MXene Quantum Dot/Zeolitic Imidazolate Framework Nanocarriers for Dual Stimulus Triggered Tumor Chemo-Phototherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
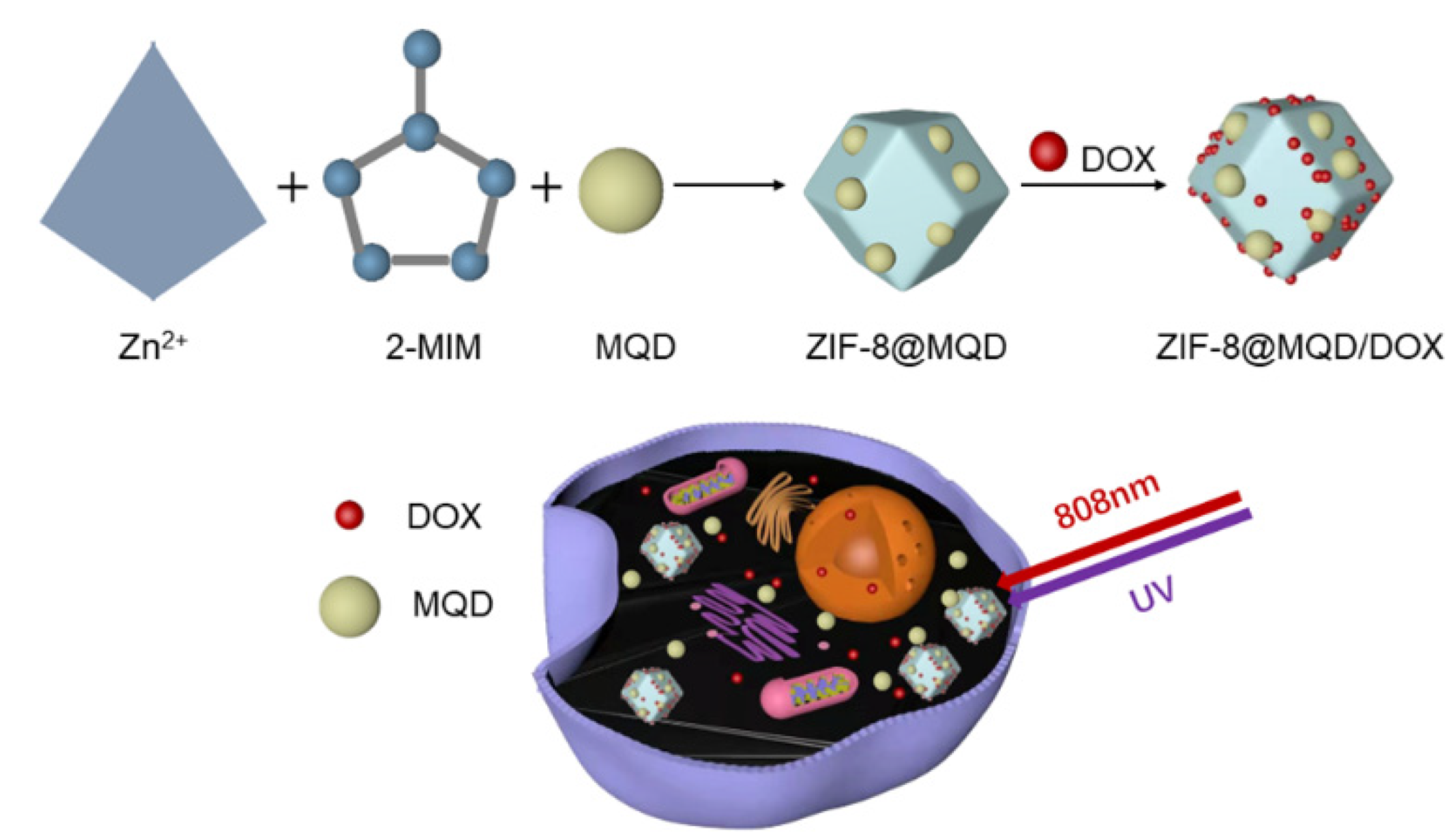
2.2. Synthesis of MQD@ZIF-8
2.3. Materials Characterization
2.4. DOX Loading and Release
2.5. Photothermal Effect of MQD@ZIF-8
2.6. Photodynamic Effect of MQD@ZIF-8
2.7. Cell Culture and Cell Cytotoxicity
2.8. Statistical Analysis
3. Results and Discussions
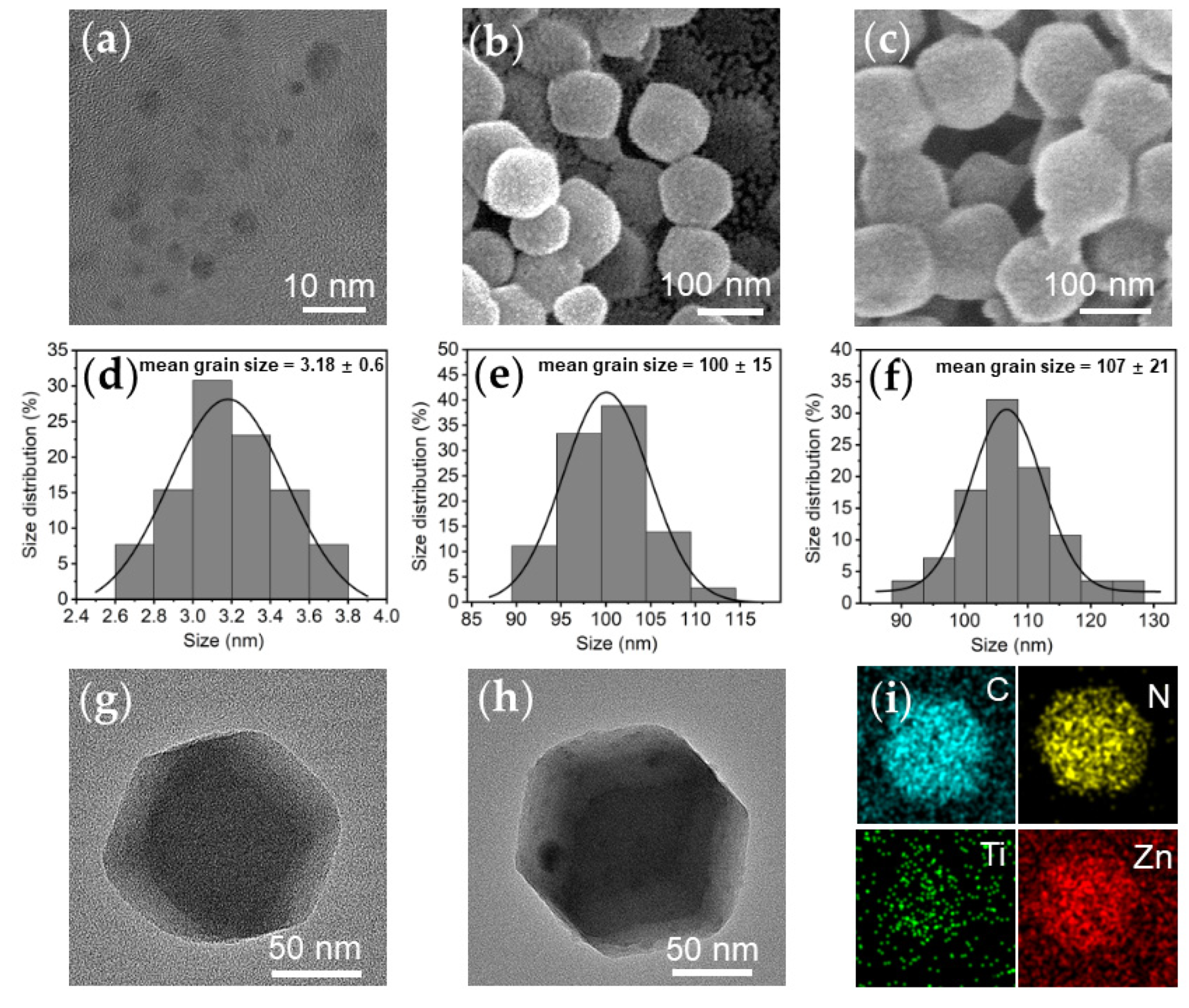
3.1. Samples Characterization
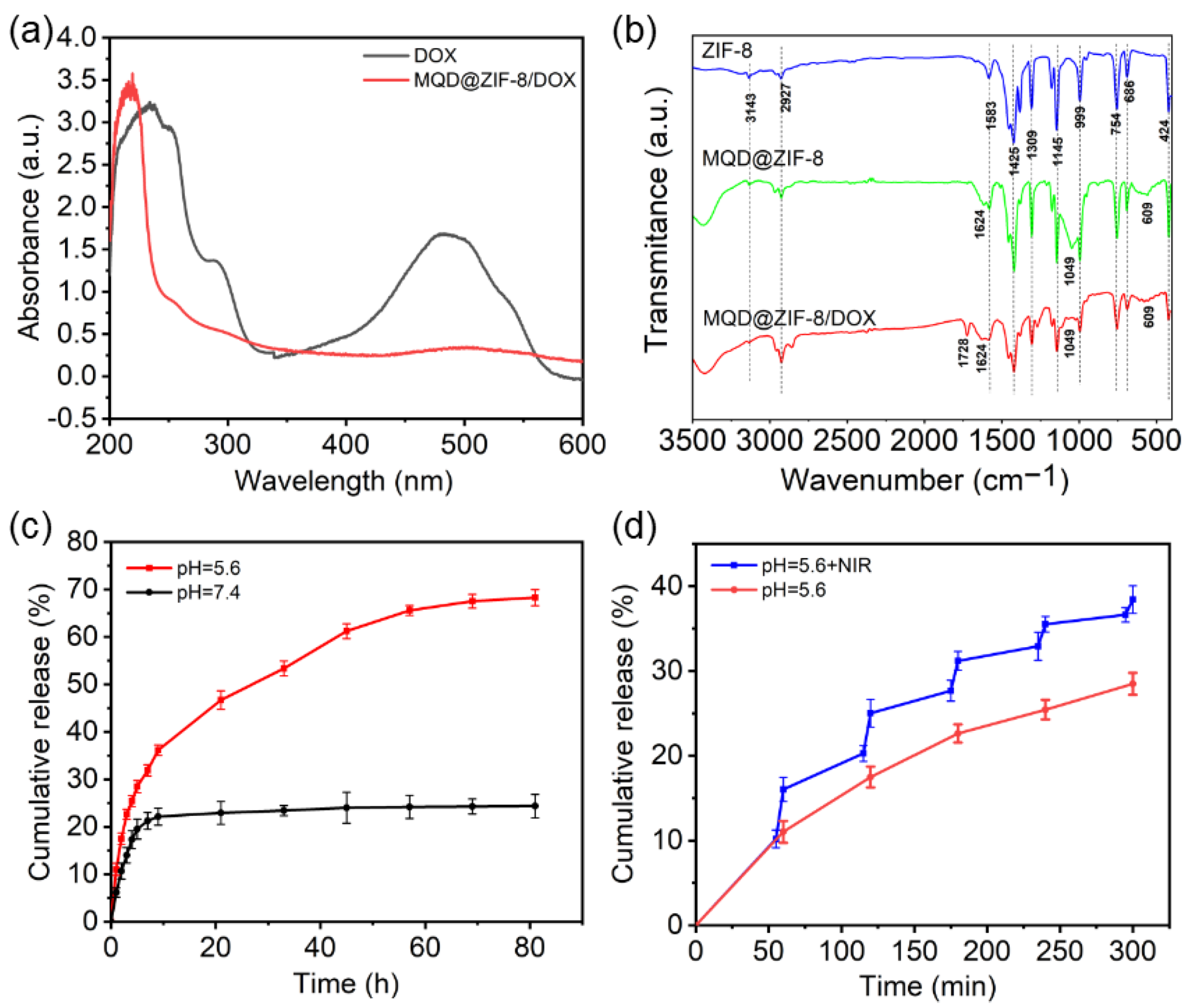
3.2. Loading and Releasing Profiles of DOX
3.3. Analysis of Photothermal Properties of MQD@ZIF-8
3.4. Photodynamic Properties of MQD@ZIF-8
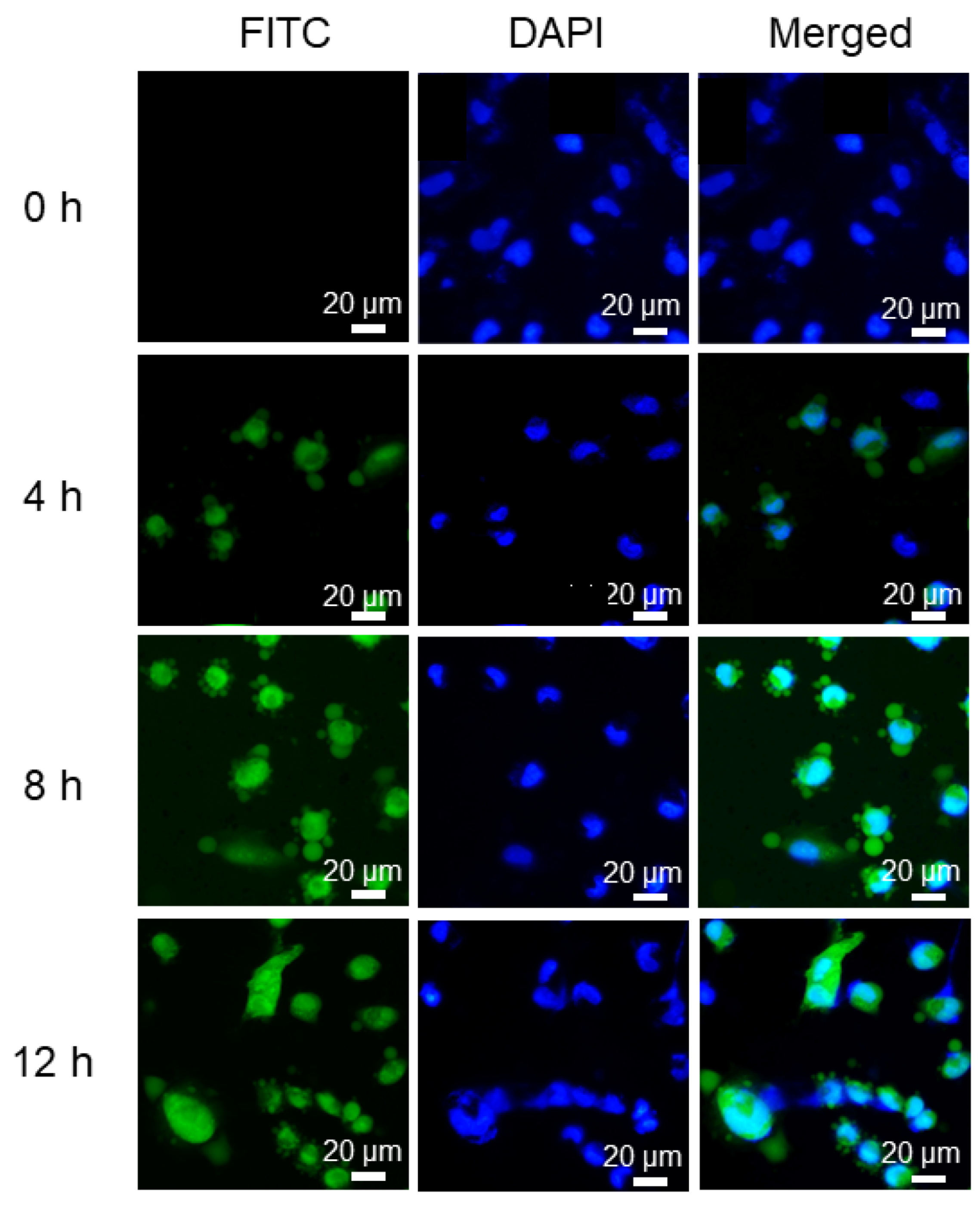
3.5. In Vitro Cellular Uptake
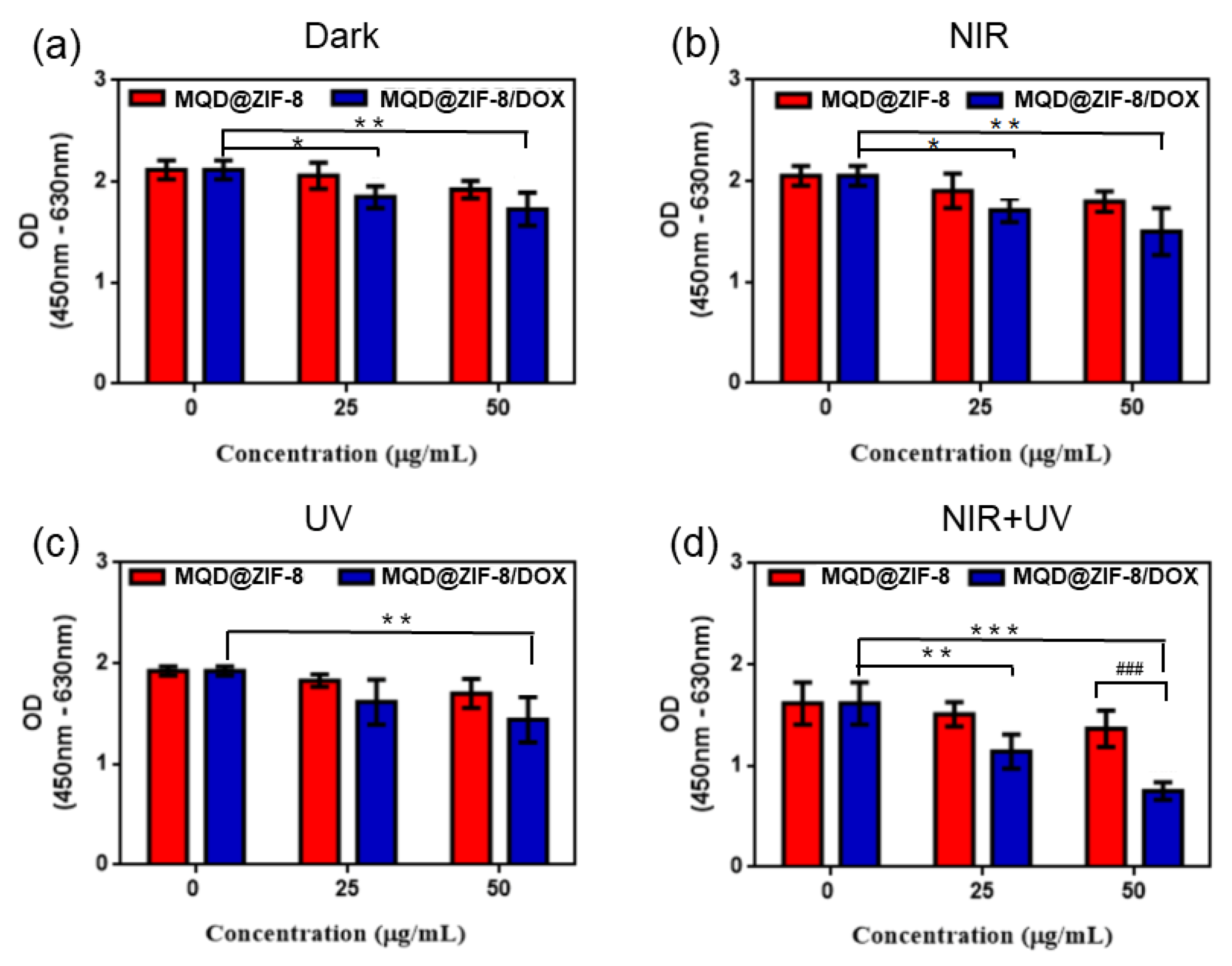
3.6. Cell Cytotoxicity of MQD@ZIF-8
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.Q.; Li, H.; Cao, M.M.; He, S.Y.; Lei, L.; Peng, J.; Chen, W.Q. Cancer burden in China: Trends, risk factors and prevention. Cancer Biol. Med. 2020, 4, 879–895. [Google Scholar] [CrossRef]
- Gao, B.X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Guo, H.; Xia, Y.; Feng, K.; Qu, X.; Wan, F. Surface Engineering of Metal–Organic Framework as pH-/NIR-Responsive Nanocarrier for Imaging-Guided Chemo-Photothermal Therapy. Int. J. Nanomed. 2020, 15, 3235–3250. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, W.; Xu, Z.; Wang, F. An intelligent ZIF-8-gated polydopamine nanoplatform for in vivo cooperatively enhanced combination phototherapy. Chem. Sci. 2020, 11, 1649–1656. