Influence Factors in the Wide Application of Alkali-Activated Materials: A Critical Review about Efflorescence
Abstract
:1. Introduction
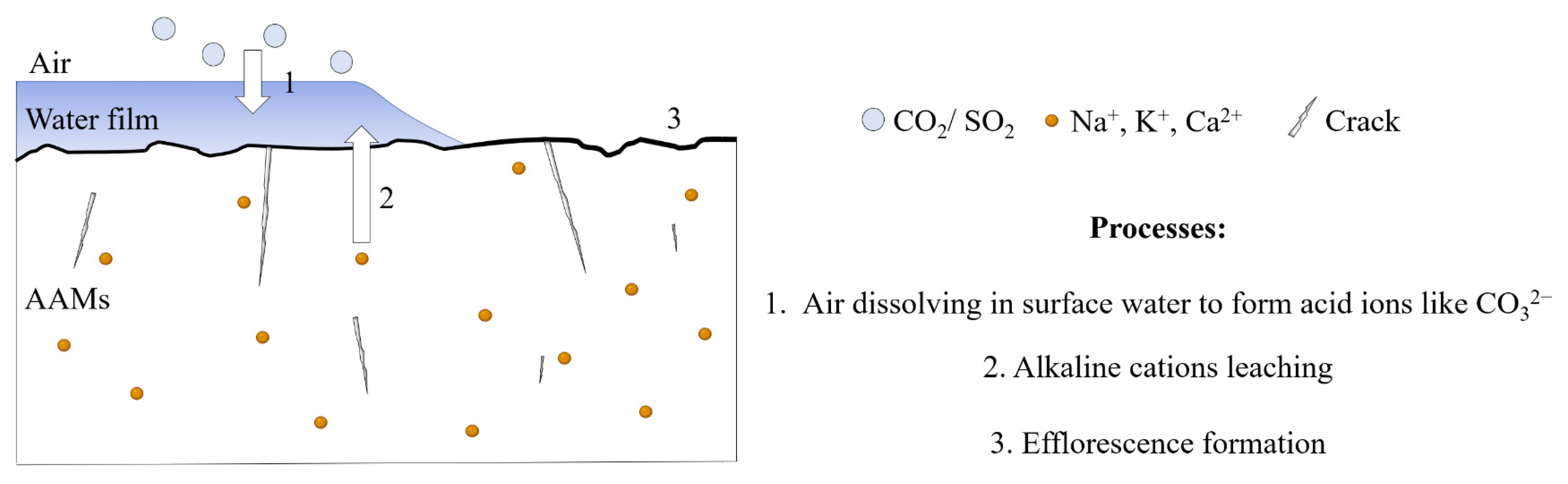
2. Investigations on the Influence Factors of Efflorescence
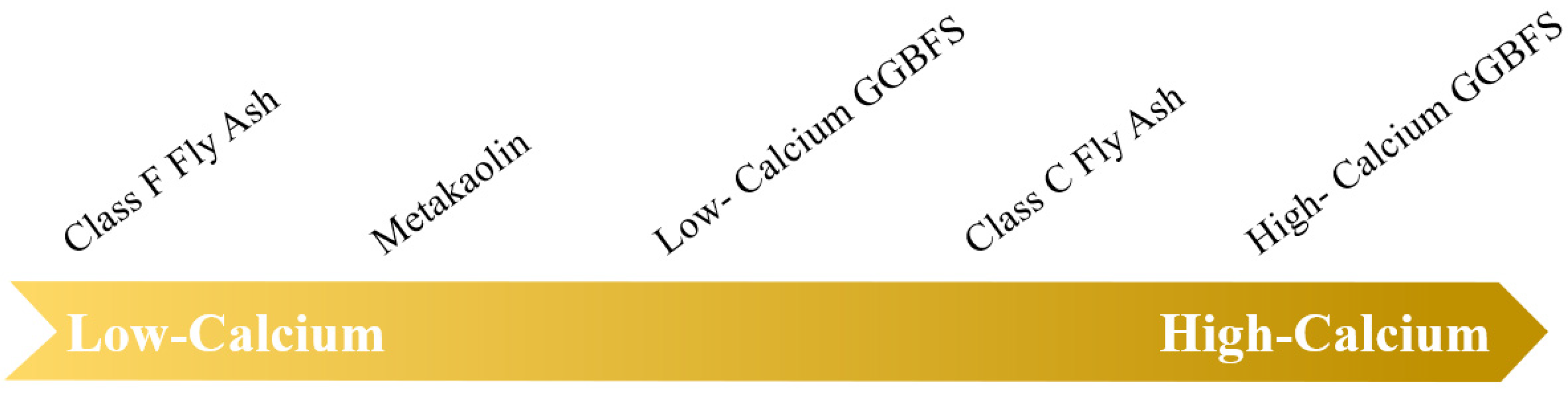
2.1. Raw Materials
2.1.1. Solid Aluminosilicate Precursors
2.1.2. Alkaline Activators
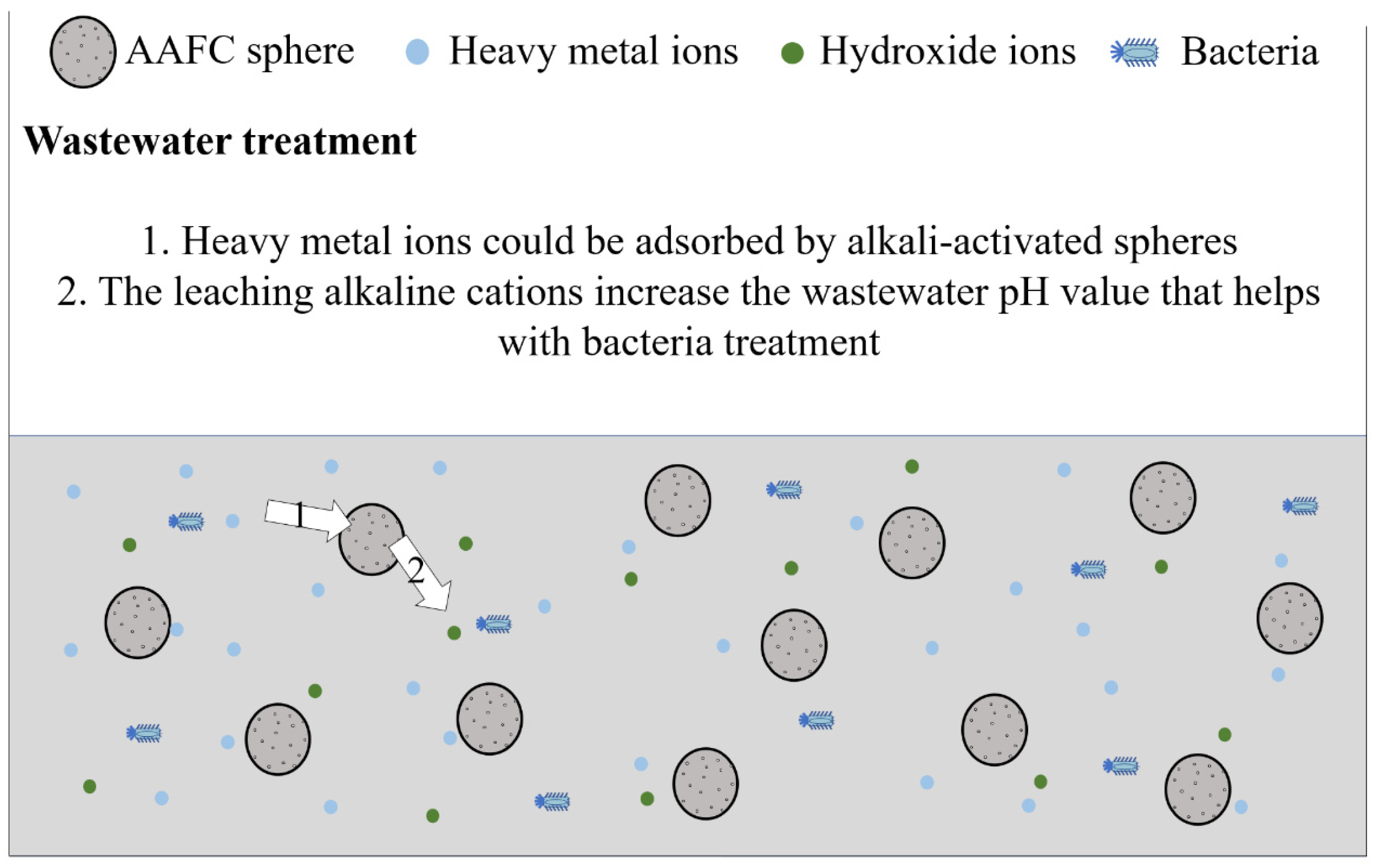
2.2. AAMs Modalities
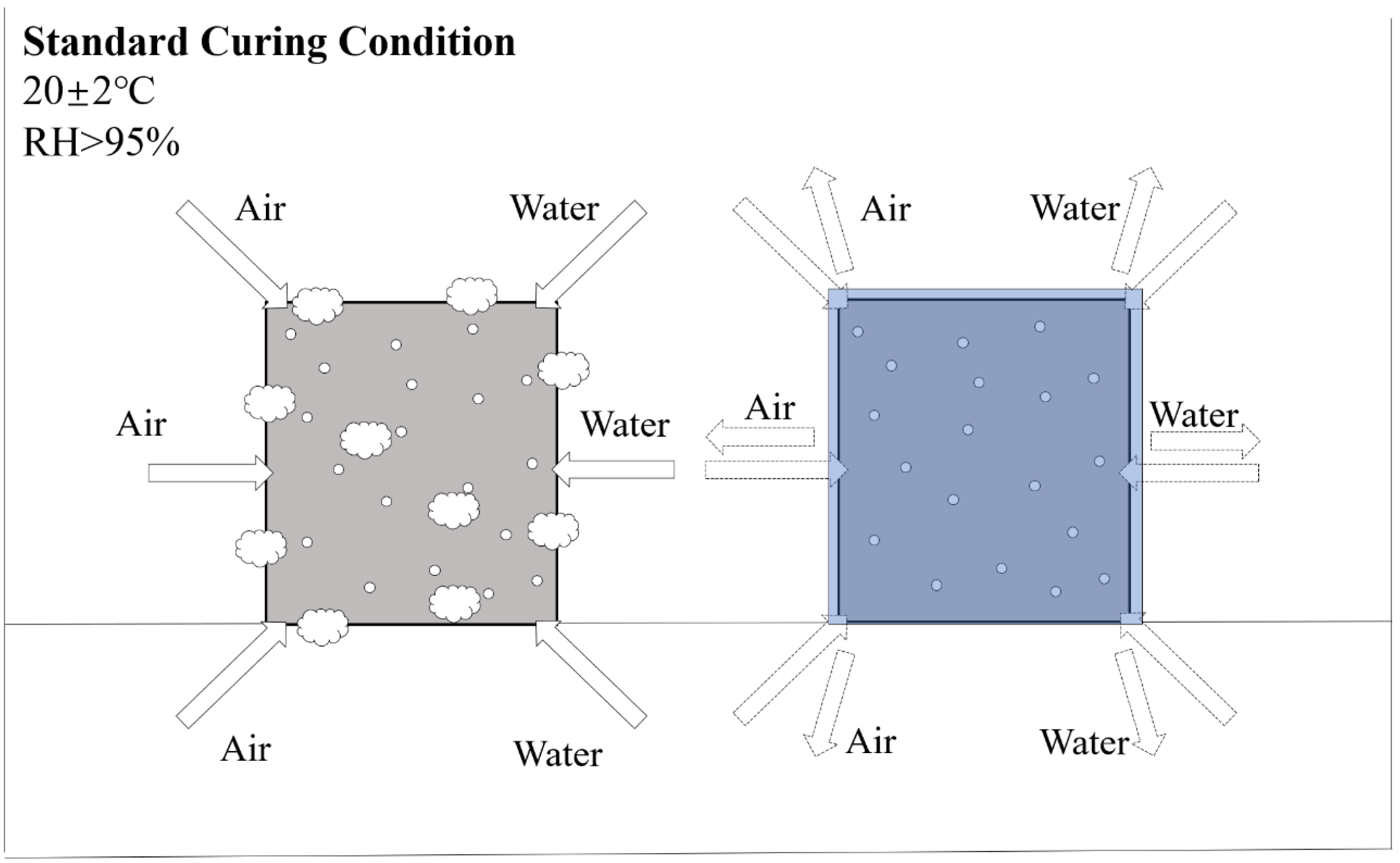
2.3. Curing Conditions
3. Impact on the Properties
4. Solution Methods
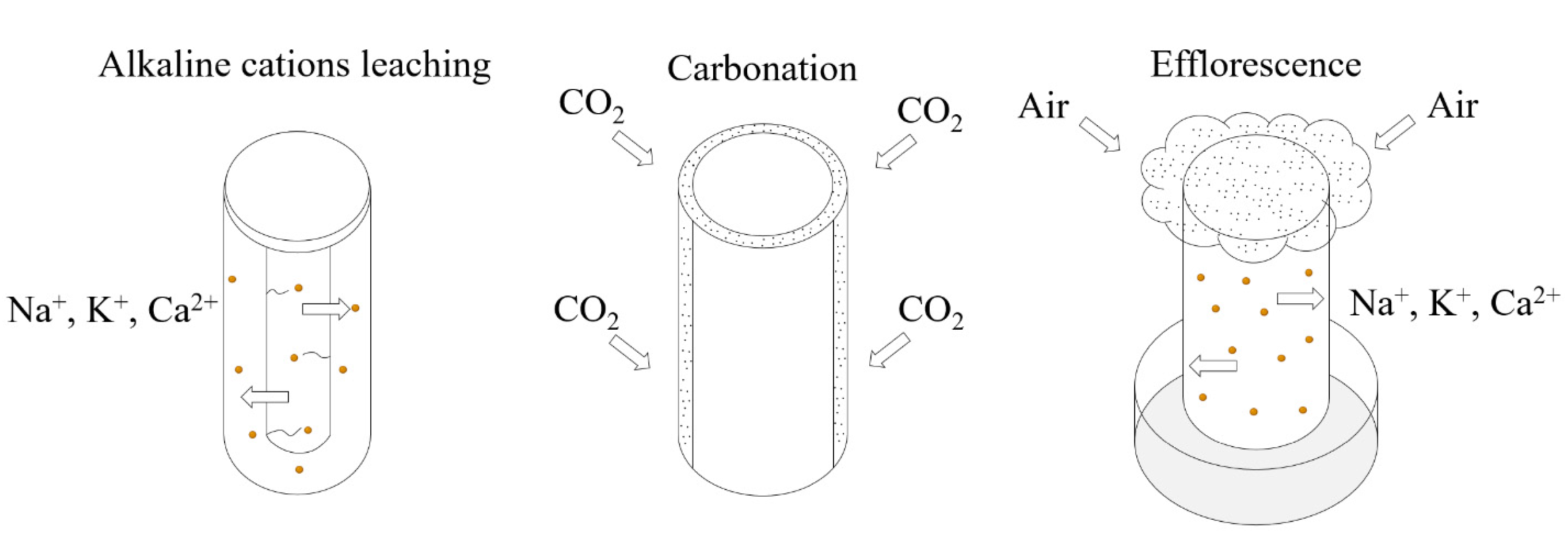
4.1. Prevention of Alkaline Cation Leaching
4.1.1. Promotion of the Alkali-Activated Reaction
4.1.2. Increase in Material Compactness
4.2. Reduction in the CO2 Adsorption
5. Prospects and Challenges
5.1. Wide Practical Application
5.2. Challenges in Composite Mix Ratio
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Sanjayan, J.G.; Nazari, A.; Chen, L.; Nguyen, G.H. Physical and mechanical properties of lightweight aerated geopolymer. Constr. Build. Mater. 2015, 79, 236–244. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 emission assessments of alkali-activated concrete and Ordinary Portland Cement concrete: A comparative analysis of different grades of concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Blanco, I.; Poggetto, G.D.; Morrone, B.; Tranquillo, E.; Barrino, F.; Catauro, M. Fly Ash Filled Geopolymers: Preparation and Thermal Study. Macromol. Symp. 2020, 389, 1900052. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Luo, W. A novel waterproof, fast setting and high early strength repair material derived from metakaolin geopolymer. Constr. Build. Mater. 2016, 124, 69–73. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Facile preparation of slag or fly ash geopolymer composite coatings with flame resistance. Constr. Build. Mater. 2019, 203, 655–661. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.; Yao, G.; Wang, Z.; Lyu, X. Preparation and characterization of an alkali-activated cementitious material with blast-furnace slag, soda sludge, and industrial gypsum. Constr. Build. Mater. 2022, 340, 127735. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Ma, X.; Reid, A.; Wang, H. Efflorescence and subflorescence induced microstructural and mechanical evolution in fly ash-based geopolymers. Cem. Concr. Compos. 2018, 92, 165–177. [Google Scholar] [CrossRef]
