Nettle, a Long-Known Fiber Plant with New Perspectives
Abstract
:1. Introduction
2. Historical Perspective
2.1. Clone Selection
2.2. Fiber Processing
3. Biology, Physiology, and Genetic of Urtica dioica L.
3.1. Biology, Ecology, and Reproduction
3.2. Stem and Fiber Morphology and Fiber Composition
3.3. Phylogeny and Genetic Features
3.4. Nettle Phytochemistry
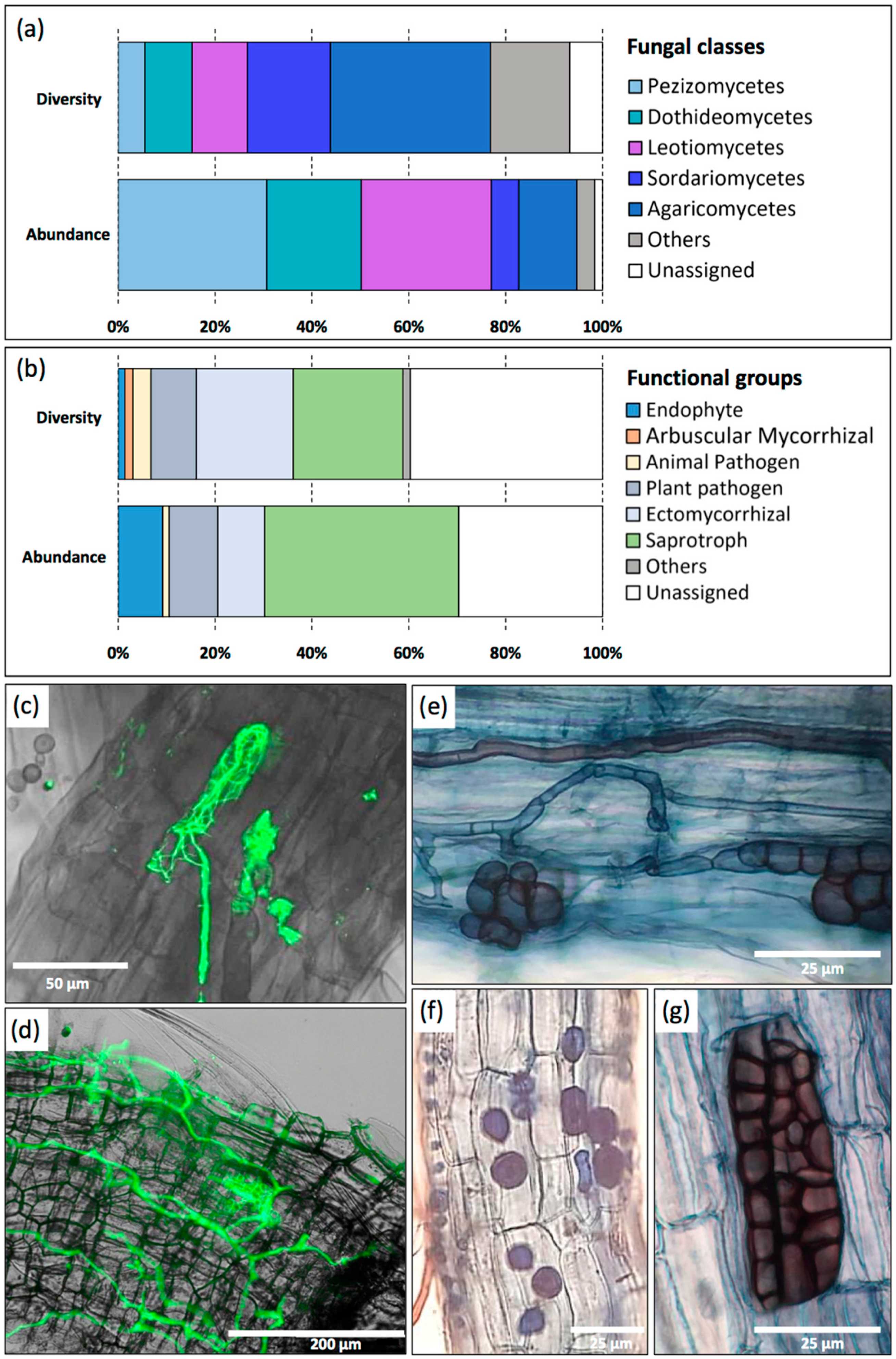
3.5. Nettle-Associated Organisms
4. Nettle Cultivation, Harvest, and Fiber Processing
- Can the cultivation of nettle be improved to increase bast fiber yield?
- Are there processes on the market for processing bast fibers that can be used for nettle processing without major adaptations?
- Which processes can be chosen to obtain nettle fibers for high-quality fiber composites?
- What still needs to be optimized to turn nettle into a valuable fiber for composites?
4.1. Agronomic Practices for Fiber from Nettles
4.2. Fiber Yield Improvement
4.3. Fiber Extraction and Processing
5. Nettle as a Multipurpose Crop
5.1. Potential Industrial Uses of Nettle
5.2. Fiber-Based Applications
5.3. Recent Application Developments for Nettle Fibers
5.4. Nettle Use in Phytomanagement Strategies
6. Conclusions
- Recovery of natural fibers for use in composite materials and technical textiles
- Recovery of ingredients and nutraceutical
- Co-cropping with trees
- Risk management for sites affected by land contamination
- Recovery of use for marginal land
- Soil improvement and soil carbon sequestration
- Providing a wide range of ecological niches for many native species (so supporting wider ecosystem service delivery).
- A greater range of nettle fiber compositional information and functionality testing for modern applications (for example, in reinforcement for composite materials)
- A greater understanding of the variability of these fibers and how this variability impacts their use
- A greater understanding of climate effects on nettle growth and nettle fiber properties
- A deeper understanding of nutraceutical and ingredient products available from nettles and the potential to deliver these products in parallel with natural fibers from the same harvested biomass
- Greater functional understanding of the ecological consequences of nettle production and use
- A greater effort in piloting and demonstrating nettle production and use, and in particular on marginal areas, including those affected by land contamination
- A more robust basis for understanding the economic and wider sustainability consequences of nettle production and use and the potential contribution this might have for addressing the current two leading challenges to humankind: climate change and chemical contamination.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartl, A.; Vogl, C. Dry Matter and Fiber Yields, and the Fiber Characteristics of Five Nettle Clones (Urtica dioica L.) Organically Grown in Austria for Potential Textile Use. Am. J. Altern. Agric. 2002, 17, 195–200. [Google Scholar] [CrossRef]
- Bacci, L.; Baronti, S.; Predieri, S.; Di Virgilio, N. Fiber Yield and Quality of Fiber Nettle (Urtica dioica L.) Cultivated in Italy. Ind. Crops Prod. 2009, 29, 480–484. [Google Scholar] [CrossRef]
- Harwood, J.; Horne, M.; Waldron, D. Cultivating Stinging Nettle (Urtica dioica) for Fibre Production in the UK. Asp. Appl. Biol. 2010, 101, 133–138. [Google Scholar]
- Jankauskienė, Z.; Gruzdevienė, E. Changes in the Productivity of Wild and Cultivated Stinging Nettle (Urtica dioica L.) as Influenced by the Planting Density and Crop Age. Zemdirb. Agric. 2015, 102, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica Spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, J.; Dhakal, H. Sustainable Biobased Composites for Advanced Applications: Recent Trends and Future Opportunities—A Critical Review. Compos. Part C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Shah, D. Developing Plant Fibre Composites for Structural Applications by Optimising Composite Parameters: A Critical Review. J. Mater. Sci. 2013, 48, 6083–6107. [Google Scholar] [CrossRef]
- Meirhaeghe, C. Évaluation de la Disponibilité et de L’accessibilité de Fibres Végétales à Usage Matériaux en France; Report Fibres Recherche Développement ADEME; French Environment and Energy Management Agency: Angers, France, 2011; 84p.
- Di Virgilio, N.; Papazoglou, E.; Jankauskiene, Z.; Lonardo, S.; Praczyk, M.; Wielgusz, K. The Potential of Stinging Nettle (Urtica dioica L.) as a Crop with Multiple Uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Vogl, C.; Hartl, A. Production and Processing of Organically Grown Fiber Nettle (Urtica dioica L.) and Its Potential Use in the Natural Textile Industry: A Review. Am. J. Altern. Agric. 2003, 18, 119–128. [Google Scholar] [CrossRef]
- Jeannin, T.; Yung, L.; Evon, P.; Labonne, L.; Ouagne, P.; Lecourt, M.; Cazaux, D.; Chalot, M.; Placet, V. Native Stinging Nettle (Urtica dioica L.) Growing Spontaneously under Short Rotation Coppice for Phytomanagement of Trace Element Contaminated Soils: Fibre Yield, Processability and Quality. Ind. Crops Prod. 2020, 145, 111997. [Google Scholar] [CrossRef]
- Taylor, K. Biological Flora of the British Isles: Urtica dioica L. J. Ecol. 2009, 97, 1436–1458. [Google Scholar] [CrossRef]
- Wonglersak, R.; Cronk, Q.; Percy, D. Salix Transect of Europe: Structured Genetic Variation and Isolation-by-Distance in the Nettle Psyllid, Trioza urticae (Psylloidea, Hemiptera), from Greece to Arctic Norway. Biodivers. Data J. 2017, 5, e10824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windler, R.; Rast-Eicher, A.; Mannering, U. Nessel und Flachs: Textilfunde aus einem frühmittelalterlichen Mädchengrab in Flurlingen (Kanton Zürich). Archeol. Svizz. 1995, 18, 155–161. [Google Scholar] [CrossRef]
- Edom, G. From Sting to Spin—A History of Nettle Fibre, Revised ed.; Urtica Books: Bognor Regis, UK, 2019. [Google Scholar]
- Bredemann, G. Die Große Brennessel Urtica dioica L.; Akademie-Verlag: Berlin, Germany, 1959; 137p. [Google Scholar]
- Dreyer, J. Die Fasernessel als Nachwachsender Rohstoff. Leistungsprüfung von Fasernesseln (Urtica dioica L., Große Brennessel) Unter Besonderer Berücksichtigung der Phänotypischen Differenzierung Anbauwürdiger Klone; Schriftenreihe Naturwissenschaftliche Forschungsergebnisse; Verlag Dr. Kovac: Hamburg, Germany, 1999. [Google Scholar]
- Harwood, J.; Edom, G. Nettle Fibre: Its Prospects, Uses and Problems in Historical Perspective. Text. Hist. 2012, 43, 107–119. [Google Scholar] [CrossRef]
- Dreyling, G. Die Fasernessel (Urtica dioica L.), eine wiederentdeckte alte Kulturpflanze. Umweltwiss. Schadst. Forsch. 2002, 14, 125. [Google Scholar] [CrossRef]
- Bredemann, G. Die Bestimmung des Fasergehaltes dei Massenuntersuchungen von Hanf, Flachs, Fasernesseln und anderen Bastfaserpflanzen. Faserforsch 1942, 16, 14–39. [Google Scholar]
- Dreyer, J.; Dreyling, G.; Feldmann, F. Cultivation of Stinging Nettle Urtica dioica L. with High Fibre Content as a Raw Material for the Production of Fibre and Cellulose: Qualitative and Quantitative Differentiation of Ancient Clones. J. Appl. Bot. 1996, 70, 28–39. [Google Scholar]
- Francken-Welz, H. Vergleichende Bewertung der Ertragsfähigkeit und Faserqualität von Lein (Linum usitatissimum L.), Hanf (Cannabis sativa L.) und Fasernessel (Urtica dioica L.) zur Produktion Hochwertiger Industriefasern; Shaker Verlag: Düren, Germany, 2003. [Google Scholar]
- Wurl, G.; Graf, T.; Vetter, A.; Biertümpfel, A. 10 Years agrotechnical Trials to Fibre Nettle (Urtica dioica L.) in Thuringia. Prod. Process. Use Nat. Fibres Potsdam. Bornim 2002, 30, 95–96. [Google Scholar]
- Biskupek-Korrell, B.; Schneider, C. Schlussbericht Zum Vorhaben “Entwicklung eines Kostengünstigen Vermehrungsverfahrens für Fasernesseln über Somatische Embryogenese und Erzeugung von Verkapselten, Synthetischen Samen”; Final Report DBU Reg.; Deutsche Bundesstiftung Umwelt (DBU): Hannover, Germany, 2012; 57p.
