Influence of Post-Bond Heat Treatment on Microstructure and Creep Behavior of the Brazed Single-Crystal Nickel Superalloy
Abstract
:1. Introduction
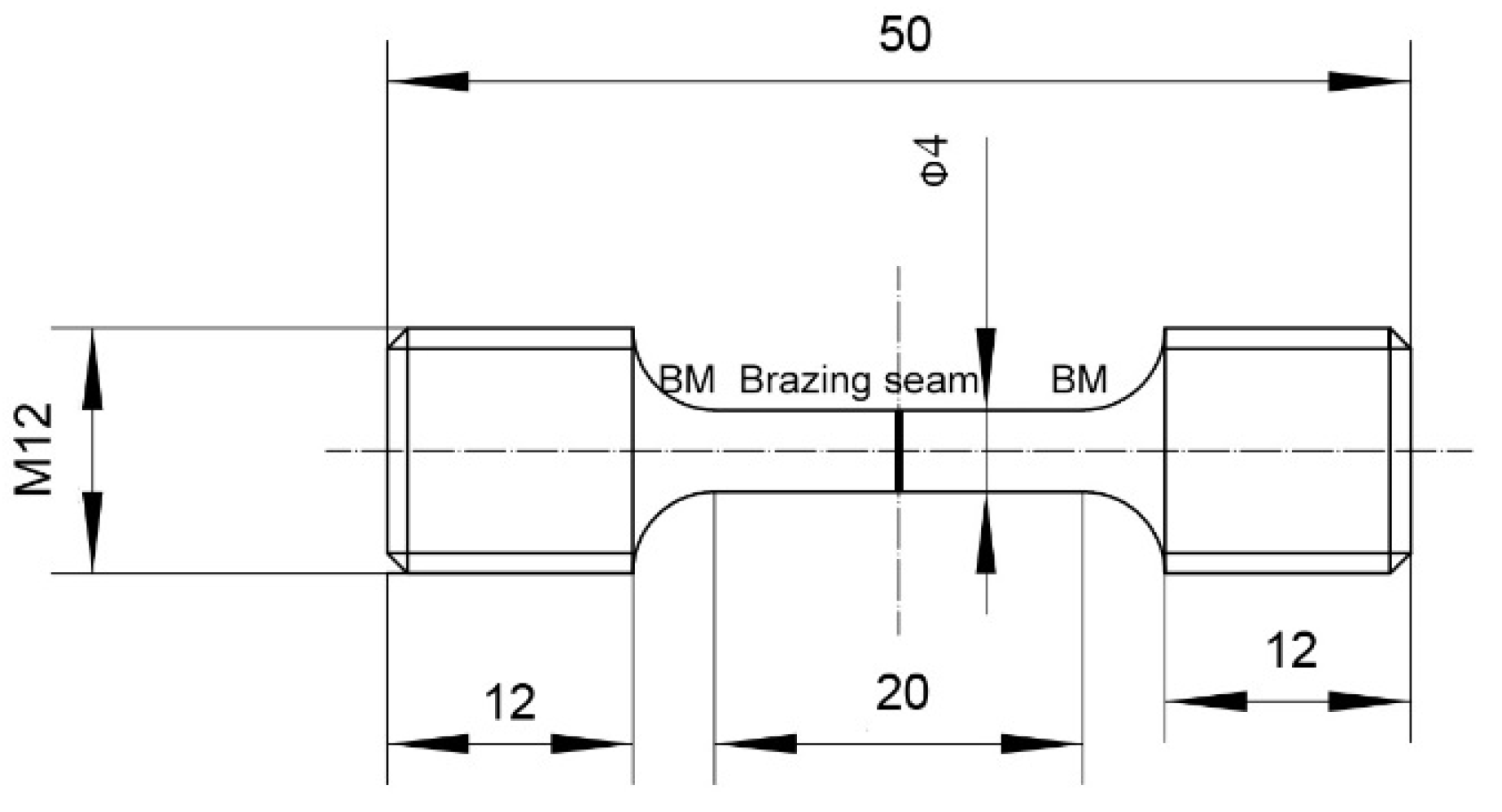
2. Experimental Procedure
3. Results and Discussion
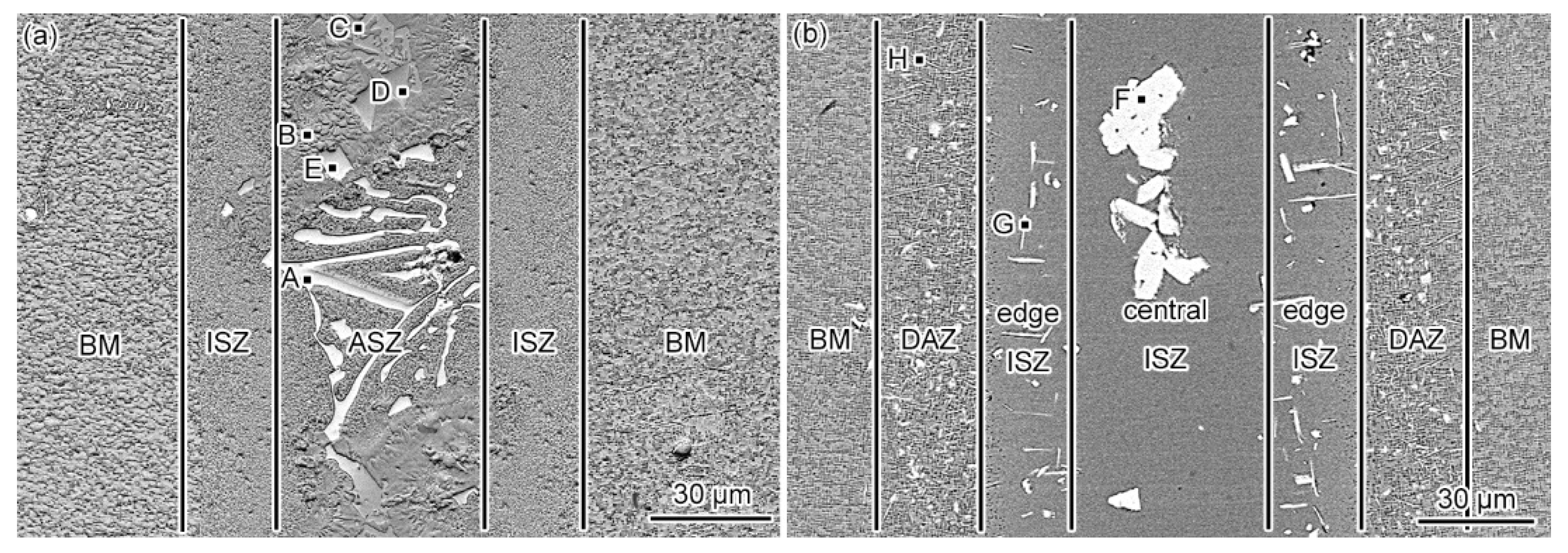
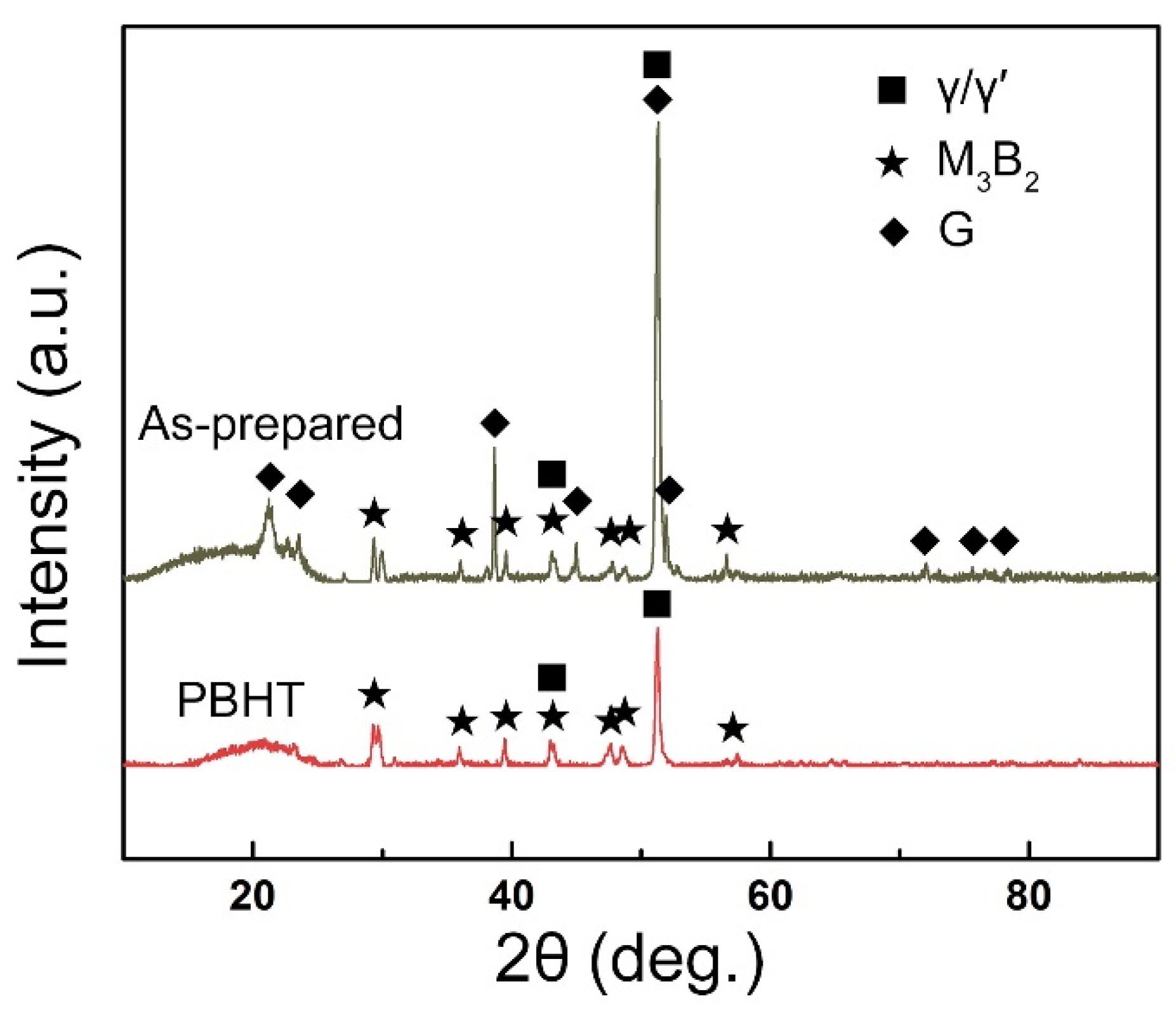
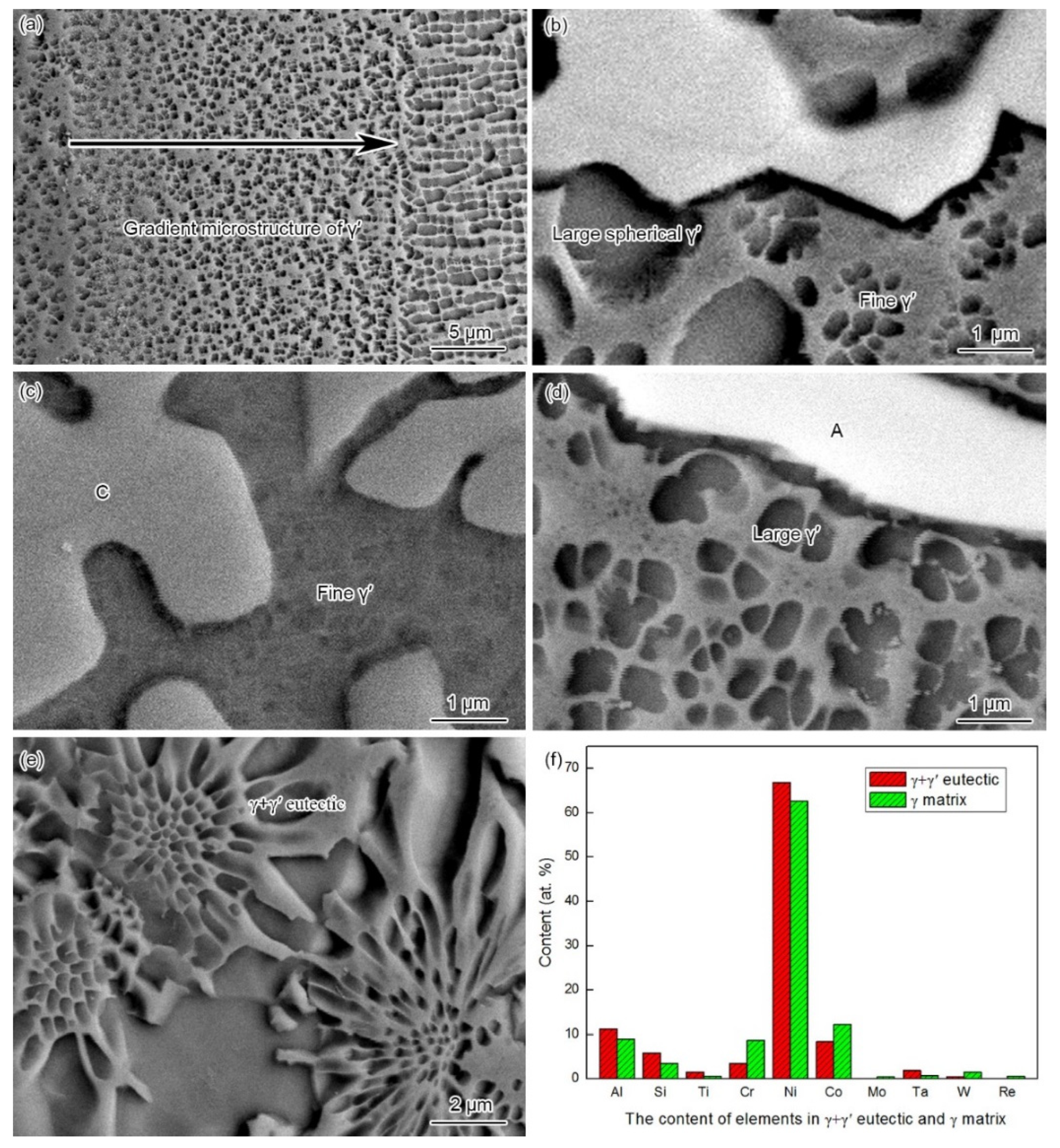
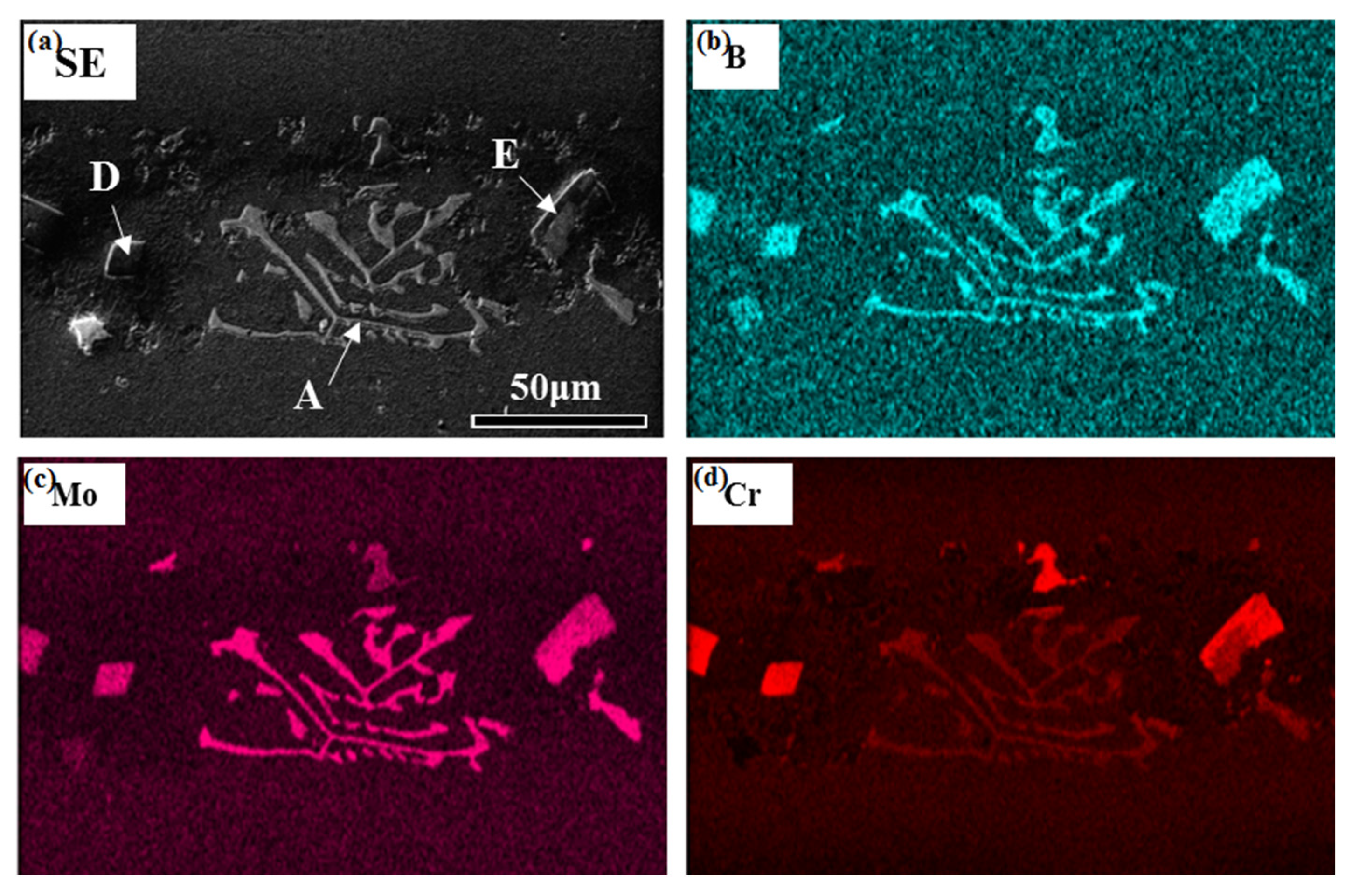