- Walkley, B.; Rees, G.J.; San Nicolas, R.; van Deventer, J.S.J.; Hanna, J.V.; Provis, J.L. New Structural Model of Hydrous Sodium Aluminosilicate Gels and the Role of Charge-Balancing Extra-Framework Al. J. Phys. Chem. C 2018, 122, 5673–5685. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Eckhard, D.; Zhang, Z.; Provis, J.L.; Kirchheim, A.P. Metakaolin-based geopolymers: Efflorescence and its effect on microstructure and mechanical properties. Ceram. Int. 2022, 48, 2212–2229. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Wang, S.; Zeng, L.; Ran, P.; Ji, G. Effect of nano-SiO2 on the efflorescence of an alkali-activated metakaolin mortar. Constr. Build. Mater. 2020, 253, 118952. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, G.; Liang, K. Development of fly ash and slag based high-strength alkali-activated foam concrete. Cem. Concr. Compos. 2022, 128, 104447. [Google Scholar] [CrossRef]
- Najafi Kani, E.; Allahverdi, A.; Provis, J.L. Efflorescence control in geopolymer binders based on natural pozzolan. Cem. Concr. Compos. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- Bernal, S.A. Microstructural Changes Induced by CO2 Exposure in Alkali-Activated Slag/Metakaolin Pastes. Front. Mater. 2016, 3, 43. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I.; Arellano-Aguilar, R.; Gorokhovsky, A. Statistical Analysis of Strength Development as a Function of Various Parameters on Activated Metakaolin/Slag Cements. J. Am. Ceram. Soc. 2010, 93, 541–547. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, C.; Wang, W.; Shi, Y.; Gao, X. Immobilization of MSWI fly ash through geopolymerization: Effects of water-wash. Waste Manag. 2011, 31, 311–317. [Google Scholar] [CrossRef]
- Firdous, R.; Stephan, D.; Djobo, J.N.Y. Natural pozzolan based geopolymers: A review on mechanical, microstructural and durability characteristics. Constr. Build. Mater. 2018, 190, 1251–1263. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.-L.; Dai, J.-G.; Poon, C.-S.; Zhang, W.-D.; Zhang, P. Inhibiting efflorescence formation on fly ash–based geopolymer via silane surface modification. Cem. Concr. Compos. 2018, 94, 43–52. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Effect of hydrophobic surface-modified fine aggregates on efflorescence control in geopolymer. Cem. Concr. Compos. 2022, 126, 104337. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jitsangiam, P.; Rattanasak, U. Hydrophobicity and efflorescence of lightweight fly ash geopolymer incorporated with calcium stearate. J. Clean. Prod. 2022, 364, 132449. [Google Scholar] [CrossRef]