- Biskupek-Korrell, B.; Knapwost, C.; Schneider, C.; Wartenberg, S. Schlussbericht Zum Verbundvorhaben “Züchtung Faserreicher, Ertragreicher und Widerstandsfähiger Fasernesselklone Mit Guten Faserqualitäten und Entwicklung eines Effizienten und Kostengünstigen Vermehrungsverfahrens, Teilvorhaben 1: Fasernesselzüchtung und Entwicklung eines Effizienten Vermehrungsverfahrens”; Final Report FNR Reg.; Agency for Renewable Resources (FNR): Gülzow, Germany, 2013; 58p.
- Fischer, H.; Gusovius, H.; Lühr, C.; Rödel, P.; Schneider, C.; Kreye, S.; Machmüller, A.; Rottmann-Meyer, M.; Beckhaus, H. Schlussbericht zum Verbundvorhaben “InBeNeFa—Verbundvorhaben: Entwicklung einer Industriellen Bereitstellungskette von Brennnesseljungpflanzen bis zur Nesselfaser”; Research Report of the Faserinstitut Bremen; Faserinstitut Bremen: Bremen, Germany, 2019; 117p. [Google Scholar]
- Böhmer, G. Technische Geschichte der Pflanzen; Weidmannsche Buchhandlung: Leipzig, Germany, 1794. [Google Scholar]
- Richter, O. Alte und neue Textilpflanzen. In Vorträge des Vereins zur Verbreitung Naturwissenschaftlicher Kenntnisse; Kalisynditat: Berlin, Germany, 1915. [Google Scholar]
- Sethmann, A. Girardinia Diversifolia (LINK) FRIIS (Urticaceae)-Eine Neue Faserpflanze—Untersuchungen zu den Morphologischen und Mechanischen Fasercharakteristika. Ph.D. Thesis, Universität Hamburg, Hamburg, Germany, 2004. [Google Scholar]
- Schnegelsberg, G. Handbuch der Faser—Theorie und Systematik der Faser; Deutscher Fachverlag: Frankfurt, Germany, 1999. [Google Scholar]
- Subedee, B.; Chaudhary, R.; Uprety, Y.; Dorji, T. Socio-Ecological Perspectives of Himalayan Giant Nettle (Girardinia diversifolia (Link) Friis) in Nepal. J. Nat. Fibers 2020, 17, 9–17. [Google Scholar] [CrossRef]
- von Roeßler-Ladé, A. Die Nessel eine Gespinstpflanze: Mit Anleitung zu deren Anbau und Weiteren Bearbeitung; August Schroeter’s Verlag: Stuttgart, Germany, 1916. [Google Scholar]
- Bouché, C.; Grothe, H. Ramie, Rheea, Chinagras und Nesselfaser; Verlag von Julius Springer: Berlin, Germany, 1884. [Google Scholar]
- Ganswindt, A. Die Bastfasern und ihre Technische Verarbeitung—Zum Gebrauche an Färbereischulen, Technischen Hochschulen, sowie zum Selbstunterricht; Chemisch-Technische Bibliothek—Band 371; A. Hartleben’s Verlag: Leipzig, Germany, 1922. [Google Scholar]
- Schulz, W. Referat über die Erfahrungen in der Verarbeitung der Brennnessel; Druck von H. Scherokosz: Berlin, Germany, 1920. [Google Scholar]
- Lüdtke, M.; Kling, R.; Scheithauer, G. Aufbereitung und Verspinnung der Wildnesselfaser. Dtsch. Leinen-Ind. 1944, 4, 41–43. [Google Scholar]
- Elster, J. Nesselstängelschälmaschine—Mit zwei verschiedenen Geschwindigkeit umlaufenden endlosen Schindtüchern. German Patent 158675, 10 May 1940. [Google Scholar]
- Elster, J. Verfahren Zum Anführen und Naßbehandeln der von Stengeln abgelösten Nesselrindenbänder. German Patent 158676, 10 May 1940. [Google Scholar]
- Dreyer, J.; Müssig, J.; Koschke, N.; Ibenthal, W.; Harig, H. Comparison of Enzymatically Separated Hemp and Nettle Fibre to Chemically Separated and Steam Exploded Hemp Fibre. J. Ind. Hemp 2002, 7, 43–59. [Google Scholar] [CrossRef]
- Müssig, J.; Amaducci, S.; Bourmaud, A.; Beaugrand, J.; Shah, D. Transdisciplinary Top-down Review of Hemp Fibre Composites: From an Advanced Product Design to Crop Variety Selection—A Critical Review. Compos. Part C Open Access 2020, 2, 100010. [Google Scholar] [CrossRef]
- Schlüter, M.; Meyer, M.; Risse, S.; Räbiger, N.; Fischer, H.; Müssig, J.; Bluhm, C. Sustainable Production of High Quality Hemp Fibres by Enzymatic Modification. In Proceedings of the 27th International Exhibition-Congress on Chemical Engineering, Environmental Protection and Biotechnology, Frankfurt, Germany, 22–23 May 2003. [Google Scholar]
- Henning, T.; Quandt, D.; Große-Veldmann, B.; Monro, A.; Weigend, M. Weeding the Nettles II: A Delimitation of “Urtica dioica L.” (Urticaceae) Based on Morphological and Molecular Data, Including a Rehabilitation of Urtica gracilis Ait. Phytotaxa 2014, 162, 61–83. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Provan, J.; Wang, H.; Chen, C.; Cadotte, M.; Luo, Y.; Amorim, B.; Li, D.; Milne, R. Testing Darwin’s Transoceanic Dispersal Hypothesis for the Inland Nettle Family (Urticaceae). Ecol. Lett. 2018, 21, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Šrutek, M.; Teckelmann, M. Review of Biology and Ecology of Urtica dioica. Preslia Praha 1998, 70, 1–19. [Google Scholar]
- Cronk, Q.; Hidalgo, O.; Pellicer, J.; Percy, D.; Leitch, I. Salix Transect of Europe: Variation in Ploidy and Genome Size in Willow-Associated Common Nettle, Urtica dioica L. Sens. Lat., from Greece to Arctic Norway. Biodivers. Data J. 2016, 4, e10003. [Google Scholar] [CrossRef] [Green Version]
- Klimešová, J. The Effects of Timing and Duration of Floods on Growth of Yound Plants of Phalaris Arundinacea L. and Urtica dioica L.: An Experimental Study. Aquat. Bot. 1994, 48, 21–29. [Google Scholar] [CrossRef]
- Bisht, S.; Bhandari, S.; Bisht, N. Urtica dioica L.: An Undervalued, Economically Important Plant. Agric. Sci. Res. J. 2012, 2, 250–252. [Google Scholar]
- Rutto, L.; Ansari, M.; Brandt, M. Biomass Yield and Dry Matter Partitioning in Greenhouse-Grown Stinging Nettle under Different Fertilization Regimes. HortTechnology 2012, 22, 751–756. [Google Scholar] [CrossRef]
- Nkhabu, K.; Liphoto, M.; Ntahane, T.; Senoko, K. Genetic Diversity of Stinging Nettle (Urtica dioica) by Agro Morphological Markers. Eur. J. Bot. Plant Sci. Phytol. 2021, 6, 51–68. [Google Scholar]
- Guil-Guerrero, J.; Rebolloso-Fuentes, M.; Isasa, M. Fatty Acids and Carotenoids from Stinging Nettle (Urtica dioica L.). J. Food Compos. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Reaume, T. Stinging Nettle Urtica dioica Urticaceae—Nettle Family. Nat. Manit. 2010, 3. Available online: https://www.google.fr/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwia6u-v7LH4AhXD4KQKHVpNClwQFnoECAYQAQ&url=https%3A%2F%2Fwww.naturemanitoba.ca%2Fsites%2Fdefault%2Ffiles%2FStingingNettle.pdf&usg=AOvVaw3ARsU0bSiMyfjbd1jAbFW6 (accessed on 1 May 2022).
- Shannon, R.; Holsinger, K. The Genetics of Sex Determination in Stinging Nettle (Urtica dioica). Sex. Plant Reprod. 2007, 20, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Große-Veldmann, B.; Weigend, M. The Geometry of Gender: Hyper-Diversification of Sexual Systems in Urtica L. (Urticaceae). Cladistics 2017, 34, 131–150. [Google Scholar] [CrossRef]
- Wheeler, K. A Natural History of Nettles; Trafford Publishing: Victoria, BC, Canada, 2004; 318p. [Google Scholar]
- Draghi, F. L’ortie Dioïque (Urtica dioica L.): Étude Bibliographique. Ph.D. Thesis, University of Lorraine, Nancy, France, 2005. [Google Scholar]
- Popay, I. Urtica dioica (Stinging Nettle): Invasive Species Compedium; Centre for Agriculture and Bioscience International (CABI): Wallingford, UK, 2014. [Google Scholar]
- Rutto, L.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral Properties and Dietary Value of Raw and Processed Stinging Nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013, 857120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Kali, G. Study on Morpho-Anatomical and Histo-Chemical Characterisation of Stinging Nettle, Urtica dioica L. in Uttarakhand, India. J. Pharmacogn. Phytochem. 2019, 8, 4325–4331. [Google Scholar]
- Thurston, E. Morphology, Fine Structure, and Ontogeny of the Stinging Emergence of Urtica dioica. Am. J. Bot. 1974, 61, 809–817. [Google Scholar] [CrossRef]
- Emmelin, N.; Feldberg, W. The Mechanism of the Sting of the Common Nettle (Urtica urens). J. Physiol. 1947, 106, 440–455. [Google Scholar] [CrossRef]
- Collier, H.; Chesher, G. Identification of 5-Hydroxytryptamine in the Sting of the Nettle (Urtica dioica). Br. J. Pharmacol. Chemother. 1956, 11, 186–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, A.; Olsen, M. Mechanism of Action of Stinging Nettles. Wilderness Environ. Med. 2011, 22, 136–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuberville, T.; Dudley, P.; Pollard, A. Responses of Invertebrate Herbivores to Stinging Trichomes of Urtica dioica and Laportea canadensis. Oikos 1996, 75, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Pullin, A.; Gilbert, J. The Stinging Nettle, Urtica dioica, Increases Trichome Density after Herbivore and Mechanical Damage. Oikos 1989, 54, 275–280. [Google Scholar] [CrossRef]
- Pollard, A.; Briggs, D. Genecological Studies of Urtica dioica L. I. The Nature of Intraspecific Variation in U. dioica. New Phytol. 1982, 92, 453–470. [Google Scholar] [CrossRef]
- Pollard, A.; Briggs, D. Genecological Studies of Urtica dioica L, II, Patterns of Variation at Wicken Fen, Cambridgeshire, England. New Phytol. 1984, 96, 483–499. [Google Scholar] [CrossRef]
- Luna, T. Propagation Protocol for Stinging Nettle (Urtica dioica). Nativ. Plants J. 2001, 2, 110–111. [Google Scholar] [CrossRef]
- Rosnitschek-Schimmel, I. Biomass and Nitrogen Partitioning in a Perennial and an Annual Nitrophilic Species of Urtica. Z. Pflanzenphysiol. 1983, 109, 215–225. [Google Scholar] [CrossRef]
- Basset, I.; Crompton, C.; Woodland, D. The Biology of Canadian Weeds: 21. Urtica dioica L. Can. J. Plant Sci. 1977, 57, 491–498. [Google Scholar] [CrossRef]
- Grime, J.; Hodgson, J.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species; Castlepoint Press: Hoboken, NJ, USA, 1988; 752p. [Google Scholar]
