Surgery Training System Supported by Organic Materials
Abstract
:1. Introduction
- -
- The material of the fixture to be implanted (implant VXI300 is commercially pure grade 1 unalloyed titanium (ASTM standard F-67) [10]);
- -
- -
- The microstructure (implant covered with titanium oxide: TiO blast structure);
- -
- The bone bed where the implant is anchored, the surgical non-traumatic technique, and the load of the implant (preferably in the longitudinal direction);
- -
- The surgical technique (following an appropriate surgical procedure, including preventing bone overheating and avoiding applying biofilm to the implant [12]);
- -
- The implant load (implant diameter, i.e., the contact surface of the implant with the bone [3]).
2. Materials and Methods
2.1. Composite
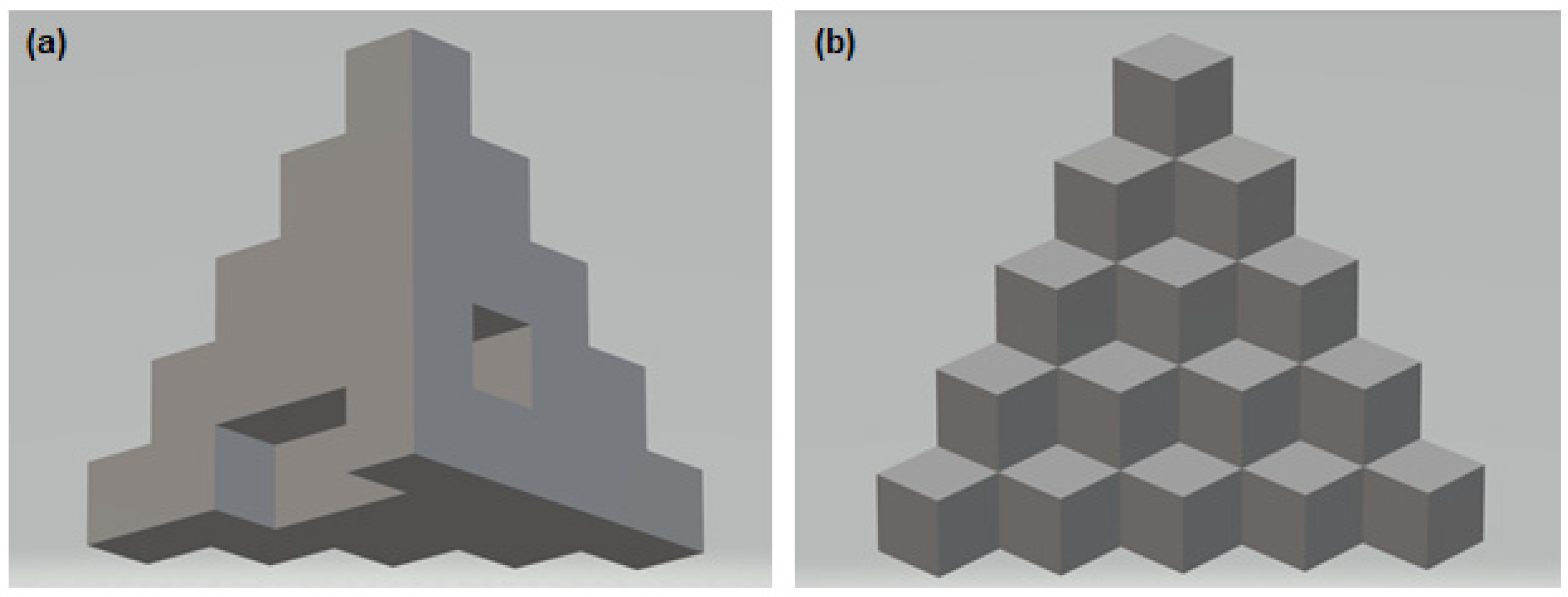
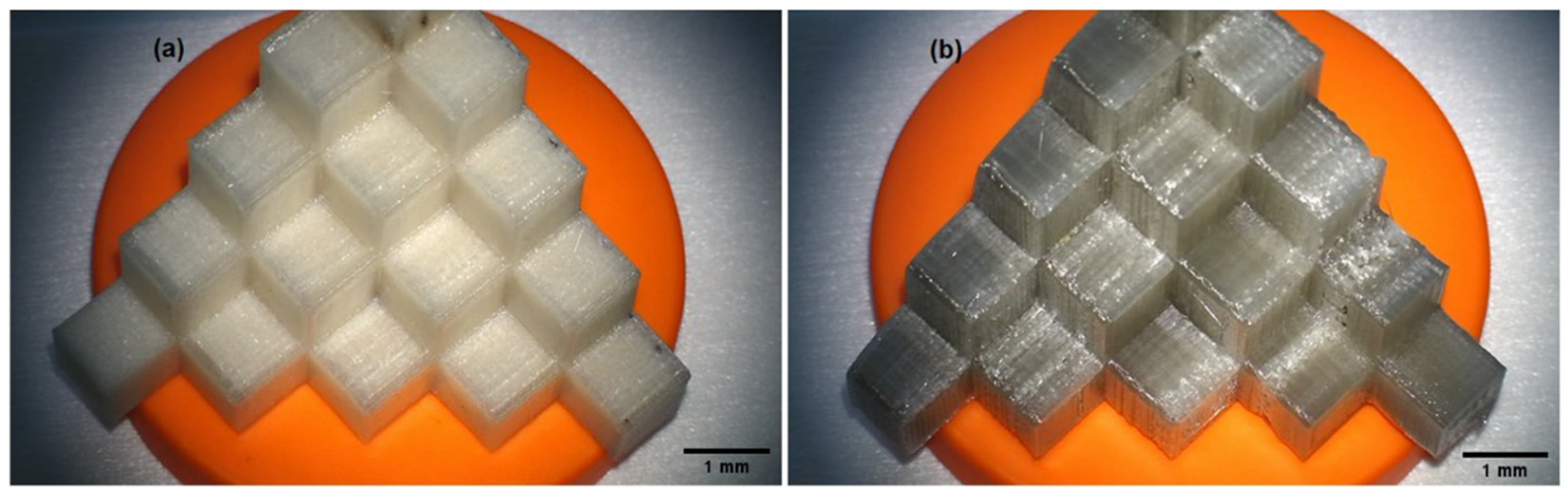
2.2. 3D Printing and Visual Assessment of the Printing Effect
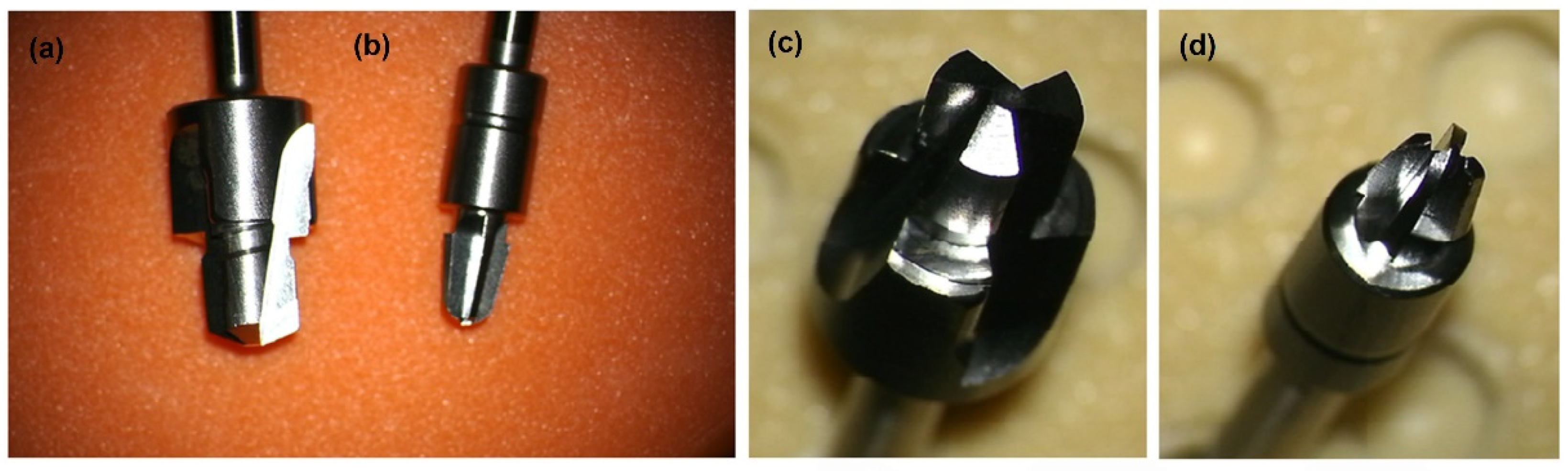
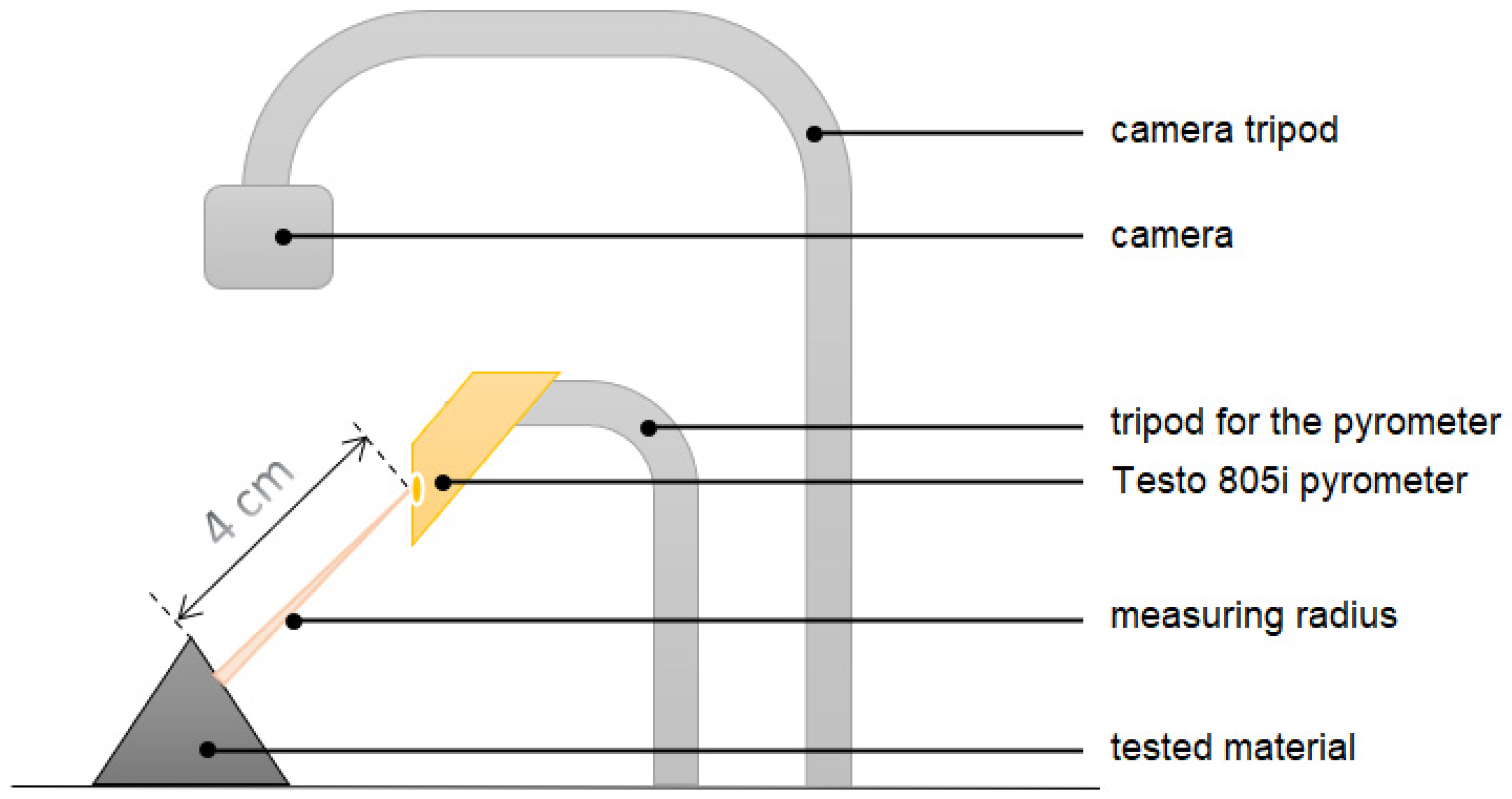
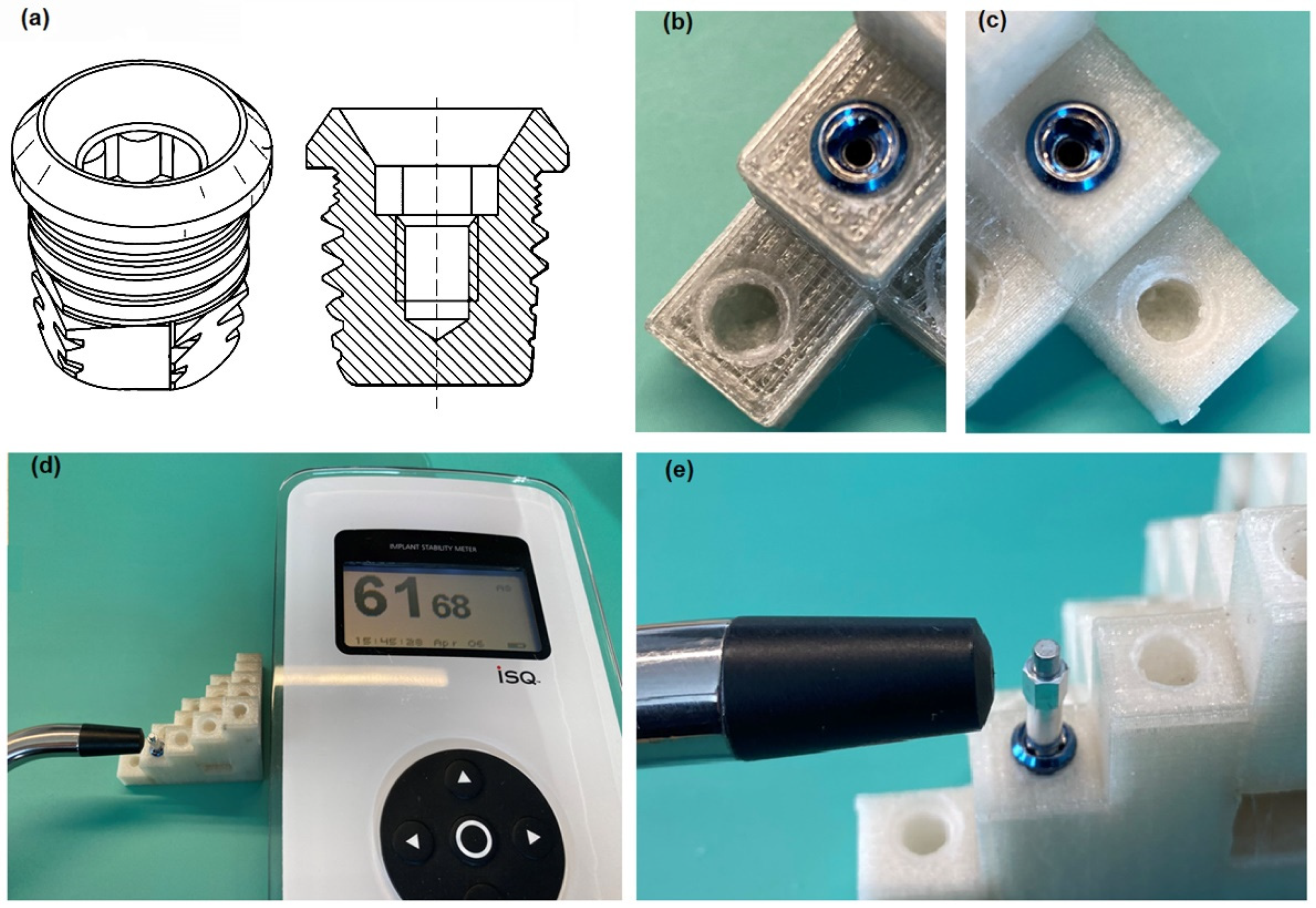