- Tan, J.; Cizer, Ö.; Vandevyvere, B.; De Vlieger, J.; Dan, H.; Li, J. Efflorescence mitigation in construction and demolition waste (CDW) based geopolymer. J. Build. Eng. 2022, 58, 105001. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Zhou, Z.; Du, P.; Xu, D.; Xie, N.; Cheng, X.; Liu, Y. Effect of zeolite on waste based alkali-activated inorganic binder efflorescence. Constr. Build. Mater. 2018, 158, 683–690. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, T.; Xu, D.; Zhou, Z.; Du, P.; Xie, N.; Cheng, X.; Liu, Y. Effect of nano-silica on the efflorescence of waste based alkali-activated inorganic binder. Constr. Build. Mater. 2018, 167, 381–390. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Longhi, M.A.; Zhang, Z.; Walkley, B.; Rodríguez, E.D.; Kirchheim, A.P. Strategies for control and mitigation of efflorescence in metakaolin-based geopolymers. Cem. Concr. Res. 2021, 144, 106431. [Google Scholar] [CrossRef]
- Tiwari, M.; Sahu, S.K.; Bhangare, R.C.; Ajmal, P.Y.; Pandit, G.G. Elemental characterization of coal, fly ash, and bottom ash using an energy dispersive X-ray fluorescence technique. Appl. Radiat. Isot. 2014, 90, 53–57. [Google Scholar] [CrossRef]
- Chen-Tan, N.W.; van Riessen, A.; Ly, C.V.; Southam, D.C. Determining the Reactivity of a Fly Ash for Production of Geopolymer. J. Am. Ceram. Soc. 2009, 92, 881–887. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM: West Conshohocken PA, USA, 2015; Volume C618-15. [Google Scholar]
- Abdulkareem, O.A.; Mustafa Al Bakri, A.M.; Kamarudin, H.; Khairul Nizar, I.; Saif, A.E.A. Effects of elevated temperatures on the thermal behavior and mechanical performance of fly ash geopolymer paste, mortar and lightweight concrete. Constr. Build. Mater. 2014, 50, 377–387. [Google Scholar] [CrossRef]
- Tang, D.; Yang, C.; Li, X.; Zhu, X.; Yang, K.; Yu, L. Mitigation of efflorescence of alkali-activated slag mortars by incorporating calcium hydroxide. Constr. Build. Mater. 2021, 298, 123873. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing Precursors for Geopolymer Cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Megat Johari, M.A.; Ahmad, Z.A.; Maslehuddin, M. Strength and microstructure of alkali-activated binary blended binder containing palm oil fuel ash and ground blast-furnace slag. Constr. Build. Mater. 2014, 52, 504–510. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Megat Johari, M.A.; Ahmad, Z.A.; Maslehuddin, M. Effects of H2O/Na2O molar ratio on the strength of alkaline activated ground blast furnace slag-ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 56, 158–164. [Google Scholar] [CrossRef]
