Surface and Structural Studies of Age-Related Changes in Dental Enamel: An Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
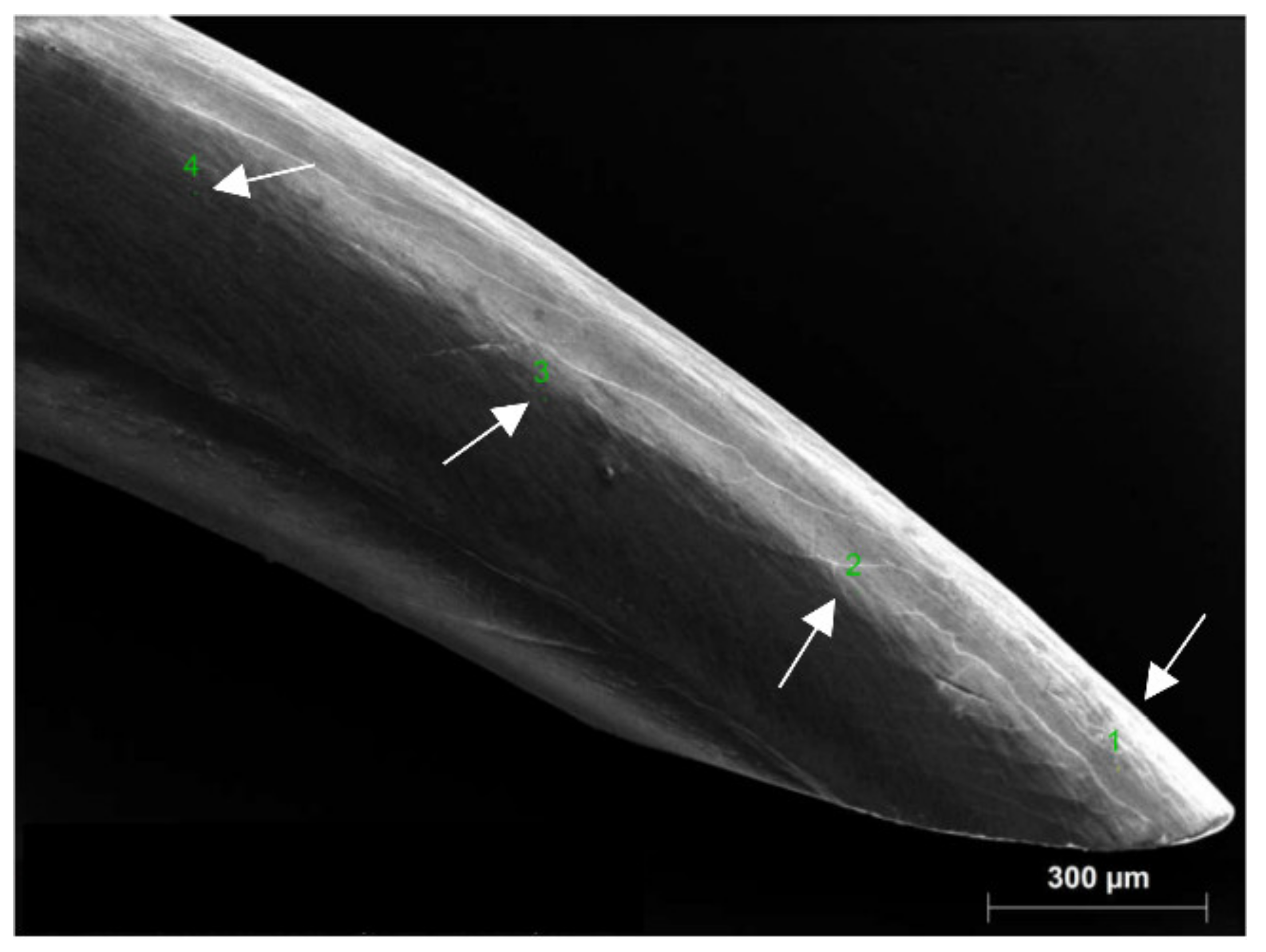
2.2. SEM Imaging and EDS Measurements
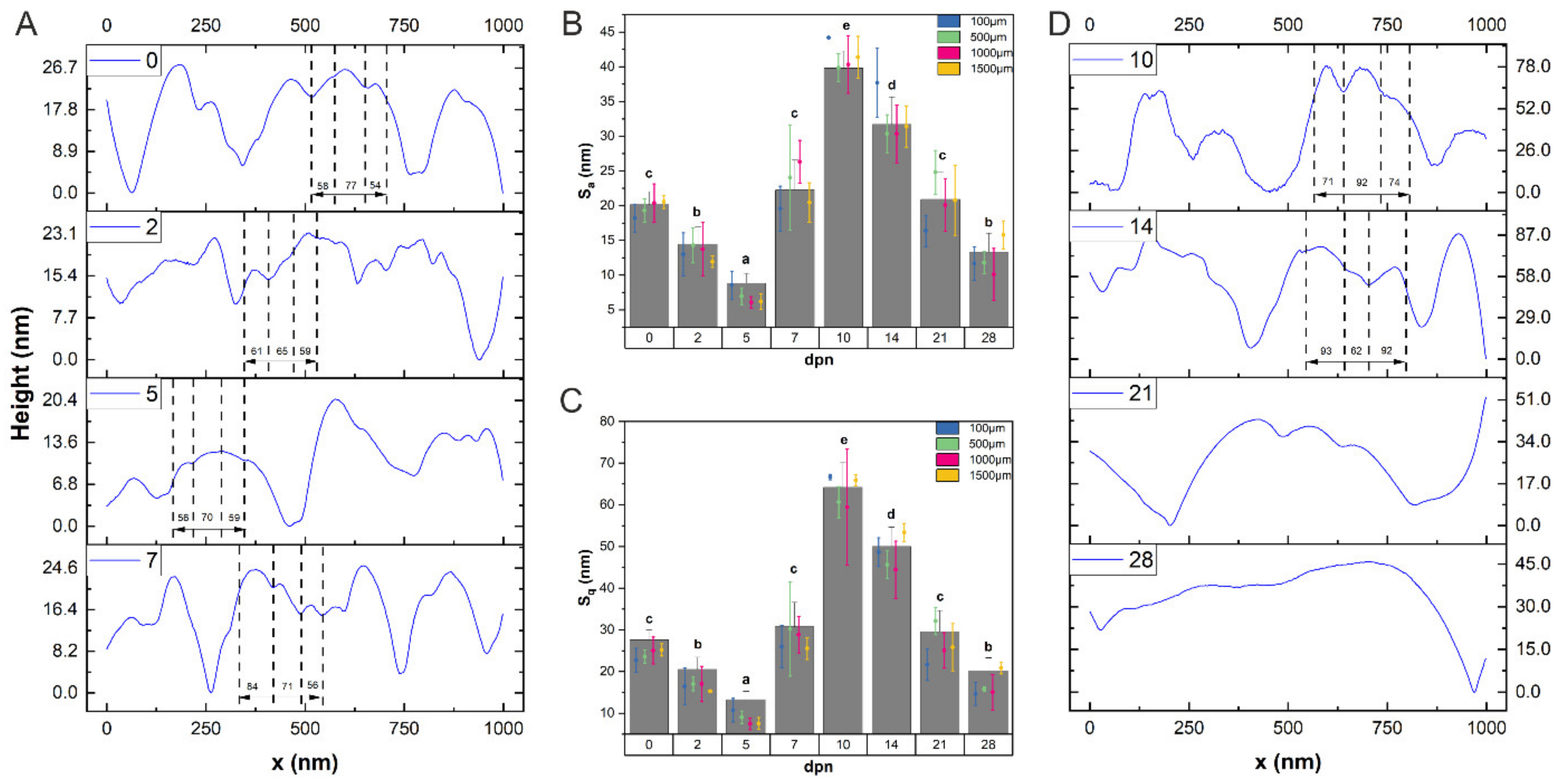
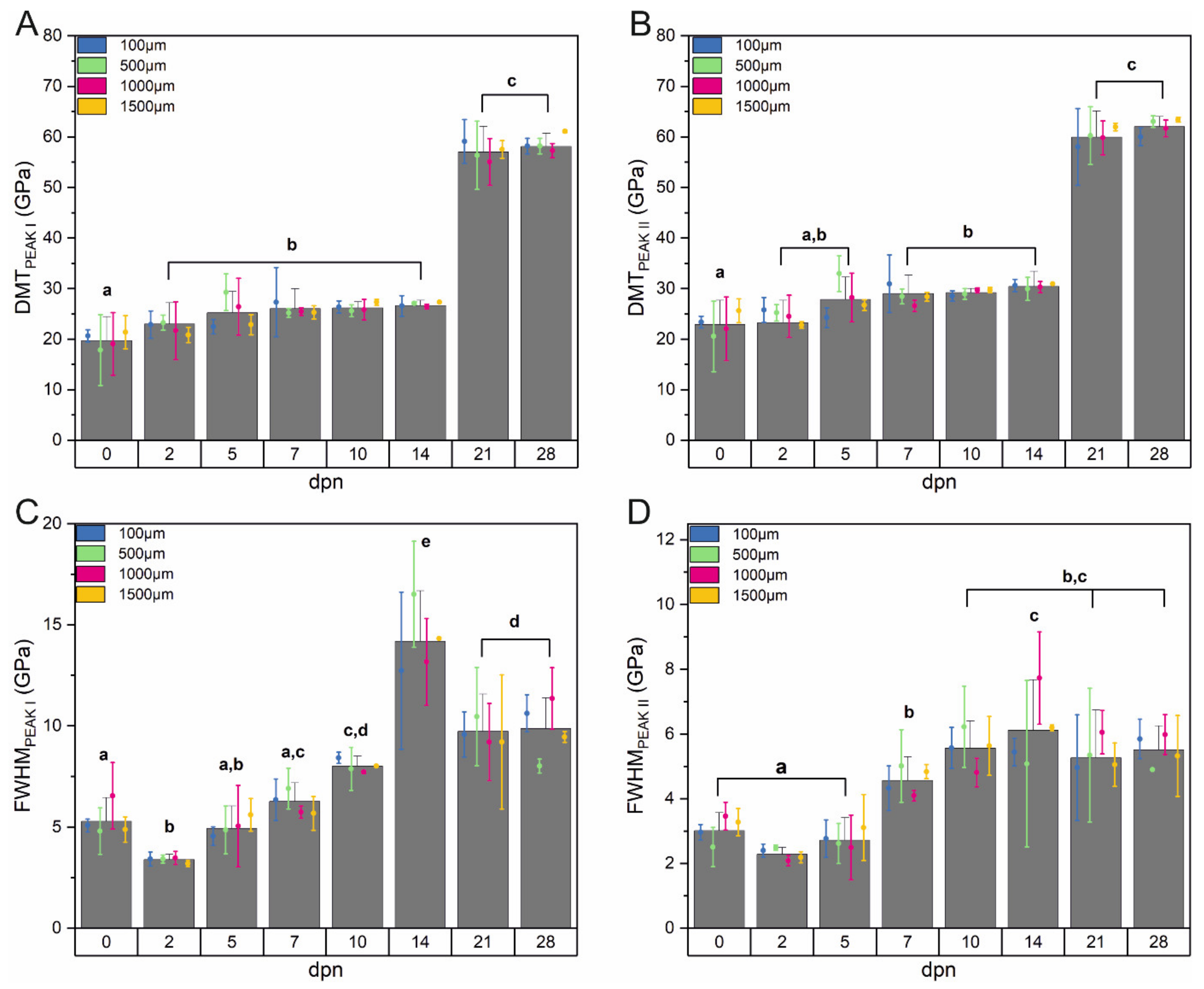
2.3. AFM Measurements and Data Analysis
2.4. X-ray Diffraction
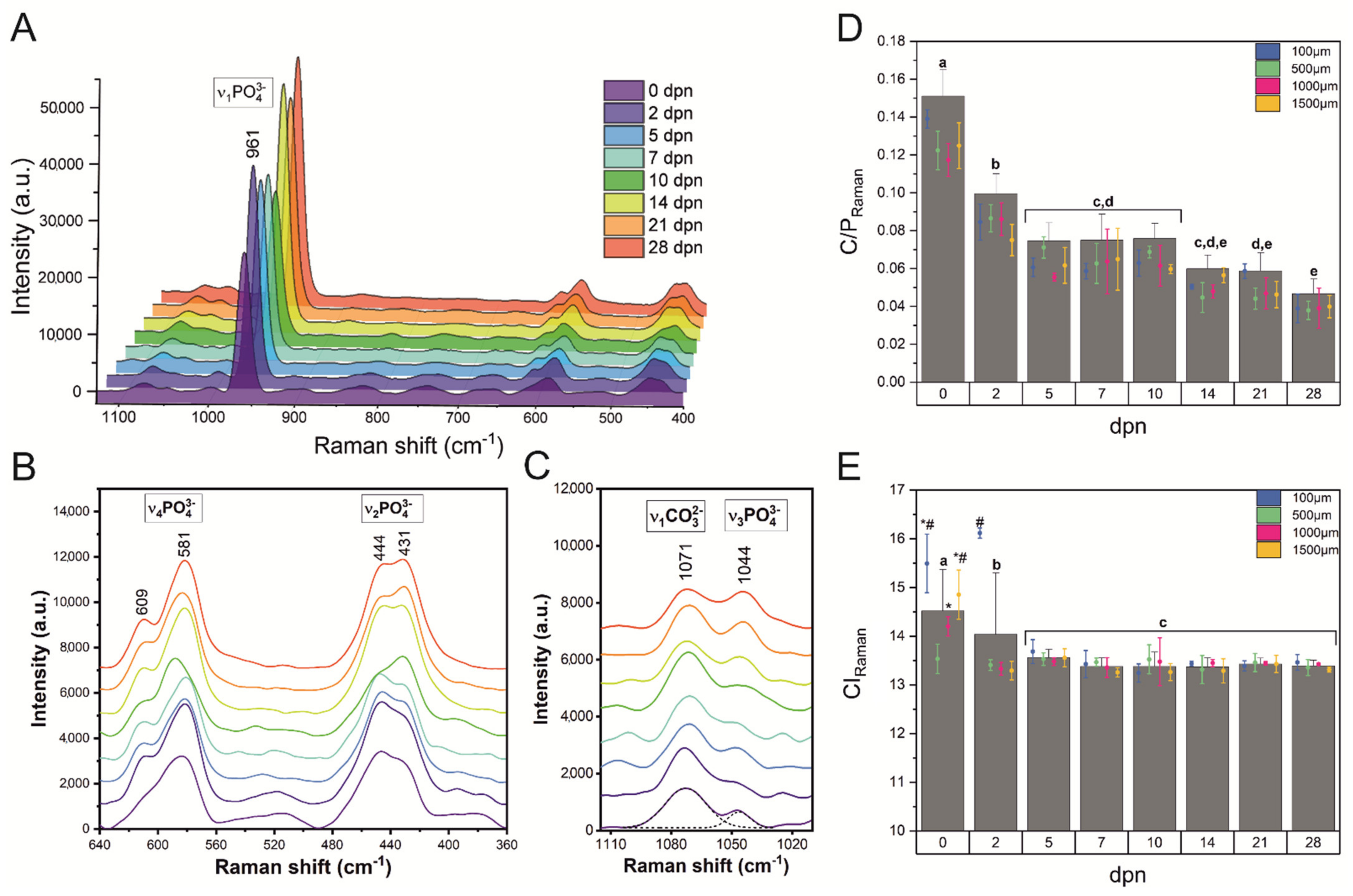
2.5. Raman Spectroscopy
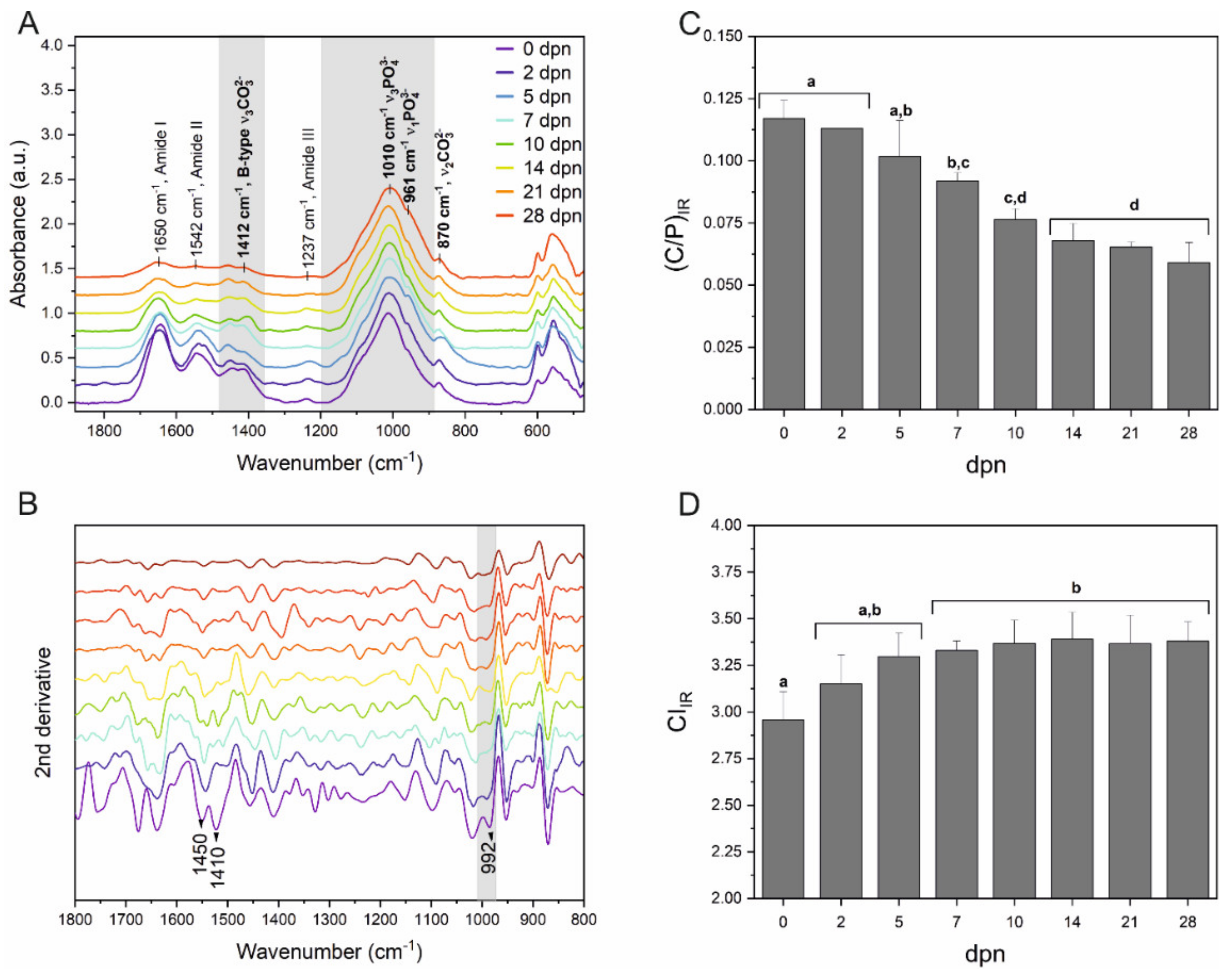
2.6. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.7. Data Structure and Analysis
2.8. Predictive Modelling
3. Results
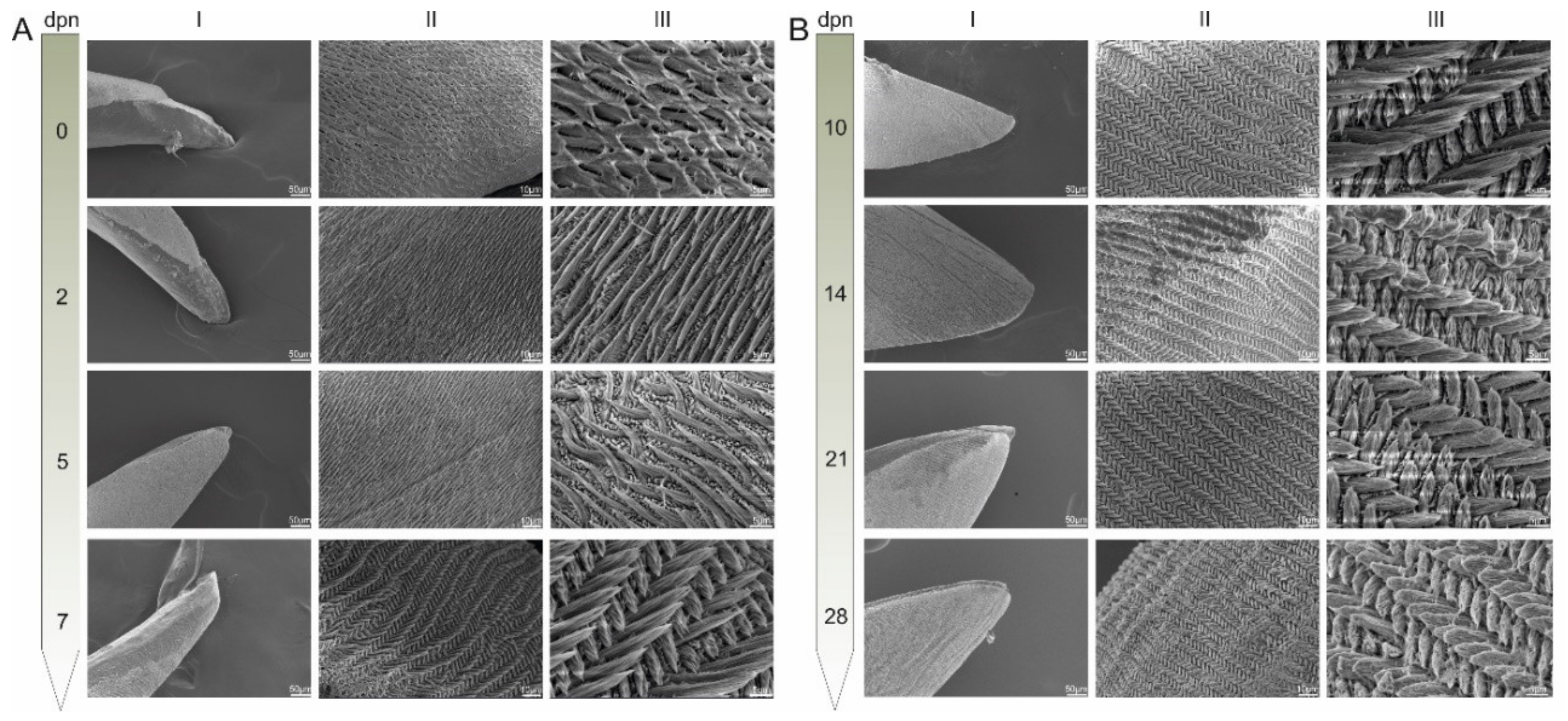
3.1. Surface Structure
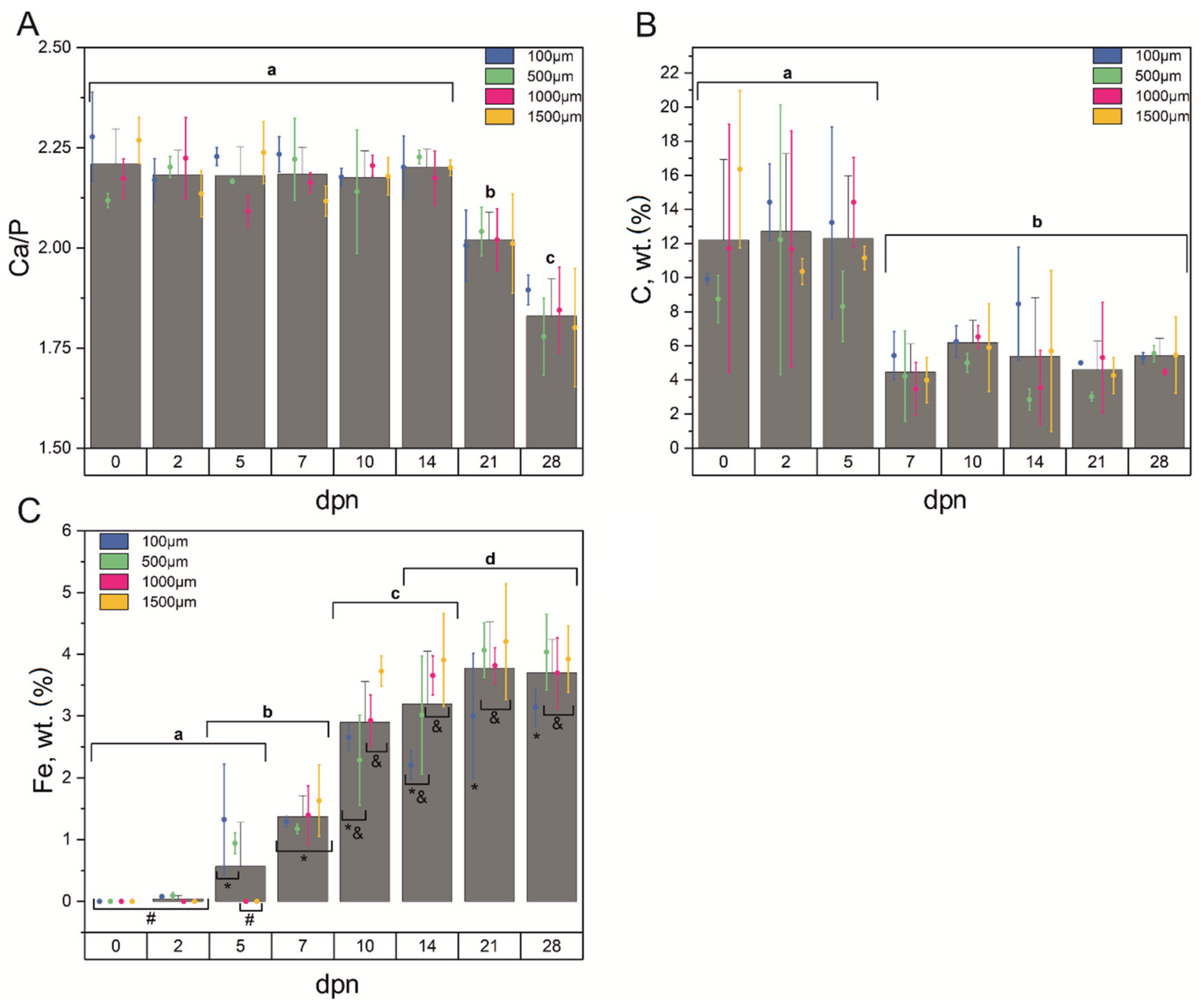
3.2. Chemical Composition
3.3. Crystallographic Organisation
3.4. Molecular Arrangement
3.5. Mechanical Features
3.6. Regression Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sa, Y.; Liang, S.; Ma, X.; Lu, S.; Wang, Z.; Jiang, T.; Wang, Y. Compositional, structural and mechanical comparisons of normal enamel and hypomaturation enamel. Acta Biomater. 2014, 10, 5169–5177. [Google Scholar] [CrossRef]
- Catón, J.; Tucker, A.S. Current knowledge of tooth development: Patterning and mineralization of the murine dentition. J. Anat. 2009, 214, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.W. The amelogenin proteins and enamel development in humans and mice. J. Oral Biosci. 2011, 53, 248–256. [Google Scholar] [CrossRef]
- Bartlett, J.D. Dental enamel development: Proteinases and their enamel matrix substrates. Int. Sch. Res. Not. 2013, 2013, 684607. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, I. The genetic basis of tooth development and dental defects. Am. J. Med. Genet. 2006, 140, 2530–2535. [Google Scholar] [CrossRef]
- Zeichner-David, M.; Vo, H.; Tan, H.; Diekwisch, T.; Berman, B.; Thiemann, F.; Alcocer, M.D.; Hsu, P.; Wang, T.; Eyna, J.; et al. Timing of the expression of enamel gene products during mouse tooth development. Int. J. Dev. Biol. 1997, 41, 27–38. [Google Scholar]
- Mestrinho, H.D.; Fonseca, J.A.C.; Gomes, R.R.; Souza, L.C.; Acevedo, A.C.; Carvalho, J.C.; Paula, L.M. Prevalence of enamel dental defects in the deciduous teeth of preschool children in Brasilia, Brazil. Eur. Cells Mater. 2007, 14 (Suppl. 2), 110. [Google Scholar]
- Arnold, W.H.; Gaengler, P. Quantitative analysis of the calcium and phosphorus content of developing and permanent human teeth. Ann. Anat.-Anatomischer Anzeiger 2007, 189, 183–190. [Google Scholar] [CrossRef]
- Farooq, I.; Ali, S.; Anderson, P. Dental Enamel. In An Illustrated Guide to Oral Histology; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 15–34. [Google Scholar]
- Goldberg, M.; Kellermann, O.; Dimitrova-Nakov, S.; Harichane, Y.; Baudry, A. Comparative studies between mice molars and incisors are required to draw an overview of enamel structural complexity. Front. Physiol. 2014, 5, 359. [Google Scholar] [CrossRef]
