Corrosion Behavior of Al Modified with Zn in Chloride Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Corrosion Tests
2.3. Microstructural Characterization
2.4. Software Methods
3. Results
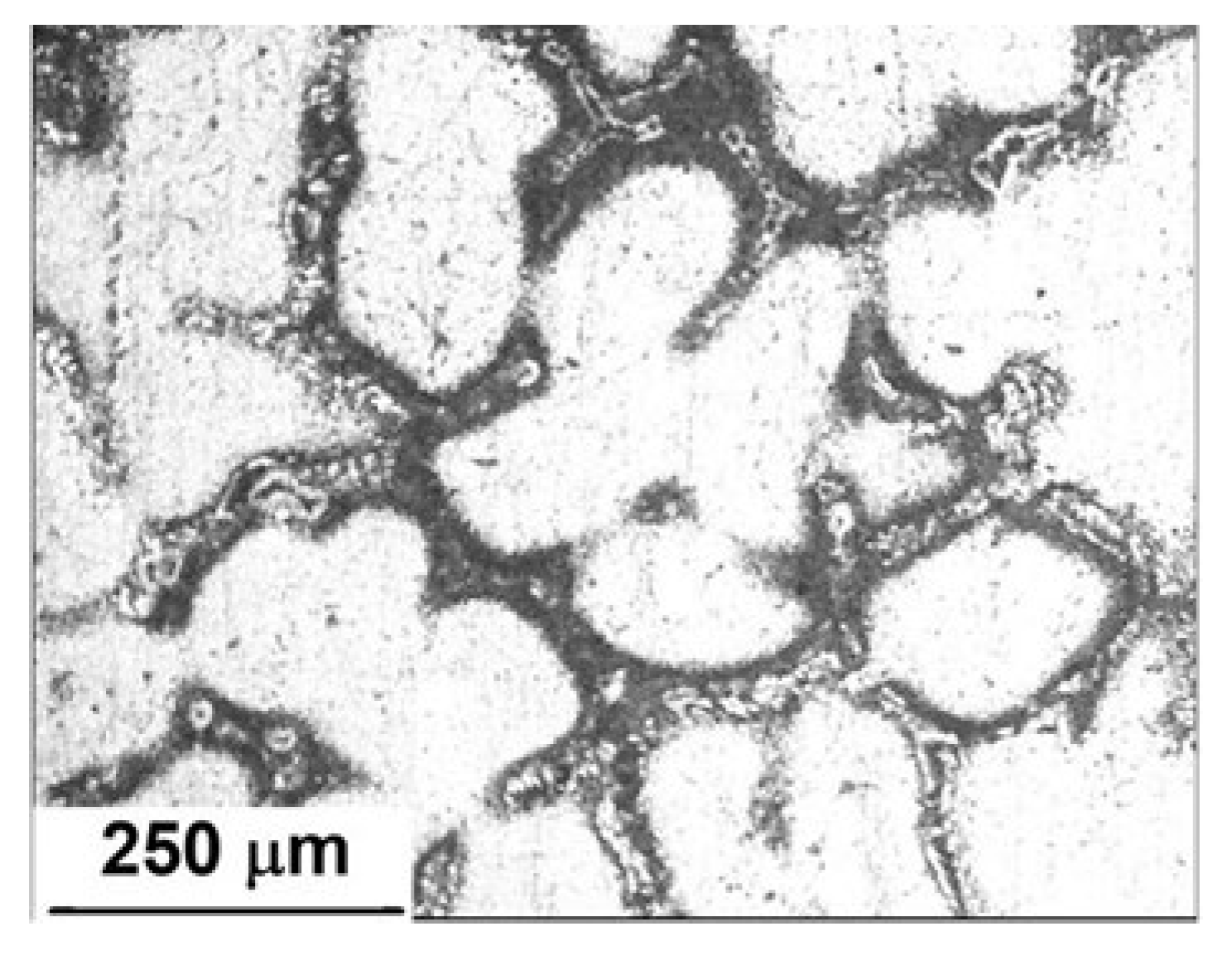
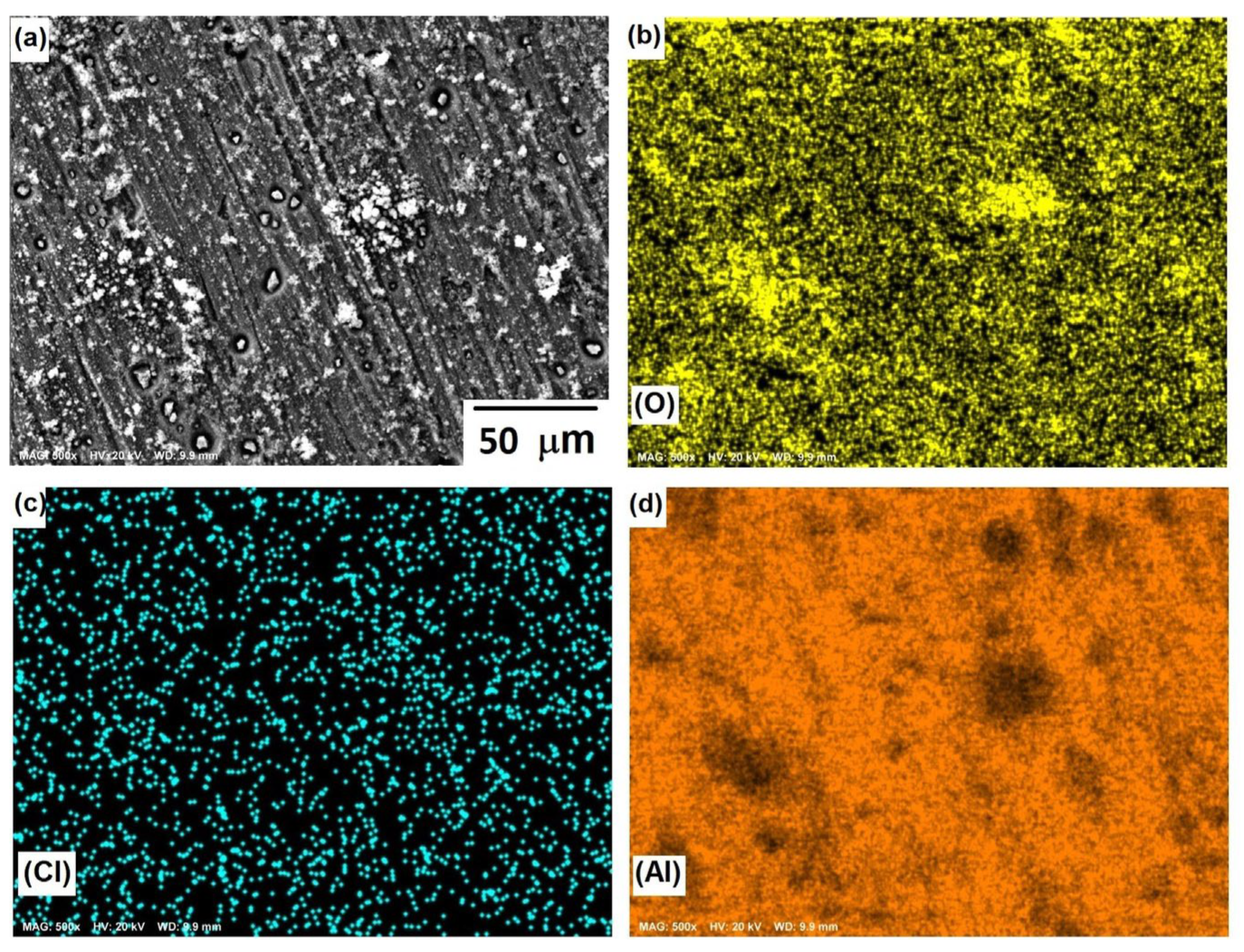
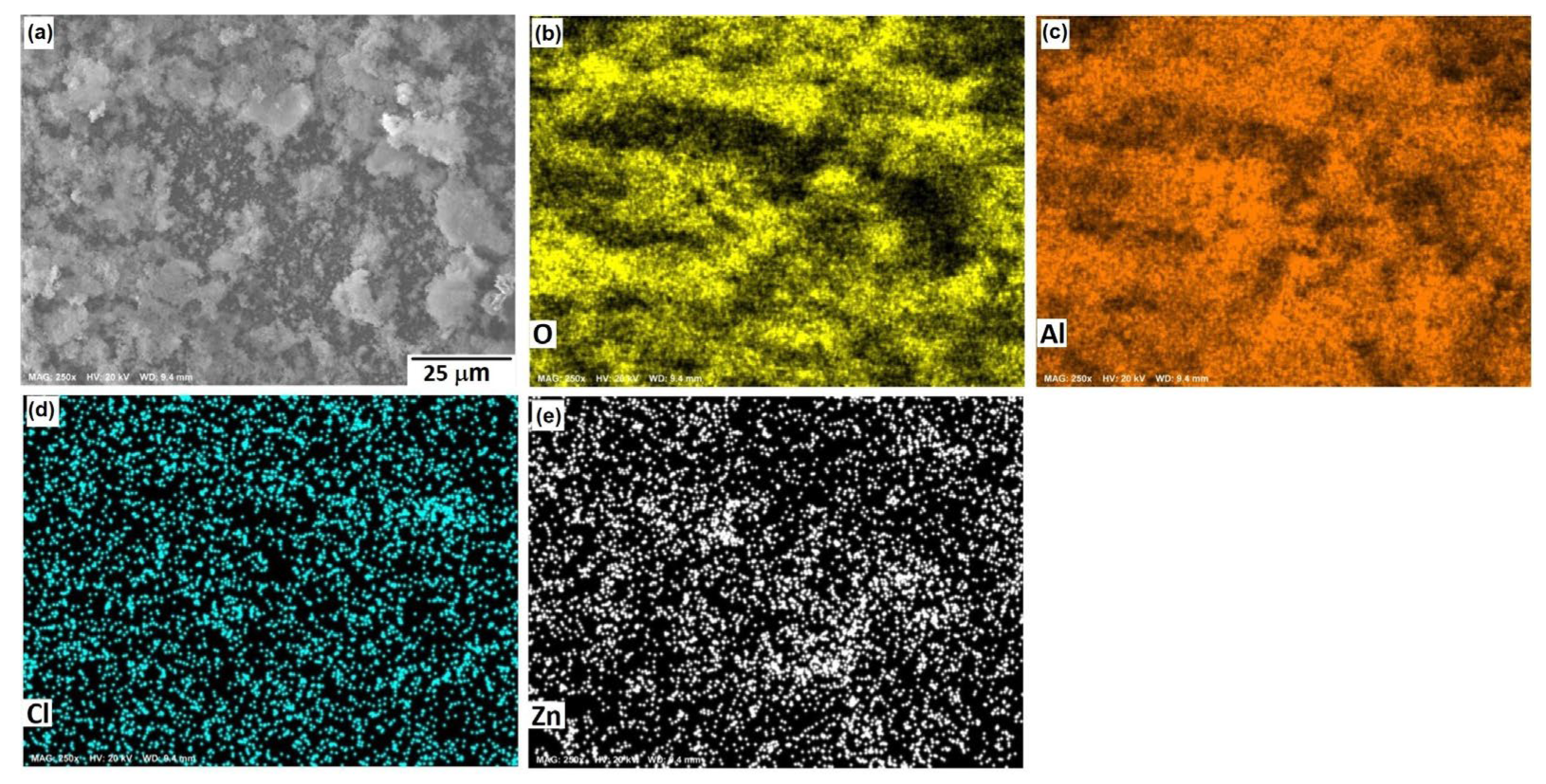
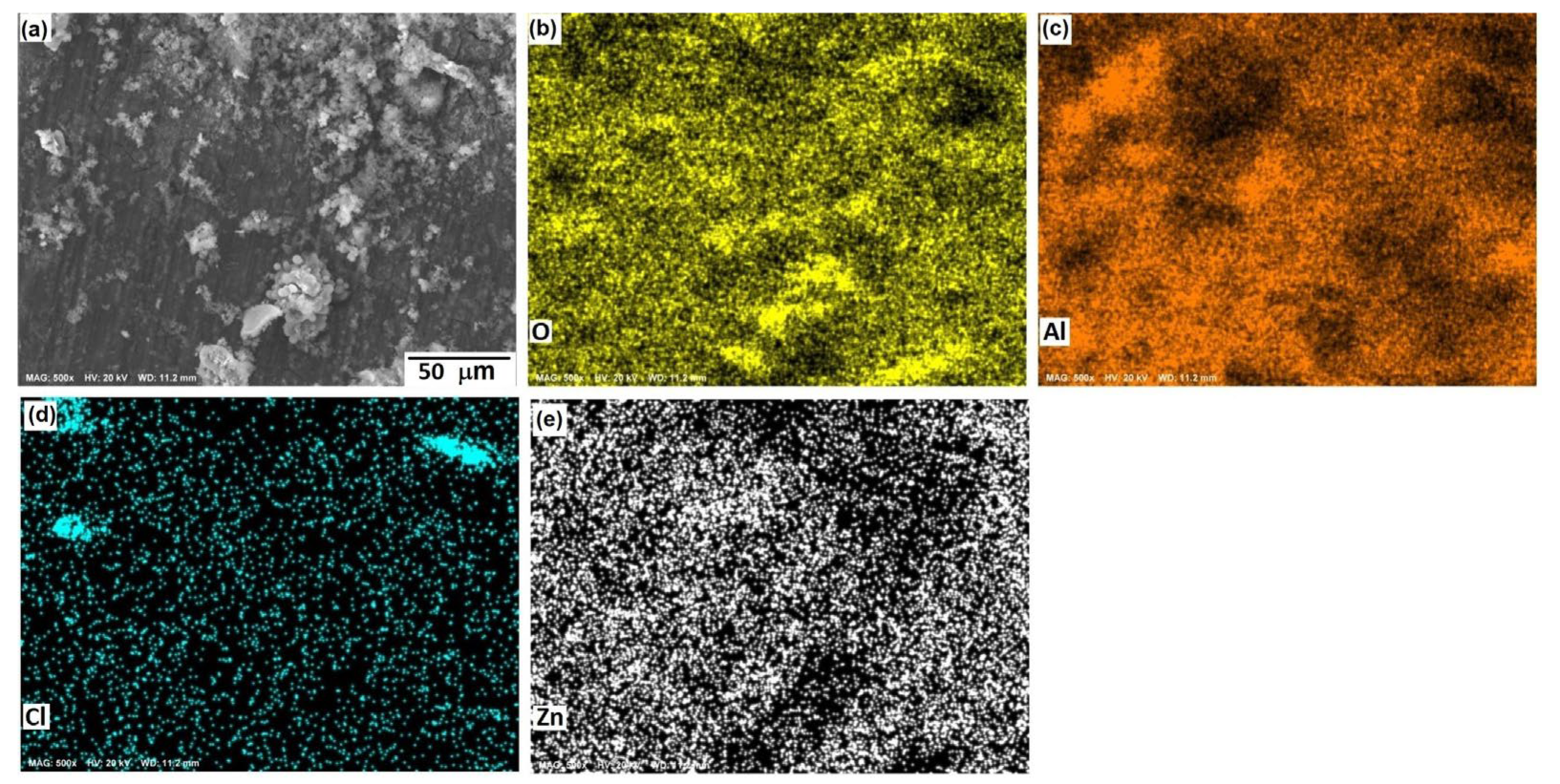
3.1. Microstructural Analysis
3.2. Potentiodynamic Polarization Curves
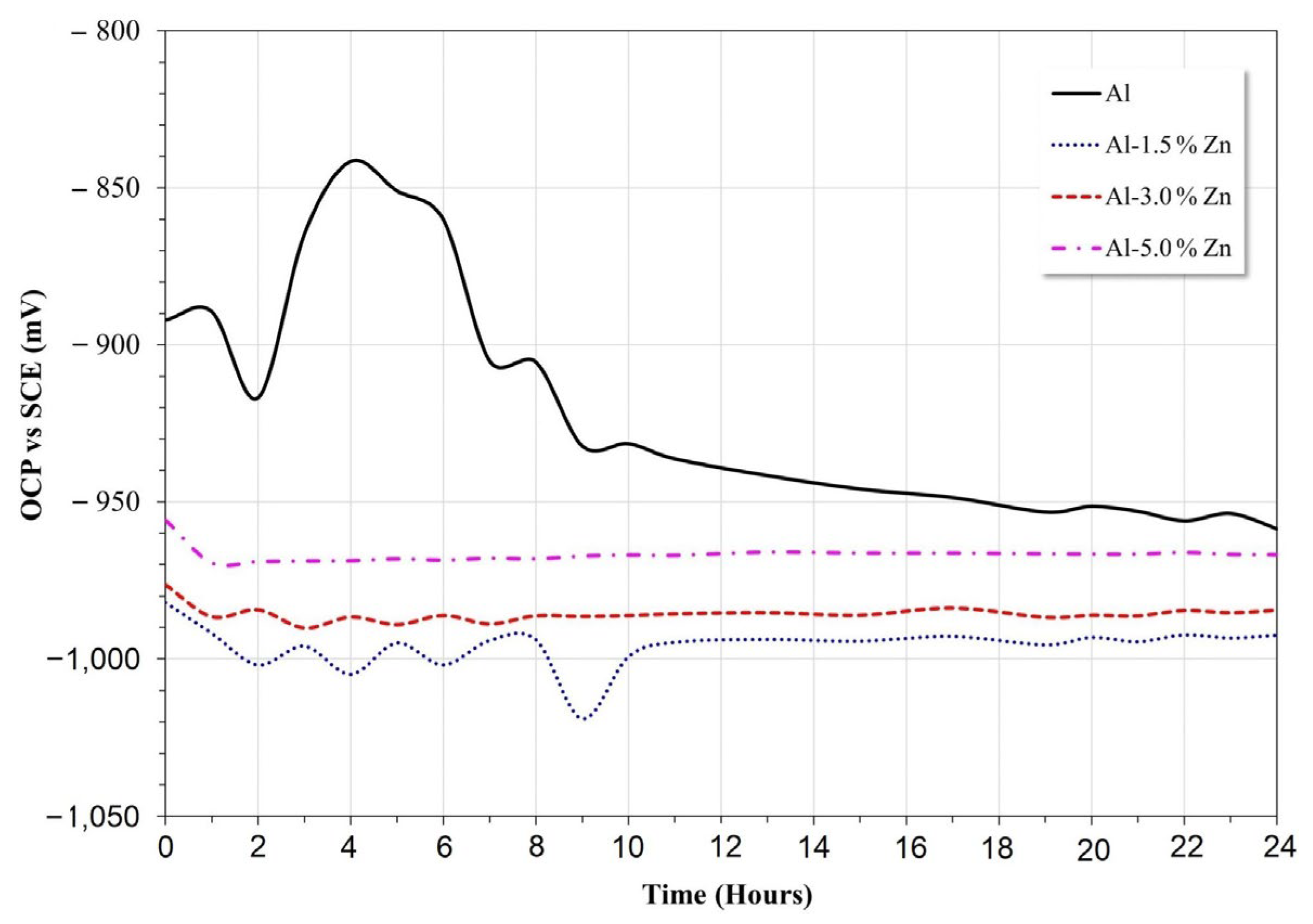
3.3. Open Circuit Potential
- (1)
- The cathodic reaction corresponds to oxygen reduction:
- (1)
- The anodic reaction implicates the dissolution of Zn:
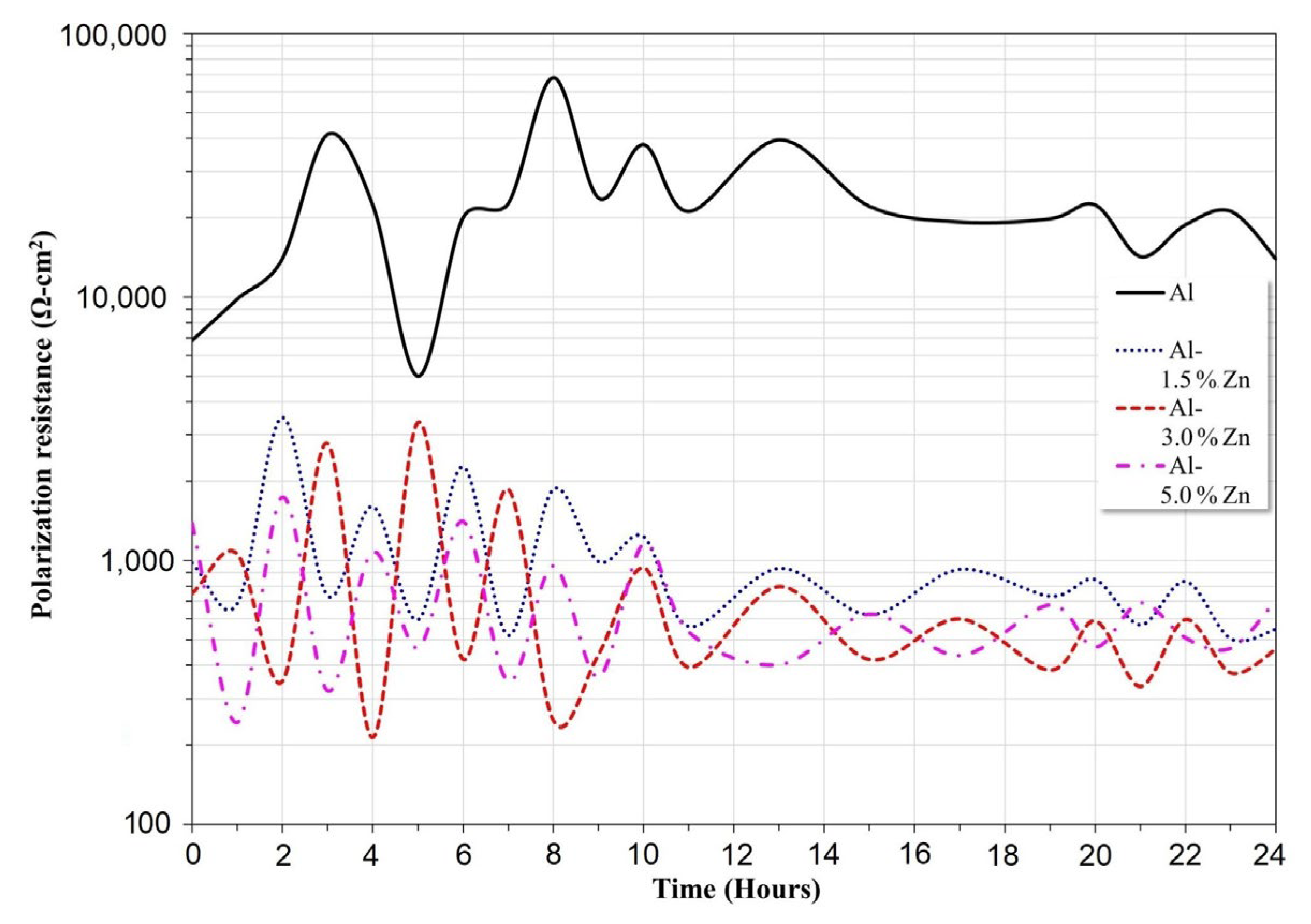
3.4. Linear Polarization Resistance
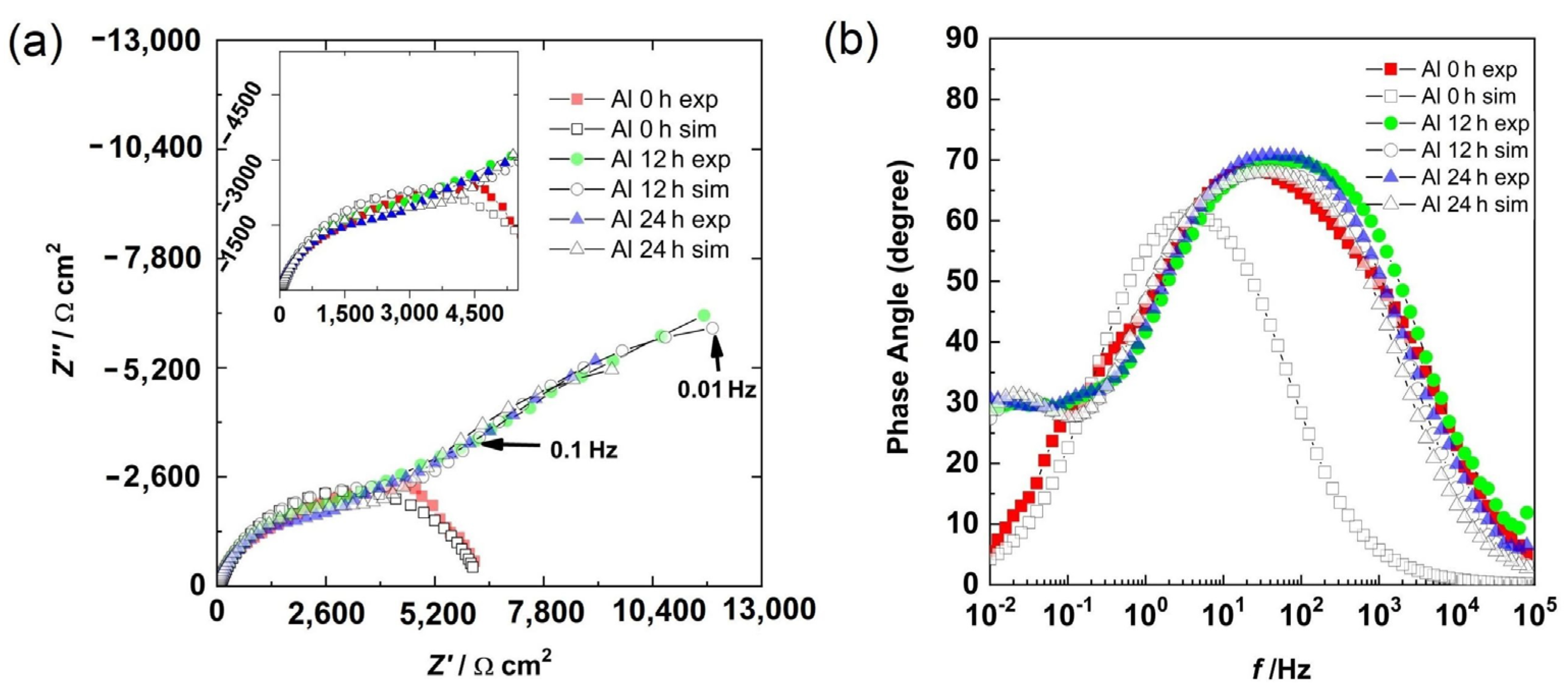
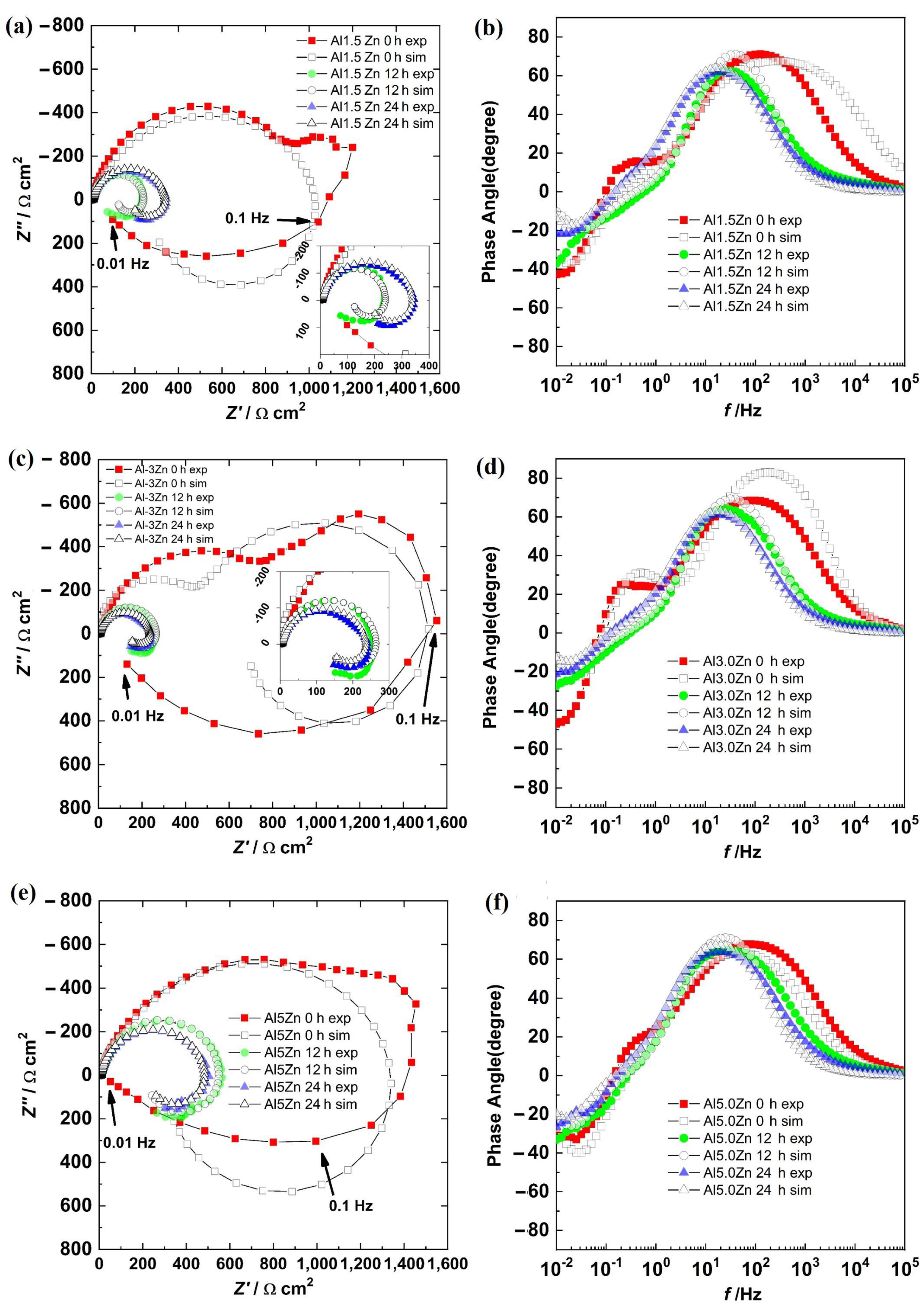
3.5. Electrochemical Impedance Spectroscopy
3.6. Impedance Spectroscopy Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, A.A. Recent Advances in Light Metals and Manufacturing for Automotive Applications. CIM J. 2021, 12, 79–87. [Google Scholar] [CrossRef]
- Berlanga-Labari, C.; Biezma-Moraleda, M.V.; Rivero, P.J. Corrosion of Cast Aluminum Alloys: A Review. Metals 2020, 10, 1384. [Google Scholar] [CrossRef]
- Googan, C. Marine Corrosion and Cathodic Protection; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-053649-2. [Google Scholar]
- Liontos, K.-P. Aluminum Based Sacrificial Anodes: Composition and Electrochemical Performance. Bachelor’s Thesis, University of Thessaly, Thessaly, Greece, July 2021. [Google Scholar]
- El-Hadad, S.; Moussa, M.E.; Waly, M. Effects of Alloying with Sn and Mg on the Microstructure and Electrochemical Behavior of Cast Aluminum Sacrificial Anodes. Int. J. Met. 2021, 15, 548–565. [Google Scholar] [CrossRef]