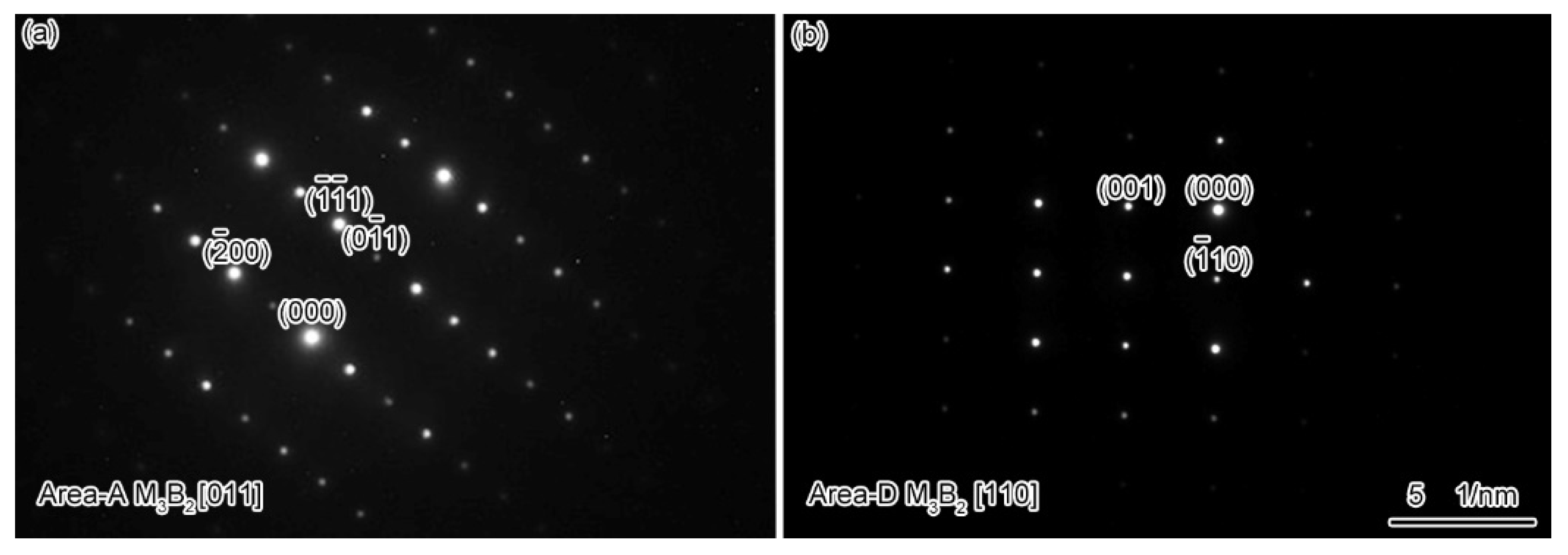
3.1. Microstructure of the As-Prepared Joint
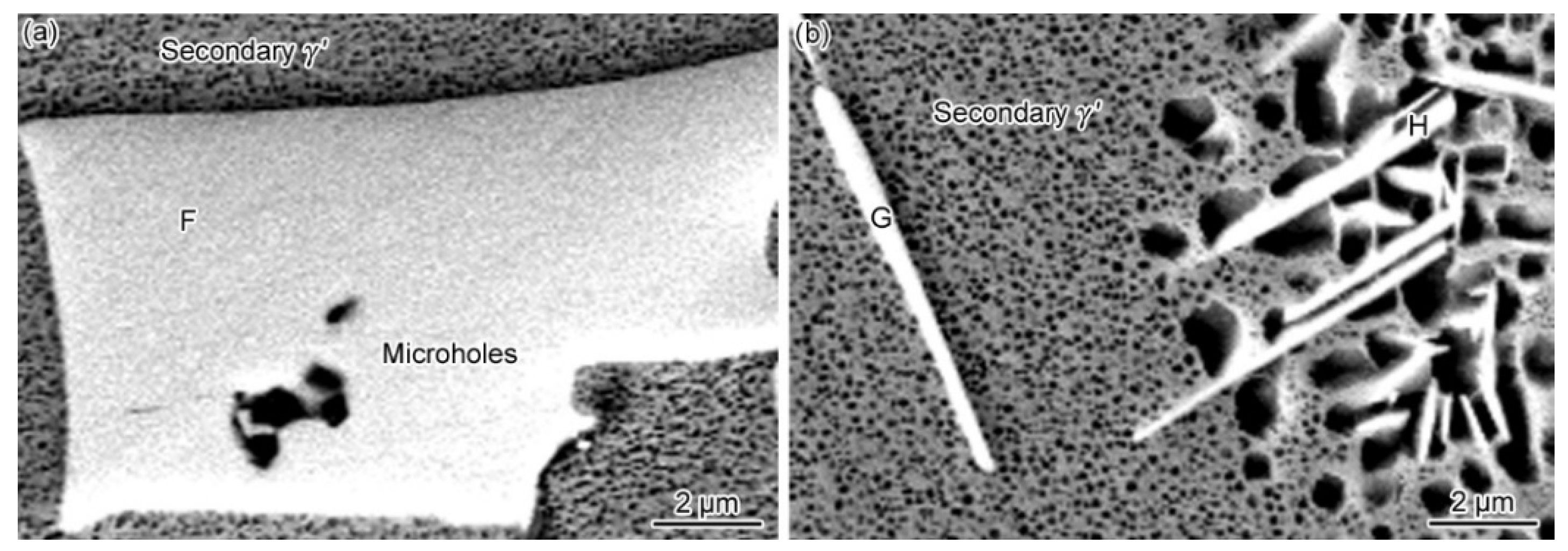
3.2. Microstructural Evolution of the PBHT-Processed Joint
3.3. Creep Properties and Fracture Mechanism of Brazed Joints
4. Conclusions
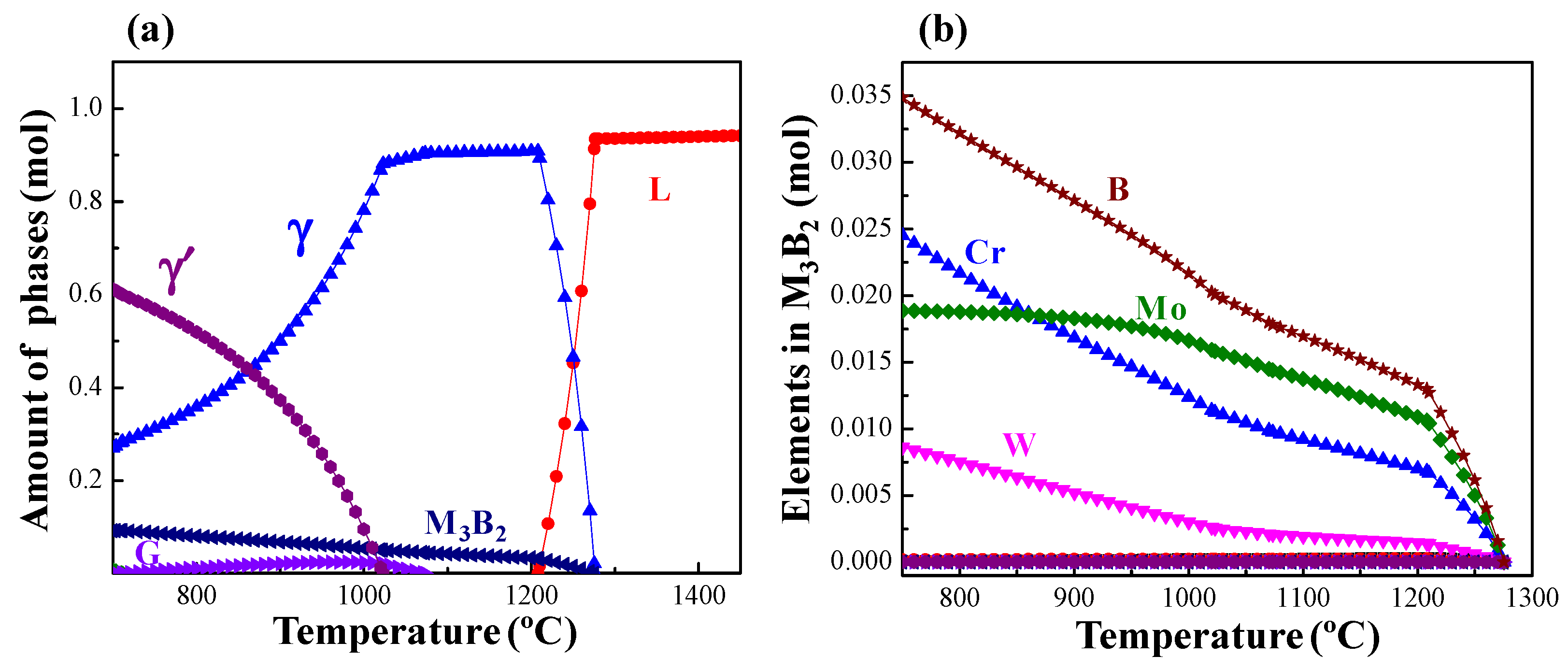
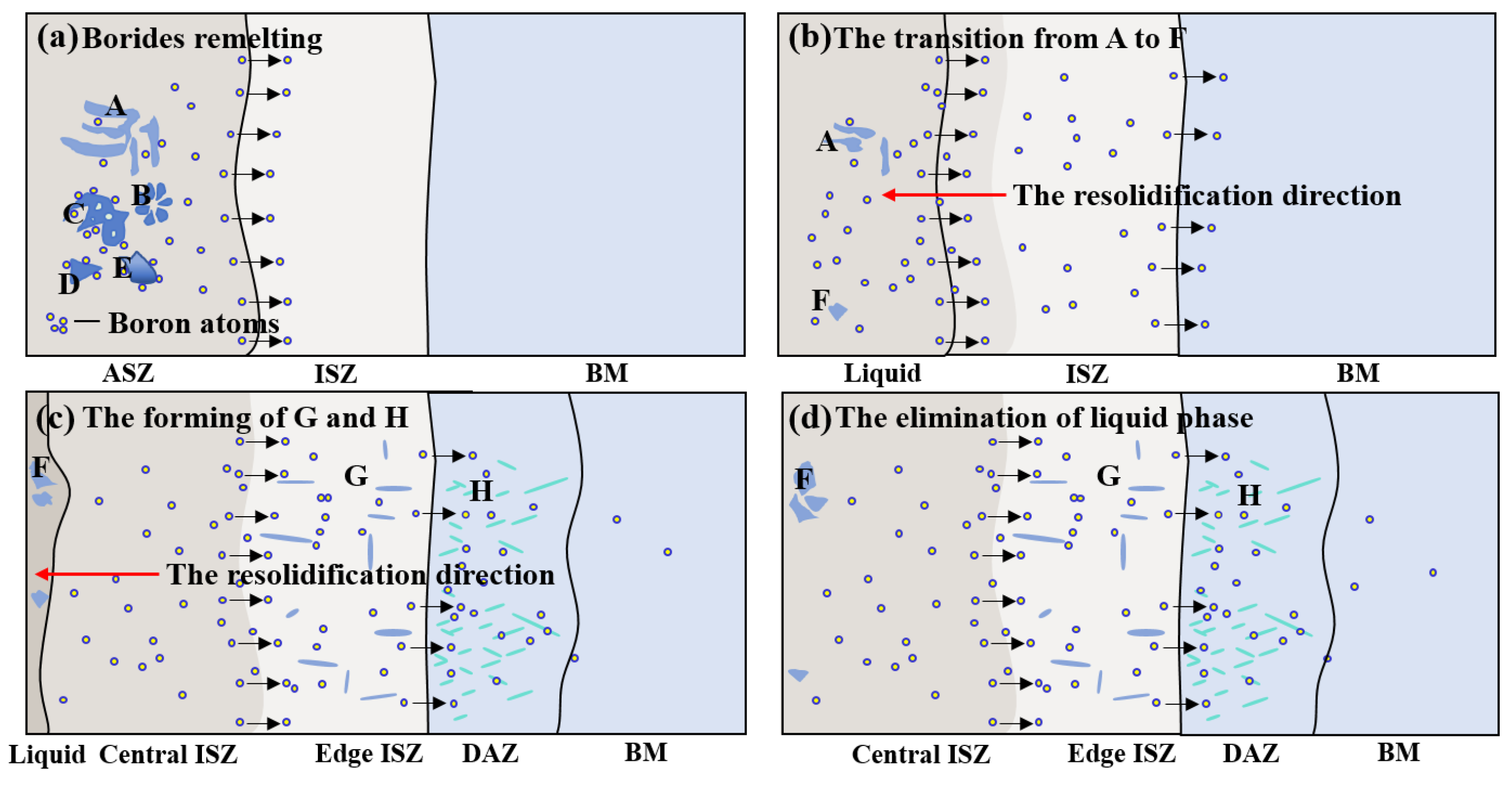
- The microstructure of the as-prepared joint, brazed at 1230 °C for 30 min, consisted of ISZ and ASZ. In the ISZ, the size and volume fraction of γ′ precipitates increased with the decreased of distance from the BM. The ASZ was mainly composed of γ matrix, skeleton-like M3B2 phase, γ + γ′ eutectic, reticular G phase, block-shaped M3B2, block-like M3B2, and precipitated γ′ phase. The size of the γ′ precipitates close to the skeleton-like M3B2 phase was obviously higher than the γ′ precipitates near the reticular G phase.
- After PBHT, the joint was composed of γ matrix and block-shaped M3B2 phase, and the low-melting-point eutectics were removed. The central ISZ was formed by the secondary isothermal solidification of the re-dissolved ASZ of the as-prepared joint. The edge ISZ, which contained lots of lath-like M3B2, was inherited from the original ISZ of the as-prepared joint. The interdiffusion of elements resulted in a fuzzy interface between the central ISZ and edge ISZ. Moreover, the size of γ′ precipitates in both ISZs was extremely finer than that in the as-prepared joint. The newly formed DAZ was located on the BM adjacent to the edge ISZ, where numerous elliptic flake-like M3B2 precipitates gradually sparsed with the increase of distance from the ISZ/DAZ interface.