- Warshawsky, H.; Josephsen, K.; Thylstrup, A.; Fejerskov, O. The development of enamel structure in rat incisors as compared to the teeth of monkey and man. Anat. Rec. 1981, 200, 371–399. [Google Scholar] [CrossRef]
- Bai, P.; Warshawsky, H. Morphological studies on the distribution of enamel matrix proteins using routine electron microscopy and freeze-fracture replicas in the rat incisor. Anat. Rec. 1985, 212, 1–16. [Google Scholar] [CrossRef]
- Klevezal, G.A.; Shchepotkin, D.V. Incisor growth rate in rodents and the record of the entire annual cycle in the incisors of Marmota baibacina centralis. Biol. Bull. 2012, 39, 684–691. [Google Scholar] [CrossRef]
- Croft, D.A.; Niemi, K.; Franco, A. Incisor morphology reflects diet in caviomorph rodents. J. Mammal. 2011, 92, 871–879. [Google Scholar] [CrossRef]
- Lozupone, E.; Favia, A. Effects of a low calcium maternal and weaning diet on the thickness and microhardness of rat incisor enamel and dentine. Arch. Oral Biol. 1989, 34, 491–498. [Google Scholar] [CrossRef]
- Bonucci, E.; Lozupone, E.; Silvestrini, G.; Favia, A.; Mocetti, P. Morphological studies of hypomineralized enamel of rat pups on calcium-deficient diet, and of its changes after return to normal diet. Anat. Rec. 1994, 239, 379–395. [Google Scholar] [CrossRef]
- Nanci, A.; Mocetti, P.; Sakamoto, Y.; Kunikata, M.; Lozupone, E.; Bonucci, E. Morphological and immunocytochemical analyses on the effects of diet-induced hypocalcemia on enamel maturation in the rat incisor. J. Histochem. Cytochem. 2000, 48, 1043–1058. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1999; Volumes 1, 2. [Google Scholar]
- Pinheiro, G.; Prata, D.F.; Araújo, I.M.; Tiscornia, G. The African spiny mouse (Acomys spp.) as an emerging model for development and regeneration. Lab. Anim. 2018, 52, 565–576. [Google Scholar] [CrossRef]
- Dickinson, H.; Ireland, Z.J.; Larosa, D.A.; O’Connell, B.A.; Ellery, S.; Snow, R.; Walker, D.W. Maternal dietary creatine supplementation does not alter the capacity for creatine synthesis in the newborn spiny mouse. Reprod. Sci. 2013, 20, 1096–1102. [Google Scholar] [CrossRef]
- Ellery, S.J.; Ireland, Z.; Kett, M.M.; Snow, R.; Walker, D.W.; Dickinson, H. Creatine pretreatment prevents birth asphyxia-induced injury of the newborn spiny mouse kidney. Pediatr. Res. 2013, 73, 201–208. [Google Scholar] [CrossRef]
- Ellery, S.J.; LaRosa, D.A.; Kett, M.M.; Gatta, P.A.D.; Snow, R.J.; Walker, D.W.; Dickinson, H. Dietary creatine supplementation during pregnancy: A study on the effects of creatine supplementation on creatine homeostasis and renal excretory function in spiny mice. Amino Acids 2016, 48, 1819–1830. [Google Scholar] [CrossRef]
