Effect of Pre-Oxidation on High-Temperature Oxidation Behavior of Al-Si Coating on Nickel-Based Superalloy
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Pre-oxidation temperature
- (2)
- Pre-oxidation time
3. Results
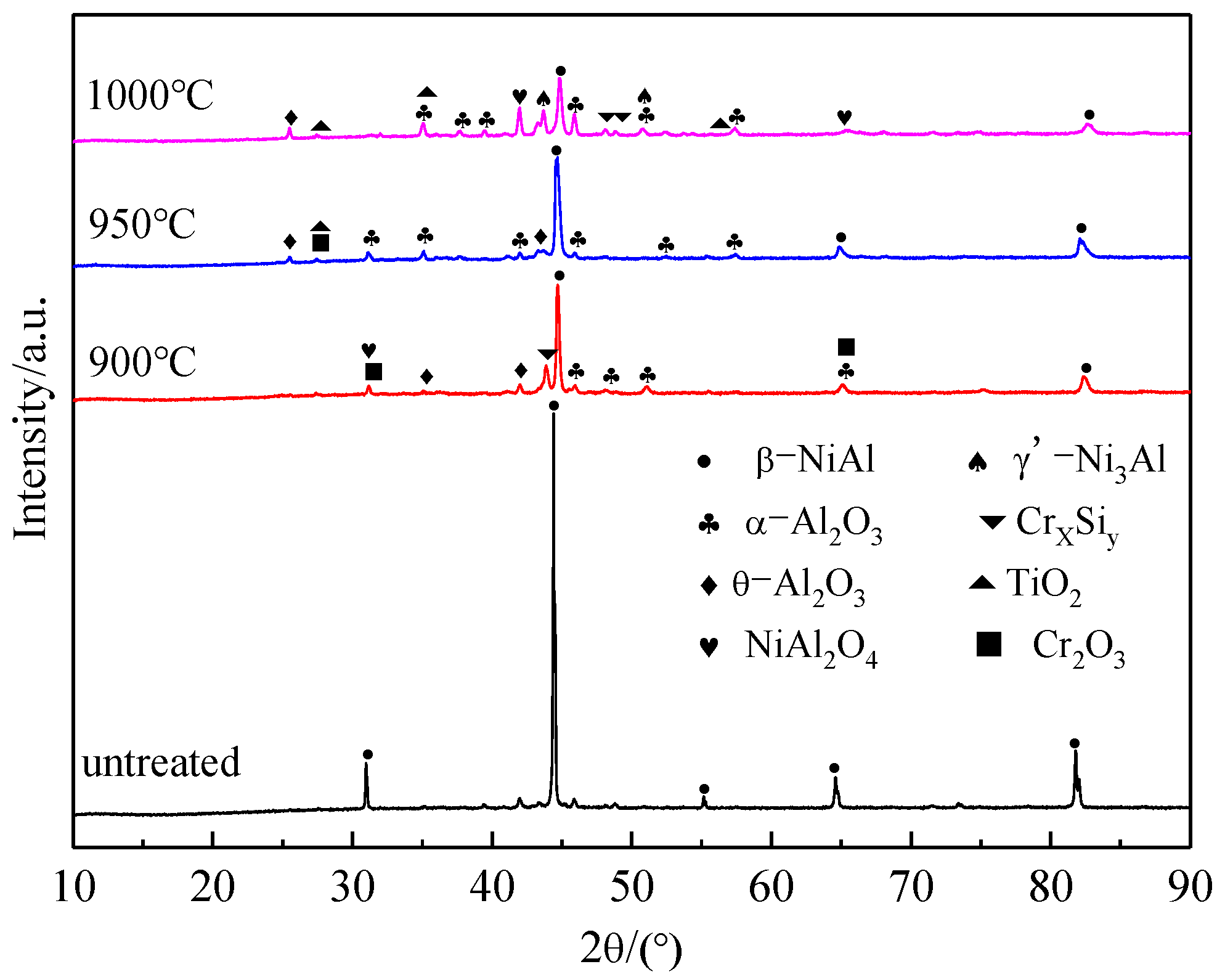
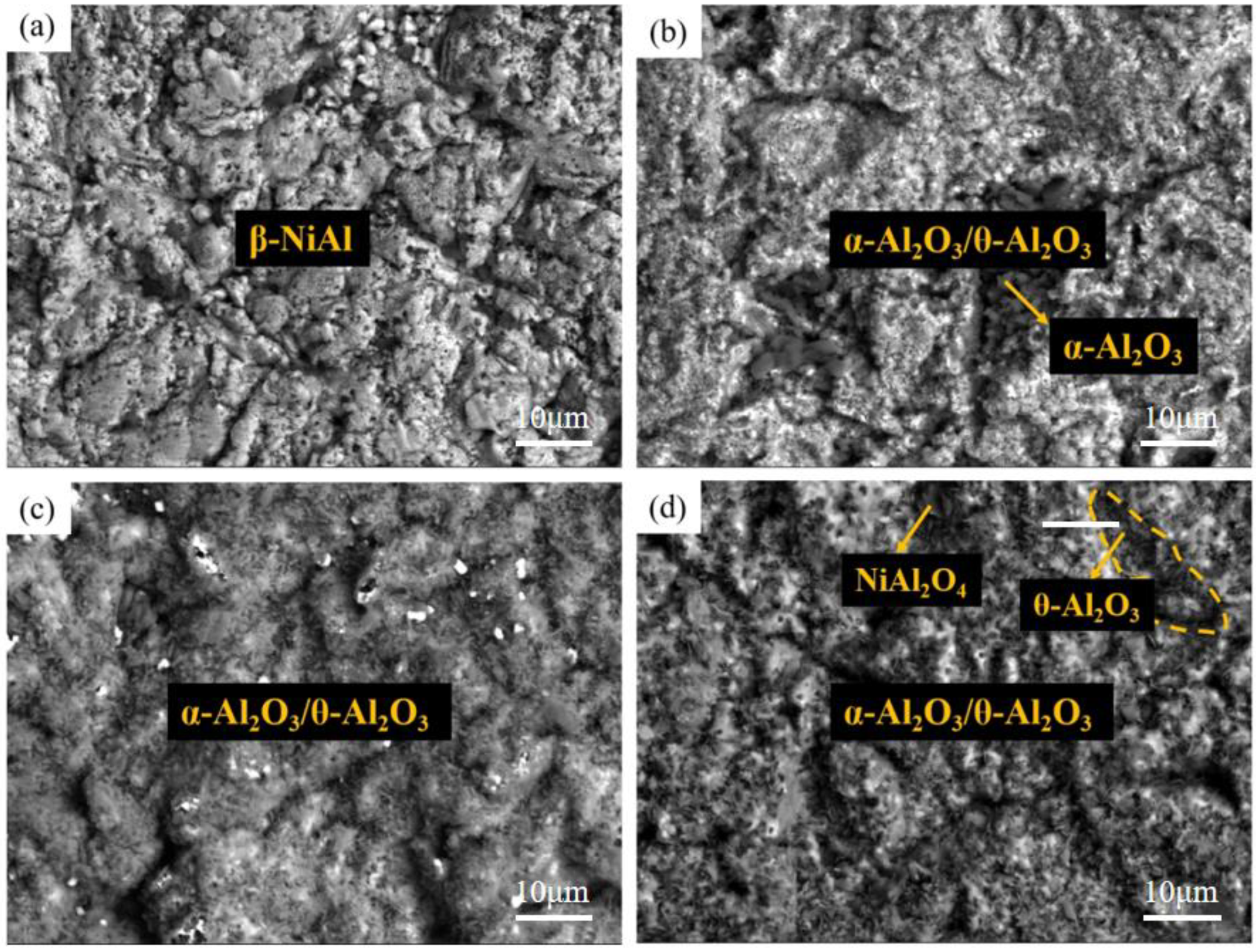
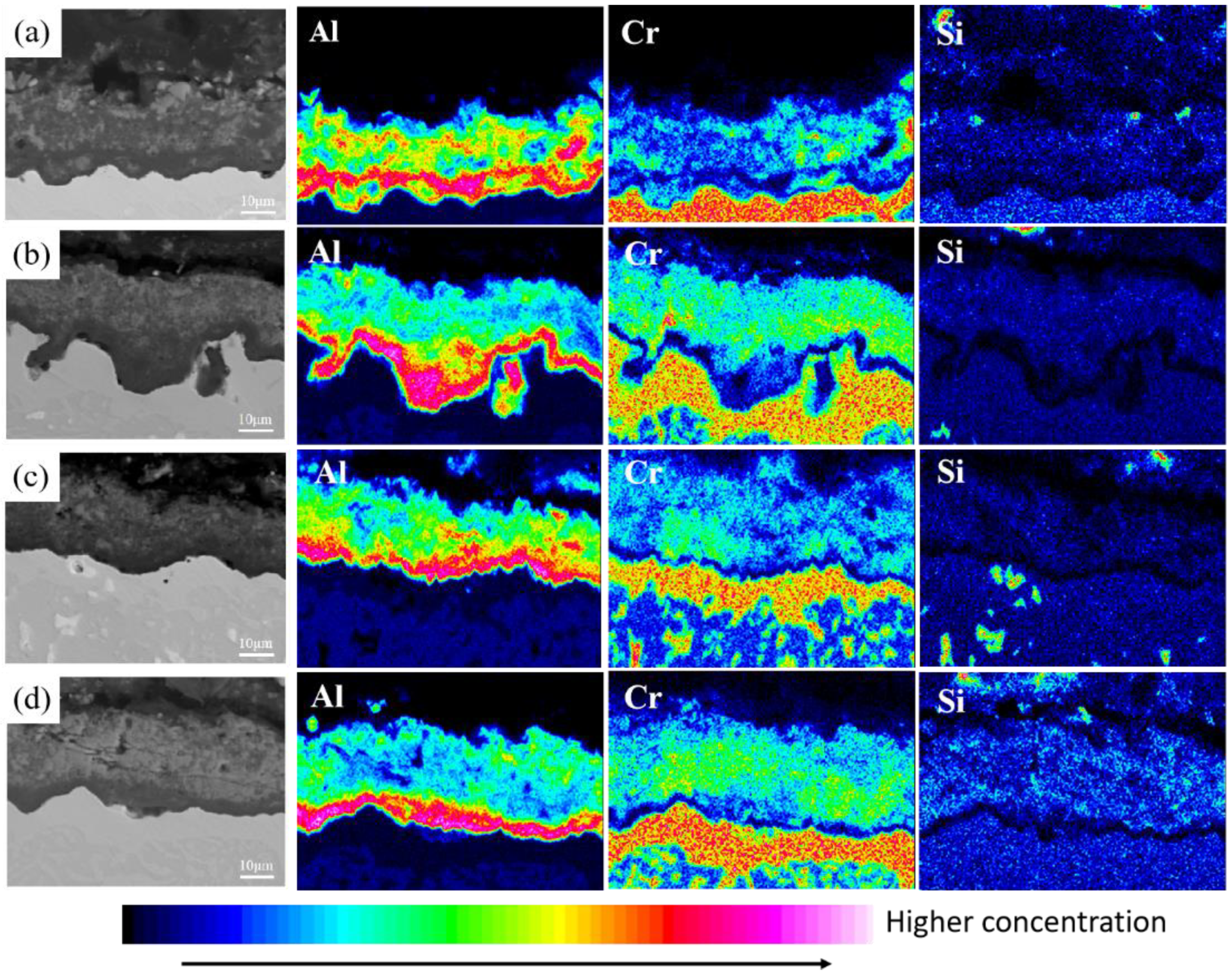
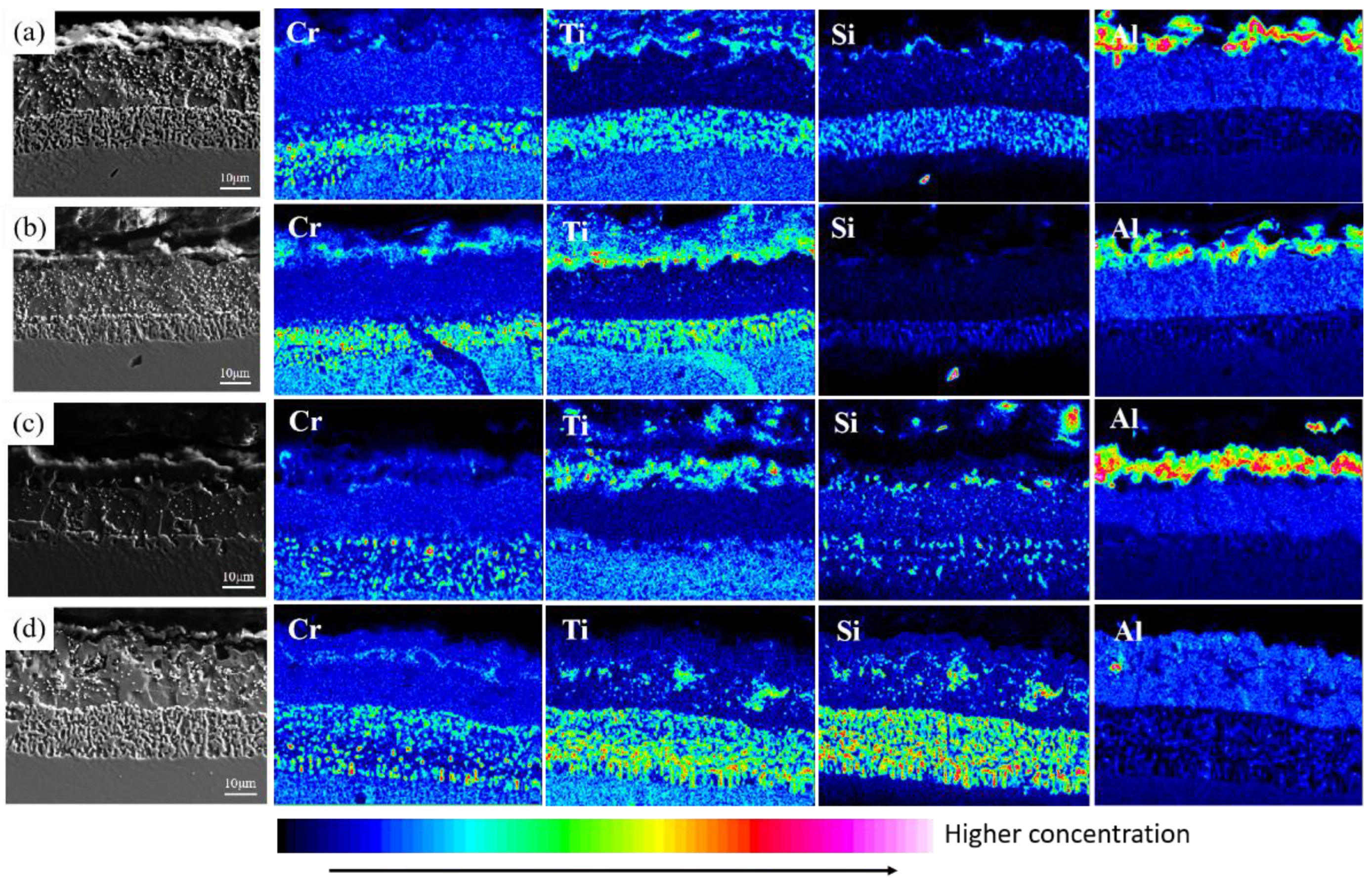