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-Loaded Gold Vesicles with Strong Plasmonic Coupling Effect for Imaging-Guided Photothermal/Photodynamic Therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, G.; Zhou, H.; Zhang, F.; Guo, Z.; Liu, C.; Zhang, X.; Zhu, L. Functional long circulating single walled carbon nanotubes for fluorescent/photoacoustic imaging-guided enhanced phototherapy. Biomaterials 2016, 103, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Fei, W.; Ding, D.; Song, C.; Liu, S. TiO2–x Based Nanoplatformfor Bimodal Cancer Imaging and NIR-Triggered Chem/Photodynamic/Photothermal Combination Therapy. Chem. Mater. 2017, 29, 9262–9274. [Google Scholar]
- Jiang, W.; Zhang, H.; Wu, J.; Zhai, G.; Li, Z.; Luan, Y.; Garg, S. CuS@MOF-Based Well-Designed Quercetin Delivery System for Chemo-Photothermal Therapy. ACS Appl. Mater. Inter. 2018, 10, 34513–34523. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Cao, M.; Zhang, X.; Lin, J.; Tian, Q.; Yang, S. pH and Glutathione Synergistically Triggered Release and Self-Assembly of Au Nanospheres for Tumor Theranostics. ACS Appl. Mater. Inter. 2020, 12, 8050–8061. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, Y.; Ji, S.; Ye, Y.; Wu, S.; Liu, J.; Chen, S.; Liang, C. Laser ablation in liquids for the assembly of Se@Au chain-oligomers with long-term stability for photothermal inhibition of tumor cells. J. Colloid Interface Sci. 2020, 566, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, Q.; Deng, G.; Zhao, H.; Zhao, L.; Lu, J.; Liu, X. Se@SiO2@Au-PEG/DOX NCs as a multifunctional theranostic agent efficiently protect normal cells from oxidative damage during photothermal therapy. Dalton Trans. 2020, 49, 2209–2217. [Google Scholar] [CrossRef]