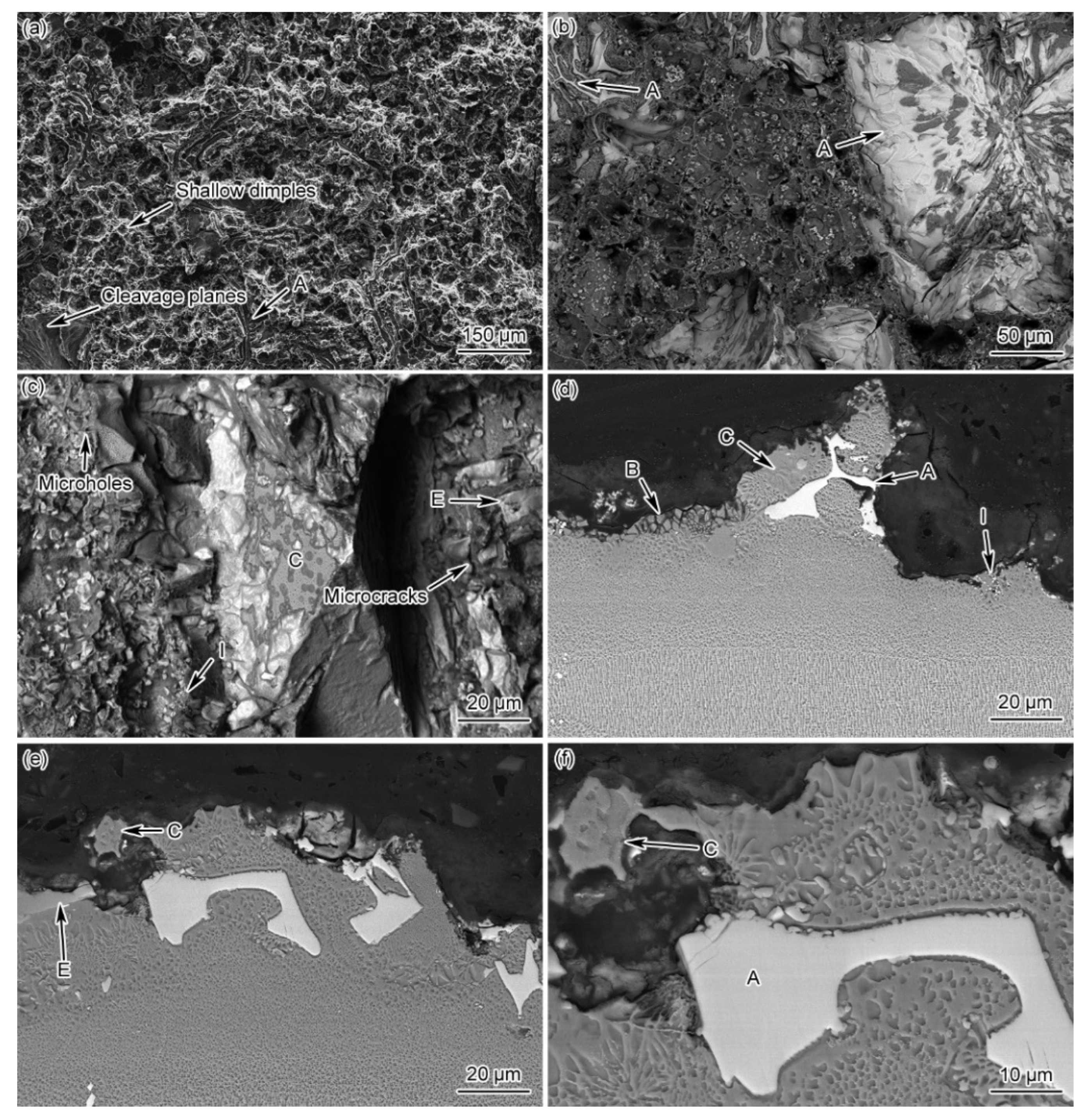
- The creep life of the as-prepared joint can be improved by 23% after PBHT. During the creep process, the cracks of as-prepared joint were mainly initiated and propagated along the γ + γ′ eutectic, as well as the skeleton-like M3B2 phase and newly-formed net-like distributed boride particles at grain boundaries, showing a mixed fracture mode, i.e., ductile and cleavage fracture. In the case of the PBHT-processed joint, the cracks were mainly nucleated from the M3B2 phase and fine boride particles, resulting in a ductile-dominant mixed fracture.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2008; ISBN 9780521070119. [Google Scholar]
- Dong, H.G.; Yang, J.; Xi, Y.Q.; Xu, X.X.; Li, P.; Dong, C.; Chen, J.Y.; Liu, N.; Zheng, L. Effect of Cr content in Ni-based amorphous filler on microstructure and shear strength of K4169 nickel-based alloy brazed joint. J. Mater. Process. Technol. 2021, 290, 116975. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.S.; Wang, L.; Li, Z.J.; Zhou, X.T.; Lou, L.H.; Zhang, J. Effect of long-term thermal exposure on microstructure and stress rupture properties of GH3535 superalloy. J. Mater. Sci. Technol. 2015, 31, 269–279. [Google Scholar] [CrossRef]
- Yue, X.; Liu, F.M.; Li, Q.; Qin, H.B.; Gao, H.T.; Li, L.K.; Yi, Y.X. Effect of post-bond heat treatment on microstructure and mechanical properties of the wide gap TLP bonded IC10 superalloy with a low boron Ni3Al-based interlayer. J. Manuf. Process. 2020, 54, 109–119. [Google Scholar] [CrossRef]
- Kong, Q.J.; Qu, S.; Shao, T.W.; Li, W.X. Application & development of brazing and diffusion welding technology in aeroengine manufacturing. Aeron. Manuf. Technol. 2010, 24, 82–84. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Liu, L.R.; Jin, T.; Sun, X.F. Development of the Ni-base single crystal superalloys. Aeroengine 2005, 31, 1–7. [Google Scholar]
- Peng, Y.; Li, J.L.; Peng, X.; Li, S.W.; Xiong, J.T.; Shi, J.M. Interfacial microstructure evolution and formation process of the joints prepared by diffusion bonding on DD6 nickel-based single crystal superalloy. J. Mater. Res. Technol. 2020, 9, 16317–16328. [Google Scholar] [CrossRef]
- Shi, D.Q.; Dong, C.L.; Yang, X.G.; Sun, Y.T.; Wang, J.K.; Liu, J.L. Creep and fatigue lifetime analysis of directionally solidified superalloy and its brazed joints based on continuum damage mechanics at elevated temperature. Mater. Des. 2013, 45, 643–652. [Google Scholar] [CrossRef]
- Dong, H.G.; Xia, Y.Q.; Xu, X.X.; Naz, G.J.; Hao, X.H.; Li, P.; Zhou, J.; Dong, C. Performance of GH4169 brazed joint using a new designed nickel-based filler metal via cluster-plus-glue-atom model. J. Mater. Sci. Technol. 2020, 39, 89–98. [Google Scholar] [CrossRef]
- Chamanfar, A.; Jahazi, M.; Cormier, J. A Review on Inertia and Linear Friction Welding of Ni-Based Superalloys. Metall. Mater. Trans. A 2015, 46, 1639–1669. [Google Scholar] [CrossRef]
- Cai, X.L.; Sun, D.Q.; Li, H.M.; Meng, C.; Wang, L.; Shen, C.J. Dissimilar joining of TiAl alloy and Ni-based superalloy by laser welding technology using V/Cu composite interlayer. Opt. Laser Technol. 2019, 111, 205–213. [Google Scholar] [CrossRef]
- Jing, Y.H.; Zheng, Z.; Liu, E.; Guo, Y. Microstructural evolution of a Ni-base alloy DZ468 joint bonded with a New Co-base filler. J. Mater. Sci. Technol. 2014, 30, 480–486. [Google Scholar] [CrossRef]
- Montazeri, M.; Ghaini, F.M. The liquation cracking behavior of IN738LC superalloy during low power Nd: Y AG pulsed laser welding. Mater. Charact. 2012, 67, 65–73. [Google Scholar] [CrossRef]
- Han, K.; Wang, H.Q.; Peng, F.; Zhang, B.G.; Shen, L. Investigation of microstructure and mechanical performance in IN738LC joint by vacuum electron beam welding. Vacuum 2019, 162, 214–227. [Google Scholar] [CrossRef]
- Ojo, O.A. Intergranular liquation cracking in heat affected zone of a welded nickel based superalloy in as cast condition. Mater. Sci. Technol. 2013, 23, 1149–1155. [Google Scholar] [CrossRef]
- Wen, M.Y.; Sun, Y.; Yu, J.J.; Yang, S.L.; Hou, X.Y.; Yang, Y.H.; Sun, X.F.; Zhou, Y.Z. Amelioration of weld-crack resistance of the M951 superalloy by engineering grain boundaries. J. Mater. Sci. Technol. 2021, 78, 260–267. [Google Scholar] [CrossRef]
