Mechanical Strength and Chloride Ions’ Penetration of Alkali-Activated Concretes (AAC) with Blended Precursor
Abstract
:1. Introduction
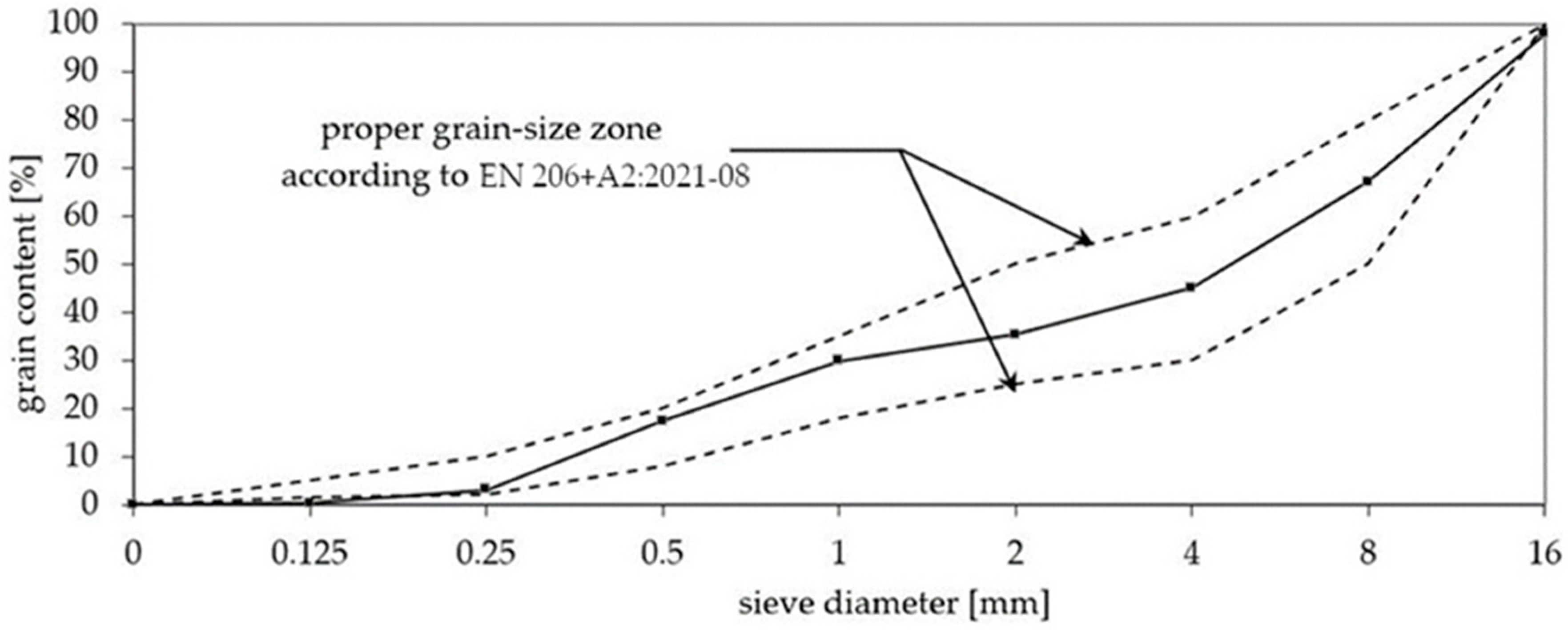
2. Materials and Methods
3. Results and Discussion
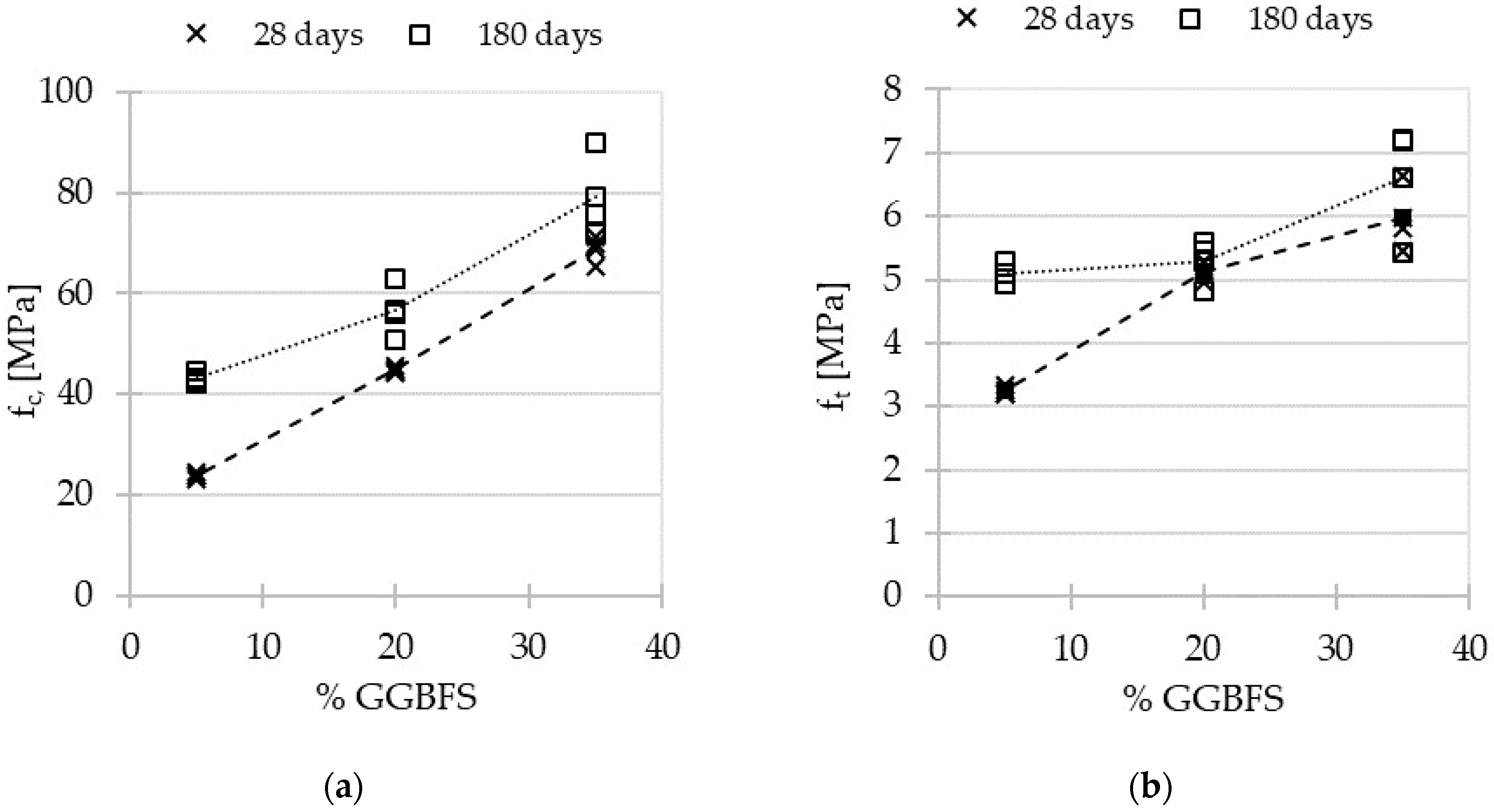
3.1. Mechanical Characteristics
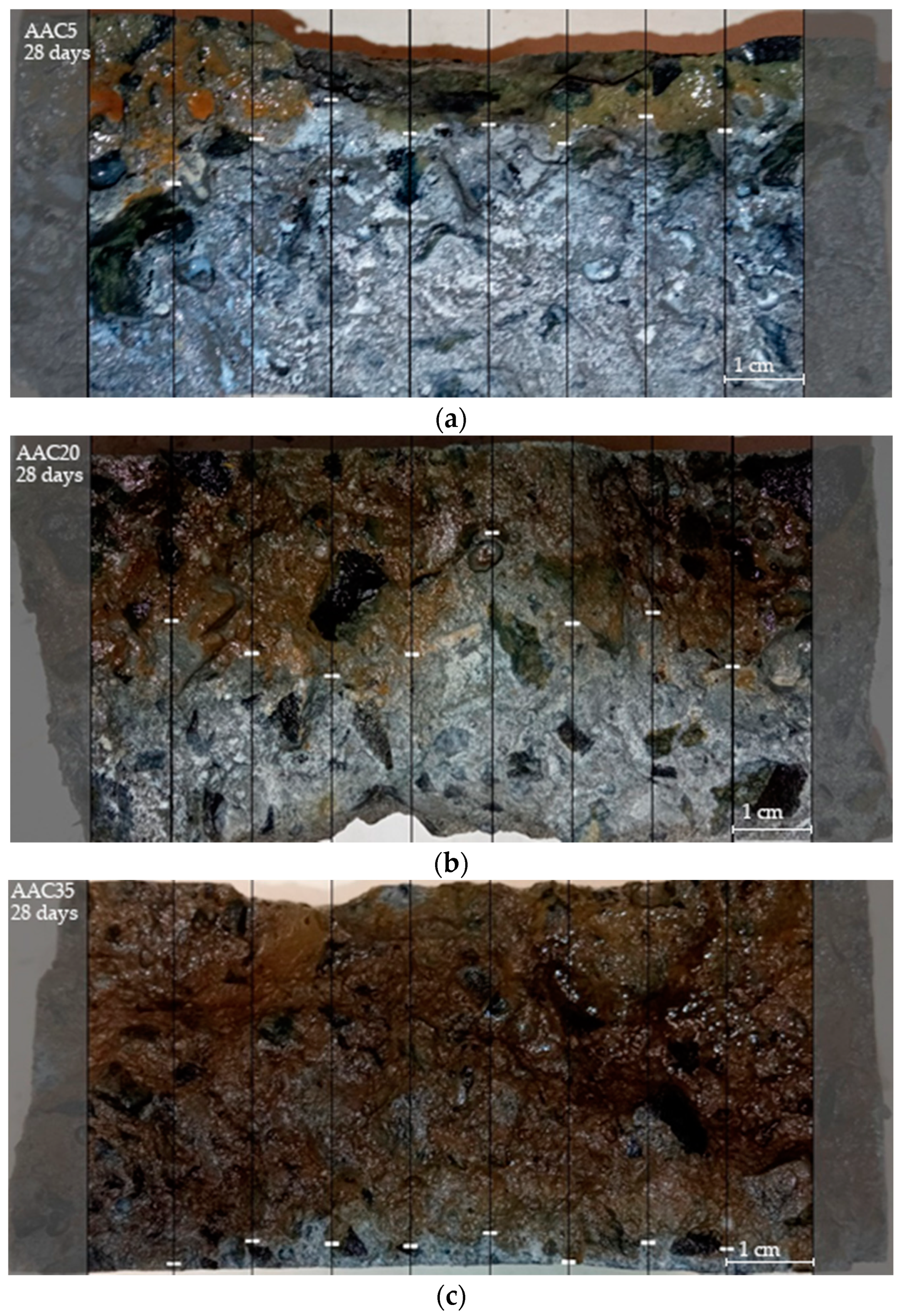
3.2. Chloride Ion Penetration and Diffusion Coefficient
- Dnssm: non-steady state migration coefficient, m2/s;
- Z: absolute value of ion valence, for chloride, z = 1;
- F: Faraday constant, F = 9.648 × 104 J/(V‧mol);
- ΔE: value of applied voltage, V;
- R: gas constant, R = 8.314 J/(K‧mol);
- T: average value of the initial and final temperatures in the anolyte solution, K;
- e: thickness of the specimen, m;
- xd: average value of the penetration depths, m;
- Δt: test duration, seconds;
- erf−1: inverse of error function;
- cd: chloride concretion at which the colour changes, cd ≈ 0.07 for OPC concrete;
- c0: chloride concentration in the catholyte solution, N.