- Shuaib-Babata, Y.; Omokheni, E.; Ambali, I.; Busari, Y.; Abdulqadir, L.; Ejiko, S.; Ibrahim, H.; Muraina, S. The Effect of Magnesium Addition on the Performance of Aluminium-Zinc–Copper Alloy as a Sacrificial Anode in Seawater. J. Mater. Environ. Sci. 2020, 11, 963–976. [Google Scholar]
- Xi, Y.; Jia, M.; Zhang, J.; Zhang, W.; Yang, D.; Sun, L. Evaluating the Performance of Aluminum Sacrificial Anodes with Different Concentration of Gallium in Artificial Sea Water. Coatings 2022, 12, 53. [Google Scholar] [CrossRef]
- Moussa, M.E.; Elkilany, H.; El-Hadad, S.; Shoeib, M. Influence of Alloying Additives on the Electrochemical Behavior of Cast Al-5Zn Alloys. In Light Metals 2020; Tomsett, A., Ed.; Springer International Publishing: Cham, Germany, 2020; pp. 1052–1055. [Google Scholar]
- Morgan, J. Cathodic Protection, 2nd ed.; NACE International: Houston, TX, USA, 1993. [Google Scholar]
- Valdez, S.; Talavera, M.A.; Genesca, J.; Juarez-Islas, J.A. Activation of an Al-Zn-Mg-Li alloy by the Presence of Precipitates to be used as Sacrificial Anode. MRS Proc. 2000, 654, 351. [Google Scholar] [CrossRef]
- Orozco, R.; Genesca, J.; Juarez-Islas, J. Effect of Mg Content on the Performance of Al-Zn-Mg Sacrificial Anodes. J. Mater. Eng. Perform. 2007, 16, 229–235. [Google Scholar] [CrossRef]
- MaCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Obot, I.B.; Onyeachu, I.B. Electrochemical frequency modulation (EFM) technique: Theory and recent practical applications in corrosion research. J. Mol. Liq. 2018, 249, 83–96. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Keddam, M.; Mottos, O.R.; Takenouti, H. Reaction Model for Iron Dissolution Studied by Electrode Impedance: I. Experimental Results and Reaction Model. J. Electrochem. Soc. 1981, 128, 257–266. [Google Scholar] [CrossRef]