2.3. Machining, Temperature Measurement and Evaluation of the Implant Stability
3. Results
3.1. Qualitative Assessment of Printed Filaments
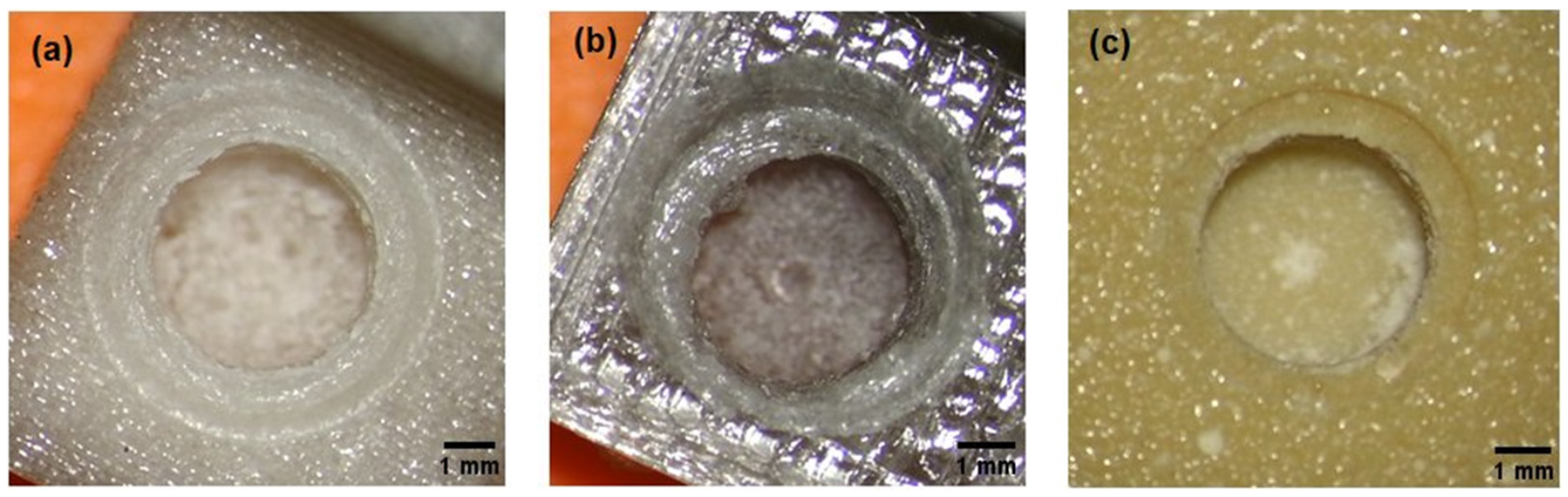
3.2. Evaluation of the Printout of Calibration Models and the Course of the Drilling Process
- -
- test model with the composition of 700 g PC/5 g PO/3.5 g talc, cream–white color, non-transparent, visible porosity of the structure (Figure 2a),
- -
- test model with the composition of 700 g PC/5 g PO/3.5 g talc, where the filament was additionally free of water before printing (Figure 2b), gray–transparent color, visible porosity of the structure.
- -
- Material no. 1: drilling in the material is easy, the chips are elastic but brittle, they disintegrate and do not remain on the drill; the drill very quickly reaches the set depth, which disturbs the sense of control over it;
- -
- Material no. 2 and 3: optimal drilling, spring-shaped chips—most of them are removed from the drill by themselves, full control during drilling; punched wells have very clear visible boundary lines of the holes;