- Brant, J.O.; Lopez, M.C.; Baker, H.V.; Barbazuk, W.B.; Maden, M. A comparative analysis of gene expression profiles during skin regeneration in mus and acomys. PLoS ONE 2015, 10, e0142931. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Kiama, S.G.; Seifert, M.G.; Goheen, J.R.; Palmer, T.M.; Maden, M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012, 489, 561–565. [Google Scholar] [CrossRef]
- Bellofiore, N.; Ellery, S.J.; Mamrot, J.; Walker, D.W.; Temple-Smith, P.; Dickinson, H. First evidence of a menstruating rodent: The spiny mouse (Acomys cahirinus). Am. J. Obstet. Gynecol. 2017, 216, 40.e1–40.e11. [Google Scholar] [CrossRef]
- Pagella, P.; Porcheri, C.; Mitsiadis, T.A. Exploiting teeth as a model to study basic features of signaling pathways. Biochem. Soc. Trans. 2020, 48, 2729–2742. [Google Scholar] [CrossRef]
- Gunier, R.B.; Mora, A.M.; Smith, D.; Arora, M.; Austin, C.; Eskenazi, B.; Bradman, A. Biomarkers of manganese exposure in pregnant women and children living in an agricultural community in California. Environ. Sci. Technol. 2014, 48, 14695–14702. [Google Scholar] [CrossRef]
- Davis, K.A.; Mountain, R.V.; Pickett, O.R.; Den Besten, P.K.; Bidlack, F.B.; Dunn, E.C. Teeth as potential new tools to measure early-life adversity and subsequent mental health risk: An interdisciplinary review and conceptual model. Biol. Psychiatry 2020, 87, 502–513. [Google Scholar] [CrossRef]
- Gil-Bona, A.; Bidlack, F.B. Tooth enamel and its dynamic protein matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef] [PubMed]
- Falster, A.U.; Yoshino, S.; Hashimoto, K.; Jr, F.J.; Simmons, W.B.; Nakamoto, T. The effect of prenatal caffeine exposure on the enamel surface of the first molars of newborn rats. Arch. Oral Biol. 1993, 38, 441–447. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Rahman, S.; Ojeh, N.; Grant, W.B.; Kaur, H.; Haq, A.; Razzaque, M.S. Oral manifestations of magnesium and vitamin D inadequacy. J. Steroid Biochem. Mol. Biol. 2020, 200, 105636. [Google Scholar] [CrossRef]
- Grazioli, G.; Valente, L.L.; Isolan, C.P.; Pinheiro, H.A.; Duarte, C.G.; Münchow, E.A. Bleaching and enamel surface interactions resulting from the use of highly-concentrated bleaching gels. Arch. Oral Biol. 2018, 87, 157–162. [Google Scholar] [CrossRef]
- Khlifi, R.; Hamza-Chaffai, A. Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: A review. Toxicol. Appl. Pharmacol. 2010, 248, 71–88. [Google Scholar] [CrossRef]
- Wychowanski, P.; Malkiewicz, K. Evaluation of metal ion concentration in hard tissues of teeth in residents of central Poland. BioMed Res. Int. 2017, 2017, 6419709. [Google Scholar] [CrossRef]
- Park, M.K.; Min, S.-Y.; Song, J.-S.; Lee, J.; Jung, H.-S.; Kim, S.-O. Estimated time of biomineralization in developing rat incisors. J. Korean Acad. Pedtatric Dent. 2017, 44, 138–146. [Google Scholar] [CrossRef]