- Klimešová, J. Population Dynamics of Phalaris arundinacea L. and Urtica dioica L. in a Floodplain during a Dry Period. Wetl. Ecol. Manag. 1995, 3, 79–85. [Google Scholar] [CrossRef]
- Gravis, A. Recherches Anatomiques Sur Les Organes Végétatifs de l’Urtica Dioica L.; Librairie Médicale & Scientifique de A. Manceaux (Bruxelles): Brussels, Belgium, 1885; Volume 1, 256p. [Google Scholar]
- Dreyer, J.; Edom, G. Pineapple, Curauá, Craua (Caroá), Macambira, Nettle, Sunn Hemp, Mauritius Hemp and Fique. In Bast and Other Plant Fibres; Woodhead Publishing Series in Textile; Woodhead Publishing: Sawston, UK, 2005; pp. 322–344. [Google Scholar]
- Franck, R. Bast and Other Plant Fibres, 1st ed.; Woodhead Publishing: Sawston, UK, 2005; ISBN 9781845690618. [Google Scholar]
- Weigend, M. Urtica dioica Subsp. cypria, with a Re-Evaluation of the U. dioica Group (Urticaceae) in Western Asia. Willdenowia 2006, 36, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Weigend, M.; Luebert, F. Weeding the Nettles I: Clarifying Species Limits in Perennial, Rhizomatous Urtica (Urticaceae) from Southern and Central Chile and Argentina. Phytotaxa 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Große-Veldmann, B.; Weigend, M. Weeding the Nettles III: Named Nonsense versus Named Morphotypes in European Urtica dioica L. (Urticaceae). Phytotaxa 2015, 208, 239–260. [Google Scholar] [CrossRef]
- Woodland, D. Biosystematics of the Perennial North American Taxa of Urtica. II. Taxonomy. Syst. Bot. 1982, 7, 282–290. [Google Scholar] [CrossRef]
- Rejlová, L.; Chrtek, J.; Trávníček, P.; Lučanová, M.; Vít, P.; Urfus, T. Polyploid Evolution: The Ultimate Way to Grasp the Nettle. PLoS ONE 2019, 14, e0218389. [Google Scholar] [CrossRef] [PubMed]
- Große-Veldmann, B. Systematics, Taxonomy, and Evolution of Urtica L. (Urticaceae). Ph.D. Thesis, Universität Bonn, Bonn, Germany, 2016. [Google Scholar]
- Rejlová, L.; Böhmová, A.; Chumová, Z.; Hořčicová, Š.; Josefiová, J.; Schmidt, P.-A.; Trávníček, P.; Urfus, T.; Vít, P.; Chrtek, J. Disparity between Morphology and Genetics in Urtica dioica (Urticaceae). Bot. J. Linn. Soc. 2021, 195, 606–621. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.; Bogarín, D.; Schley, R.; Bateman, R.; Gerlach, G.; Harpke, D.; Brassac, J.; Fernández-Mazuecos, M.; Dodsworth, S.; Hágsater, E.; et al. Resolving Relationships in an Exceedingly Young Neotropical Orchid Lineage Using Genotyping-by-Sequencing Data. Mol. Phylogenet. Evol. 2020, 144, 106672. [Google Scholar] [CrossRef]
- Escudero, M.; Eaton, D.; Hahn, M.; Hipp, A. Genotyping-by-Sequencing as a Tool to Infer Phylogeny and Ancestral Hybridization: A Case Study in Carex (Cyperaceae). Mol. Phylogenet. Evol. 2014, 79, 359–367. [Google Scholar] [CrossRef]
- Farag, M.; Weigend, M.; Luebert, F.; Brokamp, G.; Wessjohann, L. Phytochemical, Phylogenetic, and Anti-Inflammatory Evaluation of 43 Urtica Accessions (Stinging Nettle) Based on UPLC-Q-TOF-MS Metabolomic Profiles. Phytochemistry 2013, 96, 170–183. [Google Scholar] [CrossRef]
- Leitch, I.; Bennett, M. Genome Size and Its Uses: The Impact of Flow Cytometry. In Flow Cytometry with Plant Cells; John Wiley & Sons, Ltd.: Weinheim, Germany, 2007; pp. 153–176. ISBN 978-3-527-61092-1. [Google Scholar]
- Kron, P.; Suda, J.; Husband, B. Applications of Flow Cytometry to Evolutionary and Population Biology. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 847–876. [Google Scholar] [CrossRef]
- Dolezel, J.; Bartos, J.; Voglmayr, H.; Greilhuber, J. Nuclear DNA Content and Genome Size of Trout and Human. Cytometry A. 2003, 51, 127–128. [Google Scholar] [CrossRef]
- Fischer, S. Modélisation de L’évolution de la Taille des Génomes et de leur Densité en Gènes par Mutations Locales et Grands Réarrangements Chromosomiques. Ph.D. Thesis, INSA de Lyon, Villeurbanne, France, 2013. [Google Scholar]
- Garcia, S.; Leitch, I.; Anadon-Rosell, A.; Canela, M.; Gálvez, F.; Garnatje, T.; Gras, A.; Hidalgo, O.; Johnston, E.; Mas de Xaxars, G.; et al. Recent Updates and Developments to Plant Genome Size Databases. Nucleic Acids Res. 2014, 42, 1159–1166. [Google Scholar] [CrossRef]
- Barow, M.; Meister, A. Endopolyploidy in Seed Plants Is Differently Correlated to Systematics, Organ, Life Strategy and Genome Size. Plant Cell Environ. 2003, 26, 571–584. [Google Scholar] [CrossRef]
- Bainard, J.; Bainard, L.; Henry, T.; Fazekas, A.; Newmaster, S. A Multivariate Analysis of Variation in Genome Size and Endoreduplication in Angiosperms Reveals Strong Phylogenetic Signal and Association with Phenotypic Traits. New Phytol. 2012, 196, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Bainard, J.; Husband, B.; Baldwin, S.; Fazekas, A.; Gregory, T.; Newmaster, S.; Kron, P. The Effects of Rapid Desiccation on Estimates of Plant Genome Size. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2011, 19, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, J.; Leitch, I. The Plant DNA C-Values Database (Release 7.1): An Updated Online Repository of Plant Genome Size Data for Comparative Studies. New Phytol. 2020, 226, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Pustahija, F.; Brown, S.; Bogunić, F.; Bašić, N.; Muratović, E.; Ollier, S.; Hidalgo, O.; Bourge, M.; Stevanović, V.; Siljak-Yakovlev, S. Small Genomes Dominate in Plants Growing on Serpentine Soils in West Balkans, an Exhaustive Study of 8 Habitats Covering 308 Taxa. Plant Soil 2013, 373, 427–453. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Mirshafa, A.; Yekta Moghaddam, N.; Birjandian, B.; Shaki, F. Mitochondrial Dysfunction Contribute to Diabetic Neurotoxicity Induced by Streptozocin in Mice: Protective Effect of Urtica Dioica and Pioglitazone. Toxicol. Mech. Methods 2018, 28, 499–506. [Google Scholar] [CrossRef]
- Al-Tameme, H.; Hadi, M.; Hameed, I. Phytochemical Analysis of Urtica dioica Leaves by Fourier-Transform Infrared Spectroscopy and Gas Chromatography-Mass Spectrometry. J. Pharmacogn. Phytother. 2015, 7, 238–252. [Google Scholar] [CrossRef] [Green Version]
- Maobe, M.A.G.; Nyarango, R.M. Fourier Transformer Infra-Red Spectrophotometer Analysis of Urtica dioica Medicinal Herb Used for the Treatment of Diabetes, Malaria and Pneumonia in Kisii Region, Southwest Kenya. World Appl. Sci. J. 2013, 8, 1128–1135. [Google Scholar]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC Analysis of Phenolic Compounds in Leaves, Stalks, and Textile Fibers of Urtica dioica L. J. Agric. Food Chem. 2008, 56, 9127–9132. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the Alimurgic Plants Taraxacum officinale, Papaver rhoeas and Urtica dioica by Combined NMR and GC–MS Analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef]
- Brahmi-Chendouh, N.; Piccolella, S.; Nigro, E.; Hamri-Zeghichi, S.; Madani, K.; Daniele, A.; Pacifico, S. Urtica dioica L. Leaf Chemical Composition: A Never-Ending Disclosure by Means of HR-MS/MS Techniques. J. Pharm. Biomed. Anal. 2021, 195, 113892. [Google Scholar] [CrossRef]
- Opačić, N.; Radman, S.; Fabek Uher, S.; Benko, B.; Voća, S.; Šic Žlabur, J. Nettle Cultivation Practices—From Open Field to Modern Hydroponics: A Case Study of Specialized Metabolites. Plants 2022, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Roslon, W.; Weglarz, Z. Polyphenolic Acids of Female and Male Forms of Urtica dioica. Acta Hortic. 2003, 597, 101–104. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Elez Garofulić, I.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Wompounds in Wild Nettle (Urtica dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Koczka, N.; Petersz, D.; Stefanovits-Banyai, E. Total Phenol Content and Antioxidant Capacity (FRAP) of Urtica dioica L. Leaf Extratcs. Acta Hortic. 2015, 1099, 207–210. [Google Scholar] [CrossRef]
- Biesiada, A.; Kucharska, A.; Sokó, A.; Kus, A. Effect of the Age of Plantation and Harvest Term on Chemical Composition and Antioxidant Activity of Stinging Nettle (Urtica dioica L.). Ecol. Chem. Eng. 2010, 17, 1061–1067. [Google Scholar]
- Marotti, I.; Frassineti, E.; Trebbi, G.; Alpi, M.; D’Amen, E.; Dinelli, G. Health-Promoting Phytochemicals of Stinging Nettle (Urtica dioica L.) Grown under Organic Farming in Italian Environments. Ind. Crops Prod. 2022, 182, 114903. [Google Scholar] [CrossRef]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef]
- Kőszegi, K.; Békássy-Molnár, E.; Koczka, N.; Kerner, T.; Stefanovits-Bányai, E. Changes in Total Polyphenol Content and Antioxidant Capacity of Stinging Nettle (Urtica dioica L.) from Spring to Autumn. Period. Polytech. Chem. Eng. 2020, 64, 548–554. [Google Scholar] [CrossRef]
- Kosolapov, V.M.; Cherniavskih, V.I.; Zarudny, V.A.; Mazur, K.; Konieczna, A.; Tseiko, L.; Dumacheva, E.V.; Dumachev, D.V. Observations on the Productivity of Breeding Specimens of Urtica dioica L. from European Russian Ecotopes in Comparison with the Breeding Variety under Field Crop Conditions. Agronomy 2022, 12, 76. [Google Scholar] [CrossRef]
- Sadowska, A.; Świderski, F. Sources, Bioavailability, and Safety of Silicon Derived from Foods and Other Sources Added for Nutritional Purposes in Food Supplements and Functional Foods. Appl. Sci. 2020, 10, 6255. [Google Scholar] [CrossRef]