- Ismail, N.; El-Hassan, H. Development and Characterization of Fly Ash–Slag Blended Geopolymer Mortar and Lightweight Concrete. J. Mater. Civ. Eng. 2018, 30, 04018029. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Buruberri, L.H.; Senff, L.; Labrincha, J.A. Influence of blowing agent on the fresh- and hardened-state properties of lightweight geopolymers. Mater. Des. 2016, 108, 551–559. [Google Scholar] [CrossRef]
- Suh, J.-I.; Jeon, D.; Yoon, S.; Oh, J.E.; Park, H.-G. Development of strong lightweight cementitious matrix for lightweight concrete simply by increasing a water-to-binder ratio in Ca(OH)2-Na2CO3-activated fly ash system. Constr. Build. Mater. 2017, 152, 444–455. [Google Scholar] [CrossRef]
- Longhi, M.A.; Zhang, Z.; Rodríguez, E.D.; Kirchheim, A.P.; Wang, H. Efflorescence of Alkali-Activated Cements (Geopolymers) and the Impacts on Material Structures: A Critical Analysis. Front. Mater. 2019, 6, 89. [Google Scholar] [CrossRef]
- Srinivasamurthy, L.; Chevali, V.S.; Zhang, Z.; Wang, H. Phase changes under efflorescence in alkali activated materials with mixed activators. Constr. Build. Mater. 2021, 283, 122678. [Google Scholar] [CrossRef]
- Allahverdi, A.; Najafi Kani, E.; Shaverdi, B. Carbonation Versus Efflorescence in Alkali-Activated Blast-Furnace Slag in Relation with Chemical Composition of Activator. Int. J. Civ. Eng. 2017, 15, 565–573. [Google Scholar] [CrossRef]
- Maghsoodloorad, H.; Allahverdi, A. Efflorescence Formation and Control in Alkali-Activated Phosphorus Slag Cement. Int. J. Civ. Eng. 2016, 14, 425–438. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Pore solution in alkali-activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 139–148. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, D.; Yang, K.; Zhang, Z.; Li, Q.; Pan, Q.; Yang, C. Effect of Ca(OH)2 on shrinkage characteristics and microstructures of alkali-activated slag concrete. Constr. Build. Mater. 2018, 175, 467–482. [Google Scholar] [CrossRef]
- Che, Y.W.; Cheng, J.; Lei, X.; Wu, H. Preparation and properties of geopolymer foam materials. Non-Metall 2015, 38. [Google Scholar]
- Phavongkham, V.; Wattanasiriwech, S.; Cheng, T.-W.; Wattanasiriwech, D. Effects of surfactant on thermo-mechanical behavior of geopolymer foam paste made with sodium perborate foaming agent. Constr. Build. Mater. 2020, 243, 118282. [Google Scholar] [CrossRef]
- Prasittisopin, L.; Termkhajornkit, P.; Kim, Y.H. Review of concrete with expanded polystyrene (EPS): Performance and environmental aspects. J. Clean. Prod. 2022, 366, 132919. [Google Scholar] [CrossRef]