- Macdonald, D.; Real, S.; Smedley, S.; Urquidi, M. Evaluation of Alloy Anodes for Aluminum-Air Batteries IV. Electrochemical Impedance Analysis of Pure Aluminum in 4M-KOH at 25 °C. J. Electrochem. Soc. 1988, 135, 2410–2414. [Google Scholar] [CrossRef]
- Epelboin, I.; Wiart, R. Mechanism of the Electrocrystallization of Nickel and Cobalt in Acidic Solution. J. Electrochem. Soc. 1971, 118, 1577. [Google Scholar] [CrossRef]
- Pourgharibshahi, M.; Lambert, P. The role of indium in the activation of aluminum alloy galvanic anodes. Mater. Corros. 2016, 67, 857–866. [Google Scholar] [CrossRef]
- Hernandez-Maya, R.; Rosas, O.; Saunders, J.; Castaneda, H. Dynamic Characterization of Dendrite Deposition and Growth in Li-Surface by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2015, 162, A686–A696. [Google Scholar] [CrossRef]
- Taylor, M.L.; Sharifi, S.; MacDonald, D.D. Optimization of Impedance with Differential Evolution. ECS Trans. 2013, 50, 131–139. [Google Scholar] [CrossRef]
- Taylor, M.L. Technological Aspects of Corrosion Control in Metallic Systems. Ph.D. Thesis, Pennsylvania State University, Philadelphia, PA, USA, 2012. [Google Scholar]
- Nelson, A. Co-refinement of multiple-constrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl. Crystallogr. 2006, 39, 273–276. [Google Scholar] [CrossRef]
- Murray, J.L. The Al−Zn (Aluminum-Zinc) system. Bull. Alloy Phase Diagr. 1983, 4, 55–73. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Sctrucure and Properties; BUTTER WOETHS: London, UK; Boston, MA, USA, 1976. [Google Scholar]
- Khireche, S.; Boughrara, D.; Kadri, A.; Hamadou, L.; Benbrahim, N. Corrosion mechanism of Al, Al–Zn and Al–Zn–Sn alloys in 3 wt.% NaCl solution. Corros. Sci. 2014, 87, 504–516. [Google Scholar] [CrossRef]