- Dong, L.; Ji, G.; Liu, Y.; Xu, X.; Lei, P.; Du, K.; Song, S.; Feng, J.; Zhang, H. Multifunctional Cu-Ag2S nanoparticles with high photothermal conversion efficiency for photo-acoustic imaging-guided photothermal therapy in vivo. Nanoscale 2018, 10, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Kong, C.; Jiang, C.; Hou, R.; Zhao, X.; Li, J.; Wang, Y.; Gao, Y.; Zhang, H.; Yang, B.; et al. Polydopamine-coated Au-Ag nanoparticle-guided photothermal colorectal cancer therapy through multiple cell death pathways. Acta Biomater. 2019, 83, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Luo, K.; Yin, X.; Lin, X.; Zhu, C. Biomimetic Synthesis of Ag2Se Quantum Dots with Enhanced Photothermal Properties and as "Gatekeepers" to Cap Mesoporous Silica Nanoparticles for Chemo-Photothermal Therapy. Chemi. Asian J. 2019, 14, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Yang, X.; Yang, W.; Guo, Y.; Wu, M.; Liu, Y.; Li, X.; Zhang, X.; Chang, J. PB@Au Core-Satellite Multifunctional Nanotheranostics for Magnetic Resonance and Computed Tomography Imaging in Vivo and Synergetic Photothermal and Radiosensitive Therapy. ACS Appl. Mater. Inter. 2017, 9, 1263–1272. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Ding, L.; Li, X.W.; Wu, Y.; Tang, J.H. Prussian blue-modified ferritin nanoparticles for effective tumor chemo-photothermal combination therapy via enhancing reactive oxygen species production. J. Biomater. Appl. 2019, 33, 1202–1213. [Google Scholar] [CrossRef]
