Investigation of MAO Coatings Characteristics on Titanium Products Obtained by EBM Method Using Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A., Jr. Biomaterial properties of titanium dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Singh, K. Review on titanium and titanium-based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M. Additive Manufacturing Process in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Buj-Corral, I.; Tejo-Otero, A.; Fenollosa-Artes, F. Development of AM Technologies for Metals in the Sector of Medical Implants. Metals 2020, 10, 686. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Porous Titanium by Additive Manufacturing: A Focus on Surfaces for Bone Integration. Metals 2021, 11, 1343. [Google Scholar] [CrossRef]
- Tamayo, J.A.; Riascos, M.; Vargas, C.A.; Baena, L.M. Additive manufacturing of Ti6Al4V alloy via electron beam melting for the development of implants for the biomedical industry. Heliyon 2021, 7, e06892. [Google Scholar] [CrossRef] [PubMed]
- Kolamroudi, M.K.; Asmael, M.; Ilkan, M.; Kordani, N. Developments on Electron Beam Melting (EBM) of Ti-6Al-4V: A Review. Trans. Indian Inst. Met. 2021, 74, 783–790. [Google Scholar] [CrossRef]
- Casadebaigt, A.; Hugues, J.; Monceau, D. High temperature oxidation and embrittlement at 500-600 °C of Ti-6Al-4V alloy fabricated by Laser and electron Beam Melting. Corros. Sci. 2020, 175, 108875. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Kravenja, A.K.; Finsgar, M. Analytical Techniques for the Characterization of Bioactive Implants. Biomedicines 2021, 9, 1936. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Andreev, N.; Bublikov, J.; Sitnikov, N.; Sotova, C.; Kutina, N. Investigation of wear mechanisms of multilayer nanostructured wear-resistant coatings during turning of steel. Part 2: Diffusion, oxidation processes and cracking in Ti-TiN-(Ti,Cr,Mo,Al)N coating. Wear 2021, 486–487, 204096. [Google Scholar] [CrossRef]