- Świetlicka, I.; Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Kwaśniewska, A.; Świetlicki, M.; Skic, A.; Gołacki, K. Prenatally administered HMB modifies the enamel surface roughness in spiny mice offspring: An Atomic Force Microscopy study. Arch. Oral Biol. 2016, 70, 24–31. [Google Scholar] [CrossRef]
- Świetlicka, I.; Kuc, D.; Świetlicki, M.; Arczewska, M.; Muszyński, S.; Tomaszewska, E.; Prószyński, A.; Gołacki, K.; Błaszczak, J.; Cieślak, K.; et al. Near-surface studies of the changes to the structure and mechanical properties of human enamel under the action of fluoride varnish containing CPP–ACP compound. Biomolecules 2020, 10, 765. [Google Scholar] [CrossRef]
- Fava, M.; Myaki, S.I.; Arana-Chavez, V.E.; Fava-de-Moraes, F. Effects of a non-rinse conditioner on the enamel of primary teeth. Braz. Dent. J. 2003, 14, 168–171. [Google Scholar] [CrossRef][Green Version]
- Świetlicki, M.; Chocyk, D.; Klepka, T.; Prószyński, A.; Kwaśniewska, A.; Borc, J.; Gładyszewski, G. The structure and mechanical properties of the surface layer of polypropylene polymers with talc additions. Materials 2020, 13, 698. [Google Scholar] [CrossRef]
- Loparic, M.; Wirz, D.; Daniels, A.U.; Raiteri, R.; Vanlandingham, M.R.; Guex, G.; Martin, I.; Aebi, U.; Stolz, M. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: Validation with a gel-microfiber composite. Biophys J. 2010, 98, 2731–2740. [Google Scholar] [CrossRef]
- Feninat, F.L.; Elouatik, S.; Ellis, T.H.; Sacher, E.; Stangel, I. Quantitive assessment of surface roughness as measured by AFM: Application to polished human dentin. Appl. Surf. Sci. 2001, 183, 205–215. [Google Scholar] [CrossRef]
- Meia, L.; Busscher, H.J.; Mei, H.C.; Ren, Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent. Mater. 2011, 27, 770–778. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size controlled hydroxyapatite nanoparticles as self-organised organic-inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef]
- Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Wiącek, D.; Świetlicka, I.; Skibińska, M.; Szymańska-Chargot, M.; Orzelska, J.; Świetlicki, M.; Arczewska, M.; et al. Analysis of bone osteometry, mineralization, mechanical and histomorphometrical properties of tibiotarsus in broiler chickens demonstrates an influence of dietary chickpea seeds (Cicer arietinum L.) inclusion as a primary protein source. PLoS ONE 2018, 13, e0208921. [Google Scholar]
- Hong, H.; Tie, L.; Jian, T. The crystal characteristics of enamel and dentin by XRD method. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2006, 21, 9–12. [Google Scholar]
- Yerramshetty, J.S.; Lind, C.; Akkus, O. The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone 2006, 39, 1236–1243. [Google Scholar] [CrossRef]
- Świetlicka, I.; Arczewska, M.; Muszyński, S.; Tomaszewska, E.; Świetlicki, M.; Kuc, D.; Mielnik-Błaszczak, M.; Gołacki, K.; Cieślak, K. Surface analysis of etched enamel modified during the prenatal period. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117271. [Google Scholar] [CrossRef]