- Su, Y.Y.; Teng, Z.; Yao, H.; Wang, S.J.; Tian, Y.; Zhang, Y.L.; Liu, W.F.; Tian, W.; Zheng, L.J.; Lu, N.; et al. A Multifunctional PB@mSiO2-PEG/DOX Nanoplatform for Combined Photothermal-Chemotherapy of Tumor. ACS Appl. Mater. Inter. 2016, 8, 17038–17046. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Ma, D.; Xu, S.; Wu, W.; Xu, L.; Panahandeh-Fard, M.; Zhu, X.; Wang, B.; Liu, B. Tumor-Activated and Metal-Organic Framework Assisted Self-Assembly of Organic Photosensitizers. ACS Nano 2020, 14, 13056–13068. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Gong, J.; Ye, W.; Huang, X.; Chen, J. Polyhydroxy Fullerene-loaded ZIF-8 Nanocomposites for Better Photodynamic Therapy. Z. Fur Anorg. Allg. Chem. 2020, 646, 1900–1903. [Google Scholar] [CrossRef]
- Mou, J.; Lin, T.; Huang, F.; Chen, H.; Shi, J. Black titania-based theranostic nanoplatform for single NIR laser induced dual-modal imaging-guided PTT/PDT. Biomaterials 2016, 84, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, G.; Chen, L.; Cheng, L.; Wang, L.; Ge, C.; Liu, Z. FeS nanoplates as a multifunctional nano-theranostic for magnetic resonance imaging guided photothermal therapy. Biomaterials 2015, 38, 1–9. [Google Scholar] [CrossRef]
- Shu, G.; Wang, H.; Zhao, H.X.; Zhang, X. Microwave-Assisted Synthesis of Black Titanium Monoxide for Synergistic Tumor Phototherapy. ACS Appl. Mater. Inter. 2019, 11, 3323–3333. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2-xS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper(I) Sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, J.; Liu, X.; Wang, Y.; Li, C.; Fang, J.; Chang, B.; Qi, Y.; Li, Y.; Ning, G. Titanium carbide/zeolite imidazole framework-8/polylactic acid electrospun membrane for near-infrared regulated photothermal/photodynamic therapy of drug-resistant bacterial infections. J. Colloid Interface Sci. 2021, 599, 390–403. [Google Scholar] [CrossRef]