- Kaewmaneekul, T.; Lothongkum, G. Effect of aluminium on the passivation of zinc–aluminium alloys in artificial seawater at 80 °C. Corros. Sci. 2013, 66, 67–77. [Google Scholar] [CrossRef]
- Pyun, S.i.; Moon, S.M.; Ahn, S.H.; Kim, S.S. Effects of Cl−, NO− 3 and SO2− 4 ions on anodic dissolution of pure aluminum in alkaline solution. Corros. Sci. 1999, 41, 653–667. [Google Scholar] [CrossRef]
- Foley, R.T. Localized Corrosion of Aluminum Alloys—A Review. Corrosion 1986, 42, 277–288. [Google Scholar] [CrossRef]
- Pourbaix, M.; Yang, X.-Z.; Zhang, H.-M. Electrode Potential-Temperatura Diagram. In Equilibrium Diagrams Localized Corrosion; The Electrochemical Society: Philadelphia, PA, USA, 1984; pp. 15–44. [Google Scholar]
- Mouanga, M.; Berçot, P.; Rauch, J.Y. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I Corros. Layer Charact. Corros. Sci. 2010, 52, 3984–3992. [Google Scholar] [CrossRef]
- Muñoz, A.G.; Saidman, S.B.; Bessone, J.B. Corrosion of an Al–Zn–In alloy in chloride media. Corros. Sci. 2002, 44, 2171–2182. [Google Scholar] [CrossRef]
- Matthews, D.B. The Stern-Geary and Related Methods for Determining Corrosion Rates. Aust. J. Chem. 1975, 28, 243–251. [Google Scholar] [CrossRef]
- Flamini, D.O.; Saidman, S.B.; Bessone, J.B. Aluminium activation produced by gallium. Corros. Sci. 2006, 48, 1413–1425. [Google Scholar] [CrossRef]
- Wood, G.C.; Richardson, J.A.; Abd Rabbo, M.F.; Mapa, L.M.; Sutton, W.H. Passivity of Metals. In Proceedings of the Fourth International Symposium on Passivity, Pennington, NJ, USA; 1978; p. 973. [Google Scholar]
- Vijh, A.K. The pitting corrosion potentials of metals and surface alloys in relation to their solid state cohesion. Mater. Chem. Phys. 1988, 20, 371–380. [Google Scholar] [CrossRef]