- -
- Material no. 4: drilling is like rubber, feel resistance on the drill; the shavings stick to the drill, the residual material must be manually removed from the drill; punched wells have very clear visible boundary lines of the holes,
- -
- Material no. 5: no control over drill depth; the drill has resistance, it takes a long time to drill the hole and reach the set depth, the material seems very dense; the chips stick to the drill bit, the drill should be cleaned mechanically; coastlines invisible through melted edges and lumpy bone chips.
3.3. Stability Measurement Results
4. Discussion
- -
- in appearance and behavior of chips;
- -
- in the ISQ study, the lowest results were observed for the non-dried model, although it was not statistically significant (Table 4).
- -
- in the color and the way of reflecting the light of the printed models (Figure 7), where the PC can be colored in any color because this material can be dyed very easily according to the safety data sheets;
- -
- The material is easy to thermoform, which means that we can freely recreate the angles and planes that interest us;
- -
- The model can be a 1:1 mapping of selected bone fragments of the patient, which will facilitate the planning of the stages of the actual surgery; we obtain a faithful representation of the patient’s anatomy;
- -
- Models made of such a material can be successfully personalized according to the training needs, the surgeon’s skills, or the degree of advancement in surgical training;
- -
- Model renewal (the ability to easily print with exactly the same shapes) does not limit the surgeon to one attempt; it makes it possible to consider many methods of carrying out the procedure and to test them in practice; it also has a didactic value, as the surgeon can test many options, including the risky ones, and on their basis reduce the risk of error;
- -
- There are no contraindications for the model made of the tested material to be sterilized or disinfected, in addition to using various available methods; the use of the material in a sterile field is not excluded.