- Xu, D.; You, Y.; Zeng, F.; Wang, Y.; Liang, C.; Feng, H.; Ma, X. Disassembly of Hydrophobic Photosensitizer by Bio-degradable Zeolitic Imidazolate Framework-8 for Photodynamic Cancer Therapy. ACS Appl. Mater. Inter. 2018, 10, 15517–15523. [Google Scholar] [CrossRef]
- Zhang, J.F.; Li, G.R.; Zhang, Y.G.; Zhang, W.; Wang, X.; Zhao, Y.; Li, J.D.; Chen, Z.W. Vertically rooting multifunctional tentacles on carbon scaffold as efficient polysulfide barrier toward superior lithium-sulfur batteries. Nano Energy 2019, 64, 103905. [Google Scholar] [CrossRef]
- Li, H.P.; Sun, L.C.; Zhang, Y.G.; Tan, T.Z.; Wang, G.K.; Bakenov, Z. Enhanced cycle performance of Li/S battery with the reduced graphene oxide/activated carbon functional interlayer. J. Energy Chem. 2017, 26, 1276–1281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.G.; Zhao, Y.; Konarov, A.; Lin, Z.; Chen, P. Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulfur cathode performance in Li/S batteries. J. Alloy. Compd. 2015, 619, 298–302. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Bakenov, Z.; Tuiyebayeva, M.; Konarov, A.; Chen, P. Synthesis of Hierarchical Porous Sulfur/Polypyrrole/Multiwalled Carbon Nanotube Composite Cathode for Lithium Batteries. Electrochim. Acta 2014, 143, 49–55. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Zhang, Y.G.; Ricardez-Sandoval, L.; Wang, X.; Li, J.D. Construction of Oxygen-Deficient La(OH)3 Nanorods Wrapped by Reduced Graphene Oxide for Polysulfide Trapping toward High-Performance Lithium/Sulfur Batteries. ACS Appl. Mater. Inter. 2019, 11, 23271–23279. [Google Scholar] [CrossRef] [PubMed]