- Tiotiu, A.; Brazdova, A.; Longé, C.; Gallet, P.; Morisset, M.; Leduc, V.; Hilger, C.; Broussard, C.; Couderc, R.; Sutra, J.-P.; et al. Urtica dioica Pollen Allergy: Clinical, Biological, and Allergomics Analysis. Ann. Allergy Asthma Immunol. 2016, 117, 527–534. [Google Scholar] [CrossRef]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Petronijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical Composition of Stinging Nettle Leaves Obtained by Different Analytical Approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Jaja, N.; Codling, E.E.; Rutto, L.K.; Timlin, D.; Reddy, V.R. Poultry Litter and Inorganic Fertilization: Effects on Biomass Yield, Metal and Nutrient Concentration of Three Mixed-Season Perennial Forages. Agronomy 2022, 12, 570. [Google Scholar] [CrossRef]
- Kara, D. Evaluation of Trace Metal Concentrations in Some Herbs and Herbal Teas by Principal Component Analysis. Food Chem. 2009, 114, 347–354. [Google Scholar] [CrossRef]
- Rafajlovska, V.; Kavrakovski, Z.; Simonovska, J.; Srbinoska, M. Determination of Protein and Mineral Contents in Stinging Nettle. Qual. Life 2013, 7, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-9368-1. [Google Scholar]
- Tack, F.; Verloo, M. Metal Contents in Stinging Nettle (Urtica dioica L.) as Affected by Soil Characteristics. Sci. Total Environ. 1996, 192, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Joergensen, R. Decomposition of Heavy Metal Contaminated Nettles (Urtica dioica L.) in Soils Subjected to Heavy Metal Pollution by River Sediments. Chemosphere 2006, 65, 981–987. [Google Scholar] [CrossRef]
- Toubal, S.; Bouchenak, O.; Elhaddad, D.; Yahiaoui, K.; Boumaza, S.; Arab, K. MALDI-TOF MS Detection of Endophytic Bacteria Associated with Great Nettle (Urtica dioica L.), Grown in Algeria. Pol. J. Microbiol. 2018, 67, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Wenneker, M.; Verdel, M.; Groeneveld, R.; Kempenaar, C.; van Beuningen, A.; Janse, J. Ralstonia (Pseudomonas) Solanacearum Race 3 (Biovar 2) in Surface Water and Natural Weed Hosts: First Report on Stinging Nettle (Urtica dioica). Eur. J. Plant Pathol. 1999, 105, 307–315. [Google Scholar] [CrossRef]
- Mojicevic, M.; D’Agostino, P.; Nikodinovic-Runic, J.; Vasiljevic, B.; Gulder, T.; Vojnovic, S. Antifungal Potential of Bacterial Rhizosphere Isolates Associated with Three Ethno-Medicinal Plants (Poppy, Chamomile, and Nettle). Int. Microbiol. 2019, 22, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Bert, V.; Perlein, A.; Tisserant, B.; Ferrant, P.; Lounès-Hadj Sahraoui, A. In Situ Cultivation of Aromatic Plant Species for the Phytomanagement of an Aged-Trace Element Polluted Soil: Plant Biomass Improvement Options and Techno-Economic Assessment of the Essential Oil Production Channel. Sci. Total Environ. 2021, 789, 147944. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.; Bertheau, C.; Tafforeau, F.; Zappelini, C.; Valot, B.; Maillard, F.; Selosse, M.-A.; Viotti, C.; Chiapusio, G.; Chalot, M. Partial Overlap of Fungal Communities Associated with Nettle and Poplar Roots When Co-Occurring at a Trace Metal Contaminated Site. Sci. Total Environ. 2021, 782, 146692. [Google Scholar] [CrossRef]
- Davis, B.N.K. The Colonization of Isolated Patches of Nettles (Urtica dioica L.) by Insects. J. Appl. Ecol. 1975, 12, 1–14. [Google Scholar] [CrossRef]
- Davis, B.N.K. The European Distribution of Insects on Stinging Nettles, Urtica dioica L.: A Field Survey. Boll. Zool. 1989, 56, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Perrin, R.M. The Role of the Perennial Stinging Nettle, Urtica dioica, as a Reservoir of Beneficial Natural Enemies. Ann. Appl. Biol. 1975, 81, 289–297. [Google Scholar] [CrossRef]
- Davis, B. The Hemiptera and Coleoptera of Stinging Nettle (Urtica dioica L.) in East Anglia. J. Appl. Ecol. 1973, 10, 213–237. [Google Scholar] [CrossRef]
- James, D.; Lauby, G.; Seymour, L.; Buckley, K. Beneficial Insects Associated with Stinging Nettle, Urtica dioica Linnaeus, in Central Washington State. Pan-Pac. Entomol. 2015, 91, 82–90. [Google Scholar] [CrossRef]
- Alhmedi, A.; Haubruge, E.; Bodson, B.; Francis, F. Aphidophagous Guilds on Nettle (Urtica dioica) Strips Close to Fields of Green Pea, Rape and Wheat. Insect Sci. 2007, 14, 419–424. [Google Scholar] [CrossRef]
- Alhmedi, A.; Haubruge, E.; Francis, F. Effect of Stinging Nettle Habitats on Aphidophagous Predators and Parasitoids in Wheat and Green Pea Fields with Special Attention to the Invader Harmonia Axyridis Pallas (Coleoptera: Coccinellidae). Entomol. Sci. 2009, 12, 349–358. [Google Scholar] [CrossRef]
- Alhmedi, A.; Haubruge, E.; D’hoedt, S.; Francis, F. Quantitative Food Webs of Herbivore and Related Beneficial Community in Non-Crop and Crop Habitats. Biol. Control 2011, 58, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Zabel, J.; Tscharntke, T. Does Fragmentation of Urtica Habitats Affect Phytophagous and Predatory Insects Differentially? Oecologia 1998, 116, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ekesi, S.; Shah, P.; Clark, S.; Pell, J. Conservation Biological Control with the Fungal Pathogen Pandora neoaphidis: Implications of Aphid Species, Host Plant and Predator Foraging. Agric. For. Entomol. 2005, 7, 21–30. [Google Scholar] [CrossRef]
- Perrin, R.M. The Population Dynamics of the Stinging Nettle Aphid, Microlophium Carnosum (Bukt.). Ecol. Entomol. 1976, 1, 31–40. [Google Scholar] [CrossRef]
- Baverstock, J.; Porcel, M.; Clark, S.; Copeland, J.; Pell, J. Potential Value of the Fibre Nettle Urtica dioica as a Resource for the Nettle Aphid Microlophium carnosum and Its Insect and Fungal Natural Enemies. BioControl 2010, 56, 215–223. [Google Scholar] [CrossRef]
- Callaway, R. Positive Interactions among Plants. Bot. Rev. 1995, 61, 306–349. [Google Scholar] [CrossRef]
- Franks, S. Facilitation in Multiple Life-History Stages: Evidence for Nucleated Succession in Coastal Dunes. Plant Ecol. 2003, 168, 1–11. [Google Scholar] [CrossRef]
- Roberts, R.; Marrs, R.; Skeffington, R.; Bradshaw, A. Ecosystem Development on Naturally Colonized China Clay Wastes: I. Vegetation Changes and Overall Accumulation of Organic Matter and Nutrients. J. Ecol. 1981, 69, 153–161. [Google Scholar] [CrossRef]
- Prach, K.; Wade, P. Population Characteristics of Expansive Perennial Herbs. Preslia 1992, 64, 45–51. [Google Scholar]
- Ruckenbauer, P.; Burstmayr, H.; Sturtz, A. The Stinging Nettle: Its Reintroduction for Fibre Production; IENICA Project Report 2002; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Puntieri, J.; Pyšek, P. The Effects of Physical Support and Density on Biomass Production and Size Hierarchies of Galium aparine Populations. Oikos 1993, 67, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Ivins, J. Concerning the Ecology of Urtica dioica L. J. Ecol. 1952, 40, 380–382. [Google Scholar] [CrossRef]
- Bojović, B.; Dragana, J.; Stankovic, M. Allelopathic Effect of Aqueous Extracts of Urtica dioica L. on Germination and Growth of Some Cereals. In Proceedings of the 2nd International Conference on Plant Biology—21st Symposium of the Serbian Plant Physiology Society, Petnica, Serbia, 17–20 June 2015. [Google Scholar]
- Synowiec, A.; Nowicka-Połeć, A. Effect of Aqueous Extracts of Selected Medicinal Plants on Germination of Windgrass [Apera spica-venti (L.) P. Beauv.] and Lambsquarters (Chenopodium album L.) Seeds. Acta Agrobot. 2016, 69, 1668. [Google Scholar] [CrossRef] [Green Version]
- Dziamski, A.; Stypczyńska, Z. Allelopathic Effect of Preparations of Betula pendula Roth., Chamomilla recutita L. and Urtica dioica L. on the Initial Growth of Hordeum vulgare L. Acta Agrobot. 2015, 68, 3–8. [Google Scholar] [CrossRef]
- Khan, A.; Qureshi, R.; Ullah, F.; Gilani, S. Phytotoxic Effects of Selected Medicinal Plants Collected from Margalla Hills, Islamabad Pakistan. J. Med. Plants Res. 2011, 5, 5. [Google Scholar]
- Gatti, E.; Di Virgilio, N.; Baronti, S.; Bacci, L. Development of Urtica dioica L. Propagation Methods for Organic Production of Fiber. In Proceedings of the 16th IFOAM Organic World Congress, Modena, Italy, 16–20 June 2008. [Google Scholar]
- Jankauskienė, Z.; Gruzdevienė, E. Investigation of Stinging Nettle (Urtica dioica L.) in Lithuania. Latg. Natl. Econ. Res. 2010, 1, 176–186. [Google Scholar] [CrossRef]
- Ammarellou, A.; Kazemeitabar, K.; Najafei, H.; Mortazavei, N.; Ammarellou, N. Effects of Different Culture Media on Rooting of Urtica dioica L. Stem Cuttings. J. Soil Sci. Environ. Manag. 2012, 3, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Weglarz, Z.; Roslon, W. Developmental and Chemical Variability of Female and Male Forms of Nettle Urtica dioica L. Acta Hortic. 2000, 523, 75–80. [Google Scholar] [CrossRef]
- Vetter, A.; Wieser, P.; Wurl, G. Untersuchungen zum Anbau der Großen Brennessel (Urtica dioica L.) und deren Eignung als Verstärkungsfaser für Kunststoffe. In Final Report of the Project Plants for Energy and Industry; Thüringer Landesamt für Landwirtschaft und Ländlichen Raum: Dornburg, Germany, 1996. [Google Scholar]
- Kohler, K.; Schmidtke, K.; Rauber, R.; Hoffmann, H.; Muller, S. Eignung verschiedener Pflanzenarten zur Untersaat in Fasernesseln (Urtica dioica L.). In Proceedings of the Beiträge zur 5. Wissenschaftstagung Zum ökologischen Landbau (Conference), Berlin, Germany, 23–25 February 1999; pp. 496–500. [Google Scholar]
- Schmidtke, K.; Rauber, R.; Kohler, K. Ertragsbildung von Fasernsseln (Urtica dioica L.). Mitt. Ges. Pflanzenbauwiss. 1998, 11, 107–108. [Google Scholar]
- Rexen, F. The Stinging Nettle: Its Reintroduction for Fibre Production. Interact. Eur. Netw. Ind. Crops Their Appl. 2002. Available online: https://www.yumpu.com/en/document/view/11410932 (accessed on 1 May 2022).