- Ahmido, A.; Sabbar, A.; Zouihri, H.; Dakhsi, K.; Guedira, F.; Serghini-Idrissi, M.; El Hajjaji, S. Effect of bismuth and silver on the corrosion behavior of Sn–9Zn alloy in NaCl 3wt.% solution. Mater. Sci. Eng. B 2011, 176, 1032–1036. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Lin, K.-L. Electrochemical corrosion behaviour of lead-free Sn–8.5 Zn–X Ag–0.1 Al–0.5 Ga solder in 3.5% NaCl solution. Mater. Sci. Eng. A 2005, 406, 34–42. [Google Scholar] [CrossRef]
- Zhang, X.; Leygraf, C.; Odnevall Wallinder, I. Atmospheric corrosion of Galfan coatings on steel in chloride-rich environments. Corros. Sci. 2013, 73, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Roselli, S.; Deyá, C.; Revuelta, M.; Di Sarli, A.R.; Romagnoli, R. Zeolites as reservoirs for Ce(III) as passivating ions in anticorrosion paints. Corros. Rev. 2018, 36, 305–322. [Google Scholar] [CrossRef]
- Chiu, T.-M.; Barraza-Fierro, J.I.; Castaneda, H. Comprehensive Interfacial Mechanisms of LiMnPO4-MWCNT Composite ratios in Acidic Aqueous Electrolyte. Electrochim. Acta 2017, 253, 93–103. [Google Scholar] [CrossRef]
- Macdonald, D.D. Application of Electrochemical Impedance Spectroscopy in Electrochemistry and Corrosion Science. In Techniques for Characterization of Electrodes and Electrochemical Processes; Varma, R., Selman, J.R., Eds.; Wiley: Hoboken, NY, USA, 1991; pp. 515–580. [Google Scholar]
- Breslin, C.B.; Friery, L.P. The synergistic interaction between indium and zinc in the activation of aluminium in aqueous electrolytes. Corros. Sci. 1994, 36, 231–240. [Google Scholar] [CrossRef]
- Barraza-Fierro, J.I.; Chiu, T.-M.; Castaneda, H. Electrochemical Impedance Characterization of LiMnPO4 Electrodes with Different Additions of MWCNTs in an Aqueous Electrolyte. J. Mex. Chem. Soc. 2019, 63, 39–55. [Google Scholar] [CrossRef]