- Ce´line, C.; Sandrine, L.; Delphine, B.; Fabien, B.; Vincent, L.; Emmanuel, S.; Anne-Agathe, Q.; Nicolas, B. Catalysis of transesterification by a nonfunctionalized metal-organic framework: Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar]
- Wu, X.K.; Yin, Z.H.; Yang, Y.W.; Wang, Z.B. ZIF-8/GO sandwich composite membranes through a precursor conversion strategy for H2/CO2 separation. J. Membr. Sci. 2022, 647, 120291. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Wang, X.; Yang, D.; Yang, C.; Lou, Y.; Chen, J.; He, N. Near-infrared light-induced dissociation of zeolitic imidazole framework-8 (ZIF-8) with encapsulated CuS nanoparticles and their application as a therapeutic nanoplatform. Chem. Comm. 2016, 52, 12210–12213. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, H.; Tan, L.; Huang, Z.; Wu, Q.; Ren, X.; Ren, J.; Meng, X. Microwave-Activated Mn-Doped Zirconium Metal Organic Framework Nanocubes for Highly Effective Combination of Microwave Dynamic and Thermal Therapies Against Cancer. Acs Nano 2018, 12, 2201–2210. [Google Scholar] [CrossRef]
- Chen, W.H.; Luo, G.F.; Vazquez-Gonzalez, M.; Cazelles, R.; Sohn, Y.S.; Nechushtai, R.; Mandel, Y.; Willner, I. Glucose-Responsive Metal-Organic-Framework Nanoparticles Act as “Smart” Sense-and-Treat Carriers. ACS Nano 2018, 12, 7538–7545. [Google Scholar] [CrossRef]
- Li, B.; Ma, J.G.; Cheng, P. Silica-Protection-Assisted Encapsulation of Cu2O Nanocubes into a Metal-Organic Framework (ZIF-8) To Provide a Composite Catalyst. Angew. Chem. Int. Ed. 2018, 130, 6950–6953. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Gao, Y.; Sun, S.J.; Li, Z.H.; Wu, A.G.; Zeng, L.Y. pH-Responsive metal-organic framework encapsulated gold nanoclusters with modulated release to enhance photodynamic therapy/chemotherapy in breast cancer. J. Mater. Chem. B 2020, 8, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Comm. 2011, 47, 12578–12591. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, X.; Wang, S.; Lei, C.; Huang, Y.; Nie, Z.; Yao, S. Fluorescent Ti3C2 MXene quantum dots for an alkaline phosphatase assay and embryonic stem cell identification based on the inner filter effect. Nanoscale 2018, 10, 19579–19585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ru, J.; Zhou, P.; Wang, Y.; Shan, C.; Yang, X.; Cao, J.; Liu, W.; Guo, H.; Tang, Y. A smart nanoprobe based on a gadolinium complex encapsulated by ZIF-8 with enhanced room temperature phosphorescence for synchronous oxygen sensing and photodynamic therapy. Dalton Trans. 2019, 48, 16952–16960. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Z.; Deng, T.; Chen, J.; Wen, Y.; Fu, X.; Zhou, L.; Zhu, Z.; Yu, C. A natural polysaccharide mediated MOF-based Ce6 delivery system with improved biological properties for photodynamic therapy. J. Mater. Chem. B 2020, 8, 1481–1488. [Google Scholar] [CrossRef]
- Guo, Y.P.; Wang, H.S.; Feng, X.; Zhao, Y.B.; Liang, C.Y. 3D MXene microspheres with honeycomb architecture for tumor photothermal/photodynamic/chemo combination therapy. Nanotechnology 2021, 32, 195701. [Google Scholar] [CrossRef]
- Wang, S.; Shang, L.; Li, L.; Yu, Y.; Chi, C.; Wang, K.; Zhang, J.; Shi, R.; Shen, H.; Waterhouse, G.I.N.; et al. Metal-Organic-Framework-Derived Mesoporous Carbon Nanospheres Containing Porphyrin-Like Metal Centers for Conformal Phototherapy. Adv. Mater. 2016, 28, 8379–8387. [Google Scholar] [CrossRef]