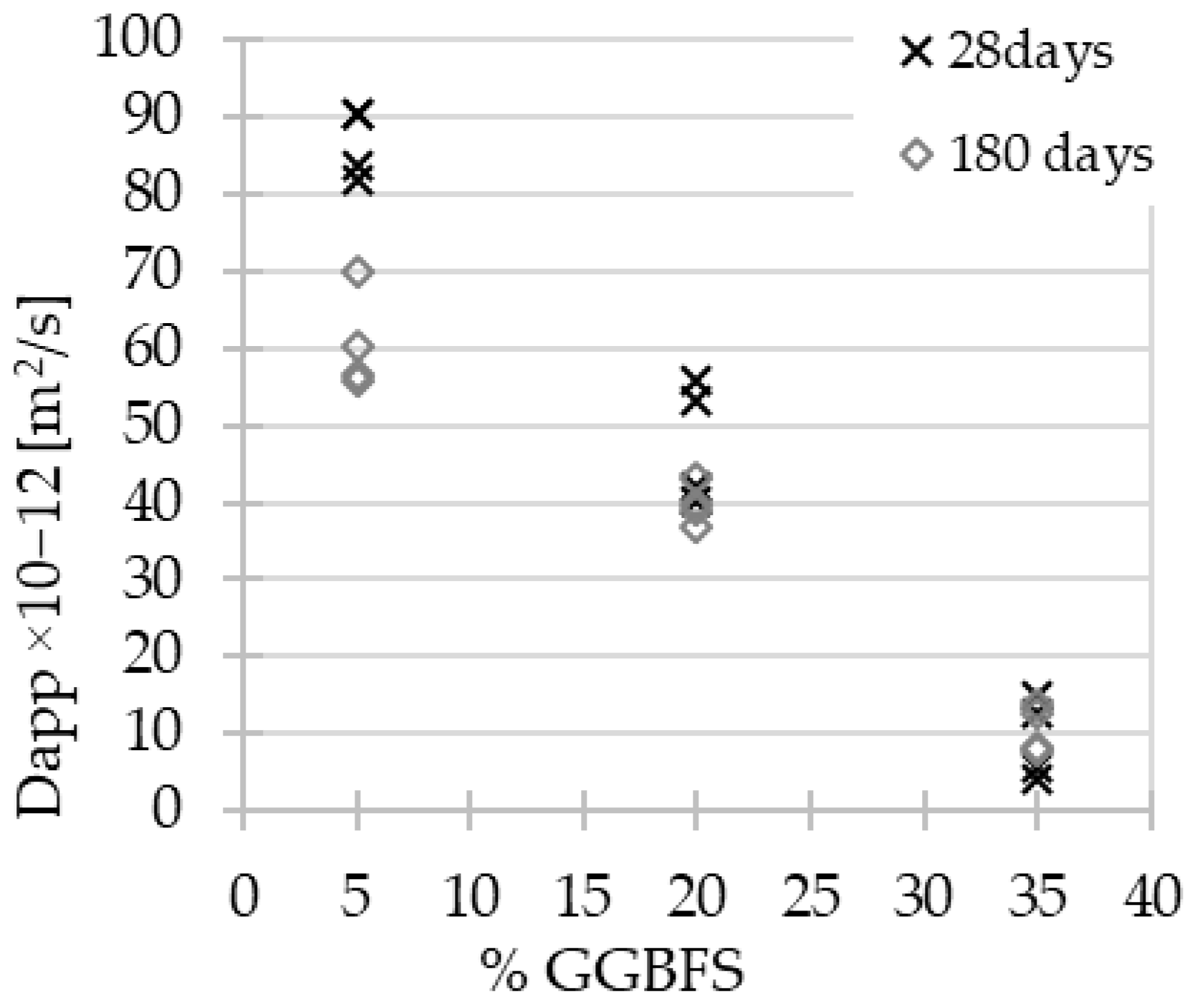
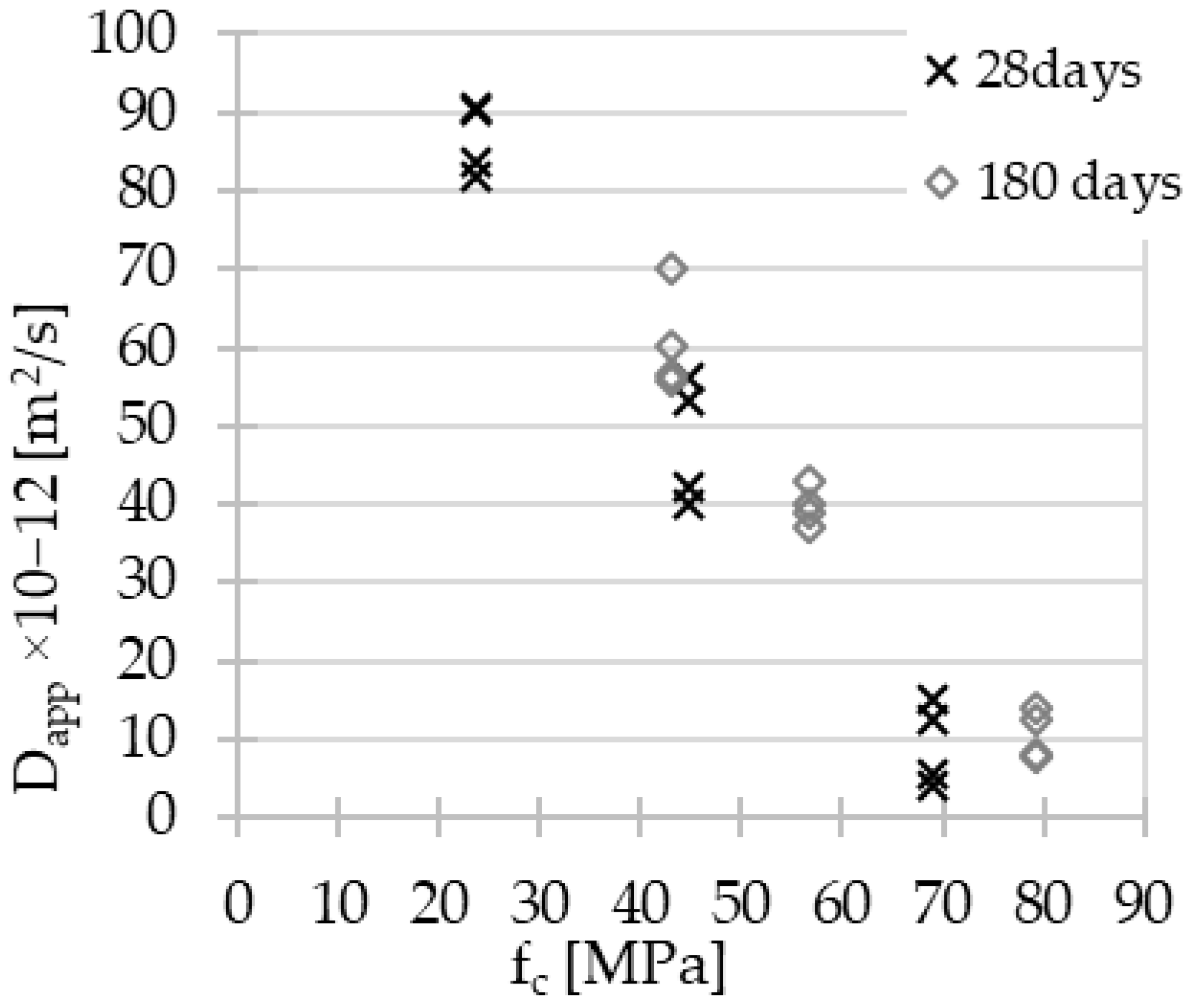
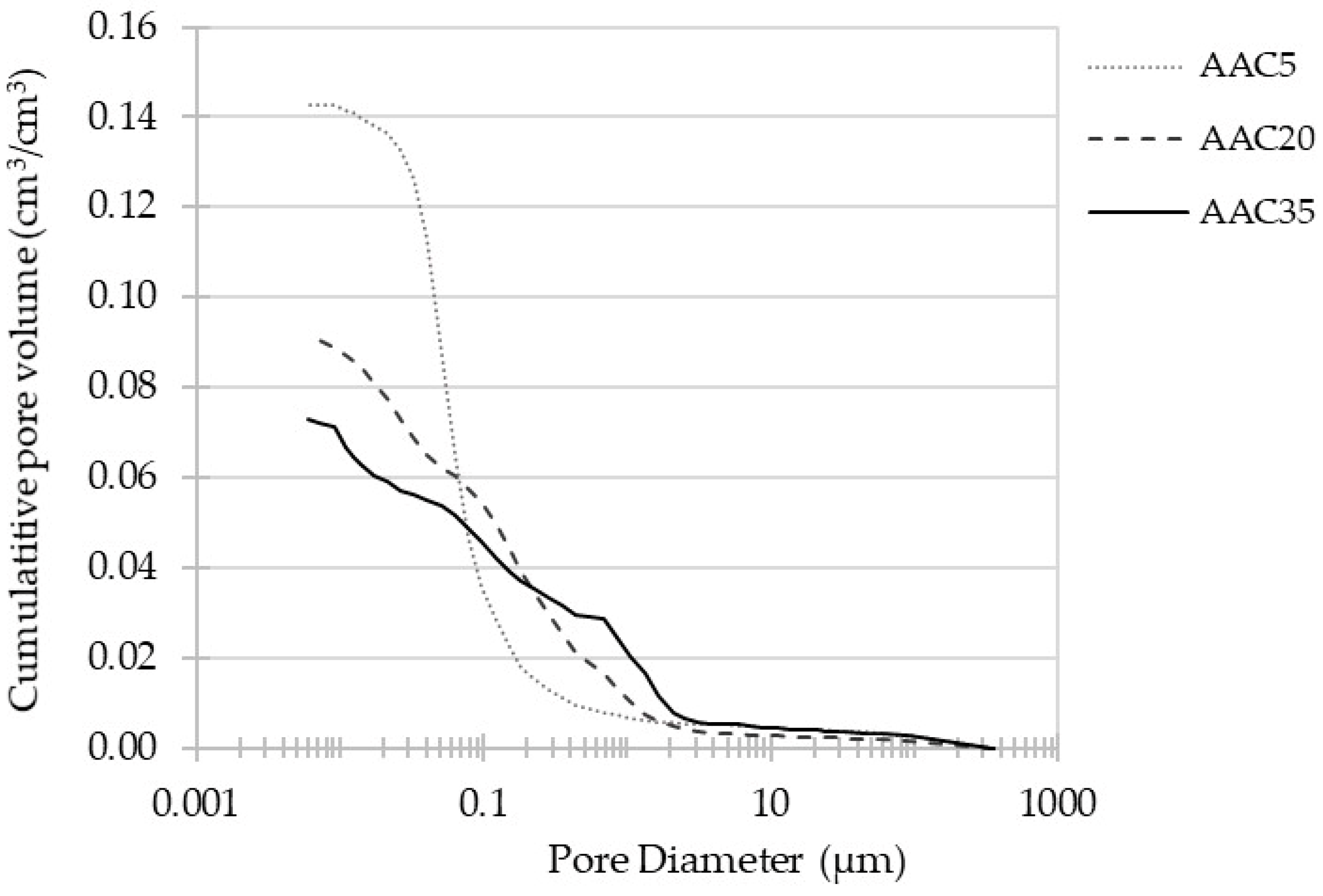