- Yamanouglu, R.; Bahador, A.; Kondoh, K. Fabrication Methods on Porous Titanium Implants by Powder Metallurgy. Trans. Indian Inst. Met 2021, 74, 2555–2567. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Migranov, M.S.; Minin, I.V.; Shekhtman, S.R.; Suhova, N.A.; Gurin, V.D.; Pivkin, P.M. Nanostructured biocompatible Ti-TiN coating for implants with improved functional properties. Proc. SPIE 2021, 11867, 1186708. [Google Scholar]
- Yadroitsev, I.; Bertrand, P.; Antonenkova, G.; Grigoriev, S.; Smurov, I. Use of track/layer morphology to develop functional parts by selective laser melting. J. Laser Appl. 2013, 25, 052003. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Fedorov, S.V.; Okunkova, A.A.; Pivkin, P.M.; Peretyagin, P.Y.; Ershov, A. Development of dlc-coated solid sialon/tin ceramic end mills for nickel alloy machining: Problems and prospects. Coatings 2021, 11, 532. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.; Wang, X.; Qin, L. Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation. Coatings 2017, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Podrabinnik, P.; Gershman, I.; Mironov, A.; Kuznetsova, E.; Peretyagin, P. Mechanisms Involved in the Formation of Secondary Structures on the Friction Surface of Experimental Aluminium Alloys for Monometallic Journal Bearings. Lubricants 2018, 6, 104. [Google Scholar] [CrossRef] [Green Version]
- Doubenskaia, M.; Pavlov, M.; Grigoriev, S.; Tikhonova, E.; Smurov, I. Comprehensive Optical Monitoring of Selective Laser Melting. J. Laser Micro Nanoeng. 2012, 7, 236–243. [Google Scholar] [CrossRef]
- Sova, A.; Okunkova, A.; Grigoriev, S.; Smurov, I. Velocity of the Particles Accelerated by a Cold Spray Micronozzle: Experimental Measurements and Numerical Simulation. J. Therm. Spray Technol. 2013, 22, 75–80. [Google Scholar] [CrossRef]