- Wu, Q.; Niu, M.; Chen, X.; Tan, L.; Fu, C.; Ren, X.; Ren, J.; Li, L.; Xu, K.; Zhong, H.; et al. Biocompatible and biodegradable zeolitic imidazolate framework/polydopamine nanocarriers for dual stimulus triggered tumor thermo-chemotherapy. Biomaterials 2018, 162, 132–143. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Liu, C.; Han, B. Preparation of a one-dimensional nanorod/metal organic framework Janus nanoplatform via side-specific growth for synergistic cancer therapy. Biomater. Sci. 2019, 7, 1696–1704. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Li, M.; Wang, J.; Zou, X.; Wang, H.; Wang, D.; Zhou, H.; Yang, L.; Gao, W.; Liang, C. MXene Quantum Dot/Zeolitic Imidazolate Framework Nanocarriers for Dual Stimulus Triggered Tumor Chemo-Phototherapy. Materials 2022, 15, 4543. https://doi.org/10.3390/ma15134543
Feng X, Li M, Wang J, Zou X, Wang H, Wang D, Zhou H, Yang L, Gao W, Liang C. MXene Quantum Dot/Zeolitic Imidazolate Framework Nanocarriers for Dual Stimulus Triggered Tumor Chemo-Phototherapy. Materials. 2022; 15(13):4543. https://doi.org/10.3390/ma15134543
Chicago/Turabian StyleFeng, Xin, Mingjun Li, Jianming Wang, Xianrui Zou, Hongshui Wang, Donghui Wang, Huan Zhou, Lei Yang, Wei Gao, and Chunyong Liang. 2022. "MXene Quantum Dot/Zeolitic Imidazolate Framework Nanocarriers for Dual Stimulus Triggered Tumor Chemo-Phototherapy" Materials 15, no. 13: 4543. https://doi.org/10.3390/ma15134543
APA StyleFeng, X., Li, M., Wang, J., Zou, X., Wang, H., Wang, D., Zhou, H., Yang, L., Gao, W., & Liang, C. (2022). MXene Quantum Dot/Zeolitic Imidazolate Framework Nanocarriers for Dual Stimulus Triggered Tumor Chemo-Phototherapy. Materials, 15(13), 4543. https://doi.org/10.3390/ma15134543