- Francken-Welz, H.; Scherr-Triebel, M.; Léon, J. Ertrags- und Qualitätsbildung von Lein, Hanf und Fasernessel in Abhängigkeit von Bestandesdichte und N-Düngung. Mitt. Ges. FuÈr Pflanzenbauwiss. 1999, 2, 177–178. [Google Scholar]
- Lehne, P.; Schmidtke, K.; Rauber, R. Ertrag von Fasernesseln im ökologischen Landbau bei unterschiedlicher Nährstoffversorgung. Mitt Ges Pflanzenbauwiss 2001, 13, 158–159. [Google Scholar]
- Kakabouki, I.; Zisi, C.; Karydogianni, S.; Priniotakis, G.; Darawsheh, M.; Tselia, Z. Effect of Nettle (Urtica dioca L.) Density on Fiber Yield and Quality in a Natural Ecosystem under East Mediterranean Conditions. J. Phytol. 2020, 12, 73–76. [Google Scholar] [CrossRef]
- Radman, S.; Zutic, C.; Coga, L.; Fabek, S.; Benko, B.; Toth, N. Yield and Mineral Content of Stinging Nettle as Affected by Nitrogen Fertilization. J. Agric. Sci. Technol. 2016, 18, 1117–1128. [Google Scholar]
- Tavano, D.; Gallucci, T.; Camaggio, G.; Lagioia, G. Potential of Dyeing and Fiber Plants in Apulia Region. J. Commod. Sci. Technol. Qual. 2011, 50, 207–224. [Google Scholar]
- Šrutek, M. Growth Responses of Urtica dioica L. to Different Water Table Depth. Plant Ecol. 1997, 130, 163–169. [Google Scholar] [CrossRef]
- Peruzzi, A.; Martelloni, L.; Frasconi, C.; Fontanelli, M.; Pirchio, M.; Raffaelli, M. Machines for Non-Chemical Intra-Row Weed Control in Narrow and Wide-Row Crops: A Review. J. Agric. Eng. 2017, 48, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Gazoulis, I.; Kanatas, P.; Papastylianou, P.; Tataridas, A.; Alexopoulou, E.; Travlos, I. Weed Management Practices to Improve Establishment of Selected Lignocellulosic Crops. Energies 2021, 14, 2478. [Google Scholar] [CrossRef]
- Rosnitschek-Schimmel, I. Seasonal Dynamics of Nitrogenous Compounds in a Nitrophilic Weed I. Changes in Inorganic and Organic Nitrogen Fractions of the Different Plant Parts of Urtica dioica. Plant Cell Physiol. 1985, 26, 169–176. [Google Scholar] [CrossRef]
- Fodor, F.; Cseh, E. Effect of Different Nitrogen-forms and Iron-chelates on the Development of Stinging Nettle. J. Plant Nutr. 1993, 16, 2239–2253. [Google Scholar] [CrossRef]
- Biesiada, A.; Woloszczak, E.; Sokol-Letowska, A.; Kucharska, A.; Nawirska-Olszańska, A. The Effect of Nitrogen Form and Dose on Yield, Chemical Composition and Antioxidant Activity of Stinging Nettle (Urtica dioica L.). Herba Pol. 2009, 55, 84–93. [Google Scholar]
- Radman, S.; Zutic, I.; Fabek, S.; Zlabur, J.; Benko, B.; Toth, N.; Coga, L. Influence of Nitrogen Fertilization on Chemical Composition of Cultivated Nettle. Emir. J. Food Agric. 2015, 27, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Weiß, F. Effects of Varied Nitrogen Fertilization and Cutting Treatments on the Development and Yield Components of Cultivated Stinging Nettles. Acta Hortic. 1993, 331, 137–144. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in Vegetables: Toxicity, Content, Intake and EC Regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Hofstra, R.; Lanting, L.; de Visser, R. Metabolism of Urtica dioica as Dependent on the Supply of Mineral Nutrients. Physiol. Plant. 1985, 63, 13–18. [Google Scholar] [CrossRef]
- Radman, S.; Fabek Uher, S.; Opačić, N.; Ivanka, Ž.; Benko, B.; Jurčić, B.; Šic Žlabur, J. Application of biostimulants in nettle cultivation. Glasnik Zaštite Bilja 2022, 45, 22–28. [Google Scholar] [CrossRef]
- Bacci, L.; Di Lonardo, S.; Albanese, L.; Mastromei, G.; Perito, B. Effect of Different Extraction Methods on Fiber Quality of Nettle (Urtica dioica L.). Text. Res. J. 2011, 81, 827–837. [Google Scholar] [CrossRef]
- Lützkendorf, R.; Mieck, K.; Reußmann, T.; Dreyling, G.; Dreyer, J.; Lück, M. Nesselfaser-Verbundwerkstoffe für Fahrzeuginnenteile—Was können sie? Tech. Text. 2000, 30–32. [Google Scholar]
- Amaducci, S.; Scordia, D.; Liu, F.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S. Key Cultivation Techniques for Hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Baumgardner, D. Stinging Nettle: The Bad, the Good, the Unknown. J. Patient-Cent. Res. Rev. 2016, 3, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Kalia, A.; Joshi, B.; Mukhija, M. Pharmacognostical Review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201. [Google Scholar] [CrossRef]
- Suryawan, I.; Suardana, N.; Suprapta Winaya, I.; Budiarsa Suyasa, I.; Tirta Nindhia, T. Study of Stinging Nettle (Urtica dioica L.) Fibers Reinforced Green Composite Materials: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 201, 012001. [Google Scholar] [CrossRef] [Green Version]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.; Ksouda, K. Screening of Pharmacological Uses of Urtica dioica and Others Benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, M.; Outlaw, R.; Zhao, X.; Manos, D.; Holloway, B.; Mammana, V. Free-Standing Subnanometer Graphite Sheets. Appl. Phys. Lett. 2004, 85, 1265–1267. [Google Scholar] [CrossRef]
- Fan, H.; Shen, W. Carbon Nanosheets: Synthesis and Application. ChemSusChem 2015, 8, 2004–2027. [Google Scholar] [CrossRef] [PubMed]
- Veca, L.; Meziani, M.; Wang, W.; Wang, X.; Lu, F.; Zhang, P.; Lin, Y.; Fee, R.; Connell, J.; Sun, Y. Carbon Nanosheets for Polymeric Nanocomposites with High Thermal Conductivity. Adv. Mater. 2009, 21, 2088–2092. [Google Scholar] [CrossRef]
- Nuilek, K.; Simon, A.; Baumli, P. Synthesis and Characterization of Carbon Nanosheets from Stinging Nettle (Urtica dioica). IOP Conf. Ser. Mater. Sci. Eng. 2019, 613, 012017. [Google Scholar] [CrossRef]
- Bodros, E.; Baley, C. Study of the Tensile Properties of Stinging Nettle Fibres (Urtica dioica). Mater. Lett. 2008, 62, 2143–2145. [Google Scholar] [CrossRef] [Green Version]
- Bogard, F.; Bach, T.; Abbes, B.; Bliard, C.; Maalouf, C.; Bogard, V.; Beaumont, F.; Polidori, G. A Comparative Review of Nettle and Ramie Fiber and Their Use in Biocomposites, Particularly with a PLA Matrix. J. Nat. Fibers 2021, 1–25. [Google Scholar] [CrossRef]
- Fischer, H.; Werwein, E.; Graupner, N. Nettle Fibre (Urtica dioica L.) Reinforced Poly(Lactic Acid): A First Approach. J. Compos. Mater. 2012, 46, 3077–3087. [Google Scholar] [CrossRef]
- Ketema, A.; Worku, A. Antibacterial Finishing of Cotton Fabric Using Stinging Nettle (Urtica dioica L.) Plant Leaf Extract. J. Chem. 2020, 2020, 4049273. [Google Scholar] [CrossRef] [Green Version]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Common Nettle (Urtica dioica L.) as an Active Filler of Natural Rubber Biocomposites. Materials 2021, 14, 1616. [Google Scholar] [CrossRef]
- Mudoi, M.P.; Sinha, S.; Parthasarthy, V. Polymer Composite Material with Nettle Fiber Reinforcement: A Review. Bioresour. Technol. Rep. 2021, 16, 100860. [Google Scholar] [CrossRef]
- Suomela, J.A.; Vajanto, K.; Räisänen, R. Seeking Nettle Textiles—Utilizing a Combination of Microscopic Methods for Fibre Identification. Stud. Conserv. 2018, 63, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Suryawan, I.; Suardana, N.; Winaya, I.; Suyasa, I. A Study on Correlation between Hardness and Thermal Conductivity of Polymer Composites Reinforced with Stinging Nettle Fiber. Int. J. Civ. Eng. Technol. 2020, 11, 94–104. [Google Scholar] [CrossRef]
- Delahaye, J. Utilisations de L’ortie-Urtica dioïca L. Ph.D. Thesis, University of Rouen, Rouen, France, 2015; 228p. [Google Scholar]
- De Vico, G.; Guida, V.; Carella, F. Urtica dioica (Stinging Nettle): A Neglected Plant with Emerging Growth Promoter/Immunostimulant Properties for Farmed Fish. Front. Physiol. 2018, 9, 285. [Google Scholar] [CrossRef]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef] [Green Version]
- Gülçin, I.; Küfrevioglu, O.; Oktay, M.; Büyükokuroglu, M. Antioxidant, Antimicrobial, Antiulcer and Analgesic Activities of Nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Mukundi, M.; Mwaniki, N.; Piero, N.; Murugi, N.; Kelvin, J.; Yusuf, A.; Mwonjoria, J.; Ngetich, A.; Agyirifo, D.; Gathumbi, P.; et al. Potential Anti-Diabetic Effects and Safety of Aqueous Extracts of Urtica dioica Collected from Narok County, Kenya. Pharm. Anal. Acta 2017, 8, 1000548. [Google Scholar] [CrossRef]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Prakash Mishra, A.; Sener, B.; et al. Urtica dioica-Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evid. Based Complement. Altern. Med. 2022, 2022, e4024331. [Google Scholar] [CrossRef]
- Bourgeois, C.; Leclerc, E.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.; Seigneuret, J.; Auguin, D.; Pichon, C.; Lainé, E.; et al. Nettle (Urtica dioica L.) as a Source of Antioxidant and Anti-Aging Phytochemicals for Cosmetic Applications. C. R. Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Fischer, A.; Brodziak-Dopierała, B.; Loska, K.; Stojko, J. The Assessment of Toxic Metals in Plants Used in Cosmetics and Cosmetology. Int. J. Environ. Res. Public Health 2017, 14, 1280. [Google Scholar] [CrossRef] [Green Version]
- Knoth, D.; Alnemari, R.M.; Wiemann, S.; Keck, C.M.; Brüßler, J. Fingerprint of Nature—Skin Penetration Analysis of a Stinging Nettle Plant Crystals Formulation. Cosmetics 2021, 8, 21. [Google Scholar] [CrossRef]
- Ankarcrona, J. Urtica dioica, a Weed with Many Possibilities. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2019. [Google Scholar]