- Landolf, D. Corrosion and Surface Chemistry of Metals, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Lausanne, Switzerland, 2007. [Google Scholar]
- Ható, Z.; Boda, D.; Kristóf, T. Simulation of steady-state diffusion: Driving force ensured by dual control volumes or local equilibrium Monte Carlo. J. Chem. Phys. 2012, 137, 054109. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Liang, X.; Liu, L.; Cai, G.; Yang, P.; Pei, Y.; Liu, C. Evidence for Pseudocapacitance and Faradaic Charge Transfer in High-Mobility Thin-Film Transistors with Solution-Processed Oxide Dielectrics. J. Phys. Chem. Lett. 2020, 11, 2765–2771. [Google Scholar] [CrossRef] [PubMed]




| Material | Ecorr (mV) | ba (mV/Dec) | bc (mV/Dec) | Icorr (mA/cm2) |
|---|---|---|---|---|
| Al | −874 | 192 | 342 | 0.00467 |
| Al-1.5%Zn | −980 | 36 | 400 | 0.01991 |
| Al-3.0%Zn | −970 | 36 | 366 | 0.01919 |
| Al-5.0%Zn | −952 | 16 | 331 | 0.01413 |
| Time | |||
|---|---|---|---|
| Parameter | 0 h | 12 h | 24 h |
| k1 | 6081 | -- | -- |
| k2 | 2359 | -- | -- |
| k3 | 6139 | -- | -- |
| k4 | 3738 | -- | -- |
| k5 | 4544 | -- | -- |
| b1 | 5 | -- | -- |
| b2 | 3 | -- | -- |
| OH | 1 × 10−10 | -- | -- |
| Cl | 0.008 | -- | - |
| AlCl | 8.77 × 10−7 | -- | -- |
| H | 1 × 10−10 | -- | -- |
| O | 2.08 × 10−5 | -- | -- |
| β1 | 3.72 × 10−8 | -- | -- |
| β2 | 6.78 × 10−8 | -- | -- |
| Rs | 69.54 | 7 | 7 |
| RCT | 5500 | 15,712 | 13,519 |
| Qdl | 6.36 × 10−6 | 6.53 × 10−4 | 8.41 × 10−4 |
| ndl | 0.99 | 0.8 | 0.8 |
| Rfilm | 6222.5 | 5861 | 4773 |
| Qfilm | 1.19 × 10−4 | 6.24 × 10−5 | 7.82 × 10−5 |
| nfilm | 0.8 | 0.8 | 0.8 |