- Corrêa-Afonso, A.; Bachmann, L.; Almeida, C.; Dibb, R.; Borsatto, M. Loss of structural water and carbonate of Nd:YAG laser-irradiated human enamel. Lasers Med. Sci. 2015, 30, 1183–1187. [Google Scholar] [CrossRef]
- Bachmann, L.; Diebolder, R.; Hibst, R.; Zezell, D.M. Infrared absorption bands of enamel and dentin tissues from human and bovine teeth. Appl. Spectrosc. Rev. 2003, 38, 1–14. [Google Scholar] [CrossRef]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the Near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Castellano, G.; Fanelli, A.M.; Pelillo, M. An iterative pruning algorithm for feedforward neural networks. IEEE Trans. Neural Netw. 1997, 8, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.M. Neural Networks for Pattern Recognition; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Kirkham, J.; Brookes, S.J.; Shore, R.C.; Bonass, W.A.; Smith, D.A.; Wallwork, M.L.; Robinson, C. Atomic force microscopy studies of crystal surface topology during enamel development. Connect. Tissue Res. 1998, 38, 91–100, discussion 139–145. [Google Scholar] [CrossRef] [PubMed]
- Solaymani, S.; Nezafat, N.B.; Ţălu, Ş.; Shafiekhani, A.; Dalouji, V.; Amiri, A.; Rezaee, S.; Morozov, I.A. Atomic force microscopy studies of enamel, inner enamel, dentin, and cementum in canine teeth. Microsc. Res. Tech. 2021, 84, 1098–1105. [Google Scholar] [CrossRef]
- Besnard, C.; Harper, R.A.; Salvati, E.; Moxham, T.E.J.; Romano Brandt, L.; Landini, G.; Shelton, R.M.; Korsunsky, A.M. Analysis of in vitro demineralised human enamel using multi-scale correlative optical and scanning electron microscopy, and high-resolution synchrotron wide-angle X-ray scattering. Mater. Des. 2021, 206, 109739. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Koudriavtseva, O.; Herrera-Becerra, R.; Escobosa, A. XRD characterization of crystallinity of human tooth enamel under influence of mechanical grinding. Mater. Sci. Appl. 2015, 6, 464. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Susi, H.; Byler, D.M. Resolution-enhanced fourier transform infrared spectroscopy of enzymes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1986; Volume 130, pp. 290–311. [Google Scholar]
- Akiva, A.; Kerschnitzki, M.; Pinkas, I.; Wagermaier, W.; Yaniv, K.; Fratzl, P.; Addadi, L.; Weiner, S. Mineral formation in the larval zebrafish tail bone occurs via an acidic disordered calcium phosphate phase. J. Am. Chem. Soc. 2016, 138, 14481–14487. [Google Scholar] [CrossRef]
- Xia, J.; Tian, Z.; Hua, L.; Chen, L.; Zhou, Z.; Qian, L.; Ungar, P. Enamel crystallite strength and wear: Nanoscale responses of teeth to chewing loads. J. R. Soc. Interface 2017, 14, 20170456. [Google Scholar] [CrossRef]
