 | b = 5.224 | ||
| 222 | 1.509 | 20 | c = 5.224 |
| No. | 1–2 μm | 2–3 μm | 3–4 μm | 4–5 μm | 5–6 μm | 6–7 μm | 7–8 μm | 8–9 μm | >9 μm | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Al-Steel | 10.37 | 6.07 | 3.85 | 1.04 | 0.30 | 0.15 | 0.30 | 0.15 | 0.15 | 22.38 |
| Zr-Steel | 43.01 | 18.63 | 3.73 | 1.36 | 0.68 | 0.34 | 0.68 | 0 | 0.34 | 68.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Li, Y.; Wang, P.; Zhu, F.; Yang, Y.; Xiao, B. Effect of Oxide Metallurgy on Inclusions in 125 ksi Grade OCTG Steel with Sulfide Stress Corrosion Resistance. Materials 2022, 15, 4544. https://doi.org/10.3390/ma15134544
Zhang S, Li Y, Wang P, Zhu F, Yang Y, Xiao B. Effect of Oxide Metallurgy on Inclusions in 125 ksi Grade OCTG Steel with Sulfide Stress Corrosion Resistance. Materials. 2022; 15(13):4544. https://doi.org/10.3390/ma15134544
Chicago/Turabian StyleZhang, Si, Yanmei Li, Ping Wang, Fuxian Zhu, Yulong Yang, and Bang Xiao. 2022. "Effect of Oxide Metallurgy on Inclusions in 125 ksi Grade OCTG Steel with Sulfide Stress Corrosion Resistance" Materials 15, no. 13: 4544. https://doi.org/10.3390/ma15134544
APA StyleZhang, S., Li, Y., Wang, P., Zhu, F., Yang, Y., & Xiao, B. (2022). Effect of Oxide Metallurgy on Inclusions in 125 ksi Grade OCTG Steel with Sulfide Stress Corrosion Resistance. Materials, 15(13), 4544. https://doi.org/10.3390/ma15134544