- Zhang, X.J.; Guo, S.Q.; Zhang, W.Y. Aviation Arc Welding Technology; Aviation Industry Press: Beijing, China, 2014; ISBN 978-7-5165-0369-0. [Google Scholar]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Diffusion brazing metallurgy of IN718/Ni-Cr-Si-B-Fe/IN718. Weld. J. 2014, 93, 60–68. [Google Scholar]
- Bridges, D.; Xu, R.; Hu, A. Microstructure and mechanical properties of Ni nano-particle-bonded Inconel 718. Mater. Des. 2016, 174, 107784. [Google Scholar] [CrossRef]
- Ruiz-Vargas, J.; Siredey-Schwaller, N.; Gey, N.; Bocher, P.; Hazotte, A. Microstructure development during isothermal brazing of Ni/BNi-2 couples. J. Mater. Process. Technol. 2013, 213, 20–29. [Google Scholar] [CrossRef]
- Xiong, H.P. Aeronautical and Aerospace Brazing & Diffusion Bonding Technologies; Aviation Industry Press: Beijing, China, 2021; ISBN 978-7-5165-2500-5. [Google Scholar]
- Liu, D.; Song, Y.Y.; Shi, B.; Zhang, Q.; Song, X.G.; Niu, H.W.; Feng, J.C. Vacuum brazing of GH99 superalloy using graphene reinforced BNi-2 composite filler. J. Mater. Sci. Technol. 2018, 34, 1843–1850. [Google Scholar] [CrossRef]
- Ma, C.W.; Shi, K.; Yu, Z.S.; Xu, P.Q. Correlation between brazed joint elevated strength, microstructure and brazing processing parameter of Inconel superalloy. Acta Metall. Sin. (Engl. Lett.) 2011, 24, 205–212. [Google Scholar]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Microstructure evolution mechanism during post-bond heat treatment of transient liquid phase bonded wrought IN718 superalloy: An approach to fabricate boride-free joints. J. Alloys Compd. 2017, 723, 84–91. [Google Scholar] [CrossRef]
- Wang, G.L.; Sun, Y.; Wang, X.G.; Liu, J.D.; Liu, J.L.; Li, J.G.; Yu, J.J.; Zhou, Y.Z.; Jin, T.; Sun, X.D.; et al. Microstructure evolution and mechanical behavior of Ni-based single crystal superalloy joint brazed with mixed powder at elevated temperature. J. Mater. Sci. 2017, 33, 1219–1226. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Hou, X.Y.; Zhang, H.Y.; Zhou, Y.Z.; Ding, Y.T. Effect of Orientation Deviation on Microstructure and Mechanical Properties of Nickel-Based Single Crystal Superalloy Brazing Joints. Acta Metall. Sin. 2020, 56, 171–181. [Google Scholar] [CrossRef]
- Hinchy, E.P.; Barron, D.; Pomeroy, M.J.; Tanner, D.A. Diffusion braze homogenisation and contraction during re-repair heat treatments of a single crystal nickel-based superalloy. J. Alloys Compd. 2021, 857, 157560. [Google Scholar] [CrossRef]
- Amiri, D.; Sajjadi, S.A.; Kamyabi-Gol, A. Pre- and post-TLP bond solution treatments: Effects on the microstructure and mechanical properties of GTD-111 superalloy. J. Manuf. Process. 2020, 57, 36–47. [Google Scholar] [CrossRef]
- Wang, S.Y.; Sun, Y.; Hou, X.Y.; Cui, C.Y.; Sun, X.F.; Zhou, Y.Z. Investigation on microstructure and mechanical properties of a vacuum brazed joint of γ′-strengthened co-based single crystal superalloy before and after the post-bond heat treatment. Vacuum 2020, 177, 109413. [Google Scholar] [CrossRef]
- Liu, M.C.; Sheng, G.M.; He, H.J.; Jiao, Y.J. Microstructural evolution and mechanical properties of TLP bonded joints of Mar-M247 superalloys with Ni-Cr-Co-W-Ta-B interlayer. J. Mater. Process. Technol. 2017, 246, 245–251. [Google Scholar] [CrossRef]
- Arhami, F.; Mirsalehi, S.E.; Sadeghian, A. Effect of bonding time on microstructure and mechanical properties of diffusion brazed IN-939. J. Mater. Process. Technol. 2019, 265, 219–229. [Google Scholar] [CrossRef]
- Yuan, L.; Xiong, J.T.; Du, Y.J.; Ren, J.; Shi, J.M.; Li, J.L. Microstructure and mechanical properties in the TLP joint of FeCoNiTiAl and Inconel 718 alloys using BNi2 filler. J. Mater. Process. Technol. 2021, 61, 176–185. [Google Scholar] [CrossRef]
- Li, S.W.; Li, J.L.; Shi, J.M.; Peng, Y.; Peng, X.; Sun, X.J.; Jin, F.; Xiong, J.T.; Zhang, F.S. Microstructure and mechanical properties of transient liquid phase bonding DD5 single-crystal superalloy to CrCoNi-based medium-entropy alloy. J. Mater. Sci. Technol. 2022, 96, 140–150. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. TLP bonding of cast IN718 nickel based superalloy: Process-microstructure-strength characteristics. Mater. Sci. Eng. A 2013, 568, 76–82. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.; Li, C.F.; Yang, G.; Ren, Y.H.; Qin, L.Y. Effect of post heat treatment on microstructure and mechanical properties of Ti-6Al-4V jointing parts proceeded by laser additive manufacturing. Mater. Sci. Eng. A 2020, 788, 139544. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Solidification and solid state phenomena during TLP bonding of IN718 superalloy using Ni-Si-B ternary filler alloy. J. Alloys Compd. 2013, 563, 143–149. [Google Scholar] [CrossRef]