- Nodehi, M.; Ozbakkaloglu, T.; Gholampour, A.; Mohammed, T.; Shi, X. The effect of curing regimes on physico-mechanical, microstructural and durability properties of alkali-activated materials: A review. Constr. Build. Mater. 2022, 321, 126335. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Qiu, J.; Yang, E.-H. Micromechanics-based investigation of a sustainable ambient temperature cured one-part strain hardening geopolymer composite. Constr. Build. Mater. 2017, 131, 552–563. [Google Scholar] [CrossRef]
- Wu, B.; Li, L.; Deng, H.; Zheng, Z.; Xiang, Y.; Li, Y.; Ma, X. Characteristics and mechanism of efflorescence in fly ash-based geopolymer mortars under quasi-natural condition. J. Build. Eng. 2022, 55, 104708. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.P.; van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Processing Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A. Development of high strength one-part geopolymer mortar using sodium metasilicate. Constr. Build. Mater. 2020, 236, 117611. [Google Scholar] [CrossRef]
- Okoye, F.N.; Durgaprasad, J.; Singh, N.B. Mechanical properties of alkali activated flyash/Kaolin based geopolymer concrete. Constr. Build. Mater. 2015, 98, 685–691. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Taebuanhuad, S. Role of microwave radiation in curing the fly ash geopolymer. Adv. Powder Technol. 2013, 24, 703–707. [Google Scholar] [CrossRef]
- Preethi, R.K.; Venkatarama Reddy, B.V. Experimental investigations on geopolymer stabilised compressed earth products. Constr. Build. Mater. 2020, 257, 119563. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. (Eds.) Geopolymers: Structures, Processing, Properties and Industrial Application; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slg. J. Mater. Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Niş, A.; Altındal, İ. Compressive Strength Performance of Alkali Activated Concretes under Different Curing Conditions. Period. Polytech. Civ. Eng. 2021, 65, 17016. [Google Scholar] [CrossRef]
- Rostami, M.; Behfarnia, K. The effect of silica fume on durability of alkali activated slag concrete. Constr. Build. Mater. 2017, 134, 262–268. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.; Peethamparan, S. Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag: Comparison with ordinary Portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Chang, J.; Lau, D. Investigation of the macro performance, mechanism, and durability of multiscale steel fiber reinforced low-carbon ecological UHPC. Constr. Build. Mater. 2022, 327, 126921. [Google Scholar] [CrossRef]