- Chakravartula, N.S.S.; Moscetti, R.; Farinon, B.; Vinciguerra, V.; Merendino, N.; Bedini, G.; Neri, L.; Pittia, P.; Massantini, R. Stinging Nettles as Potential Food Additive: Effect of Drying Processes on Quality Characteristics of Leaf Powders. Foods 2021, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Maietti, A.; Tedeschi, P.; Catani, M.; Stevanin, C.; Pasti, L.; Cavazzini, A.; Marchetti, N. Nutrient Composition and Antioxidant Performances of Bread-Making Products Enriched with Stinging Nettle (Urtica dioica) Leaves. Foods 2021, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Zeipina, S.; Alsina, I.; Lepse, L. Stinging Nettle—The Source of Biologically Active Compounds as Sustainable Daily Diet Supplement. Res. Rural Dev. 2014, 1, 34–38. [Google Scholar]
- Grela, E.; Krusiński, R.; Matras, J. Efficacy of Diets with Antibiotic and Herb Mixture Additives in Feeding of Growing-Finishing Pigs. J. Anim. Feed Sci. 1998, 7, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Lötscher, Y.; Kreuzer, M.; Messikommer, R. Utility of Nettle (Urtica dioica) in Layer Diets as a Natural Yellow Colorant for Egg Yolk. Anim. Feed Sci. Technol. 2013, 186, 158–168. [Google Scholar] [CrossRef]
- Milosevic, B.; Omerovic, I.; Savic, Z.; Andjusic, L.; Milanovic, V.; Ciric, S. Stinging Nettle (Urtica dioica) in Broiler Nutrition. Worlds Poult. Sci. J. 2021, 77, 901–912. [Google Scholar] [CrossRef]
- Nasiri, M.; Azizi, K.; Hamzehzarghani, H.; Ghaderi, R. Studies on the Nematicidal Activity of Stinging Nettle (Urtica dioica) on Plant Parasitic Nematodes. Arch. Phytopathol. Plant Prot. 2014, 47, 591–599. [Google Scholar] [CrossRef]
- Bensadoun, F.; Verpoest, I.; Baets, J.; Müssig, J.; Graupner, N.; Davies, P.; Gomina, M.; Kervoelen, A.; Baley, C. Impregnated Fibre Bundle Test for Natural Fibres Used in Composites. J. Reinf. Plast. Compos. 2017, 36, 942–957. [Google Scholar] [CrossRef]
- Maričić, B.; Radman, S.; Romić, M.; Perković, J.; Major, N.; Urlić, B.; Palčić, I.; Ban, D.; Zorić, Z.; Ban, S.G. Stinging Nettle (Urtica dioica L.) as an Aqueous Plant-Based Extract Fertilizer in Green Bean (Phaseolus vulgaris L.) Sustainable Agriculture. Sustainability 2021, 13, 4042. [Google Scholar] [CrossRef]
- Bos, H. The Potential of Flax Fibres as Reinforcement for Composite Materials. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, X.; Gao, C.; Li, Z.; Chen, J.; Guo, L.; Wang, T.; Xu, J. Molecular Diagnostics and Pathogenesis of Fungal Pathogens on Bast Fiber Crops. Pathogens 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Gangil, B.; Singh Mer, K.; Gupta, M.; Patel, V. Bast Fiber-Based Polymer Composites. In Hybrid Fiber Composites; John Wiley & Sons, Ltd.: Weinheim, Germany, 2020; pp. 147–167. ISBN 978-3-527-82457-1. [Google Scholar]
- Paukszta, D.; Mańkowski, J.; Kołodziej, J.; Szostak, M. Polypropylene (PP) Composites Reinforced with Stinging Nettle (Urtica dioica L.) Fiber. J. Nat. Fibers 2013, 10, 147–158. [Google Scholar] [CrossRef]
- Lanzilao, G.; Goswami, P.; Blackburn, R. Study of the Morphological Characteristics and Physical Properties of Himalayan Giant Nettle (Girardinia diversifolia L.) Fibre in Comparison with European Nettle (Urtica dioica L.) Fibre. Mater. Lett. 2016, 181, 200–203. [Google Scholar] [CrossRef]
- Bourmaud, A.; Beaugrand, J.; Shah, D.; Placet, V.; Baley, C. Towards the Design of High-Performance Plant Fibre Composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Baley, C. Analysis of the Flax Fibres Tensile Behaviour and Analysis of the Tensile Stiffness Increase. Compos. Part A Appl. Sci. Manuf. 2002, 33, 939–948. [Google Scholar] [CrossRef]
- Baley, C. Fibres Naturelles de Renfort Pour Matériaux Composites. Tech. Ing. 2004, am5130. [Google Scholar] [CrossRef]
- Placet, V.; Trivaudey, F.; Cisse, O.; Gucheret-Retel, V.; Boubakar, M. Diameter Dependence of the Apparent Tensile Modulus of Hemp Fibres: A Morphological, Structural or Ultrastructural Effect? Compos. Part A Appl. Sci. Manuf. 2012, 43, 275–287. [Google Scholar] [CrossRef]
- Cherrett, N.; Barrett, J.; Clemett, A.; Chadwick, M.; Chadwick, M. Ecological Footprint and Water Analysis of Cotton, Hemp and Polyester; Stockholm Environmental Institute: Stockholm, Sweden, 2005; ISBN 978-91-975238-2-0. [Google Scholar]
- Parraga-Aguado, I.; Querejeta, J.; González-Alcaraz, M.; Jiménez-Cárceles, F.; Conesa, H. Usefulness of Pioneer Vegetation for the Phytomanagement of Metal(Loid)s Enriched Tailings: Grasses vs. Shrubs vs. Trees. J. Environ. Manag. 2014, 133, 51–58. [Google Scholar] [CrossRef]
- Parmar, S.; Singh, V. Phytoremediation Approaches for Heavy Metal Pollution: A Review. J. Plant Sci. Res. 2015, 2, 139. [Google Scholar]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.-J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation Potential of Twelve Wild Plant Species for Toxic Elements in a Contaminated Soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Shams, K.; Tichy, G.; Fischer, A.; Sager, M.; Peer, T.; Bashar, A.; Filip, K. Aspects of Phytoremediation for Chromium Contaminated Sites Using Common Plants Urtica dioica, Brassica napus and Zea mays. Plant Soil 2010, 328, 175–189. [Google Scholar] [CrossRef]
- Krystofova, O.; Adam, V.; Babula, P.; Zehnalek, J.; Beklova, M.; Havel, L.; Kizek, R. Effects of Various Doses of Selenite on Stinging Nettle (Urtica dioica L.). Int. J. Environ. Res. Public Health 2010, 7, 3804–3815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koblar, A.; Tavčar, G.; Ponikvar-Svet, M. Stress Syndrome Response of Nettle (Urtica dioica L.) Grown in Fluoride Contaminated Substrate to Fluoride and Fluorine Accumulation Pattern. J. Fluor. Chem. 2015, 172, 7–12. [Google Scholar] [CrossRef]
- Codling, E.; Rutto, K. Stinging Nettle (Urtica dioica L.) Growth and Mineral Uptake from Lead-Arsenate Contaminated Orchard Soils. J. Plant Nutr. 2014, 37, 393–405. [Google Scholar] [CrossRef]
- Sharifi, K.; Rahnavard, A.; Saeb, K.; Gholamreza Fahimi, F.; Tavana, A. Ability of Urtica dioica L. to Adsorb Heavy Metals (Pb, Cd, As, and Ni) from Contaminated Soils. Soil Sediment Contam. Int. J. 2022, 1–34. [Google Scholar] [CrossRef]
- Dimitrijević, V.; Stanković, M.; Djordjevic, D.; Krstić, I.; Nikolić, M.; Bojić, A.; Krstić, N. The Preliminary Adsorption Investigation of Urtica dioica L. Biomass Material as a Potential Biosorbent for Heavy Metal Ions. Stud. Univ. Babeș-Bolyai Chem. 2019, 64, 19–39. [Google Scholar] [CrossRef]
- Ertan, B.; Efe, D. The Adsorption Performance of Urtica dioica on the Removal of Cadmium from Aqueous Solutions. Asian J. Biotechnol. Genet. Eng. 2019, 2, 1–7. [Google Scholar]
- Bislimi, K.; Halili, J.; Sahiti, H.; Bici, M.; Mazreku, I. Effect of Mining Activity in Accumulation of Heavy Metals in Soil and Plant (Urtica dioica L). J. Ecol. Eng. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Edwards, S.; MacLeod, C.; Lester, J. The Bioavailability of Copper and Mercury to the Common Nettle (Urtica dioica) and the Earthworm Eisenia Fetida from Contaminated Dredge Spoil. Water Air Soil Pollut. 1998, 102, 75–90. [Google Scholar] [CrossRef]
- Hiller, E.; Jurkovič, Ľ.; Majzlan, J.; Kulikova, T.; Faragó, T. Environmental Availability of Trace Metals (Mercury, Chromium and Nickel) in Soils from the Abandoned Mine Area of Merník (Eastern Slovakia). Pol. J. Environ. Stud. 2021, 30, 5013–5025. [Google Scholar] [CrossRef]
- Murtić, S.; Zahirović, Ć.; Čivić, H.; Sijahović, E.; Jurković, J.; Avdić, J.; Šahinović, E.; Podrug, A. Phytoaccumulation of Heavy Metals in Native Plants Growing on Soils in the Spreča River Valley, Bosnia and Herzegovina. Plant Soil Environ. 2021, 67, 533–540. [Google Scholar] [CrossRef]
- Paukszto, A.; Mirosławski, J. Using Stinging Nettle (Urtica dioica L.) to Assess the Influence of Long Term Emission upon Pollution with Metals of the Tatra National Park Area (Poland). Atmos. Pollut. Res. 2019, 10, 73–79. [Google Scholar] [CrossRef]
- Spongberg, A.; Hartley, L.; Neher, D.; Witter, J. Fate of Heavy Metal Contaminants in a Former Sewage Treatment Lagoon, Hancock County, Ohio. Soil Sediment Contam. Int. J. 2008, 17, 619–629. [Google Scholar] [CrossRef]
- Barboiu, G.; Radulescu, C.; Popescu, I.; Dulama, I.; Bucurică, I.; Teodorescu, S.; Ştirbescu, R.-M.; Stirbescu, N.-M.; Tanase, N. Potential Health Risk Assessment Associated with Heavy Metal Accumulation in Native Urtica dioica. Rom. Rep. Phys. 2020, 72, 711. [Google Scholar]
- Boisson, S.; Le Stradic, S.; Collignon, J.; Séleck, M.; Malaisse, F.; Ngoy Shutcha, M.; Faucon, M.; Mahy, G. Potential of Copper-Tolerant Grasses to Implement Phytostabilisation Strategies on Polluted Soils in South D.R. Congo. Environ. Sci. Pollut. Res. 2016, 23, 13693–13705. [Google Scholar] [CrossRef]
- Yung, L. Fonctionnement et Performances Du Système Agroforestier Peuplier Ortie En Contexte de Phytomanagement. Ph.D. Thesis, University of Franche-Comté, Besançon, France, 2020. [Google Scholar]
- Yung, L.; Bertheau, C.; Cazaux, D.; Regier, N.; Slaveykova, V.I.; Chalot, M. Insect Life Traits Are Key Factors in Mercury Accumulation and Transfer within the Terrestrial Food Web. Environ. Sci. Technol. 2019, 53, 11122–11132. [Google Scholar] [CrossRef] [Green Version]
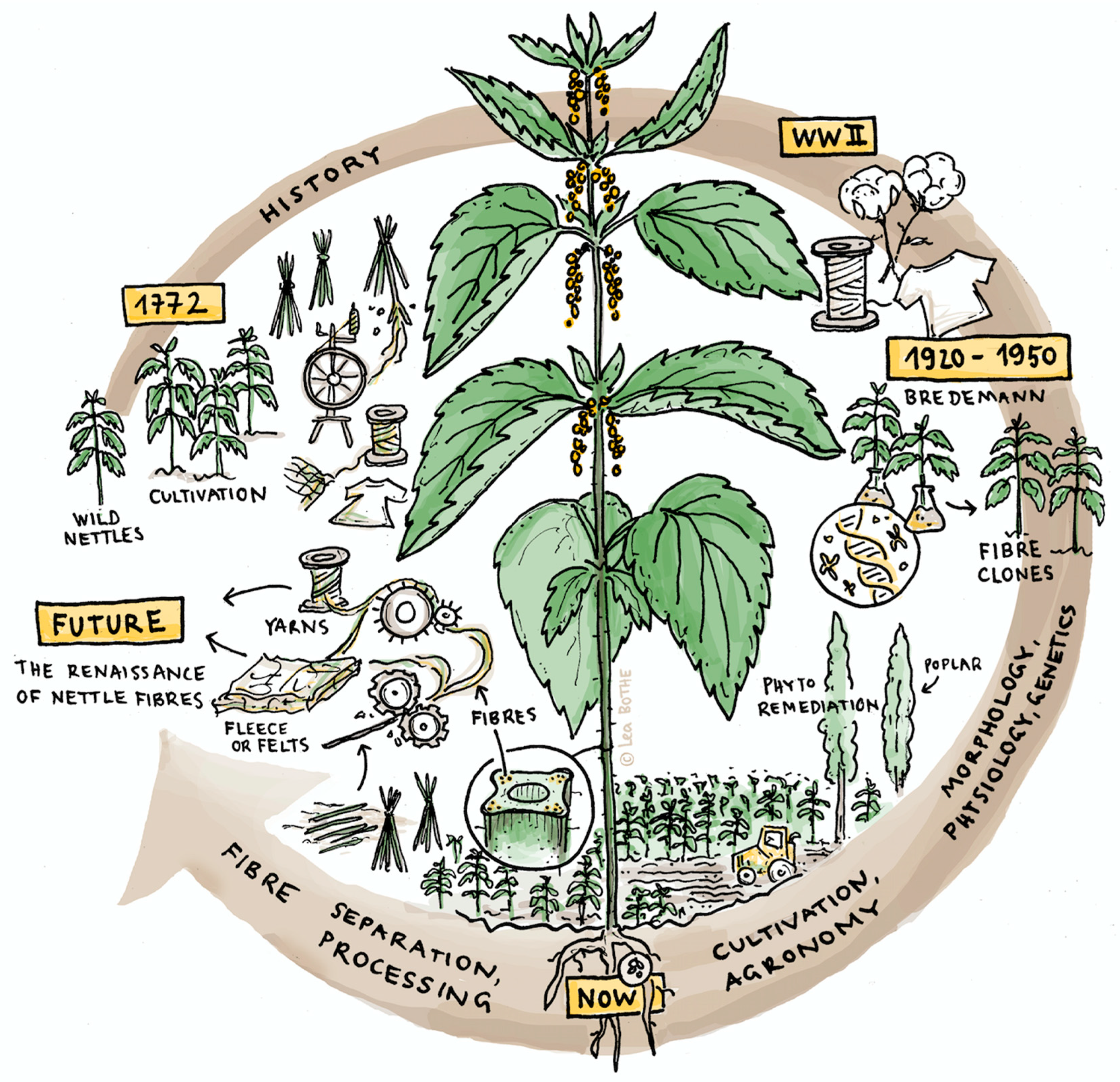
 ) and disordered (
) and disordered (  ) process lines for nettle fiber extraction and separation from 1723 until present.
) process lines for nettle fiber extraction and separation from 1723 until present.
 ) and disordered (
) and disordered (  ) process lines for nettle fiber extraction and separation from 1723 until present.