3.1. Microstructure of Coatings at Different Pre-Oxidation Temperatures
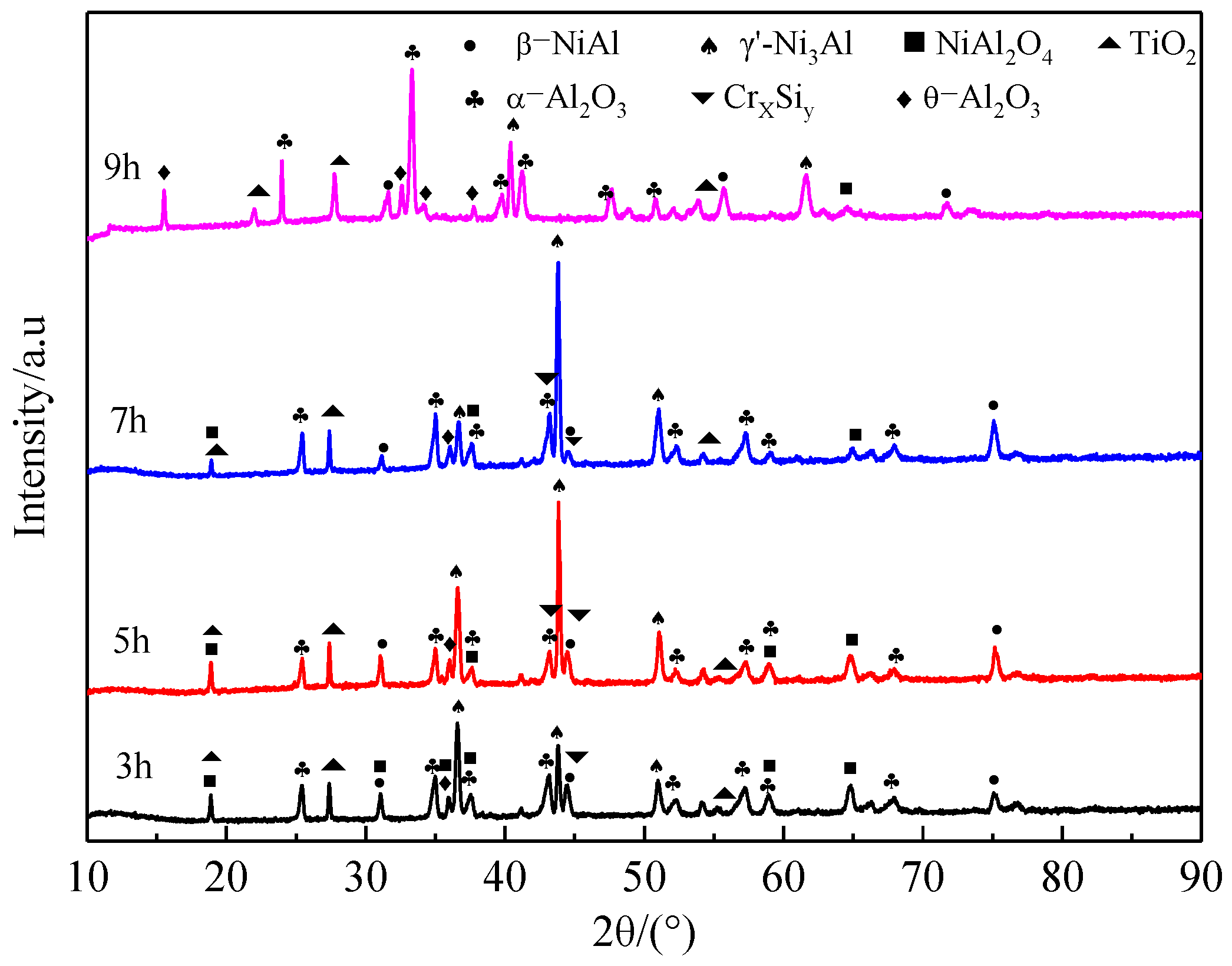
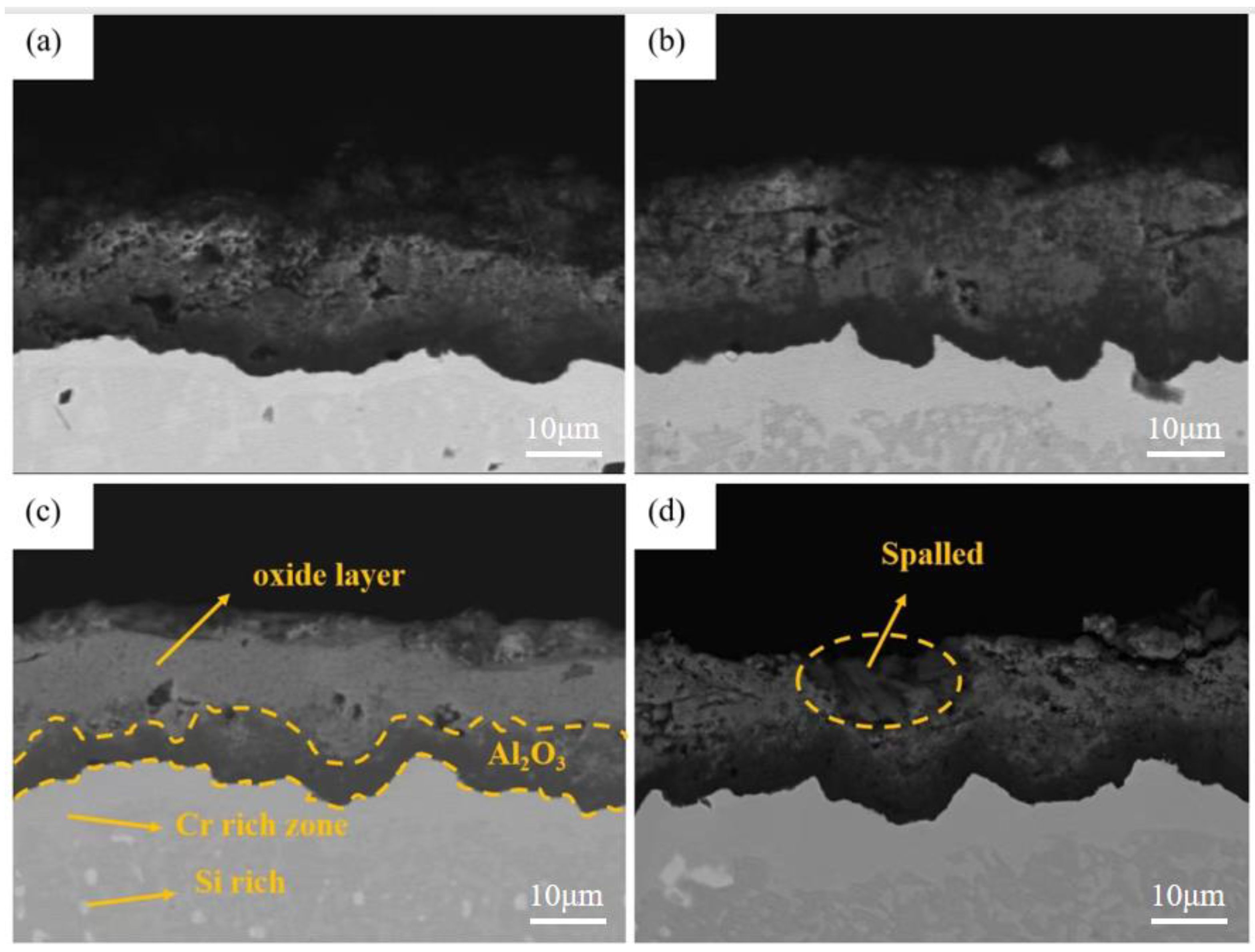
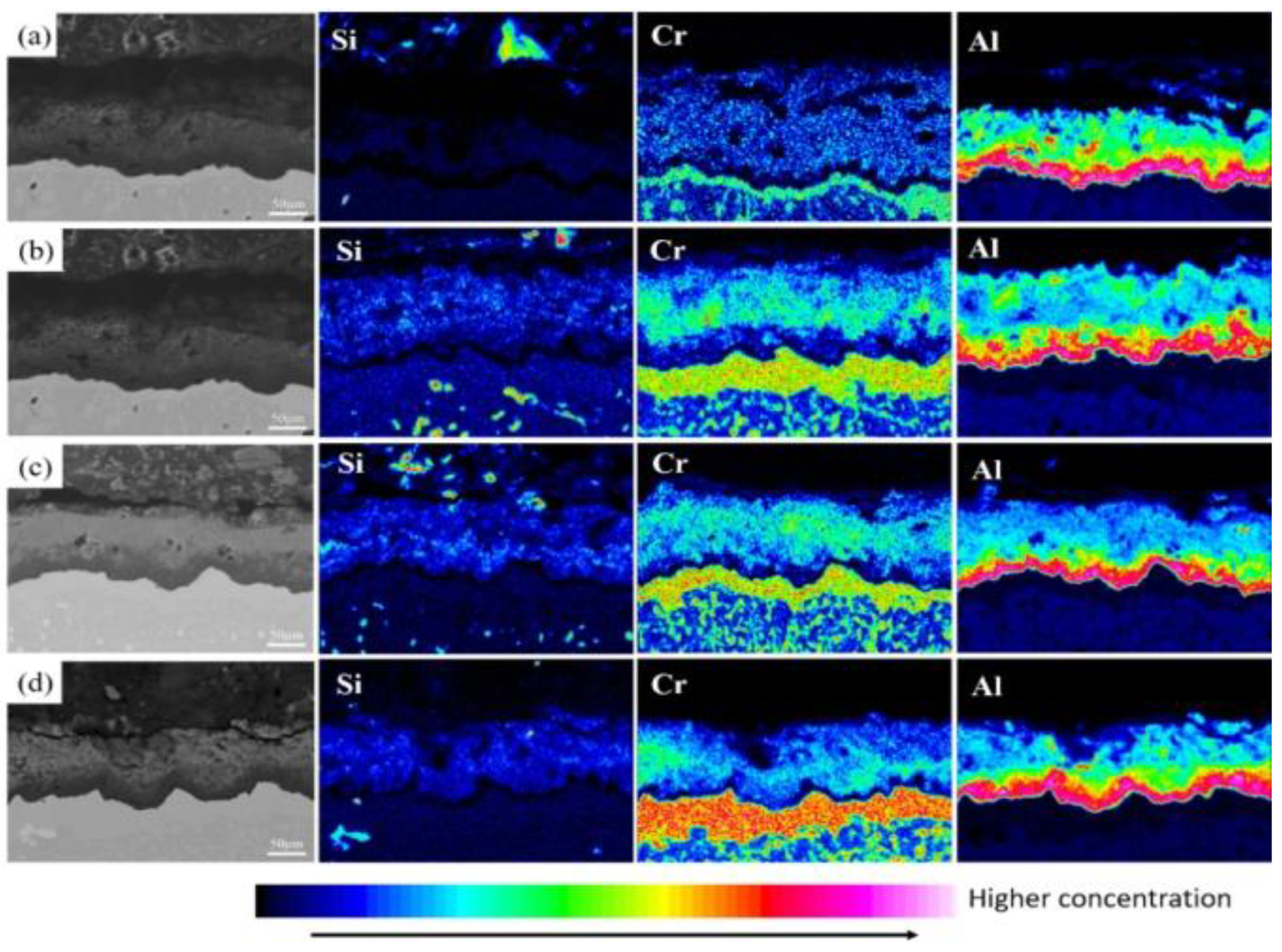
3.2. Microstructure of Coatings at Different Pre-Oxidation Times
4. Discussion
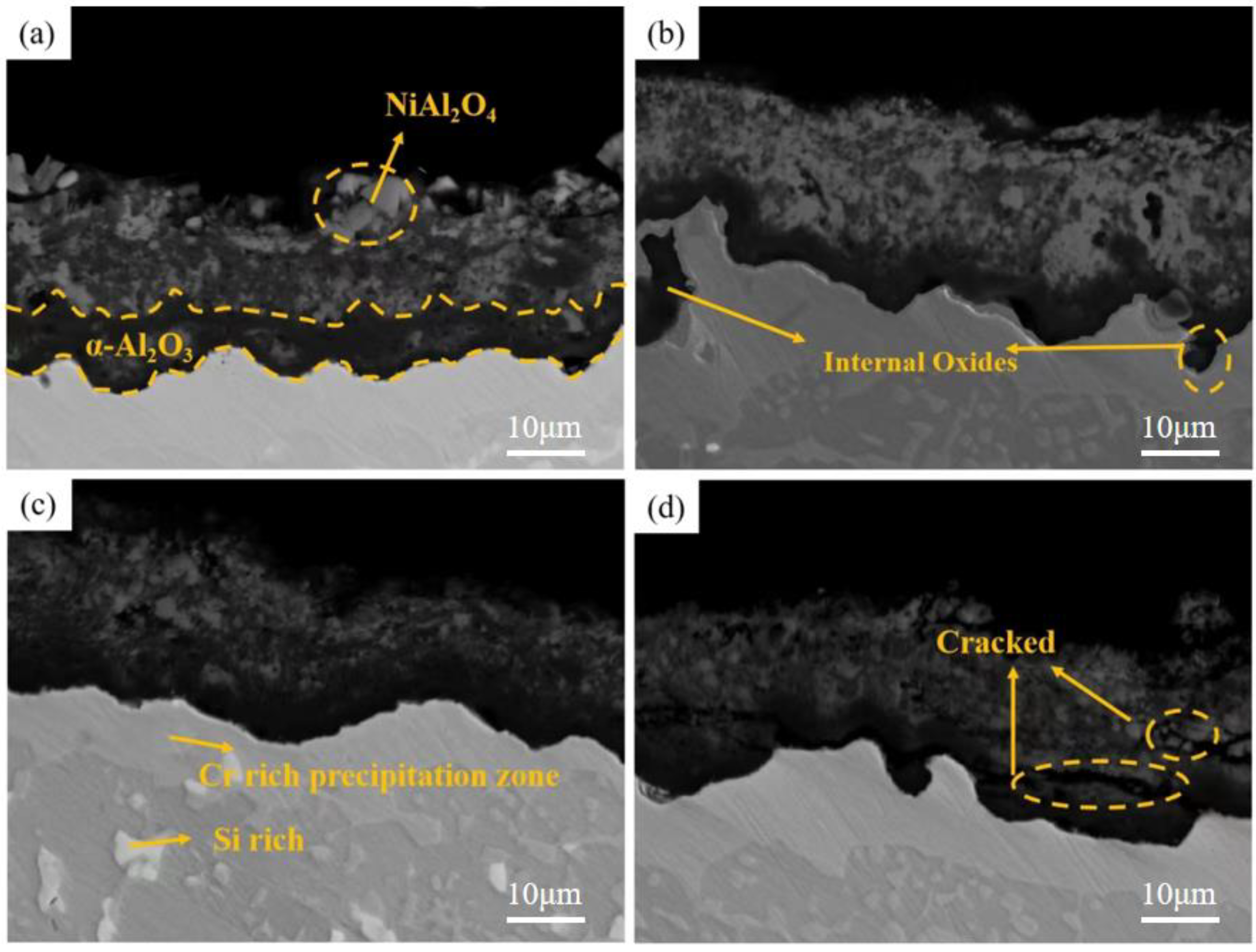
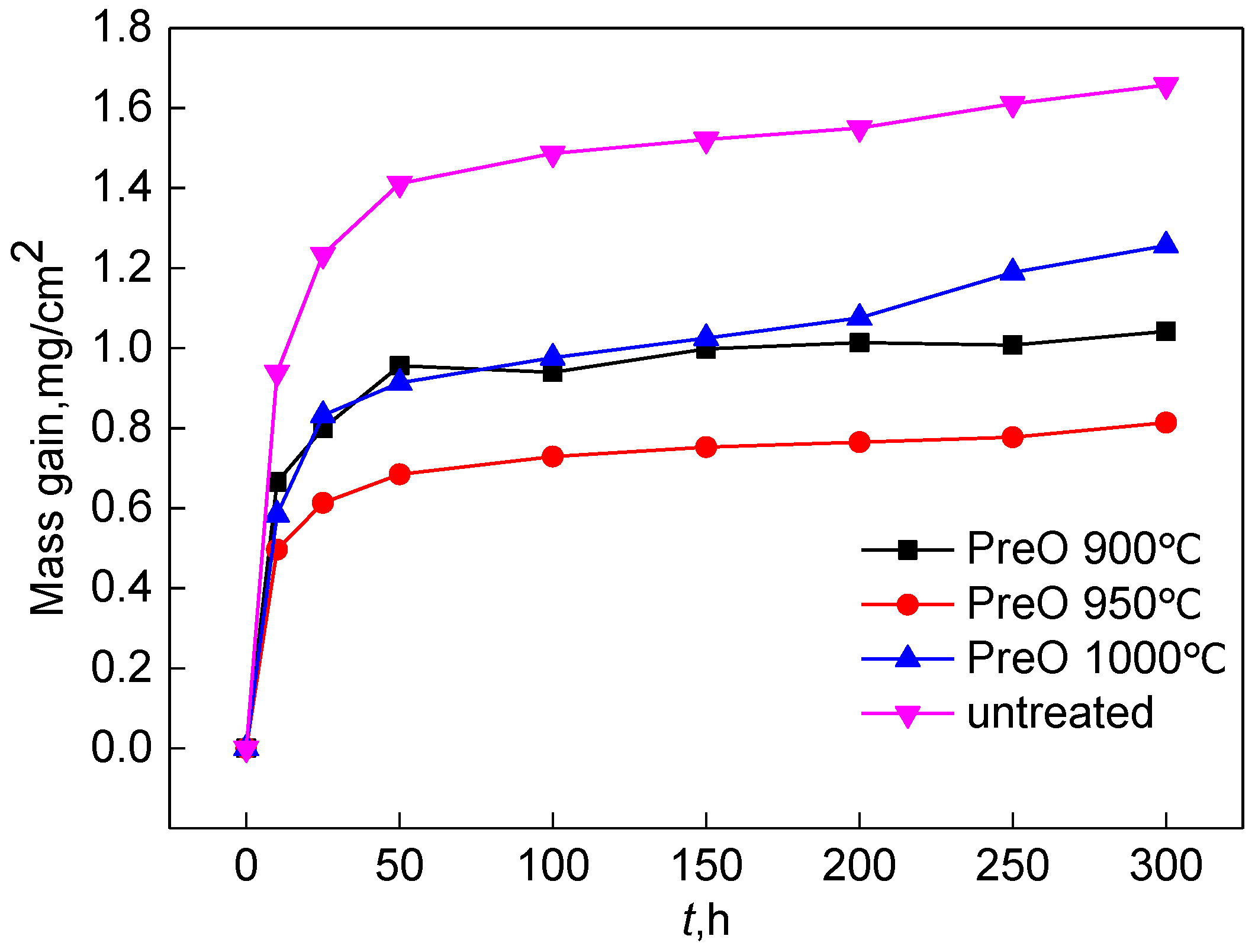
4.1. Effect of Pre-Oxidation Temperature on High-Temperature Oxidation Behavior of Al-Si Coating