- -
- The material looks aesthetic, has no random air bubbles or other undesirable artifacts that could disturb the surgical planning process or affect the overall course of training;
- -
- The material does not raise dust and does not pick up static during cutting;
- -
- The porosity of the material allows you to keep the drill in the correct position, the drill bit does not slip, it is easier to make the first drill and continue the process with the next drill bits (according to the instructions);
- -
- The material did not crumble or chip;
- -
- Quieter operation of the drill in the material;
- -
- Benefits from the economic assessment point:
- -
- Easy availability and relatively low cost of purchasing the components that make up the tested composite;
- -
- An uncomplicated method of creating a composite in the form of a filament for 3D printing (possible to be implemented in non-laboratory conditions);
- -
- The filaments obtained are ready for direct use in commonly available printers (3D printing method: FFF).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federspil, P.; Federspil, P.A. Die epithetische Versorgung von kraniofazialen Defekten. HNO 1998, 46, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.M.; de Perceval Tara, M.P.; Fernández, A.B.M.; Martínez, E.B.; Ramos, M.R.; Valadés, R.F.; López, A.E. Bilateral auricular reconstruction with osseointegrated implant-retained prostheses. Optimization of aesthetic outcomes using virtual planning. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.; Raghoebar, G.M.; van Oort, R.P.; Vissink, A. Fate of implant-retained craniofacial prostheses: Life span and aftercare. Int. J. Oral Maxillofac. Implant. 2008, 23, 89–98. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Den Besten, C.A.; Stalfors, J.; Wigren, S.; Blechert, J.I.; Flymm, M.; Eeg-Olofsson, M.; Aggarwal, R.; Green, K.; Nelissen, R.C.; Mylanus, E.A.; et al. Stability, survival, and tolerability of an auditory osseointegrated implant for bone conduction hearing: Long-term follow-up of a randomized controlled trial. Otol. Neurotol. 2016, 37, 1077–1083. [Google Scholar] [CrossRef]
- Zarb, G.A.; Albrektsson, T. Osseointegation—a requiem for the periodontal ligament? Int. J. Periodontics Restor. Dent. 1991, 11, 88–91. [Google Scholar]
- Chang, T.L.; Garrett, N.; Roumanas, E.; Beumer, J., 3rd. Treatment satisfaction with facial prostheses. J. Prosthet. Dent. 2005, 94, 275–280. [Google Scholar] [CrossRef]
- Federspil, P.A. Implant-retained craniofacial prostheses for facial defects. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg 2009, 8, Doc03. [Google Scholar] [CrossRef]
- Granström, G. Craniofacial osseointegration. Oral Dis. 2007, 13, 261–269. [Google Scholar] [CrossRef]
- Cochlear Bone Anchored Solutions AB. Cochlear Vistafix 3 System. Key Dimensions and Material Information. 2012. Available online: https://www.cochlear.com/f3fdc71c-07a1-4c44-99b0-c3e4b8dfeff5/E82458A_Cochlear_Baha_BIA400_Data_Sheet_GB.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=f3fdc71c-07a1-4c44-99b0-c3e4b8dfeff5 (accessed on 3 February 2022).
- Jinton, L.; Holgersson, E.; Elmberg, P. Bone Anchored Fixture for a Medical Prosthesis. U.S. Patent 9173042B2, 27 October 2015. [Google Scholar]
- Cochlear Bone Anchored Solutions AB. Treatment and Surgery Guide. Cochlear™ Vistafix® 3 System A Bone Anchored Prosthetic Solution. 2012. Available online: https://www.cochlear.com/29e502a1-c752-44b8-97bd-5a376c197209/VFX001+Iss1+MAY12+Vistafix+Surgery+Guide.pdf?MOD=AJPERES&CACHEID=29e502a1-c752-44b8-97bd-5a376c197209 (accessed on 17 February 2022).
- Chrcanovic, B.R.; Nilsson, J.; Thor, A. Survival and complications of implants to support craniofacial prosthesis: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1536–1552. [Google Scholar] [CrossRef]
- Tolman, D.E.; Taylor, P.F. Bone-anchored craniofacial prosthesis study: Irradiated patients. Int. J. Oral Maxillofac. Implant. 1996, 11, 612–619. [Google Scholar]
- Subramaniam, S.S.; Breik, O.; Cadd, B.; Peart, G.; Wiesenfeld, D.; Heggie, A.; Gibbons, S.D.; Nastri, A. Long-term outcomes of craniofacial implants for the restoration of facial defects. Int. J. Oral. Maxillofac. Surg. 2018, 47, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, M.A.; Verhamme, L.; van de Pol, P.; Kunst, H.P.M.; Mylanus, E.A.M.; Hol, M.K.S. Auricular prostheses attached to osseointegrated implants: Multidisciplinary work-up and clinical evaluation. Eur. Arch. Otorhinolaryngol. 2019, 276, 1017–1027. [Google Scholar] [CrossRef]
- Huang, J.J.; Ren, J.A.; Wang, G.F.; Li, Z.A.; Wu, X.W.; Ren, H.J.; Liu, S. 3D-printed “fistula stent” designed for management of enterocutaneous fistula: An advanced strategy. World J. Gastroenterol. 2017, 23, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Posa, F.; Di Benedetto, A.; Ravagnan, R.; Cavalcanti-Adam, E.A.; Muzio, L.L.; Percoco, G.; Mori, G. Bioengineering Bone Tissue with 3D Printed Scaffolds in the Presence of Oligostilbenes. Materials 2020, 13, 4471. [Google Scholar] [CrossRef] [PubMed]
- Alaboodi, A.S.; Sivasankaran, S. Experimental design and investigation on the mechanical behavior of novel 3D printed biocompatibility polycarbonate sca_olds for medical applications. J. Manuf. Process. 2018, 35, 479–491. [Google Scholar] [CrossRef]
- Flak, T.; Trejnowska, E.; Skoczyński, S.; Gabor, J.; Swinarew, B.; Grzywnowicz, K.; Okła, H.; Jasik, K.; Stanula, A.; Brożek, G.; et al. Novel Antibacterial Modification of Polycarbonate for Increment Prototyping in Medicine. Materials 2021, 14, 4725. [Google Scholar] [CrossRef]
- Covestro Macrolon® 2600 Polycarbonate Datasheet. Songhan Plastic Technology Co., Ltd. Available online: http://www.lookpolymers.com/pdf/Covestro-Makrolon-2600-Polycarbonate.pdf (accessed on 2 February 2022).