| Al-1.5%Zn | Al-3.0%Zn | Al-5.0%Zn | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | Time | Time | |||||||
| Parameter | 0 h | 12 h | 24 h | 0 h | 12 h | 24 h | 0 h | 12 h | 24 h |
| k1 | 1.79 × 10−11 | 1.39 × 10−10 | 1.44 × 10−9 | 1.88 × 10−11 | 9.97 × 10−11 | 8.28 × 10−10 | 2.72 × 10−11 | 2.72 × 10−10 | 1.32 × 10−11 |
| k2 | 6660 | 5601 | 9180 | 5264 | 423 | 828 | 7125 | 704 | 759 |
| k3 | 81,501 | 1044 | 7284 | 8331 | 29 | 983 | 3024 | 402 | 435 |
| k4 | 1982 | 3516 | 5537 | 5510 | 670 | 237 | 8045 | 710 | 531 |
| k5 | --- | 1372 | 56 | 303 | 250 | 56 | 176 | 81 | 65 |
| b1 | 5.92 | 5 | 5 | 6 | 5.38 | 4.76 | 6 | 6 | 6 |
| b2 | 7.67 | 3 | 3 | 8 | 4 | 4 | 8 | 4 | 4 |
| OH | 1 × 10−10 | 1 × 10−10 | 1 × 10−10 | 1 × 10−10 | 3.13 × 10−10 | 1.67 × 10−10 | 1 × 10−10 | 1.70 × 10−10 | 3.54 × 10−9 |
| Cl | 0.004 | 0.004 | 0.004 | 0.002 | 0.005 | 0.003 | 0.008 | 0.006 | 0.005 |
| AlCl | 7.10 × 10−7 | 6.03 × 10−7 | 4.66 × 10−7 | 6.42 × 10−7 | 3.32 × 10−7 | 8.30 × 10−7 | 9.89 × 10−7 | 1.03 × 10−7 | 3 × 10−7 |
| H | 4.49 × 10−7 | 6.92 × 10−7 | 9.04 × 10−7 | 5.57 × 10−7 | 7.32 × 10−7 | 9.77 × 10−7 | 8.88 × 10−7 | 5.71 × 10−7 | 8.33 × 10−7 |
| O | 8.99 × 10−6 | 4.06 × 10−6 | 4.66 × 10−5 | 6.71 × 10−6 | 1.29 × 10−5 | 3.82 × 10−5 | 9.35 × 10−6 | 4.26 × 10−5 | 3.15 × 10−5 |
| β1 | 3.60 × 10−8 | 9.35 × 10−8 | 7.28 × 10−8 | 6.69 × 10−8 | 7.93 × 10−8 | 5.13 × 10−8 | 7.26 × 10−8 | 2.35 × 10−8 | 8.27 × 10−8 |
| β2 | 9.94 × 10−8 | 1 × 10−7 | 1 × 10−7 | 1 × 10−7 | 1 × 10−7 | 1 × 10−7 | 9.9 × 10−8 | 1 × 10−7 | 1 × 10−7 |
| Rs | 1.80 | 6.68 | 7.68 | 1.80 | 5.47 | 5.96 | 9.62 | 9.73 | 8.48 |
| RCT | 425 | 200 | 380 | 1000 | 300 | 280 | 500 | 530 | 400 |
| Qdl | 1 × 10−7 | 1.08 × 10−4 | 3.02 × 10−4 | 6.76 × 10−4 | 1.62 × 10−4 | 3.90 × 10−4 | 1 × 10−7 | 1.02 × 10−4 | 2.12 × 10−4 |