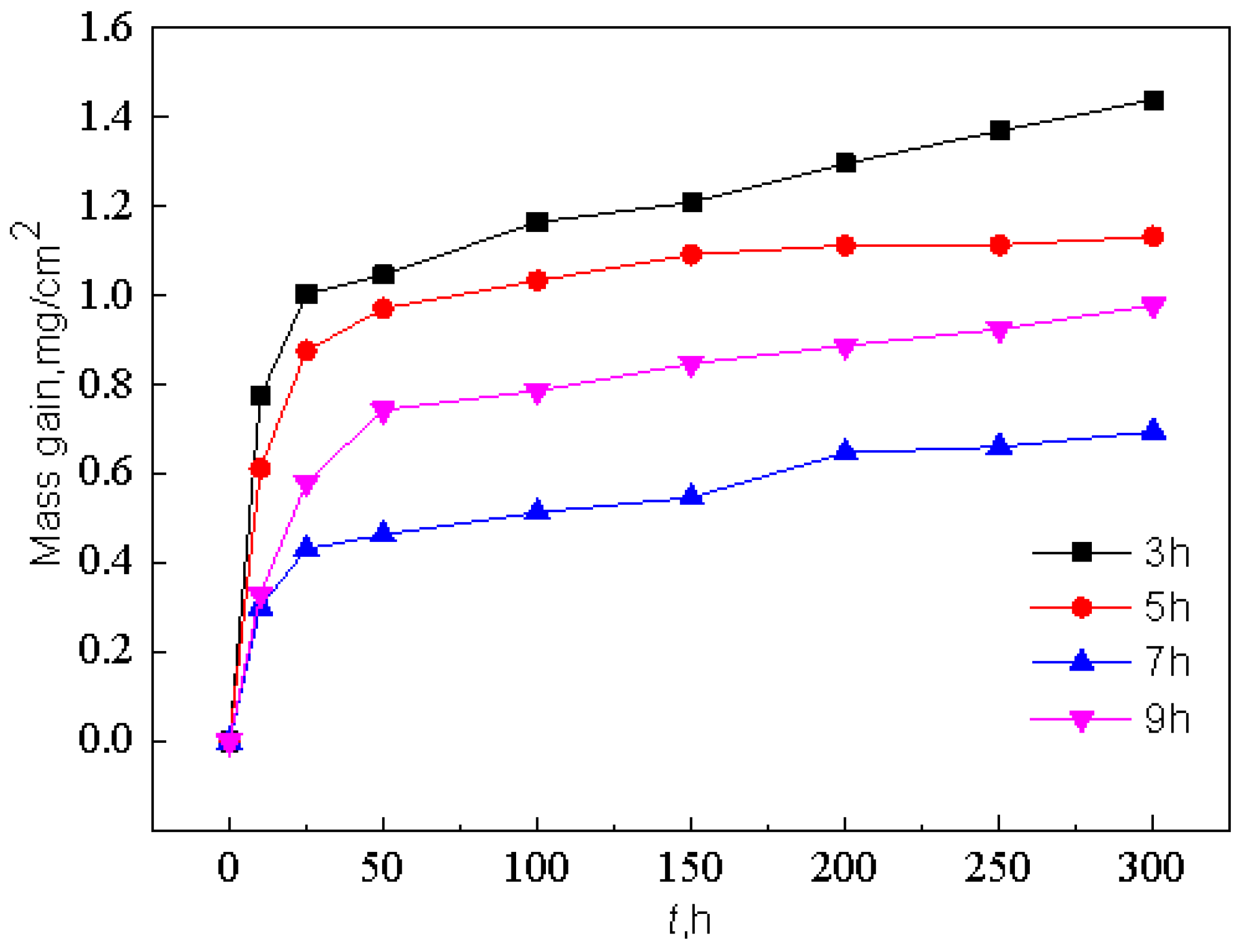
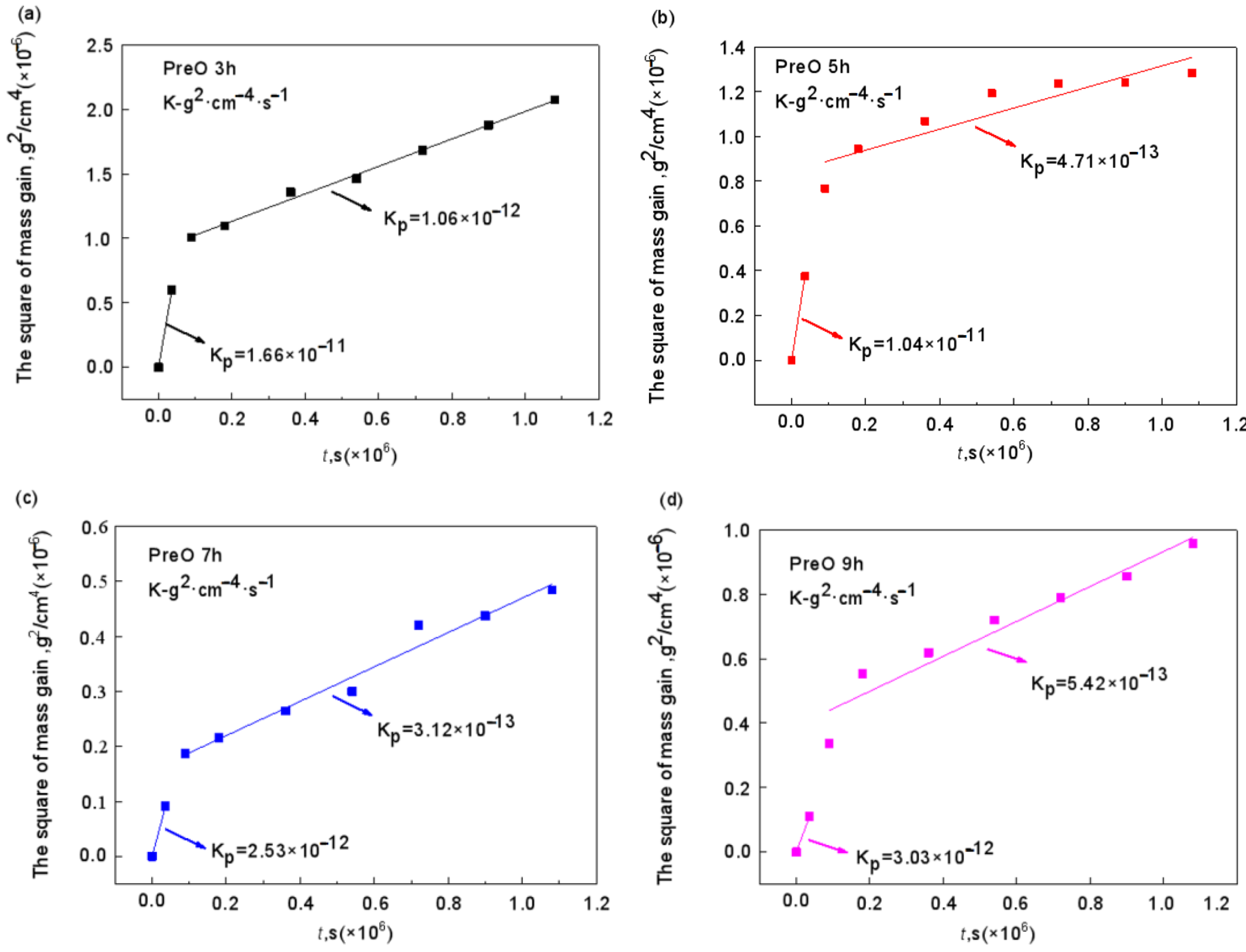
4.2. Effect of Pre-Oxidation Time on High-Temperature Oxidation Behavior of Al-Si Coating
4.3. Mechanism of Pre-Oxidation on High-Temperature Oxidation Behavior of Al-Si Coatings
5. Conclusions
- (1)
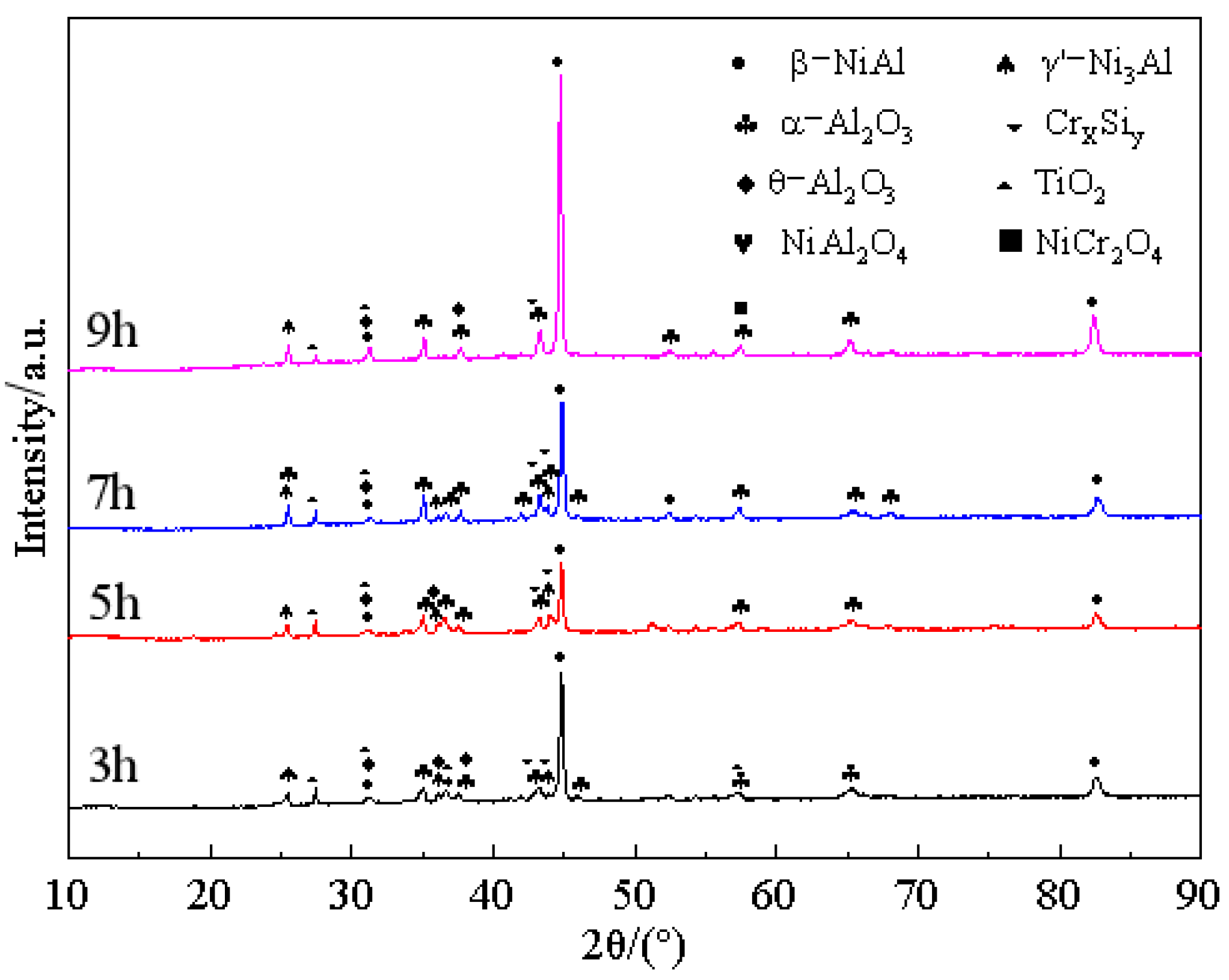
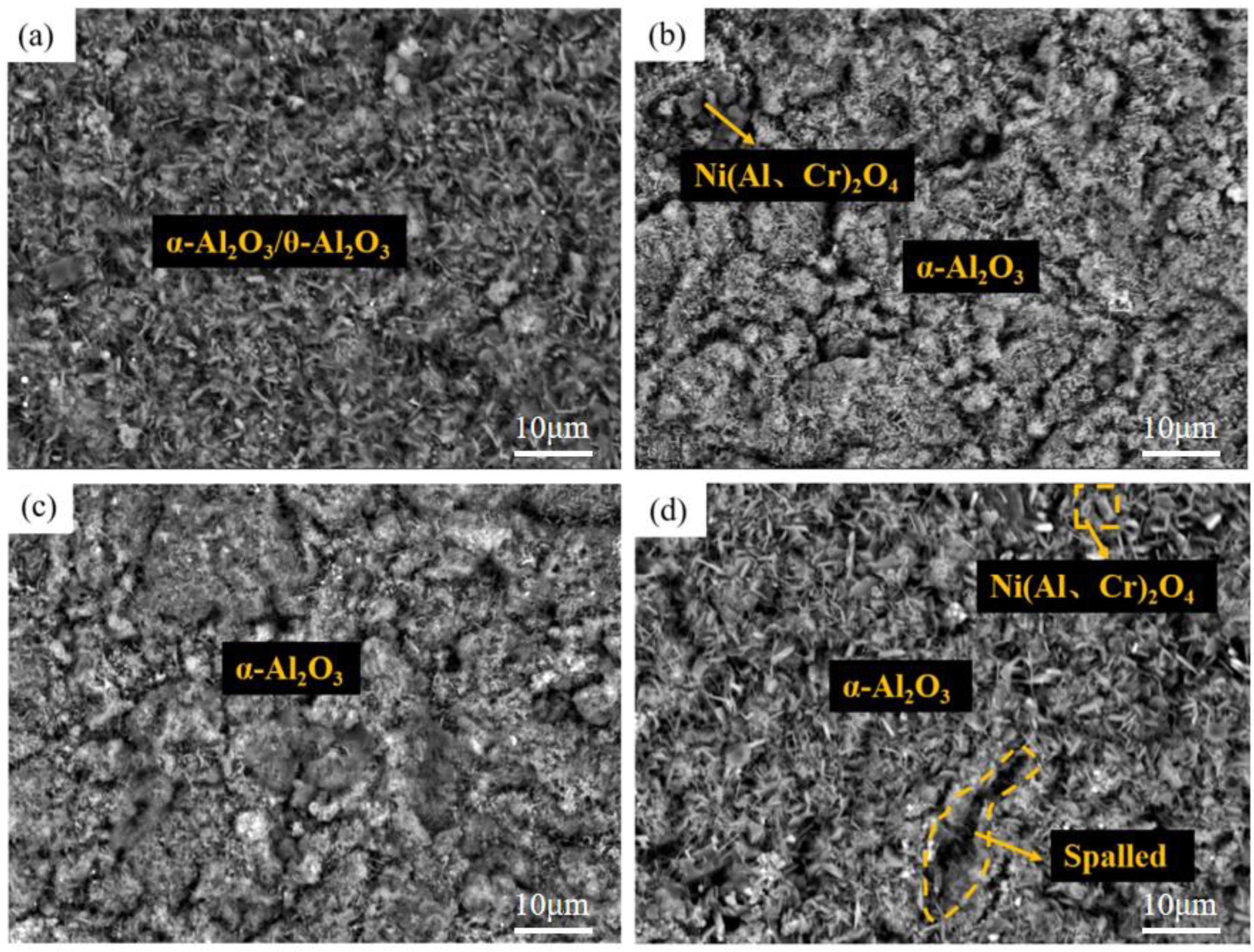