- Hassoun, M.H. Fundamentals of Artificial Neural Networks; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Holzscheck, N.; Falckenhayn, C.; Söhle, J.; Kristof, B.; Siegner, R.; Werner, A.; Schössow, J.; Jürgens, C.; Völzke, H.; Wenck, H.; et al. Modeling transcriptomic age using knowledge-primed artificial neural networks. NPJ Aging Mech. Dis. 2021, 7, 15. [Google Scholar] [CrossRef]
- Bermejillo Barrera, M.D.; Franco-Martínez, F.; Díaz Lantada, A. Artificial intelligence aided design of tissue engineering scaffolds employing virtual tomography and 3D convolutional neural networks. Materials 2021, 14, 5278. [Google Scholar] [CrossRef]
- Talaat, D.M.; Mahmoud, A. Acid resistance of enamel subsurface lesions treated with casein phosphopeptide amorphous calcium phosphate fluoride. J. Dent. Child. 2015, 82, 70–75. [Google Scholar]
- Smith, C.E.; Richardson, A.S.; Hu, Y.; Bartlett, J.D.; Hu, J.C.; Simmer, J.P. Effect of kallikrein 4 loss on enamel mineralization: Comparison with mice lacking matrix metalloproteinase 20. J. Biol. Chem. 2011, 286, 18149–18160. [Google Scholar] [CrossRef] [PubMed]
- Margolis, H.C.; Kwak, S.-Y.; Yamazaki, H. Role of mineralization inhibitors in the regulation of hard tissue biomineralization: Relevance to initial enamel formation and maturation. Front. Physiol. 2014, 5, 339. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Papagerakis, P.; Yamakoshi, Y.; Hu, J.C.-C.; Bartlett, J.D.; Simmer, J.P. Functions of KLK4 and MMP-20 in dental enamel formation. Biol. Chem. 2008, 389, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C. Enamel maturation: A brief background with implications for some enamel dysplasias. Front. Physiol. 2014, 5, 388. [Google Scholar] [CrossRef]
- Daculsi, G.; Kerebel, B. High-resolution electron microscope study of human enamel crystallites: Size, shape, and growth. J. Ultrastruct. Res. 1978, 65, 163–172. [Google Scholar] [CrossRef]
- Yilmaz, E.D.; Schneider, G.A.; Swain, M.V. Influence of structural hierarchy on the fracture behaviour of tooth enamel. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140130. [Google Scholar] [CrossRef]
- Moradian-Oldak, J. Amelogenins: Assembly, processing and control of crystal morphology. Matrix Biol. 2001, 20, 293–305. [Google Scholar] [CrossRef]
- Gordon, L.M.; Cohen, M.J.; MacRenaris, K.W.; Pasteris, J.D.; Seda, T.; Joester, D. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 2015, 347, 746–750. [Google Scholar] [CrossRef]
- Gordon, L.M.; Joester, D. Mapping residual organics and carbonate at grain boundaries and the amorphous interphase in mouse incisor enamel. Front. Physiol. 2015, 6, 57. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, H.; Witkowska, H.E.; Huang, Y.; Tanimoto, K.; Li, W. Preferential and selective degradation and removal of amelogenin adsorbed on hydroxyapatites by MMP20 and KLK4 in vitro. Front. Physiol. 2014, 5, 268. [Google Scholar] [CrossRef]