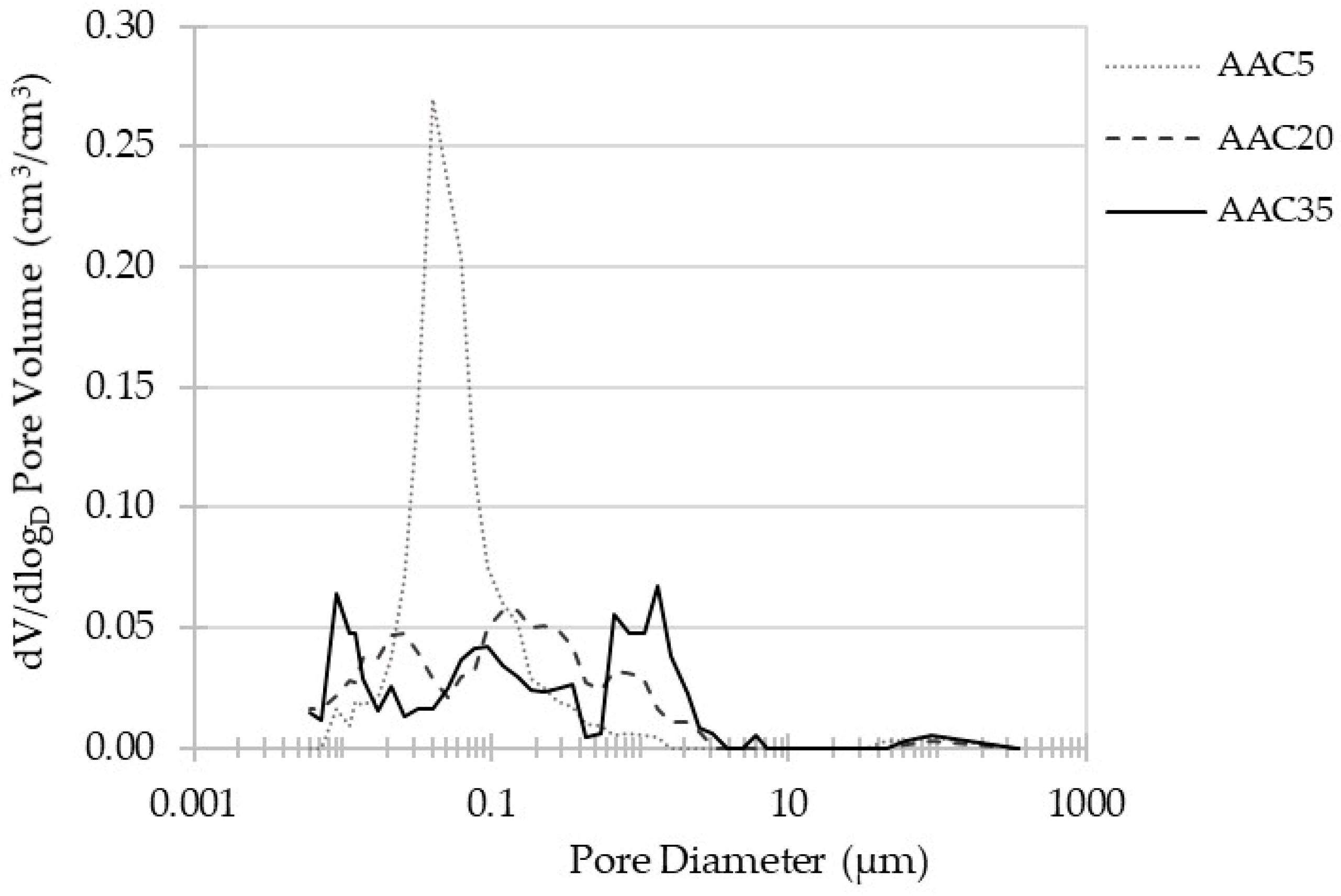
3.3. MIP Porosity
4. Conclusions
- The addition of GGBFS significantly reduces the porosity of alkali-activated concretes and changes the pore size distribution from one dominated by a specific pore diameter to continuous pore size distribution.