- Kotoban, D.; Grigoriev, S.; Okunkova, A.; Sova, A. Influence of a shape of single track on deposition efficiency of 316L stainless steel powder in cold spray. Surf. Coat. Technol. 2017, 309, 951–958. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.; Zhang, J.; Wang, X.; Qin, G.; Zhang, E. Enhanced antibacterial activity of Ti-Cu alloy by selective acid etching. Surf. Coat. Technol. 2021, 421, 127478. [Google Scholar] [CrossRef]
- Grigoriev, S.; Peretyagin, P.; Smirnov, A.; Solis, W.; Diaz, L.A.; Fernandez, A.; Torrecillas, R. Effect of graphene addition on the mechanical and electrical properties of Al2O3-SiCw ceramics. J. Eur. Ceram. Soc. 2017, 37, 2473–2479. [Google Scholar] [CrossRef]
- Gusarov, A.V.; Grigoriev, S.N.; Volosova, M.A.; Melnik, Y.A.; Laskin, A.; Kotoban, D.V.; Okunkova, A.A. On productivity of laser additive manufacturing. J. Mater. Process. Technol. 2018, 261, 213–232. [Google Scholar] [CrossRef]
- Smirnov, A.; Volosova, M.; Peretyagin, P.; Bartolome, J.F. Tribological behaviour of a 3Y-TZP/Ta ceramic-metal biocomposite against ultrahigh molecular weight polyethylene (UHMWPE). Ceram. Int. 2018, 44, 1404–1410. [Google Scholar] [CrossRef]
- Chen, T.; Deng, Z.; Liu, D.; Zhu, X.; Xiong, Y. Bioinert TiC ceramic coating prepared by laser cladding: Microstructures, wear resistance, and cytocompatibility of the coating. Surf. Coat. Technol. 2021, 423, 127635. [Google Scholar] [CrossRef]
- Chen, T.; Li, W.; Liu, D.; Xiong, Y.; Zhu, X. Effects of heat treatment on microstructure and mechanical properties of TiC/TiB composite bioinert ceramic coatings in-situ synthesized by laser cladding on Ti6Al4V. Ceram. Int. 2021, 47, 755–768. [Google Scholar] [CrossRef]
- Ren, W.; Zhuang, B.; Lei, W.; Cao, Q. Microstructure and performance evolution of Ti-6Al-4V alloy coating by laser cladding and laser shocking composite remanufacture. Opt. Laser Technol. 2021, 143, 107342. [Google Scholar] [CrossRef]