- Peterson, J.; Dechow, P.C. Material properties of the human cranial vault and zygoma. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 274, 785–797. [Google Scholar] [CrossRef]
- Gomez-Gras, G.; Abad, M.D.; Pérez, M.A. Mechanical performance of 3D-printed biocompatible polycarbonate for biomechanical applications. Polymers 2021, 13, 3669. [Google Scholar] [CrossRef]
- West, M.; Ruys, A.; Bosi, S. The Effect of the Ultraviolet Radiation Environment of LEO upon Polycarbonate Materials. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit-Meeting Papers, Reno, NV, USA, 10–13 January 2005. [Google Scholar] [CrossRef]
- Swinarew, A.S.; Swinarew, B.; Flak, T.; Okła, H.; Lenartowicz-Klik, M.; Barylski, A.; Popczyk, M.; Gabor, J.; Stanula, A. The Evaluation of Simulated Environmental Degradation of Polycarbonate Filled with Inorganic and Organic Reinforcements. Polymers 2021, 13, 3572. [Google Scholar] [CrossRef] [PubMed]
- Plastics Base. PC Polycarbonate. Available online: https://www.tworzywa.pl/wiedzopedia/baza-tworzyw/81,poliweglan-pc,polimer.html (accessed on 2 February 2022).
- Identification of plastics. Available online: https://docplayer.pl/10653085-Identyfikacja-tworzyw-sztucznych.html (accessed on 2 February 2022).
- Banasiak, A.; Sterzyński, T. Structure and properties of PE + Talc polymeric composites. Composites 2002, 3, 126–130. [Google Scholar]
- Rokpol D2002 Datasheet. PCC Rokita. Available online: https://www.products.pcc.eu/pl/id/8980/rokopol-d2002/ (accessed on 2 February 2022).
- Wodarski, K. Expertise 2.2. Analysis of the development of key technologies in the field of metallic, ceramic, polymer and composite products. Available online: http://roz4.polsl.pl/wp-content/uploads/2019/11/E-4_2.2_Analiza-rozwoju-kluczowych-technologii.pdf (accessed on 2 February 2022).
- Swinarew, A.; Grobelny, Z.; Jasik, K.; Rozwadowska, B.; Nowicki, G.; Flak, T.; Gabor, J.; Łężniak, M.; Okła, H. Sposób otrzymywania modyfikowanych poliestrów, zwłaszcza na bazie poliwęglanu, polilaktydu, lub modyfikowanych kopolimerów i modyfikowane poliestry lub modyfikowane kopolimery otrzymane tym sposobem. Patent No. 227529, 23 July 2015. [Google Scholar]
- Swinarew, A.; Grobelny, Z.; Jasik, K.; Rozwadowska, B.; Nowicki, G.; Flak, T.; Gabor, J.; Łężniak, M.; Okła, H. Sposób wytwarzania poliuretanu modyfikowanego nanokrzemionką. Polymertech spółka z ograniczoną odpowiedzialnością. Patent No. 228980, 23 July 2015. [Google Scholar]
- MCroucher. 5 mm Calibration Cube Steps. 4 June 2012. Available online: https://www.thingiverse.com/thing:24238 (accessed on 2 February 2022).
- Björn, G.; Frimanson, J. Mastoid Bone Start Drill Bit. U.S. Patent 20130218160A1, 4 April 2017. [Google Scholar]
- Nelissen, R.C.; den Besten, C.A.; Faber, H.T.; Dun, C.A.; Mylanus, E.A.; Hol, M.K. Loading of osseointegrated implants for bone conduction hearing at 3 weeks: 3-year stability, survival, and tolerability. Eur. Arch. Otorhinolaryngol. 2016, 273, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Balleri, P.; Cozzolino, A.; Ghelli, L.; Momicchioli, G.; Varriale, A. Stability measurements of osseointegrated implants using Osstell in partially edentulous jaws after 1 year of loading: A pilot study. Clin. Implant. Dent. Relat. Res. 2002, 4, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implant. Res. 1996, 7, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Schallhorn, R.A. Resonance Frequency Analysis in Implant Dentistry. Decis. Dent. 2017, 3, 34–39. [Google Scholar]
- Ostell, A.G. Osstell ISQ Quick Guide. Guidelines for Measuring Implant Stability using Osstell ISQ on Cochlear™ Baha® Implants and Abutments. Available online: https://manuals.plus/m/24c388e5f0baa1d4c0f140fbb718c8c7bfd6275d5be247abe6163c06a627a3ed (accessed on 2 February 2022).