- Increasing quasi-linear relationships between the GGBFS content in the material composition and the compressive strength, splitting tensile strength and the value of the chloride diffusion coefficient have been demonstrated.
- The effect of aging on the improvement of material properties is particularly noticeable in the case of concretes with low GGBFS content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- de Azevedo, A.R.G.; Cruz, A.S.A.; Marvila, M.T.; de Oliveira, L.B.; Monteiro, S.N.; Vieira, C.M.F.; Fediuk, R.; Timokhin, R.; Vatin, N.; Daironas, M. Natural Fibers as an Alternative to Synthetic Fibers in Reinforcement of Geopolymer Matrices: A Comparative Review. Polymers 2021, 13, 2493. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction—Recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- United Nations. Framework Convention on Climate Change Adoption of the Paris Agreement. In Proceedings of the 21st Conference of the Parties, Paris, France, 30 November–12 December 2015. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials, RILEM State Art Reports, RILEM TC 224-AAM; Springer: Berlin, Germany, 2014; Volume 13. [Google Scholar]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2014, 48, 721–731. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-ash based geopolymer mortar for high-temperature application—Effect of slag addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Sitarz, M.; Hager, I.; Choińska, M. Evolution of Mechanical Properties with Time of Fly-Ash-Based Geopolymer Mortars under the Effect of Granulated Ground Blast Furnace Slag Addition. Energies 2020, 13, 1135. [Google Scholar] [CrossRef] [Green Version]
- Duży, P.; Sitarz, M.; Adamczyk, M.; Choińska, M.; Hager, I. Chloride Ions’ Penetration of Fly Ash and Ground Granulated Blast Furnace Slags-Based Alkali-Activated Mortars. Materials 2021, 14, 6583. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.G.; de Carvalho, J.M.F.; Ribeiro, J.C.L. Application of eco-friendly alternative activators in alkali-activated materials: A review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- Kalombe, R.M.; Ojumu, V.T.; Eze, C.P.; Nyale, S.M.; Kevern, J.; Petrik, L.F. Fly Ash-Based Geopolymer Building Materials for Green and Sustainable Development. Materials 2020, 13, 5699. [Google Scholar] [CrossRef]
- Yao, X.; Yang, T.; Zhang, Z. Compressive strength development and shrinkage of alkali-activated fly ash–slag blends associated with efflorescence. Mater. Struct. 2016, 49, 2907–2918. [Google Scholar] [CrossRef]
- Hu, X.; Shi, C.; Shi, Z.; Zhang, L. Compressive strength, pore structure and chloride transport properties of alkali-activated slag/fly ash mortars. Cem. Concr. Compos. 2019, 104, 103392. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Z.; Zhu, Y.; Tian, L. Durability of alkali-activated fly ash concrete: Chloride penetration in pastes and mortars. Constr. Build. Mater. 2014, 65, 51–59. [Google Scholar] [CrossRef]
- Chen, J.; Tian, C.; Wei, X. Experimental and simulation study on chloride permeability in cement paste. Constr. Build. Mater. 2020, 262, 120600. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Huang, Y.; Yu, R.; Song, Q. Chloride binding capacity and phase modification of alumina compound blended cement paste under chloride attack. Cem. Concr. Compos. 2020, 108, 103537. [Google Scholar] [CrossRef]
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, K.S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Provis, J.L. Activating solution chemistry for geopolymers. In Geopolymers; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead Publishing Limited: Oxford, UK, 2019; pp. 50–71. [Google Scholar]
- Kaur, M.; Singh, J.; Kaur, M. Synthesis of fly ash based geopolymer mortar considering different concentrations and combinations of alkaline activator solution. Ceram. Int. 2018, 44, 1534–1537. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Hussein, O.H.; Provis, J.L. Chloride binding and mobility in sodium carbonate-activated slag pastes and mortars. Mater. Struct. 2017, 50, 252. [Google Scholar] [CrossRef] [Green Version]
- Aughenbaugh, K.L.; Stutzman, P.; Juenger, M.C.G. Identifying Glass Compositions in Fly Ash. Front. Mater. 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Palomo, A.; Alonso, S.; Fernández-Jiménez, A.; Sobrados, I.; Sanz, J. Alkaline activation of fly ashes: NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Rafeet, A.M.; Vinai, R.; Soutsos, M.; Sha, W. Effects of slag substitution on physical and mechanical properties of fly ash-based alkali activated binders (AABs). Cem. Concr. Res. 2019, 122, 118–135. [Google Scholar] [CrossRef]
- Hooton, R.D.; Thomas, M.D.A.; Standish, K. Prediction of Chloride Penetration in Concrete; Materials Science: Toronto, ON, Canada, 2001; Available online: https://trid.trb.org/view.aspx?id=690787 (accessed on 7 November 2017).