- Sheng, N.C.; Liu, J.D.; Jin, T.; Sun, X.F.; Hu, Z.Q. Isothermal solidification stage during transient liquid-phase bonding single-crystal superalloys. Philos. Mag. 2014, 94, 1219–1234. [Google Scholar] [CrossRef]
- Guo, J.T. Material Science and Engineering for Superalloys; Science Press: Beijing, China, 2010; ISBN 9787030275554. [Google Scholar]
- Wang, S.Y.; Sun, Y.; Cui, C.Y.; Sun, X.F.; Zhou, Y.Z.; Ma, Y.M.; An, H.L. Effect of post-bond heat treatment on the microstructure and high temperature mechanical property of a TLP bonded γ′-strengthened co-based single crystal superalloy. J. Mater. Sci. 2021, 80, 244–258. [Google Scholar] [CrossRef]



| Material | Cr | Co | Mo | W | Re | Al | Ti | Ta | Hf | C | Si | B | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Superalloy | 3.3–4.5 | 12–15 | 0.5–0.75 | 2–2.6 | 1–2 | 12–15 | - | 2–3 | 0.02–0.05 | 0.05–0.15 | - | - | Bal. |
| Filler metal | 12–15 | 9–11 | 1–2 | 1–2 | - | 2–4 | 1–2 | - | - | - | 5–8 | 6–9 | Bal. |
| ISZ | Area A | Area B | Area C | Area D | Dark Area of Area E | Bright Area of Area E | γ Matrix Around Area A | γ Matrix Around Area C | Area F | Area G | Needle-like Phase H | Flaky Phase H | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Joint state | As-bonded | As-bonded | As-bonded | As-bonded | As-bonded | As-bonded | As-bonded | As-bonded | As-bonded | Heat treated | Heat treated | Heat treated | Heat treated |
| B | 0.00 | 39.45 | 0.00 | 13.12 | 38.81 | 40.55 | 40.62 | 0.00 | 0.00 | 35.23 | 30.47 | 19.11 | 14.58 |
| Al | 8.61 | 0.63 | 11.21 | 0.92 | 0.12 | 0.11 | 0.11 | 9.00 | 0.45 | 0.40 | 1.21 | 3.71 | 3.12 |
| Si | 2.47 | 0.00 | 5.85 | 1.21 | 0.70 | 0.22 | 0.65 | 3.51 | 13.84 | 0.14 | 0.46 | 1.15 | 1.25 |
| Ti | 0.57 | 0.64 | 1.57 | 3.54 | 0.43 | 0.48 | 0.65 | 0.64 | 0.16 | 0.91 | 0.62 | 0.39 | 0.59 |
| Cr | 9.46 | 18.91 | 3.54 | 5.39 | 38.53 | 31.68 | 19.04 | 8.68 | 2.24 | 7.15 | 7.12 | 3.54 | 3.88 |
| Co | 12.85 | 4.19 | 8.4 | 18.05 | 5.71 | 4.38 | 4.02 | 12.20 | 9.16 | 9.54 | 10.15 | 12.51 | 12.07 |
| Ni | 61.56 | 10.61 | 66.86 | 54.44 | 6.45 | 6.53 | 7.80 | 62.69 | 73.86 | 15.32 | 28.29 | 47.78 | 51.14 |
| Mo | 0.78 | 7.58 | 0.14 | 0.13 | 3.85 | 6.54 | 8.71 | 0.46 | 0.05 | 6.85 | 3.80 | 1.21 | 1.51 |
| Hf | 0.00 | 0.05 | 0.00 | 0.25 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ta | 0.68 | 2.47 | 1.86 | 2.72 | 0.87 | 1.46 | 2.82 | 0.79 | 0.10 | 5.89 | 2.84 | 3.28 | 4.17 |
| W | 2.27 | 13.49 | 0.51 | 0.23 | 1.98 | 4.91 | 13.46 | 1.48 | 0.14 | 16.20 | 13.59 | 5.25 | 5.82 |
| Re | 0.75 | 1.98 | 0.06 | 0.00 | 2.55 | 3.10 | 2.12 | 0.55 | 0.00 | 2.37 | 1.45 | 2.07 | 1.87 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0.00 | 100 | 100 | 100 | 100 |
| at.% | Phase I in the As-Bonded Joint | Phase F in the PBHT Joint | Phase J in the PBHT Joint | Phase K in the PBHT Joint |
|---|---|---|---|---|
| O | 15.32 | 38.51 | 37.29 | 53.22 |
| Al | 7.17 | 1.56 | 2.02 | 0.00 |
| Ti | 0.00 | 1.17 | 1.24 | 0.74 |
| Cr | 32.4 | 10.14 | 21.12 | 26.08 |
| Co | 11.67 | 6.93 | 4.90 | 2.71 |
| Ni | 33.44 | 24.10 | 16.95 | 9.28 |
| Mo | 0.00 | 1.14 | 3.14 | 3.07 |
| Ta | 0.00 | 2.35 | 2.05 | 0.00 |
| W | 0.00 | 14.10 | 11.29 | 4.90 |
| Total | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Wang, S.; Qiu, K.; Sun, Y.; Yang, Y.; Zhou, Y. Influence of Post-Bond Heat Treatment on Microstructure and Creep Behavior of the Brazed Single-Crystal Nickel Superalloy. Materials 2022, 15, 4053. https://doi.org/10.3390/ma15124053
Hou X, Wang S, Qiu K, Sun Y, Yang Y, Zhou Y. Influence of Post-Bond Heat Treatment on Microstructure and Creep Behavior of the Brazed Single-Crystal Nickel Superalloy. Materials. 2022; 15(12):4053. https://doi.org/10.3390/ma15124053
Chicago/Turabian StyleHou, Xingyu, Shiyang Wang, Keqiang Qiu, Yuan Sun, Yanhong Yang, and Yizhou Zhou. 2022. "Influence of Post-Bond Heat Treatment on Microstructure and Creep Behavior of the Brazed Single-Crystal Nickel Superalloy" Materials 15, no. 12: 4053. https://doi.org/10.3390/ma15124053
APA StyleHou, X., Wang, S., Qiu, K., Sun, Y., Yang, Y., & Zhou, Y. (2022). Influence of Post-Bond Heat Treatment on Microstructure and Creep Behavior of the Brazed Single-Crystal Nickel Superalloy. Materials, 15(12), 4053. https://doi.org/10.3390/ma15124053