- Smurov, I.; Doubenskaia, M.; Grigoriev, S.; Nazarov, A. Optical Monitoring in Laser Cladding of Ti6Al4V. J. Therm. Spray Technol. 2012, 21, 1357–1362. [Google Scholar] [CrossRef]
- Ricci, V.P.; dos Santos, R.F.; Asato, G.H.; Roche, V.; Jorge, A.M.; Afonso, C.R. Assessment of anodization conditions and annealing temperature on the microstructure, elastic modulus, and wettability of β-Ti40Nb alloy. Thin Solid Films 2021, 737, 138949. [Google Scholar] [CrossRef]
- Pozhidaev, S.S.; Seleznev, A.E.; Solis Pinargote, N.W.; Peretyagin, P.Y. Spark plasma sintering of electro conductive nanocomposite Al2O3-SiCw-TiC. Mech. Ind. 2015, 16, 710. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Garcia, E.; Gutierrez-Gonzalez, C.F.; Peretyagin, P.; Solis, W.; Lopez-Esteban, S.; Torrecillas, R.; Fernandez, A. Effect of yttria-titanium shell-core structured powder on strength and ageing of zirconia/titanium composites. Mater. Sci. Eng. A 2015, 646, 96–100. [Google Scholar] [CrossRef]
- Apelfeld, A.V.; Belkin, P.N.; Borisov, A.M.; Vasin, V.A.; Krit, B.L.; Ludin, V.B.; Somov, O.V.; Sorokin, V.A.; Suminov, I.V.; Frantskevich, V.P. Modern technologies for modification of materials surface and formation of protective coatings. In Volume 1: Micro-Arc Oxidation; Renome: Moscow, Russia; St. Petersburg, Russia, 2017; 648p, ISBN 978-5-91918-832-2. [Google Scholar]
- Sobolev, A.; Kossenko, A.; Borodianskiy, K. Study of the Effect of Current Pulse Frequency on Ti-6Al-4V Alloy coating Formation by Micro Arc Oxidation. Materials 2019, 12, 3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, G.; Jiang, J.; Meletis, E.I. Investigation of the plasma electrolytic oxidation mechanism of titanium. Appl. Surf. Sci. 2019, 488, 370–382. [Google Scholar] [CrossRef]
- Zhu, L.; Petrova, R.S.; Gashinski, J.P.; Yang, Z. The effect of surface roughness on PEO-treated Ti-6Al-4V alloy and corrosion resistance. Surf. Coat. Technol. 2017, 325, 22–29. [Google Scholar] [CrossRef]