- Querido, W.; Shanas, N.A.; Bookbinder, S.; Oliveira-Nunes, M.C.; Krynska, B.; Pleshko, N. Fourier transform infrared spectroscopy of developing bone mineral: From amorphous precursor to mature crystal. Analyst 2020, 145, 764–776. [Google Scholar] [CrossRef]
- Kourkoumelis, N.; Balatsoukas, I.; Tzaphlidou, M. Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J. Biol. Phys. 2012, 38, 279–291. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Kunin, A.A.; Evdokimova, A.Y.; Moiseeva, N.S. Age-related differences of tooth enamel morphochemistry in health and dental caries. EPMA J. 2015, 6, 3. [Google Scholar] [CrossRef]
- Bronckers, A.L.; Lyaruu, D.M.; Guo, J.; Bijvelds, M.J.; Bervoets, T.J.; Zandieh-Doulabi, B.; Medina, J.F.; Li, Z.; Zhang, Y.; DenBesten, P.K. Composition of mineralizing incisor enamel in cystic fibrosis transmembrane conductance regulator-deficient mice. Eur. J. Oral Sci. 2015, 123, 9–16. [Google Scholar] [CrossRef]
- Robinson, C.; Hiller, C.R.; Weatherell, J.A. Uptake of 32P-labelled phosphate into developing rat incisor enamel. Calcif. Tissue Res. 1974, 15, 143–152. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.K.; Gilbert, P.U.P.A. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Development of a new approach to diagnosis of the early fluorosis forms by means of FTIR and Raman microspectroscopy. Sci. Rep. 2020, 10, 20891. [Google Scholar] [CrossRef]
- Dauphin, Y. Using microstructures and composition to decipher the alterations of rodent teeth in modern regurgitation pellets—A good news-bad news story. Minerals 2020, 10, 63. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Rehman, G.U.; Basavarajappa, S.; Khuraif, A.A.A.; Durgesh, B.H.; Khan, A.S.; Rehman, I.U. Applications of Raman spectroscopy in dentistry: Analysis of tooth structure. Appl. Spectrosc. Rev. 2015, 50, 332–350. [Google Scholar] [CrossRef]
- Tsuda, H.; Arends, J. Raman spectroscopy in dental research: A short review of recent studies. Adv. Dent. Res. 1997, 11, 539–547. [Google Scholar] [CrossRef]
- Carden, A.; Morris, M.D. Application of vibrational spectroscopy to the study of mineralized tissues (review). J. Biomed. Opt. 2000, 5, 259–268. [Google Scholar] [CrossRef]
- Leroy, G.; Penel, G.; Leroy, N.; Brès, E. Human tooth enamel: A Raman polarized approach. Appl. Spectrosc. 2002, 56, 1030–1034. [Google Scholar] [CrossRef]
- Reith, E.J.; Boyde, A. The enamel organ, a control gate for calcium influx into the enamel. J. Dent. Res. 1979, 58, 980. [Google Scholar] [CrossRef]
- Aoba, T. Recent observations on enamel crystal formation during mammalian amelogenesis. Anat. Rec. 1996, 245, 208–218. [Google Scholar] [CrossRef]