) process lines for nettle fiber extraction and separation from 1723 until present.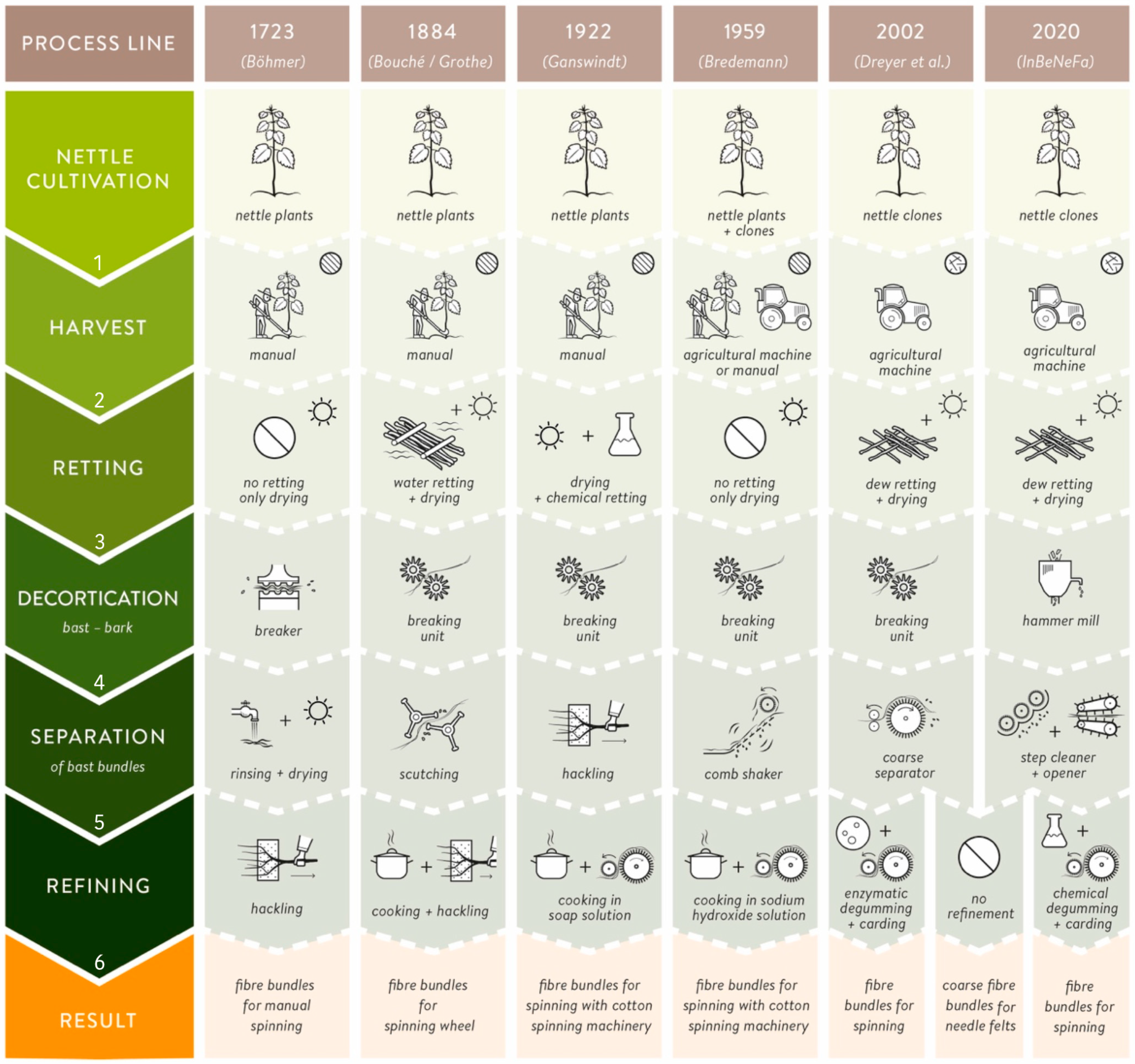
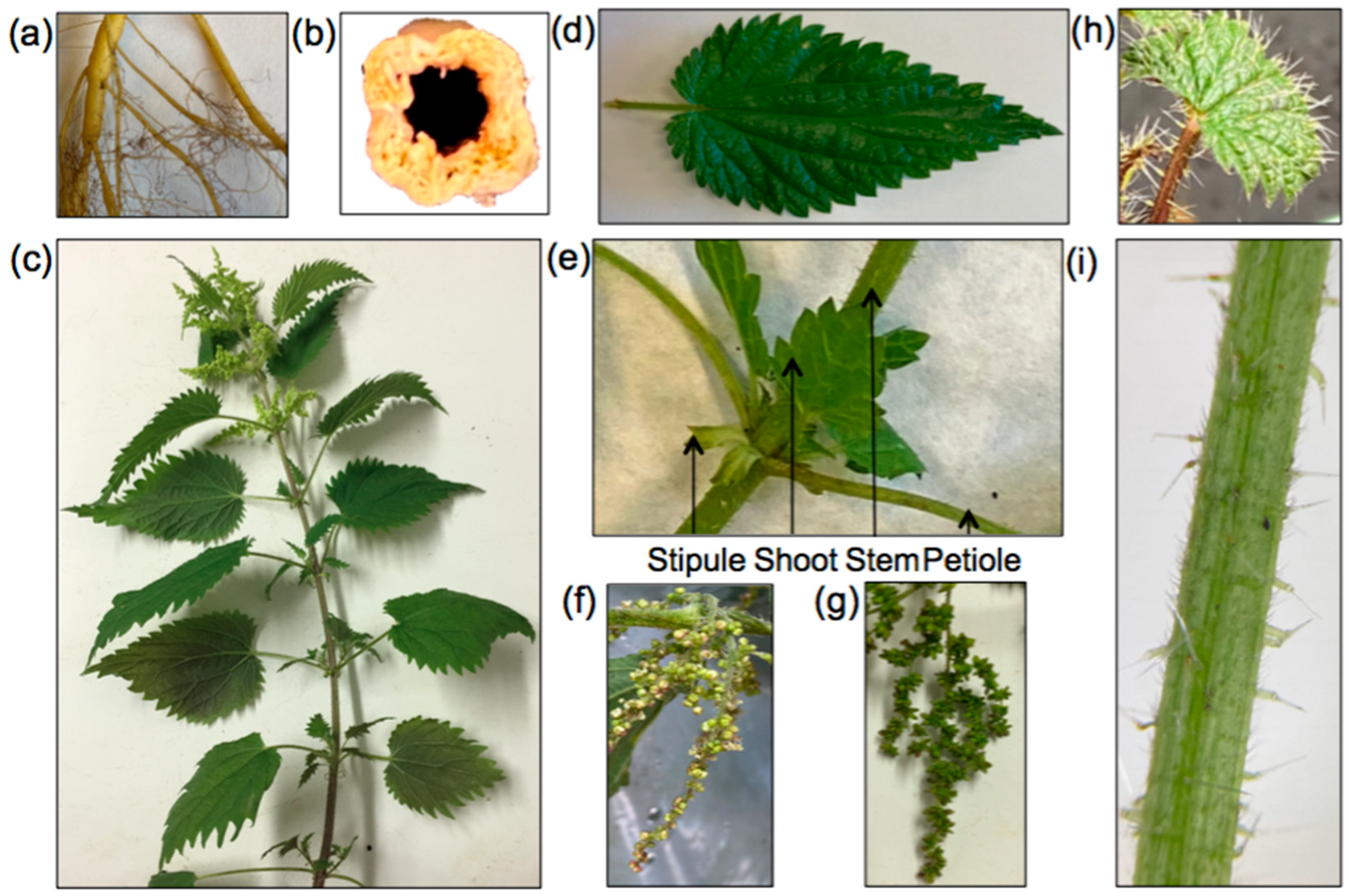
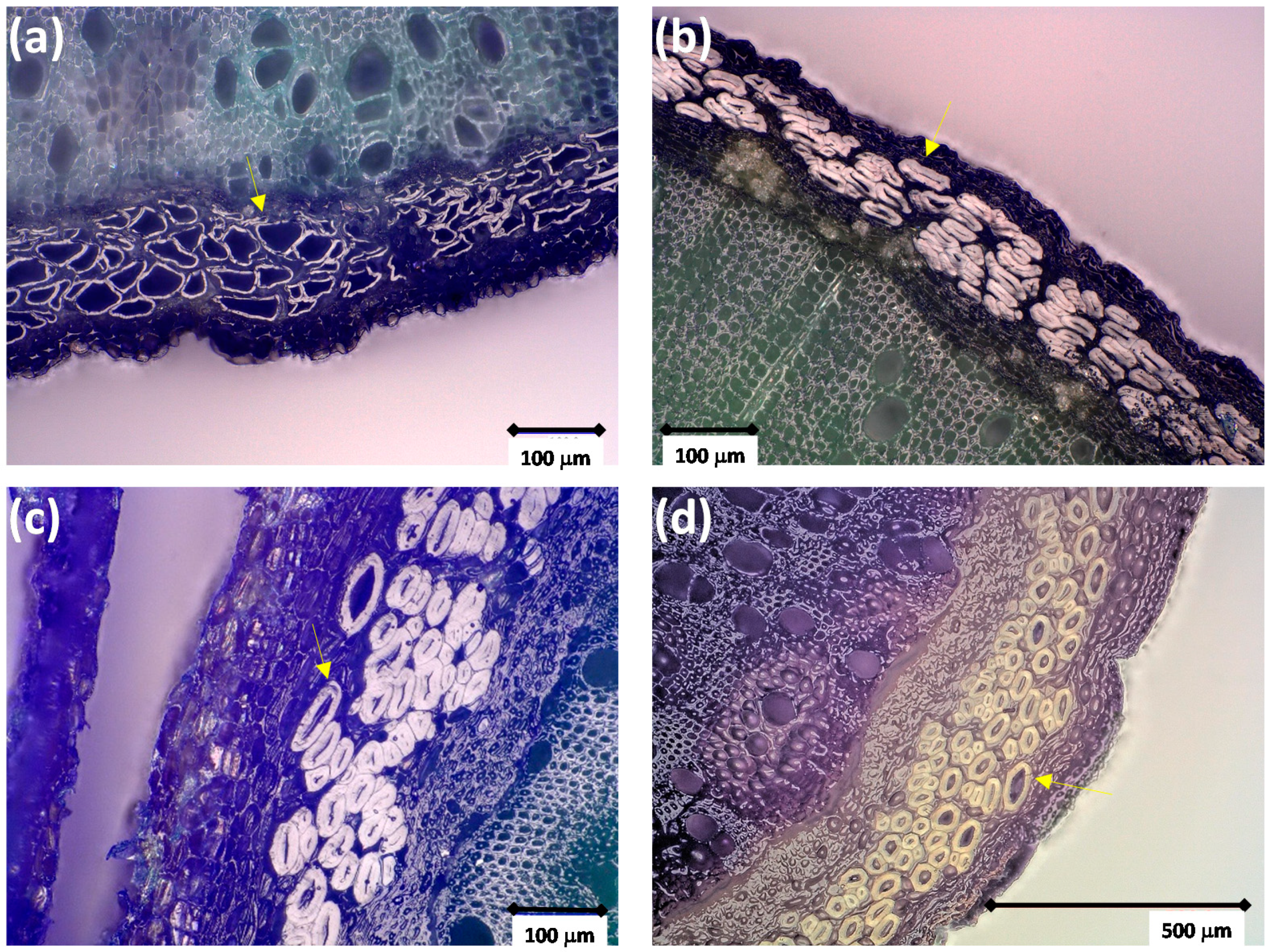

| Origin | Genome Size/pg | Haploid Genome Size/Mb | References |
|---|---|---|---|
| Germany | 2C = 2.34 | 572 | [90] |
| Canada | 2C = 1.17 | 572 | [91] |
| Canada | 1.20 < 2C < 1.30 | 611 | [92] |
| UK | 1C = 1.6 | 1564 | [93] |
| Bosnia-Herzegovina | 2C = 2.16 | 528 | [94] |
| Greece to arctic Norway | 2C = 1.33 (diploid) | 651 | [45] |
| 2C = 2.46 (tetraploid) | 602 | ||
| Europe and West Asia | 2.08 < 2C < 2.20 | 523 | [79] |
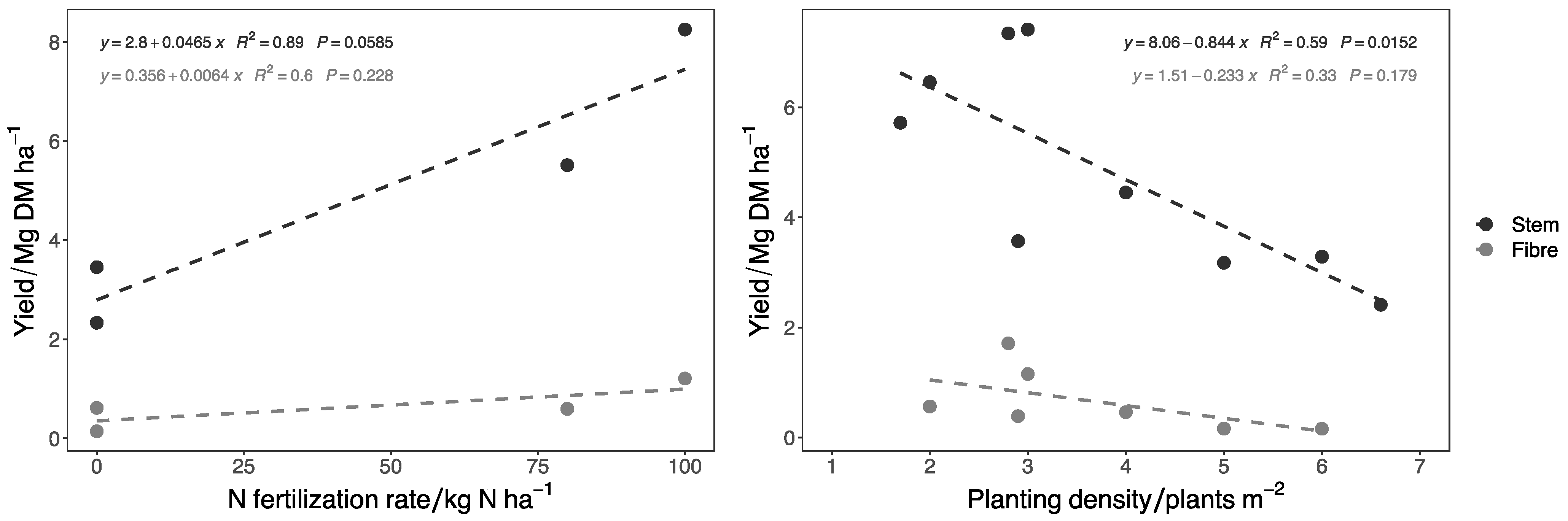
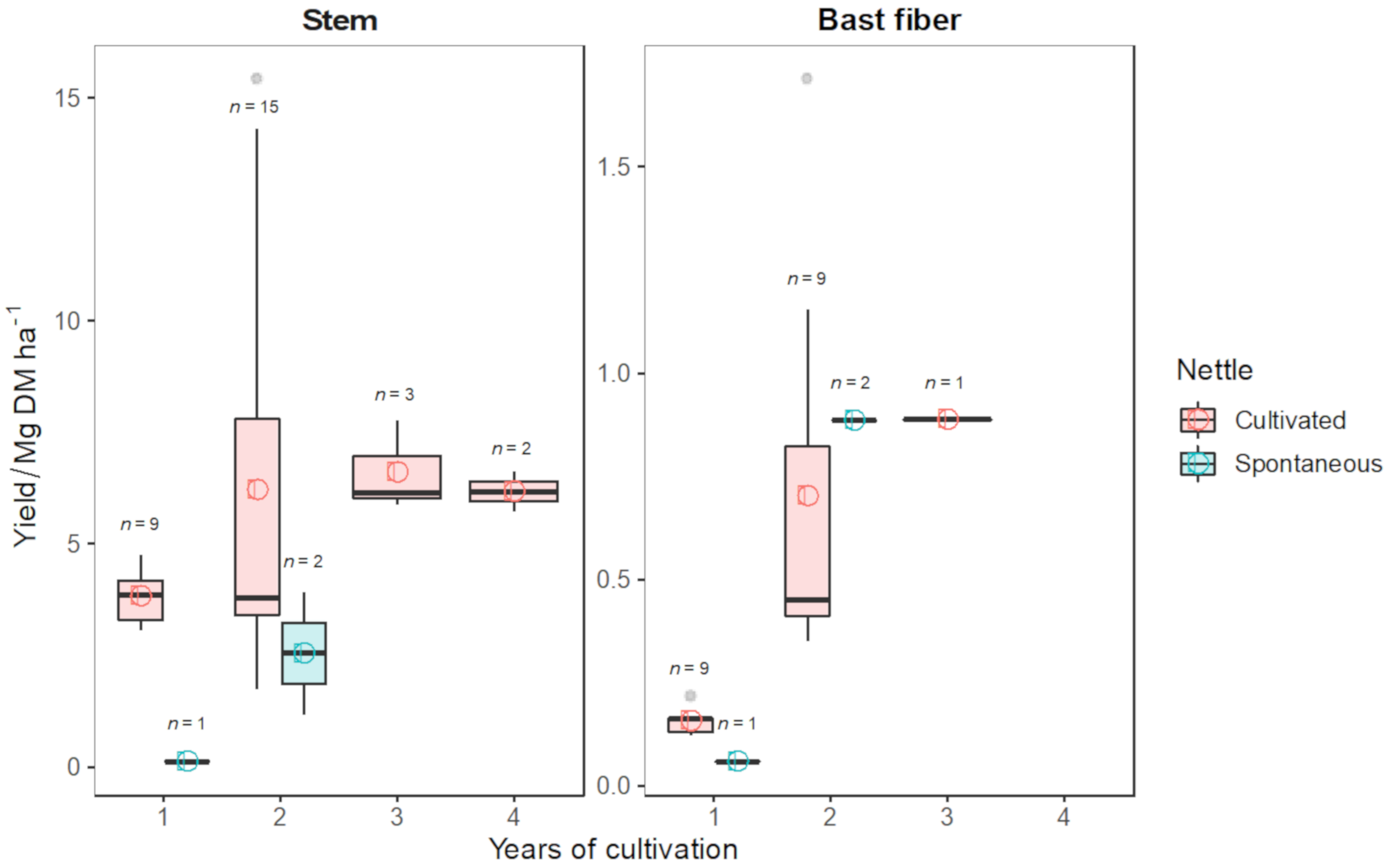
| Reference | Clone | Years of Cultivation | Country | Stem Yield/Mg DM ha−1 | Bast Fiber Yield/Mg ha−1 | Planting Density/Plant m−2 | N Fertilization Rate/kg N ha−1 |
|---|---|---|---|---|---|---|---|
| [151] | / | 2 | Germany | 5.85 | 0.71 | 4.0 | 100 |
| [21] | Clone 13 | 2 | Germany | 8.20 | 0.45 | 2.0 | 80 * |
| [152] | / | 2 | Germany | 4.09 | 0.35 | 2.9 | 0 |
| [153] | / | 2 | Germany | 3.80 | 0.45 | / | / |
| [154] | Clone 13 | 2 | Germany | 3.40 | / | / | / |
| [155] | / | 2 | Germany | 7.42 | 1.16 | 3.0 | 80 ** |
| [156] | Clone 13 | 2 | Germany | 3.05 | 0.43 | 2.9 | 80 ** |
| [1] | Clone 1-5-7-8-9 | 2 | Austria | 3.40 | 0.36 | 2.0 | 80 ** |
| [1] | Clone 1-5-7-8-9 | 3 | Austria | 7.78 | 0.89 | 2.0 | 80 ** |
| [140] | Clone 13 | 2 | Germany | 3.40 | / | 2.8 | / |
| [2] | Clone 13 | 2 | Italy | 15.42 | 1.71 | 2.8 | 100 |
| [4] | / | 2 | Lithuania | 14.30 | / | 2.8 | 32 |
| [4] | / | 2 | Lithuania | 13.61 | / | 1.7 | 32 |
| [4] | / | 3 | Lithuania | 6.15 | / | 2.8 | 32 |
| [4] | / | 3 | Lithuania | 5.88 | / | 1.7 | 32 |
| [4] | / | 4 | Lithuania | 6.63 | / | 2.8 | 32 |
| [4] | / | 4 | Lithuania | 5.72 | / | 1.7 | 32 |
| [157] | / | 1 | Greece | 3.06 | 0.22 | 4.0 | 0 |
| [157] | / | 1 | Greece | 3.18 | 0.16 | 5.0 | 0 |
| [157] | / | 1 | Greece | 3.29 | 0.16 | 6.0 | 0 |
| [157] | / | 1 | Greece | 3.69 | 0.16 | 7.0 | 0 |
| [157] | / | 1 | Greece | 4.30 | 0.17 | 8.0 | 0 |
| [157] | / | 1 | Greece | 4.76 | 0.16 | 9.0 | 0 |
| [157] | / | 1 | Greece | 3.86 | 0.12 | 10.0 | 0 |
| [157] | / | 1 | Greece | 4.14 | 0.13 | 11.0 | 0 |
| [157] | / | 1 | Greece | 4.18 | 0.13 | 12.0 | 0 |
| [158] | / | 2 | Croatia | 1.75 | / | 6.6 | 0 |
| [158] | / | 2 | Croatia | 2.00 | / | 6.6 | 50 |
| [158] | / | 2 | Croatia | 3.49 | / | 6.6 | 100 |
| [4] | Spontaneous nettle | 2 | Lithuania | 3.92 | / | 2.8 | 32 |
| [11] | Spontaneous nettle | 1 | France | 0.12 | 0.06 | / | 0 |
| [11] | Spontaneous nettle | 2 | France | 1.17 | 0.885 | / | 0 |
| Sector | Use | Part of the Plant | References |
|---|---|---|---|
| Textile/fiber | Clothes, antibacterial finishing of textiles, biobased composites, Carbon nanosheets | Leaves, stem, roots | [147,182,183,184,185,186,187,188,189] |