| ndl | 0.89 | 1.0 | 0.89 | 0.89 | 0.95 | 0.89 | 0.89 | 0.95 | 0.90 |
| Rfilm | 46.12 | --- | --- | 492.6 | --- | --- | 102.4 | --- | --- |
| Qfilm | 5.86 × 10−5 | --- | --- | 2.94 × 10−5 | --- | --- | 8.87 × 10−5 | --- | --- |
| nfilm | 0.8 | --- | --- | 1 | --- | --- | 0.8 | --- | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón, J.P.; Reyes Barragán, J.L.; Barraza Fierro, J.I.; Cruz Mejía, H.; Arrieta González, C.D.; Ravelero Vázquez, V.; Sánchez, K.P.; Torres-Mancera, M.T.; Retes-Mantilla, R.F.; Rodríguez-Díaz, R.A. Corrosion Behavior of Al Modified with Zn in Chloride Solution. Materials 2022, 15, 4229. https://doi.org/10.3390/ma15124229
Calderón JP, Reyes Barragán JL, Barraza Fierro JI, Cruz Mejía H, Arrieta González CD, Ravelero Vázquez V, Sánchez KP, Torres-Mancera MT, Retes-Mantilla RF, Rodríguez-Díaz RA. Corrosion Behavior of Al Modified with Zn in Chloride Solution. Materials. 2022; 15(12):4229. https://doi.org/10.3390/ma15124229
Chicago/Turabian StyleCalderón, Jesús Porcayo, José Luis Reyes Barragán, Jesús Israel Barraza Fierro, Héctor Cruz Mejía, Cinthya Dinorah Arrieta González, Víctor Ravelero Vázquez, Kevin Piedad Sánchez, María Teresa Torres-Mancera, Rogel Fernando Retes-Mantilla, and Roberto Ademar Rodríguez-Díaz. 2022. "Corrosion Behavior of Al Modified with Zn in Chloride Solution" Materials 15, no. 12: 4229. https://doi.org/10.3390/ma15124229
APA StyleCalderón, J. P., Reyes Barragán, J. L., Barraza Fierro, J. I., Cruz Mejía, H., Arrieta González, C. D., Ravelero Vázquez, V., Sánchez, K. P., Torres-Mancera, M. T., Retes-Mantilla, R. F., & Rodríguez-Díaz, R. A. (2022). Corrosion Behavior of Al Modified with Zn in Chloride Solution. Materials, 15(12), 4229. https://doi.org/10.3390/ma15124229