- Bernal, S.A.; Bílek, V.; Criado, M.; Fernández-Jiménez, A.; Kavalerova, E.; Krivenko, P.V.; Palacios, M.; Palomo, A.; Provis, J.L.; Puertas, F.; et al. Durability and testing—Degradation via mass transport. In RILEM State-of-the-Art Reports: Alkali Activated Materials; Provis, J.L., van Deventer, J.S.J., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014; Volume 13, pp. 223–276. [Google Scholar]
- ASTM C1556-11a; Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion. ASTM International: West Conshohocken, PA, USA, 2016.
- Kupwade-Patil, K.; Allouche, E.N. Examination of Chloride-Induced Corrosion in Reinforced Geopolymer Concretes. J. Mater. Civ. Eng. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride-induced corrosion of reinforcement in low-calcium fly ash-based geopolymer concrete. Cem. Concr. Res. 2016, 88, 96–107. [Google Scholar] [CrossRef]
- Olivia, M.; Nikraz, H. Durability of fly ash geopolymer concrete in a seawater environment. In Proceedings of the Concrete 2011 Conference, Perth, Australia, 12 October 2011; The Concrete Institute of Australia: Perth, Australia, 2011; pp. 1–9. [Google Scholar]
- Nordtest NT BUILD 443; Concrete Hardened: Accelerated Chloride Penetration. NORDTEST: Espoo, Finland, 1995.
- Fan, J.; Zhu, H.; Shi, J.; Li, Z.; Yang, S. Influence of slag content on the bond strength, chloride penetration resistance, and interface phase evolution of concrete repaired with alkali activated slag/fly ash. Constr. Build. Mater. 2020, 263, 120639. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, T.; Yang, Z.; Zhan, W.; Zhang, Y. Sustainable use of ferronickel slag in cementitious composites and the effect on chloride penetration resistance. Constr. Build. Mater. 2020, 240, 117969. [Google Scholar] [CrossRef]
- Nordtest NT BUILD 492; Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady State Migration Experiments. NORDTEST: Espoo, Finland, 1999.
- ASTM C1202-19; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM International: West Conshohocken, PA, USA, 2019.
- Noushini, A.; Castel, A. Performance-based criteria to assess the suitability of geopolymer concrete in marine environments using modified ASTM C1202 and ASTM C1556 methods. Mater. Struct. 2018, 51, 146. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Tong, K.T. Mechanical and durability properties of alkali-activated fly ash concrete with increasing slag content. Constr. Build. Mater. 2021, 301, 124330. [Google Scholar] [CrossRef]
- Osio-Norgaard, J.; Gevaudan, J.P.; Srubar, W.V. A review of chloride transport in alkali-activated cement paste, mortar, and concrete. Constr. Build. Mater. 2018, 186, 191–206. [Google Scholar] [CrossRef]
- EN 1097-6:2013-11 Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption. Available online: https://standards.iteh.ai/catalog/standards/cen/efd7df30-eac1-4445-90eb-9b2958fb2564/en-1097-6-2013 (accessed on 6 May 2022).
- EN 12620+A1:2010 Aggregates for Concrete. Available online: https://iss.rs/sr_Cyrl/project/show/iss:proj:26477 (accessed on 1 May 2022).
- EN 206+A2:2021-08 Concrete—Specification, Performance, Production and Conformity. Available online: https://www.en-standard.eu/bs-en-206-2013-a2-2021-concrete-specification-performance-production-and-conformity/ (accessed on 5 April 2022).
- EN 12390-3:2019 Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. Available online: https://standards.iteh.ai/catalog/standards/cen/7eb738ef-44af-436c-ab8e-e6561571302c/en-12390-3-2019 (accessed on 20 April 2022).
- EN 12390-6:2011 Testing Hardened Concrete—Part 6: Tensile Splitting Strength of Test Specimens. Available online: https://standards.iteh.ai/catalog/standards/cen/81ec74fb-9338-4909-a200-6a5042160640/en-12390-6-2009 (accessed on 2 May 2022).