- Pouhet, R.; Cyr, M. Carbonation in the pore solution of metakaolin-based geopolymer. Cem. Concr. Res. 2016, 88, 227–235. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S.J. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Yao, X.; Yang, T.; Zhang, Z. Compressive strength development and shrinkage of alkali-activated fly ash–slag blends associated with efflorescence. Mater. Struct. 2015, 49, 2907–2918. [Google Scholar] [CrossRef]
- Tan, J.; Cizer, Ö.; De Vlieger, J.; Dan, H.; Li, J. Impacts of milling duration on construction and demolition waste (CDW) based precursor and resulting geopolymer: Reactivity, geopolymerization and sustainability. Resour. Conserv. Recycl. 2022, 184, 106433. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Alkali leaching and mechanical performance of epoxy resin-reinforced geopolymer composite. Mater. Lett. 2021, 304, 130663. [Google Scholar] [CrossRef]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Habeeb, G.A.; Mahmud, H.B. Study on properties of rice husk ash and its use as cement replacement material. Mater. Res. 2010, 13, 185–190. [Google Scholar] [CrossRef]
- Provis, J.L.; Myers, R.J.; White, C.E.; Rose, V.; van Deventer, J.S.J. X-ray microtomography shows pore structure and tortuosity in alkali-activated binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]
- Katpady, D.N.; Takewaka, K.; Yamaguchi, T.; Akira, Y. Performance of slag based Shirasu geopolymer cured under ambient condition. Constr. Build. Mater. 2020, 234, 117210. [Google Scholar] [CrossRef]
- Ravikumar, D.; Peethamparan, S.; Neithalath, N. Structure and strength of NaOH activated concretes containing fly ash or GGBFS as the sole binder. Cem. Concr. Compos. 2010, 32, 399–410. [Google Scholar] [CrossRef]
- Zhou, Z.; Cheng, X.; Du, P.; Zhou, T. A Development Method of Nano-Alumina Modified Alkali-Activated Cement Efflorescence Inhibitor. C.N. Patent CN108751766A[P], 2018. [Google Scholar]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a blended slag-fly ash geopolymer concrete in field conditions after 8 years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Assi, L.N.; Deaver, E.; ElBatanouny, M.K.; Ziehl, P. Investigation of early compressive strength of fly ash-based geopolymer concrete. Constr. Build. Mater. 2016, 112, 807–815. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Cui, K.; Lau, D.; Zhang, Y.; Chang, J. Mechanical properties and mechanism of nano-CaCO3 enhanced sulphoaluminate cement-based reactive powder concrete. Constr. Build. Mater. 2021, 309. [Google Scholar] [CrossRef]
- Zhou, Z.; Cheng, X.; Du, P.; Zhou, T. A Development Method of Nano-TiO2 Modified Alkali-Activated Cement Efflorescence Inhibitor. C.N. Patent CN108947306A[P], 2018. [Google Scholar]