- Nelissen, R.C.; Stalfors, J.; de Wolf, M.J.; Flynn, M.C.; Wigren, S.; Eeg-Olofsson, M.; Green, K.; Rothera, M.P.; Mylanus, E.A.; Hol, M.K. Long-term stability, survival, and tolerability of a novel osseointegrated implant for bone conduction hearing: 3-year data from a multicenter, randomized, controlled, clinical investigation. Otol Neurotol. 2014, 8, 1486–1491. [Google Scholar] [CrossRef]
- Charpiot, A.; Chambres, O.; Herve, J.F.; Million, P.; Riedinger, A.M.; Hemar, P. Osteointegrated cranio-facial implants: 49 patients report. Rev. Laryngol. Otol. Rhinol. 2006, 127, 217–222. [Google Scholar]
- Gawęcki, W.; Gibasiewicz, R.; Marszał, J.; Błaszczyk, M.; Gawłowska, M.; Wierzbicka, M. The evaluation of a surgery and the short-term benefits of a new active bone conduction hearing implant—the Osia®. BJORL. Braz. J. Otorhinolaryngol. 2022, 88, 289–295. [Google Scholar] [CrossRef]
- Marszal, J.; Gibasiewicz, R.; Blaszczyk, M.; Gawlowska, M.B.; Gawecki, W. Piezoelectric bone conduction hearing implant Osia®—audiological and quality of life benefits. Otolaryngol. Pol. 2021, 75, 11–22. [Google Scholar] [CrossRef]
- Rahne, T.; Svensson, S.; Lagerkvist, H.; Holmberg, M.; Plontke, S.K.; Wenzel, C. Assessment of Temporal Bone Thickness for Implantation of a New Active Bone-Conduction Transducer. Otol. Neurotol. 2021, 42, 278–284. [Google Scholar] [CrossRef]
- Ekşi, M.S.; Güdük, M.; Usseli, M.I. Frontal Bone is Thicker in Women and Frontal Sinus is Larger in Men: A Morphometric Analysis. J. Craniofac. Surg. 2021, 32, 1683–1684. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-Printed Biosensor Arrays for Medical Diagnostics. Micromachines 2018, 9, 394. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Haryńska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing—Characterization and Fabrication. Materials 2019, 12, 887. [Google Scholar] [CrossRef]
- Bulanda, K.; Oleksy, M.; Oliwa, R.; Budzik, G.; Przeszłowski, Ł.; Fal, J.; Jesionowski, T. Polymer Composites Based on Polycarbonate (PC) Applied to Additive Manufacturing Using Melted and Extruded Manufacturing (MEM) Technology. Polymers 2021, 13, 2455. [Google Scholar] [CrossRef]

| The Material Amount [g] | |||
|---|---|---|---|
| Filament Number | PC | PO | Talc |
| 1. | 700 | 5.0 | 17.5 |
| 2. | 700 | 5.0 | 7.0 |
| 3. | 700 | 5.0 | 3.5 |
| 4. | 700 | 2.5 | 7.0 |
| 5. | 700 | 2.5 | 3.5 |
| Sample Number | Commercial Model | Model Undryed | Model Dryed | |||
|---|---|---|---|---|---|---|
| ΔT drill 1 [°C] | ΔT drill 2 [°C] | ΔT drill 1 [°C] | ΔT drill 2 [°C] | ΔT drill 1 [°C] | ΔT drill 2 [°C] | |
| 1. | 1.0 | 4.2 | 1.4 | 5.0 | 1.4 | 3.1 |
| 2. | 1.4 | 3.3 | 1.8 | 5.1 | 1.7 | 3.8 |
| 3. | 0.7 | 9.0 | 6.0 | 4.0 | 2.9 | 4.0 |
| 4. | 1.6 | 14.6 | 3.1 | 4.0 | 1.8 | 3.9 |
| 5. | 1.2 | 7.7 | 1.7 | 3.8 | 1.5 | 3.9 |
| 6. | 0.4 | 6.4 | 1.5 | 4.2 | 1.8 | 4.3 |
| 7. | 0.6 | 6.8 | 1.5 | 5.1 | 2.9 | 3.4 |
| 8. | 0.8 | 6.4 | 1.9 | 4.4 | 2.5 | 3.7 |
| 9. | 1.3 | 7.9 | 2.9 | 3.4 | 2.2 | 3.9 |
| 10. | 1.7 | 5.1 | 2.5 | 3.7 | 3.0 | 4.1 |
| 11. | 2.4 | 7.0 | 3.8 | 5.1 | 3.6 | 5.0 |
| 12. | 2.4 | 16.5 | 3.6 | 5.2 | 3.4 | 7.6 |
| 13. | 6.6 | 13.5 | 3.4 | 7.6 | 3.7 | 5.2 |
| 14. | 6.3 | 15.1 | 2.9 | 4.5 | 5.9 | 5.0 |
| 15. | 6.1 | 14.4 | 4.4 | 14.1 | 4.4 | 14.1 |
| Average | 2.3 | 9.2 | 2.8 | 5.3 | 2.8 | 5.0 |