| Medicine | Anemia, eczema, antioxidant, analgesic, diabetes, cancer, resistance to bacterial infections | Leaves, stem, roots | [98,190,191,192,193,194,195] |
| Cosmetics | Soap, shampoo | Leaves, roots | [196,197,198] |
| Food | Soup, tea, salad, food dye, food additive | Leaves, stem, roots, seeds | [50,199,200,201,202] |
| Forage crop | Dietary supplements for animals | Leaves, stem, roots | [203,204,205] |
| Crop farming | Biostimulant, green manure, nettle slurry, pest control, plant-based fertilizer | Leaves, stem, roots | [206,207,208] |
| Type of Fibers | References | Elastic Modulus/GPa | Stress at Failure/MPa | Strain at Failure/% | |
|---|---|---|---|---|---|
| Nettle (Urtica dioica L.) | Range | 36–87 | 711–2196 | 2.11–2.80 | |
| Mean values and standard deviation of datasets reported in literature | [182] | 87 ± 28 | 1594 ± 640 | 2.11 ± 0.91 | |
| [213] | 79 ± 29 | 2196 ± 801 | 2.80 ± 0.90 | ||
| [11] | 36 ± 19 | 812 ± 451 | 2.14 ± 0.81 | ||
| 53 ± 24 | 711 ± 427 | 1.37 ± 0.53 | |||
| 54 ± 17 | 1314 ± 552 | 2.62 ± 1.16 | |||
| Flax (Linum Usitatissimum L.) | Range | [214] | 37–75 | 595–1510 | 1.60–3.60 |
| Example of mean values and standard deviation for a dataset | [215,216] | 54 ± 15 | 1339 ± 486 | 3.27 ± 0.84 | |
| Hemp (Cannabis Sativa) | Range | [214] | 14–44 | 285–889 | 0.80–3.30 |
| Example of mean values and standard deviation for a dataset | [217] | 25 ± 11 | 636 ± 253 | 2.10 ± 0.70 | |
| Glass | Range | [7] | 70–85 | 2000–3700 | 2.50–5.30 |
| Carbon | Range | [7] | 150–500 | 1300–6300 | 0.30–2.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viotti, C.; Albrecht, K.; Amaducci, S.; Bardos, P.; Bertheau, C.; Blaudez, D.; Bothe, L.; Cazaux, D.; Ferrarini, A.; Govilas, J.; et al. Nettle, a Long-Known Fiber Plant with New Perspectives. Materials 2022, 15, 4288. https://doi.org/10.3390/ma15124288
Viotti C, Albrecht K, Amaducci S, Bardos P, Bertheau C, Blaudez D, Bothe L, Cazaux D, Ferrarini A, Govilas J, et al. Nettle, a Long-Known Fiber Plant with New Perspectives. Materials. 2022; 15(12):4288. https://doi.org/10.3390/ma15124288
Chicago/Turabian StyleViotti, Chloé, Katharina Albrecht, Stefano Amaducci, Paul Bardos, Coralie Bertheau, Damien Blaudez, Lea Bothe, David Cazaux, Andrea Ferrarini, Jason Govilas, and et al. 2022. "Nettle, a Long-Known Fiber Plant with New Perspectives" Materials 15, no. 12: 4288. https://doi.org/10.3390/ma15124288
APA StyleViotti, C., Albrecht, K., Amaducci, S., Bardos, P., Bertheau, C., Blaudez, D., Bothe, L., Cazaux, D., Ferrarini, A., Govilas, J., Gusovius, H.-J., Jeannin, T., Lühr, C., Müssig, J., Pilla, M., Placet, V., Puschenreiter, M., Tognacchini, A., Yung, L., & Chalot, M. (2022). Nettle, a Long-Known Fiber Plant with New Perspectives. Materials, 15(12), 4288. https://doi.org/10.3390/ma15124288