- Behfarnia, K.; Rostami, M. Effects of micro and nanoparticles of SiO2 on the permeability of alkali activated slag concrete. Constr. Build. Mater. 2017, 131, 205–213. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, M.; Zhang, J.; Ren, J.; Vatin, N.I.; Sabri, M.M.S. The Use of GA and PSO in Evaluating the Shear Strength of Steel Fiber Reinforced Concrete Beams. KSCE J. Civ. Eng. 2022, 26, 3918–3931. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, M.; Zhang, J.; Ren, J.; Vatin, N.I.; Sabri, M.M.S. Development of a New Stacking Model to Evaluate the Strength Parameters of Concrete Samples in Laboratory. Iran. J. Sci. Technol. Trans. Civ. Eng. 2022. [Google Scholar] [CrossRef]
- Liu, S.; Hao, Y.; Ma, G. Approaches to enhance the carbonation resistance of fly ash and slag based alkali-activated mortar-experimental evaluations. J. Clean. Prod. 2021, 280, 124321. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Uptake of chloride and carbonate by Mg-Al and Ca-Al layered double hydroxides in simulated pore solutions of alkali-activated slag cement. Cem. Concr. Res. 2017, 100, 1–13. [Google Scholar] [CrossRef]
- Shui, Z.H.; Yu, R.; Chen, Y.X.; Duan, P.; Ma, J.T.; Wang, X.P. Improvement of concrete carbonation resistance based on a structure modified Layered Double Hydroxides (LDHs): Experiments and mechanism analysis. Constr. Build. Mater. 2018, 176, 228–240. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.X.; Leitão, A.A. Comparative Structural, thermodynamic and electronic analyses of ZnAlAn− hydrotalcite-like compounds (An−Cl−, F−, Br−, OH−, CO32− or NO3−): An ab initio study. Appl. Clay Sci. 2012, 56, 16–22. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M.C. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Microporous Mesoporous Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]
- Bukowski, J.M.; Berger, R.L. Reactivity and strength development of CO2 activated non-hydraulic calcium silicates. Cem. Concr. Res. 1979, 9, 57–68. [Google Scholar] [CrossRef]
- He, J.; Gao, Q.; Wu, Y.; He, J.; Pu, X. Study on improvement of carbonation resistance of alkali-activated slag concrete. Constr. Build. Mater. 2018, 176, 60–67. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Lai, S.H.; Ling, T.-C. Investigation on the copper ion removal potential of a facile-fabricated foamed geopolymer sphere for wastewater remediation. Clean. Mater. 2022, 4, 100088. [Google Scholar] [CrossRef]