- Wang, J.; Liu, E. The relationship between steady-state chloride diffusion and migration coefficients in cementitious materials. Mag. Concr. Res. 2020, 72, 1016–1026. [Google Scholar] [CrossRef]
- Tang, L. Electrically accelerated methods for determining chloride diffusivity in concrete—Current development. Mag. Concr. Res. 1996, 48, 173–179. [Google Scholar] [CrossRef]
- Tang, L.; Sørensen, H.E. Precision of the Nordic test methods for measuring the chloride diffusion/migration coefficients of concrete. Mater. Struct. Mater. Constr. 2001, 34, 479–485. [Google Scholar] [CrossRef]
- Zhang, X.; Long, K.; Liu, W.; Li, L.; Long, W.J. Carbonation and Chloride Ions’ Penetration of Alkali-Activated Materials: A Review. Molecules 2020, 25, 5074. [Google Scholar] [CrossRef]
- Spragg, R.P.; Castro, J.; Li, W.; Pour-Ghaz, M.; Huang, P.-T.; Weiss, J. Wetting and drying of concrete using aqueous solutions containing deicing salts. Cem. Concr. Compos. 2011, 33, 535–542. [Google Scholar] [CrossRef]
- Sitarz, M.; Urban, M.; Hager, I. Rheology and Mechanical Properties of Fly Ash-Based Geopolymer Mortars with Ground Granulated Blast Furnace Slag Addition. Energies 2020, 13, 2639. [Google Scholar] [CrossRef]
- French National Research Project PERFDUB Internal Report. Essai Accéléré de Migration non Stationnaire. Recommandations to the XP P18462 French Standard, Working Group GT1A. 2021. Available online: https://www.omnispace.fr/perfdub/ (accessed on 2 May 2022).
- EN 12390-18:2021-08 Testing Hardened Concrete—Part 18: Determination of the Chloride Migration Coefficient. Available online: https://genorma.com/en/project/show/cen:proj:63061 (accessed on 29 April 2022).
- Fu, Q.; Bu, M.; Zhang, Z.; Xu, W.; Yuan, Q.; Niu, D. Hydration Characteristics and Microstructure of Alkali-Activated Slag Concrete: A Review. Engineering 2021, in press. [Google Scholar] [CrossRef]
- Runci, A.; Serdar, M. Effect of curing time on the chloride diffusion of alkali-activated slag. Case Stud. Constr. Mater. 2022, 16, e00927. [Google Scholar] [CrossRef]
- Dudek, M.; Sitarz, M. Analysis of Changes in the Microstructure of Geopolymer Mortar after Exposure to High Temperatures. Materials 2020, 13, 4263. [Google Scholar] [CrossRef] [PubMed]


| wt.% | SiO2 | Al2O3 | FexOy | CaO | MgO | SO3 | K2O | Na2O | P2O5 | TiO2 | Mn3O4 | Cl− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 52.30 | 28.05 | 6.32 | 3.05 | 1.71 | 0.28 | 2.51 | 0.76 | 0.69 | 1.35 | 0.07 | - |
| GGBFS | 39.31 | 7.61 | 1.49 | 43.90 | 4.15 | 0.51 | 0.36 | 0.47 | - | - | - | 0.04 |
| Characteristic | Unit | Woellner Geosil® 34417 |
|---|---|---|
| Na2O content | wt.% | 16.74 |
| SiO2 content | wt.% | 27.5 |
| Density | g/cm3 | 1.552 |
| Viscosity | mPa×s | 470 |
| Weight ratio (WR = wt.% SiO2/wt. Na2O) | - | 1.64 |
| Molar ratio (MR = mol SiO2/mol Na2O) | - | 1.70 |
| AAC5B | AAC20B | AAC35B | |
|---|---|---|---|
| FA | 336.9 | 292.3 | 244.1 |
| GGBFS | 17.7 | 73.1 | 131.4 |
| Alkaline Solution + water | 189.4 | 195.1 | 200.5 |
| Sand 0/2 | 662.4 | 662.4 | 662.4 |
| Basalt 2/8 | 708.9 | 708.9 | 708.9 |
| Basalt 8/16 | 648.4 | 648.4 | 648.4 |
| water/binder (w/b) ratio | 0.37 | ||
| alkaline solution/binder ratio | 0.53 | ||
| amount of paste in concrete | 300 dm3/m3 | ||
| AAC5 | AAC20 | AAC35 | ||
|---|---|---|---|---|
| 28 days | Penetration depth [mm] | 37.2 | 19.1 | 4.1 |
| σ—standard deviation | 1.8 | 2.3 | 1.5 | |
| 180 days | Penetration depth [mm] | 28.8 | 18.3 | 7.2 |
| σ—standard deviation | 2.3 | 1.3 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duży, P.; Choinska, M.; Hager, I.; Amiri, O.; Claverie, J. Mechanical Strength and Chloride Ions’ Penetration of Alkali-Activated Concretes (AAC) with Blended Precursor. Materials 2022, 15, 4475. https://doi.org/10.3390/ma15134475
Duży P, Choinska M, Hager I, Amiri O, Claverie J. Mechanical Strength and Chloride Ions’ Penetration of Alkali-Activated Concretes (AAC) with Blended Precursor. Materials. 2022; 15(13):4475. https://doi.org/10.3390/ma15134475
Chicago/Turabian StyleDuży, Patrycja, Marta Choinska, Izabela Hager, Ouali Amiri, and Jérôme Claverie. 2022. "Mechanical Strength and Chloride Ions’ Penetration of Alkali-Activated Concretes (AAC) with Blended Precursor" Materials 15, no. 13: 4475. https://doi.org/10.3390/ma15134475
APA StyleDuży, P., Choinska, M., Hager, I., Amiri, O., & Claverie, J. (2022). Mechanical Strength and Chloride Ions’ Penetration of Alkali-Activated Concretes (AAC) with Blended Precursor. Materials, 15(13), 4475. https://doi.org/10.3390/ma15134475