- Bogdashkina, N.L.; Gerasimov, M.V.; Zalavutdinov, R.K.; Kasatkina, I.V.; Krit, B.L.; Lyudin, V.B.; Fedichkin, I.D.; Shcherbakov, A.I.; Apelfeld, A.V. Influence of Nickel Sulfate Additives to Electrolytes Subjected to Microarc Oxidation on the Structure, Composition and Properties of Coatings Formed on Titanium. Surf. Eng. Appl. Electrochem. 2018, 54, 331–337. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Allahkaram, S.R.; Munoz, A.I.; Towhidi, N. The influence of current density and frequency on the microstructure and corrosion behavior of plasma electrolytic oxidation coatings on Ti6Al4V. J. Mater. Eng. Perform. 2017, 26, 931–944. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S.R. Effect of incorporation of nanoparticles with different composition on wear and corrosion behavior of ceramic coatings developed on pure titanium by micro arc oxidation. Surf. Coat. Technol. 2017, 309, 767–778. [Google Scholar] [CrossRef]
- Teng, F.Y.; Tai, I.C.; Wang, M.W.; Wang, Y.J.; Hung, C.C.; Tseng, C.C. The structures, electrochemical and cell performance of titania films formed on titanium by micro-arc oxidation. J. Taiwan Inst. Chem. Eng. 2014, 45, 1331–1337. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Golozar, M.A. A comparison of corrosion, tribocorrosion and electrochemical impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process. Appl. Surf. Sci. 2015, 324, 751–756. [Google Scholar] [CrossRef]
- Fan, X.; Feng, B.; Di, Y.; Lu, X.; Duan, K.; Wang, J.; Weng, J. Preparation of bioactive TiO film on porous titanium by micro-arc oxidation. Appl. Surf. Sci. 2012, 258, 7584–7588. [Google Scholar] [CrossRef]
- Yetim, A.F. Investigation of wear behavior of titanium oxide films, produced by anodic oxidation, on commercially pure titanium in vacuum conditions. Surf. Coat. Technol. 2010, 205, 1757–1763. [Google Scholar] [CrossRef]