- Daan Frenkel, B.S. Understanding Molecular Simulation from Algorithms to Applications; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Lau, D.; Jian, W.; Yu, Z.; Hui, D. Nano-engineering of construction materials using molecular dynamics simulations: Prospects and challenges. Compos. Part B Eng. 2018, 143, 282–291. [Google Scholar] [CrossRef]
| Solid Aluminosilicate Precursors | Activator SiO2/Na2O Molar Ratio | Total Na/Al Molar Ratio | Si/Al Ratio | Solution/Solid Ratio | Additive | Curing Condition | Efflorescence Degree | Reference |
|---|---|---|---|---|---|---|---|---|
| Natural pozzolan | 0.45 | 0.61 | None | None | High-alumina cements Secar 71 | 25 °C RH95% | 1.26% | [16] |
| Waste glass powder Class C FA Limestone | None | None | None | 0.4 | None | 20 °C Air curing | 2.8% | [21] |
| Class F FA Bauxite | None | None | 1.5 | None | None | 80 °C oven | None | [22] |
| FA | 1.5 | None | None | None | None | 25 ± 1 °C RH 90 ± 10% | 4.0% | [11] |
| FA GGBFS | 1.5 | None | None | None | None | 80 °C Hydrothermal | 16.0% | [10] |
| Class F FA | 1.2 | None | None | None | Octyltriethoxysilane | 20 °C RH > 95% | 3.5% | [23] |
| Class F FA GGBFS | 2.0 | None | None | None | Fumed silica | None | None | [24] |
| FA | None | None | None | None | 5% calcium stearate | 65 °C | 0.0% | [25] |
| Construction and demolition waste | 0.67 | None | None | None | 20 wt% MK | None | 10.2% | [26] |
| Steel slag and blast furnace slag | None | None | None | None | 15% 5A zeolite | 20 °C RH > 95% | None | [27] |
| Steel slag and blast furnace slag | None | None | None | None | 2.0% nano-silica | 20 °C RH > 95% | None | [28] |
| Steel slag and blast furnace slag | 1.4 | None | None | None | 3.0% nano-SiO2 | 20 °C RH > 95% | None | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.; Cui, K.; Sabri, M.M.S.; Huang, J. Influence Factors in the Wide Application of Alkali-Activated Materials: A Critical Review about Efflorescence. Materials 2022, 15, 6436. https://doi.org/10.3390/ma15186436
Liang K, Cui K, Sabri MMS, Huang J. Influence Factors in the Wide Application of Alkali-Activated Materials: A Critical Review about Efflorescence. Materials. 2022; 15(18):6436. https://doi.org/10.3390/ma15186436
Chicago/Turabian StyleLiang, Kaikang, Kai Cui, Mohanad Muayad Sabri Sabri, and Jiandong Huang. 2022. "Influence Factors in the Wide Application of Alkali-Activated Materials: A Critical Review about Efflorescence" Materials 15, no. 18: 6436. https://doi.org/10.3390/ma15186436
APA StyleLiang, K., Cui, K., Sabri, M. M. S., & Huang, J. (2022). Influence Factors in the Wide Application of Alkali-Activated Materials: A Critical Review about Efflorescence. Materials, 15(18), 6436. https://doi.org/10.3390/ma15186436