- A layer of α-Al2O3 and θ-Al2O3 oxidation film is formed on the surface of the pre-oxidized Al-Si coating. Within a certain range of pre-oxidation temperature, with an increase in the pre-oxidation temperature, the oxidation mass gain of the coating decreases continuously, and the high-temperature oxidation resistance increases. After 300 h of oxidation, the oxide film on the surface is still continuous and dense, and no peeling phenomenon occurs. When the pre-oxidation temperature is 1000 °C, the decomposition of Cr2O3 is accelerated, which leads to thinning of the oxide film, an increase in the internal stress between the oxide films and a decrease in the high-temperature oxidation resistance, but it is still higher than that of the Al-Si coating without pre-oxidation.
- (2)
- Under constant temperature oxidation at 1000 °C, the Al-Si coating pre-oxidized at 950 °C for 7 h has the best high-temperature oxidation resistance. Pre-oxidation for 7 h can promote formation of more α-Al2O3 oxides on the coating surface. After 300 h of oxidation, the oxide film on the coating surface still remains relatively continuous and dense. After 9 h of pre-oxidation, the oxidation mass gain of the coating is also significantly reduced, but, at the same time, the element diffusion between the coating and matrix and the consumption of Al elements are accelerated. After 300 h of oxidation, NiAl2O4 spinel oxide with poor oxidation resistance is generated on the surface of the coating, resulting in a reduction in the high-temperature oxidation resistance of the coating.