| SD | 2.1 | 4.1 | 1.2 | 2.6 | 1.2 | 2.7 |
| Temperature Increase during Cutting with a Drill | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Number | Commercial Model | Model 1 (700 g PC/5.0 g PO/17.5 g Talk) | Model 2 (700 g PC/5.0 g PO/7.0 g Talk) | Model 3 (700 g PC/5.0 g PO/3.5 g Talk) | Model 4 (700 g PC/2.5 g PO/7.0 g Talk) | Model 5 (700 g PC/2.5 g PO/3.5 g Talk) | ||||||
| ΔT drill 1 [°C] | ΔT drill 2 [°C] | ΔT drill 1 [°C] | ΔT drill 2 [°C] | ΔT drill 1 [°C] | ΔT drill 2 [°C] | ΔT drill 1 [°C] | ΔT drill 2 [°C] | ΔT drill 1 [°C] | ΔT drill 2 [°C] | ΔT drill 1 [°C] | ΔT drill 2 [°C] | |
| 1. | 1.0 | 4.2 | 0.3 | 1.9 | 0.9 | 2.7 | 1.4 | 3.1 | 0.8 | 2.6 | 1.2 | 1.8 |
| 2. | 1.4 | 3.3 | 1.0 | 3.3 | 1.5 | 4.3 | 1.7 | 3.8 | 1.1 | 3.3 | 2.2 | 5.1 |
| 3. | 0.7 | 9.0 | 1.8 | 3.9 | 1.0 | 7.4 | 2.9 | 4.0 | 1.3 | 3.1 | 2.1 | 6.6 |
| 4. | 1.6 | 14.6 | 1.6 | 4.2 | 1.6 | 4.9 | 1.8 | 3.9 | 1.5 | 3.9 | 2.1 | 4.0 |
| 5. | 1.2 | 7.7 | 1.6 | 5.2 | 4.7 | 8.2 | 1.5 | 3.9 | 1.5 | 3.0 | 1.9 | 4.8 |
| 6. | 0.4 | 6.4 | 3.1 | 6.9 | 4.6 | 8.5 | 1.8 | 4.3 | 0.1 | 2.6 | 3.9 | 5.0 |
| 7. | 0.6 | 6.8 | 3.4 | 3.6 | 4.9 | 6.9 | 2.9 | 3.4 | 1.7 | 3.0 | 1.8 | 4.9 |
| 8. | 0.8 | 6.4 | 3.3 | 7.2 | 5.2 | 6.3 | 2.5 | 3.7 | 1.4 | 3.1 | 5.8 | 7.4 |
| 9. | 1.3 | 7.9 | 3.2 | 5.2 | 5.1 | 10.9 | 2.2 | 3.9 | 1.6 | 3.6 | 3.4 | 5.4 |
| 10. | 1.7 | 5.1 | 2.9 | 5.4 | 5.3 | 8.7 | 3.0 | 4.1 | 1.0 | 3.3 | 4.0 | 5.1 |
| 11. | 2.4 | 7.0 | 3.5 | 3.9 | 4.4 | 9.0 | 3.6 | 5.0 | 2.4 | 3.5 | 4.0 | 5.1 |
| 12. | 2.4 | 16.5 | 1.0 | 4.6 | 4.0 | 8.8 | 3.4 | 7.6 | 2.4 | 3.7 | 2.7 | 4.1 |
| 13. | 6.6 | 13.5 | 3.0 | 6.2 | 5.9 | 5.6 | 3.7 | 5.2 | 1.6 | 3.9 | 3.6 | 5.1 |
| 14. | 6.3 | 15.1 | 3.6 | 4.4 | 4.4 | 8.3 | 5.9 | 5.0 | 1.9 | 3.8 | 4.6 | 5.6 |
| 15. | 6.1 | 14.4 | 3.1 | 4.8 | 5.1 | 8.1 | 4.4 | 14.1 | 2.6 | 3.7 | 3.8 | 5.5 |
| Average | 2.3 | 9.2 | 2.4 | 4.7 | 3.9 | 7.2 | 2.8 | 5.0 | 1.5 | 3.3 | 3.1 | 5.0 |
| SD | 2.1 | 4.2 | 1.1 | 1.3 | 1.7 | 2.1 | 1.2 | 2.6 | 0.6 | 0.4 | 1.2 | 1.2 |
| Implant Stability Quotient | |||||||
|---|---|---|---|---|---|---|---|
| Material Type | Commercial Model | Model 1 (700 g PC/5 g PO/17.5 g Talc) | Model 2 (700 g PC/5 g PO/7 g Talc) | Model 3 (700 g PC/5 g PO/3.5 g Talc) | Model 4 (700 g PC/2.5 g PO/7 g Talc) | Model 5 (700 g PC/2.5 g PO/3.5 g Talc) | Undried Model 3 (700 g PC/5 g PO/3.5 g Talc) |
| Vertical axis | 70 ± 3 | 64 ± 2 | 66 ± 4 | 67 ± 2 | 62 ± 3 | 60 ± 4 | 59 ± 2 |
| Horizontal axis | 71 ± 3 | 64 ± 2 | 65 ± 5 | 67 ± 2 | 62 ± 3 | 60 ± 4 | 59 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczyk, M.; Gabor, J.; Flak, T.; Wróbel, Z.; Swinarew, A.S. Surgery Training System Supported by Organic Materials. Materials 2022, 15, 4162. https://doi.org/10.3390/ma15124162
Błaszczyk M, Gabor J, Flak T, Wróbel Z, Swinarew AS. Surgery Training System Supported by Organic Materials. Materials. 2022; 15(12):4162. https://doi.org/10.3390/ma15124162
Chicago/Turabian StyleBłaszczyk, Magdalena, Jadwiga Gabor, Tomasz Flak, Zygmunt Wróbel, and Andrzej S. Swinarew. 2022. "Surgery Training System Supported by Organic Materials" Materials 15, no. 12: 4162. https://doi.org/10.3390/ma15124162
APA StyleBłaszczyk, M., Gabor, J., Flak, T., Wróbel, Z., & Swinarew, A. S. (2022). Surgery Training System Supported by Organic Materials. Materials, 15(12), 4162. https://doi.org/10.3390/ma15124162