- Xie, L.; Yin, G.; Yan, D.; Liao, X.; Huang, Z.; Yao, Y.; Kang, Y.; Liu, Y. Structure, morphology and fibroblasts adhesion of surface-porous titanium via anodic oxidation. J. Mater. Sci. Mater. Med. 2010, 21, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.M.; Krit, B.L.; Lyudin, V.B.; Morozova, N.V.; Suminov, I.V.; Apelfeld, A.V. Microarc oxidation in slurry electrolytes: A review. Surf. Eng. Appl. Electrochem. 2016, 52, 50–78. [Google Scholar] [CrossRef]
- Association for the Advancement of Medical Instrumentation. ANSI/AAMI ST79:2017: Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities; Association for the Advancement of Medical Instrumentation: Arlington, MA, USA, 2017. [Google Scholar]
- Ullmann, F. Chemical Encyclopedia; Soviet Encyclopedia: Moscow, Russian, 1988; Volume 4, p. 1179. [Google Scholar]
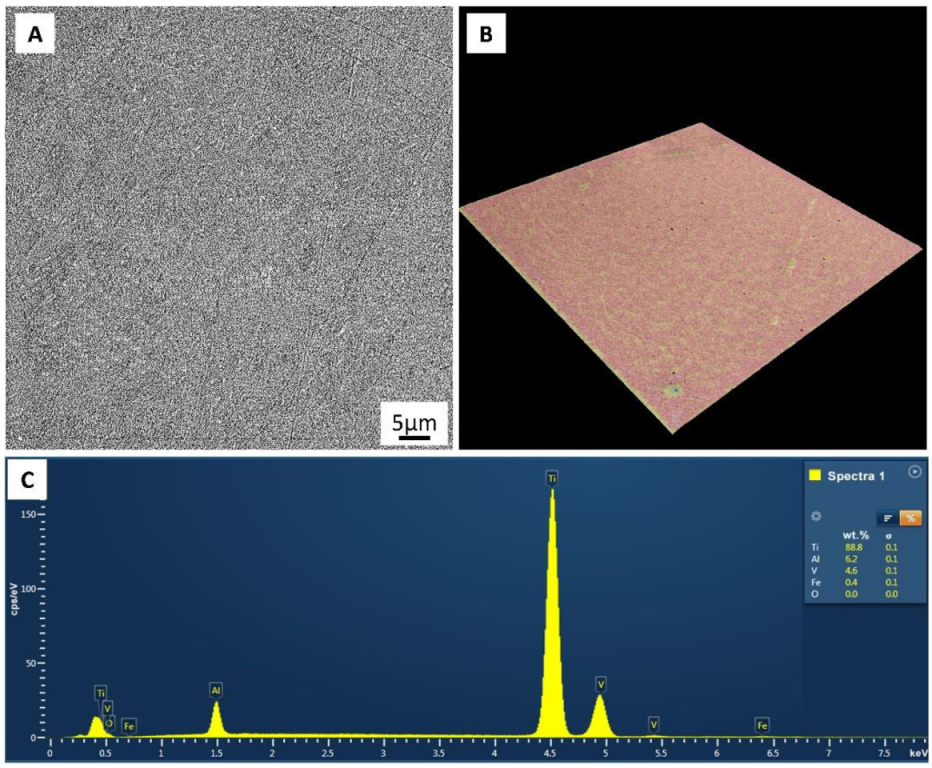

| Chemical Compositions, wt% | ||||
|---|---|---|---|---|
| Al | V | Fe | O | Ti |
| 6.37 | 4.12 | 0.29 | 0.13 | Bal. |
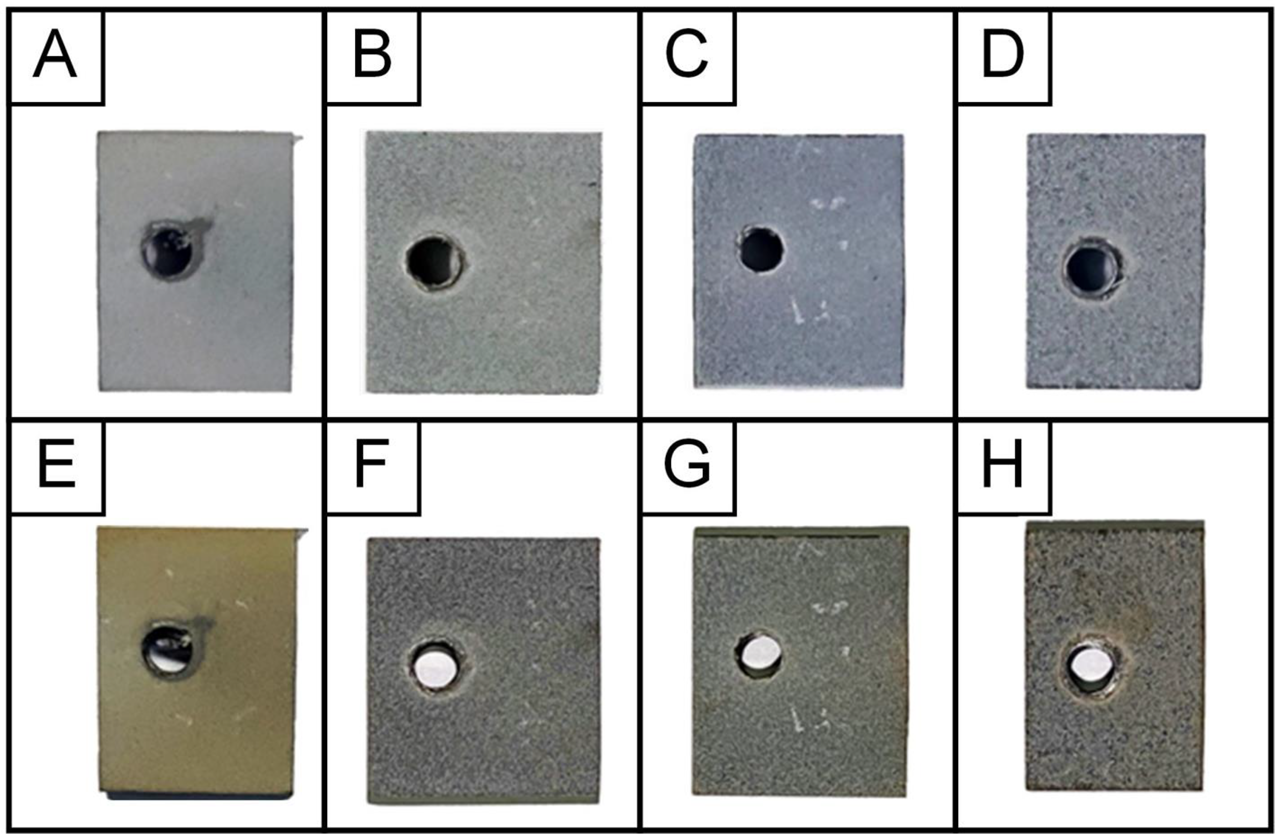
| Samples Code | Electrolyte | Electrical Mode | Sum Current Density, A/dm2 | Treatment Duration, min |
|---|---|---|---|---|
| Ti-MAO-10-30 | Na2SiO3∙9H2O + Na(PH2O2)∙H2O | 10 | 30 | |
| Ti-MAO-10-60 | 10 | 60 | ||
| Ti-MAO-20-30 | anode- cathode (50 Hz) | 20 | 30 | |
| Ti-MAO-20-60 | 20 | 60 |
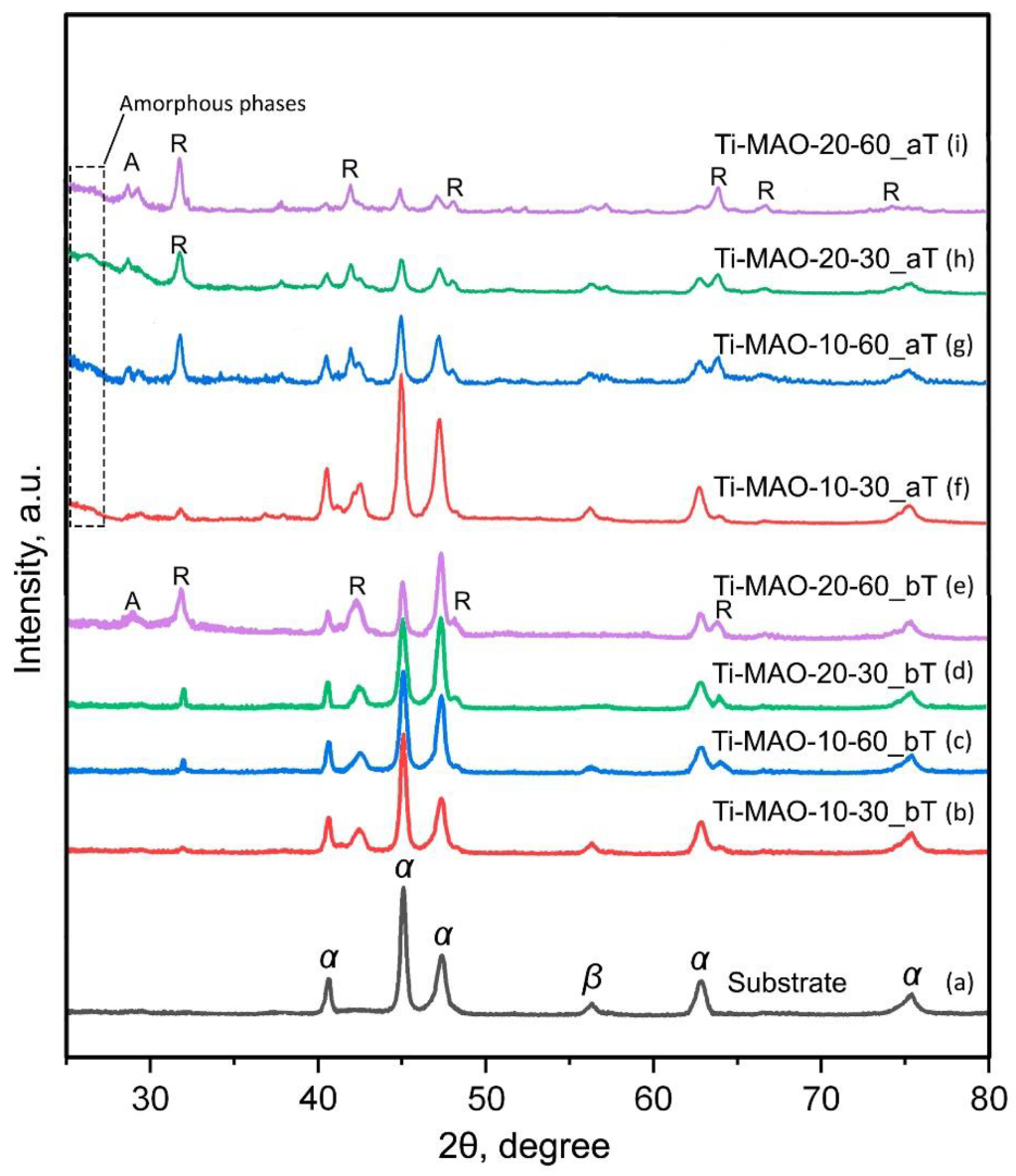
| Sample Code | Thickness, μm | Roughness, μm | |
|---|---|---|---|
| Ra | Rz | ||
| Ti-without MAO | 5.6 | 32.1 | |
| Ti-MAO-10-30_bT | 27.2 | 2.3 | 14.9 |
| Ti-MAO-10-60_bT | 42.6 | 3.7 | 22.0 |
| Ti-MAO-20-30_bT | 42.8 | 4.3 | 25.3 |
| Ti-MAO-20-60_bT | 66.4 | 4.9 | 27.4 |
| Ti-MAO-10-30_aT | 15.2 | 2.2 | 14.5 |
| Ti-MAO-10-60_aT | 36.4 | 2.7 | 15.5 |
| Ti-MAO-20-30_aT | 38.8 | 3.3 | 18.7 |
| Ti-MAO-20-60_aT | 61.6 | 4.3 | 22.1 |
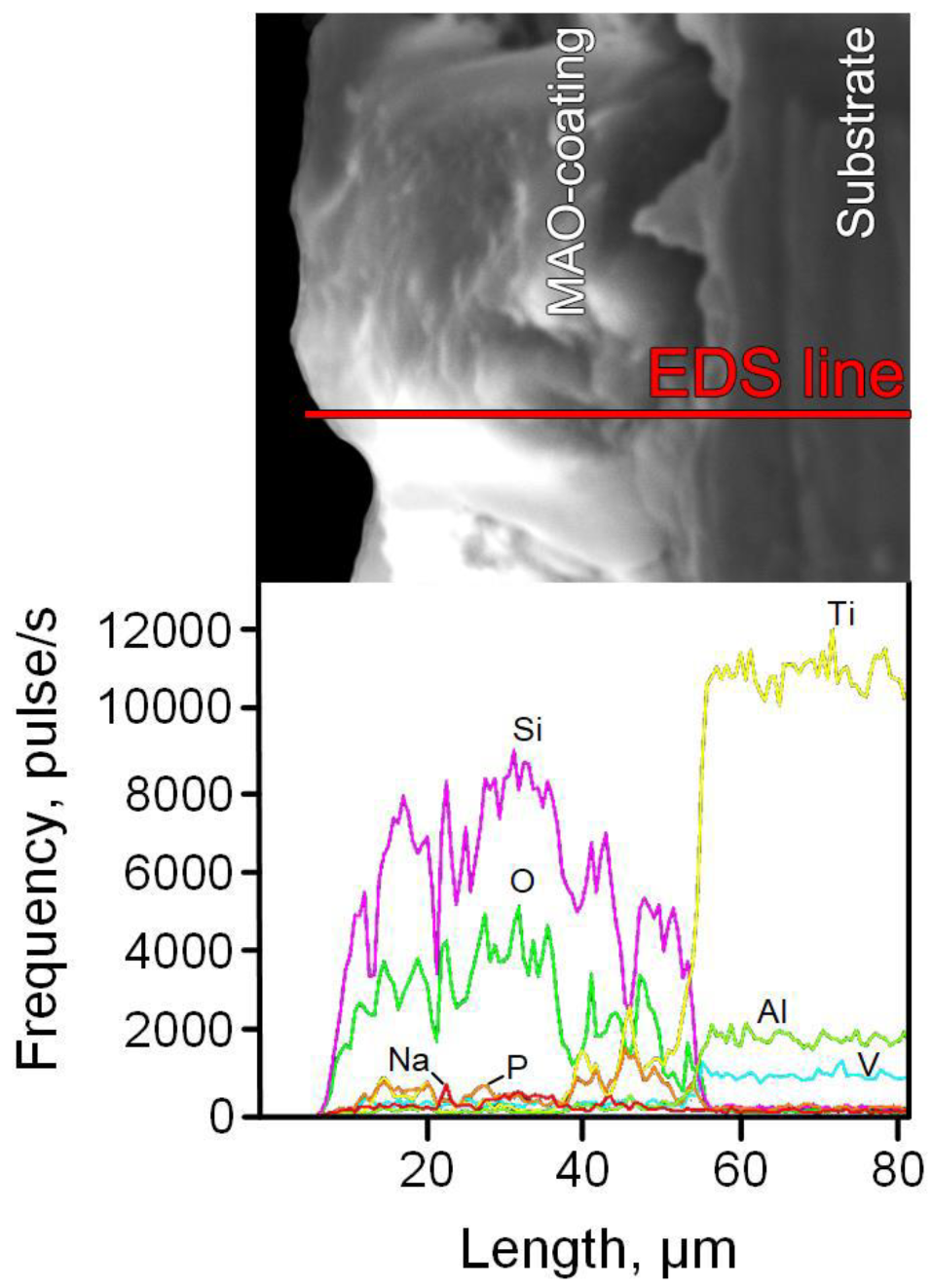
| Sample Code | Element Content, at% | ||||||
|---|---|---|---|---|---|---|---|
| O | Na | Al | Si | P | Ti | V | |