- (3)
- The pre-oxidation process can promote transformation of θ-Al2O3 to α-Al2O3 and reduce the effect of the transition of metastable θ-Al2O3 to α-Al2O3 in the early oxidation stage. After the pre-oxidation treatment of Al-Si coating, the formation of Si-rich M6C prevents interdiffusion of the elements between the substrate and coating and reduces the large consumption of Al in the oxidation process so as to ensure the continuous and dense oxide film formed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, H.R.; Li, H.R.; Cui, Q.Z.; Ren, J.W.; Zhai, Y.J.; Zhang, Q. Overview of Aeroengine and Gas Turbine Blade Coatings. Therm. Spray. Tech. 2019, 11, 82–94. [Google Scholar]
- Li, M.; Suo, J.Q.; Wu, E.P. Inspiration from foreign advanced aeroengine technology. Aviat. Manuf. Technol. 2013, 9, 66–71. [Google Scholar]
- Wang, X.Y.; Xin, L.; Wei, H.; Zhu, S.L.; Wang, F.H. Research Progress of High Temperature Protective Coatings. Corros. Sci. Prot. Technol. 2013, 25, 175–183. [Google Scholar]
- Klein, L.; Shen, Y.; Killian, M.S.; Virtanen, S. Effect of B and Cr on the high temperature oxidation behaviour of novel γ/γ′ -strengthened Cobase superalloys. Corros. Sci. 2011, 53, 2713. [Google Scholar] [CrossRef]
- Guo, J.T. Materials Science and Engineering for Superalloys; Science Press: Beijing, China, 2008; p. 664. [Google Scholar]
- Talic, B.; Molin, S.; Hendriksen, P.V.; Lein, H.L. Effect of pre-oxidation on the oxidation resistance of Crofer 22 APU. Corros. Sci. 2018, 138, 189. [Google Scholar] [CrossRef]
- Ou, D.R.; Cheng, M.J. Effect of pre-oxidation on the oxidation resistance of spinel-coated Fe-Cr ferritic alloy for solid oxide fuel cell applications. J. Power Sources 2014, 247, 84. [Google Scholar] [CrossRef]
- Li, Z.; Qian, S.; Wei, W.; Kuang, X.; Lei, Z. Effect of preoxidation temperature on high temperature cyclic oxidation resistance of GH3128 superalloy. Rare Met. Mater. Eng. 2012, 41, 1980. [Google Scholar]
- Yfya, B.; Cyja, C.; Zyz, D.; Zbb, A.; Mhc, C.; Slz, A.; Fhwa, C. Hot corrosion behaviour of single-phase platinum-modified aluminide coatings: Effect of Pt content and pre-oxidation. Corros. Sci. 2017, 127, 82–90. [Google Scholar]
- Li, Q.; Zhang, D.; Song, P.; Li, Z.; Lu, J. Influence of Pre-Oxidation on High Temperature Oxidation and Corrosion Behavior of Ni-Based Aluminide Coating in Na2SO4 Salt at 1050 °C. Front. Mater. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Peng, J.; Huang, T.; Song, P.; Huang, W.; Chen, R. Effect of platinum and pre-oxidation on the hot corrosion behavior of aluminide coating with NaCl at 1050 °C. Mater. Res. Express 2020, 7, 116–121. [Google Scholar] [CrossRef]
- Gao, B.; Wang, L.; Song, X.; Liu, Y.; Yang, S.Y.; Qian, Y.J.Y. Effect of Pre-Oxidation on High Temperature Oxidation and Corrosion Behavior of Co-Al-W-Based Superalloy. Acta Metall. Sin. 2019, 55, 9. [Google Scholar]
- Bai, Y.; Liu, Z.D.; Xie, J.X.; Bao, H.S.; Chen, Z.Z. Effect of Pre-Oxidation Treatment on the Behavior of High Temperature Oxidation in Steam of G115 Steel. Acta Metall. Sin. 2018, 54, 10. [Google Scholar]
- Tu, J.P.; Mao, Z.Y.; Li, Z.Z. High Temperature Corrosion of Ni-Cr-Ce Alloy in Chlorine Gas after Preoxidation in Air at 700 °C. J. Chin. Soc. Corros. Prot. 1994, 14, 4. [Google Scholar]
- Taniguchi, S.; Andoh, A. Improvement in the Oxidation Resistance of an Al-Deposited Fe–Cr–Al Foil by Preoxidation. Oxid. Met. 2002, 58, 545. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Lima, R.S.; Patnaik, P.C. Pre-oxidation and TGO growth behaviour of an air-plasma-sprayed thermal barrier coating. Surf. Coat. Technol. 2008, 202, 3787–3796. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Q.S.; Liu, Y.B.; Li, Y.K. Effect of Vacuum Pre-oxidation Treatment on Oxidation Behavior of Thermal Barrier Coating. J. Aeronaut. Mater. 2015, 35, 27–31. [Google Scholar]
- Mo, L.S.; Yang, Z.L. Preoxidizing effect of aluminium silicon coating. J. Chin. Soc. Corros. Pro. 1988, 8, 289–296. [Google Scholar]
- Xu, B.W.; Liu, S.Y.; Zhang, X.; Wang, H.Y.; Cui, W.L. Hot corrosion behavior of pre-alumina silicon coating in mixed sulfate at 1150 °C. Mater. Prot. 2019, 52, 5. [Google Scholar]
- Ning, X.J.; Wang, L.; Wang, Q.S.; Zhang, L.W. Effect of Vacuum Preoxidation on Microstructure and Hot Corrosion Resistance of Cold Sprayed CoNiCrAlY Coatings. J. Mater. Eng. 2013, 3, 12–16. [Google Scholar]
- Sun, Y.; Jin, L.Y.; Gong, Y.D.; Wen, X.L.; Yin, G.Q.; Wen, Q.; Tang, B.J. Experimental evaluation of surface generation and force time-varying characteristics of curvilinear grooved micro end mills fabricated by EDM. J. Manuf. Process. 2022, 73, 799–814. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.C.; Dong, J.X. Oxidation behavior and mechanism of powder metallurgy Rene95 nickel based superalloy between 800 and 1000 °C. Appl. Surf. Sci. 2010, 256, 7510. [Google Scholar] [CrossRef]
- Sun, D.J.; Liang, C.Y.; Shang, J.L.; Yin, J.H.; Song, Y.R.; Li, W.Z.; Liang, T.Q.; Zhang, X.H. Effect of Y2O3 contents on oxidation resistance at 1150 °C and mechanical properties at room temperature of ODS Ni-20Cr-5Al alloy. Appl. Surf. Sci. 2016, 385, 587. [Google Scholar] [CrossRef]
- Göbel, M.; Rahmel, A.; Schötze, M.; Schorr, M.; Wu, W.T. Interdiffusion between the platinum-modified aluminide coating RT 22 and nickel-based singlecrystal superalloys at 1000 and 1200 °C. Mater. High Temp. 1994, 12, 301–309. [Google Scholar] [CrossRef]
- Pint, B.A.; Nagaraj, B.A.; Rosenzweig, M.A. Evaluation of TBC-coated β-NiAl substrates without a bond coat. Off. Sci. Tech. Inf. Tech. Rep. 1996, 9, 51–60. [Google Scholar]
- Pint, B.A. On the formation of interfacial and internal voids in α-Al2O3 scales. Oxid. Met. 1997, 48, 303–328. [Google Scholar] [CrossRef]
- Yang, Z.L.; Yu, X.F. Effect of Si Distribution on Hot Corrosion Behavior of Al-Si Coating. J. Aeronaut. Mater. 1994, 2, 36–40. [Google Scholar]
- Yang, S.W.; Shi, Y.J.; Zhang, T.Y.; Wang, Y.H. Element Diffusion Analysis of Al-Si Coating on K4104 Nickel Base Superalloy During High Temperature Oxidation. Dev. Appl. Mater. 2010, 25, 4–8. [Google Scholar]
- Doychak, J.; Rühle, M. TEM studies of oxidized NiAl and Ni3Al cross sections. Oxid. Met. 1989, 909, 83–96. [Google Scholar] [CrossRef]
- Schumann, E. The effect of Y-ion implantation on the oxidation of β-NiAl. Oxid. Met. 1995, 43, 157–172. [Google Scholar] [CrossRef]









| Co | Cr | Mo | Al | Ti | V | C | Ni |
|---|---|---|---|---|---|---|---|
| 9–11 | 8.5–9.5 | 2.5–3.5 | 4.8–5.7 | 4.1–4.7 | 0.6–0.9 | 0.11–0.20 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lv, H.; Li, Y.; Fan, N. Effect of Pre-Oxidation on High-Temperature Oxidation Behavior of Al-Si Coating on Nickel-Based Superalloy. Materials 2022, 15, 7440. https://doi.org/10.3390/ma15217440
Li Y, Lv H, Li Y, Fan N. Effect of Pre-Oxidation on High-Temperature Oxidation Behavior of Al-Si Coating on Nickel-Based Superalloy. Materials. 2022; 15(21):7440. https://doi.org/10.3390/ma15217440
Chicago/Turabian StyleLi, Yanmei, Haishuang Lv, Yabin Li, and Naiwen Fan. 2022. "Effect of Pre-Oxidation on High-Temperature Oxidation Behavior of Al-Si Coating on Nickel-Based Superalloy" Materials 15, no. 21: 7440. https://doi.org/10.3390/ma15217440
APA StyleLi, Y., Lv, H., Li, Y., & Fan, N. (2022). Effect of Pre-Oxidation on High-Temperature Oxidation Behavior of Al-Si Coating on Nickel-Based Superalloy. Materials, 15(21), 7440. https://doi.org/10.3390/ma15217440