| Ti-MAO-10-30_bT | 57.82 | 1.11 | 0.24 | 28.98 | 4.59 | 6.87 | 0.39 |
| Ti-MAO-10-60_bT | 57.44 | 1.15 | 0.31 | 31.02 | 3.06 | 6.72 | 0.30 |
| Ti-MAO-20-30_bT | 57.49 | 0.84 | 0.44 | 31.21 | 3.12 | 6.69 | 0.21 |
| Ti-MAO-20-60_bT | 57.68 | 0.9 | 0.44 | 30.56 | 3.78 | 6.41 | 0.23 |
| Ti-MAO-10-30_aT | 56.95 | 1.72 | 0.27 | 29.93 | 4.13 | 6.58 | 0.42 |
| Ti-MAO-10-60_aT | 56.33 | 1.63 | 0.79 | 30.49 | 3.15 | 7.25 | 0.36 |
| Ti-MAO-20-30_aT | 56.27 | 1.61 | 0.81 | 30.52 | 3.18 | 7.33 | 0.28 |
| Ti-MAO-20-60_aT | 56.45 | 2.78 | 0.87 | 29.85 | 2.64 | 7.16 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, S.; Peretyagin, N.; Apelfeld, A.; Smirnov, A.; Yanushevich, O.; Krikheli, N.; Kramar, O.; Kramar, S.; Peretyagin, P. Investigation of MAO Coatings Characteristics on Titanium Products Obtained by EBM Method Using Additive Manufacturing. Materials 2022, 15, 4535. https://doi.org/10.3390/ma15134535
Grigoriev S, Peretyagin N, Apelfeld A, Smirnov A, Yanushevich O, Krikheli N, Kramar O, Kramar S, Peretyagin P. Investigation of MAO Coatings Characteristics on Titanium Products Obtained by EBM Method Using Additive Manufacturing. Materials. 2022; 15(13):4535. https://doi.org/10.3390/ma15134535
Chicago/Turabian StyleGrigoriev, Sergey, Nikita Peretyagin, Andrey Apelfeld, Anton Smirnov, Oleg Yanushevich, Natella Krikheli, Olga Kramar, Sergey Kramar, and Pavel Peretyagin. 2022. "Investigation of MAO Coatings Characteristics on Titanium Products Obtained by EBM Method Using Additive Manufacturing" Materials 15, no. 13: 4535. https://doi.org/10.3390/ma15134535
APA StyleGrigoriev, S., Peretyagin, N., Apelfeld, A., Smirnov, A., Yanushevich, O., Krikheli, N., Kramar, O., Kramar, S., & Peretyagin, P. (2022). Investigation of MAO Coatings Characteristics on Titanium Products Obtained by EBM Method Using Additive Manufacturing. Materials, 15(13), 4535. https://doi.org/10.3390/ma15134535