- Teruel Jde, D.; Alcolea, A.; Hernández, A.; Ruiz, A.J. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral Biol. 2015, 60, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Sakai, H.; Shibata, Y.; Shibata, M.; Mataki, S.; Kato, Y. Expression and localization of ferritin mRNA in ameloblasts of rat incisor. Arch. Oral Biol. 1998, 43, 367–378. [Google Scholar] [CrossRef]
- Wen, X.; Paine, M.L. Iron deposition and ferritin heavy chain (Fth) localization in rodent teeth. BMC Res. Notes 2013, 6, 1. [Google Scholar] [CrossRef]
- Takano, Y.; Ozawa, H. Cytochemical studies on the ferritin-containing vesicles of the rat incisor ameloblasts with special reference to the acid phosphatase activity. Calcif. Tissue Int. 1981, 33, 51–55. [Google Scholar] [CrossRef]
- Sparks, N.H.; Motta, P.J.; Shellis, R.P.; Wade, V.J.; Mann, S. An analytical electron microscopy study of iron-rich teeth from the butterflyfish (Chaetodon ornatissimus). J. Exp. Biol. 1990, 151, 371–385. [Google Scholar] [CrossRef]
- Raue, L.; Gersdorff, N.; Rödiger, M.; Klein, H. New insights in prism orientation within human enamel. Arch. Oral Biol. 2012, 57, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Takagi, S. Structures of bological minerals in dental research. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1035–1044. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Padmanabhan, V.P.; Kulandaivelu, R.; Sankara Narayanan Nellaiappan, T.S.; Sagadevan, S.; Paiman, S.; Mohammad, F.; Al-Lohedan, H.A.; Obulapuram, P.K.; Oh, W.C. Influence of iron doping towards the physicochemical and biological characteristics of hydroxyapatite. Ceram. Int. 2021, 47, 5061–5070. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Intrinsically superparamagnetic Fe-hydroxyapatite nanoparticles positively influence osteoblast-like cell behaviour. J. Nanobiotechnol. 2012, 10, 32. [Google Scholar] [CrossRef]
- Gamal, G.A.; al-Mufadi, F.A.; Said, A.H. Effect of iron additives on the microstructure of hydroxyapatite. Eng. Technol. Appl. Sci. Res. 2013, 3, 532–539. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.-A.; Buton, N.; Megier, C.; Beuran, M. Development of iron-doped hydroxyapatite coatings. Coatings 2021, 11, 186. [Google Scholar] [CrossRef]
- Ghadimi, E.; Eimar, H.; Marelli, B.; Nazhat, S.N.; Asgharian, M.; Vali, H.; Tamimi, F. Trace elements can influence the physical properties of tooth enamel. Springerplus 2013, 2, 499. [Google Scholar] [CrossRef]
- Alkattan, R.; Lippert, F.; Tang, Q.; Eckert, G.J.; Ando, M. The influence of hardness and chemical composition on enamel demineralization and subsequent remineralization. J. Dent. 2018, 75, 34–40. [Google Scholar] [CrossRef]
- Brito, J.S.; Neto, A.S.; Silva, L.; Menezes, R.; Araújo, N.; Carneiro, V.; Moreno, L.M.; Miranda, J.; Álvares, P.; Nevares, G.; et al. Analysis of dental enamel surface submitted to fruit juice plus soymilk by micro X-Ray fluorescence: In vitro study. Scietific World J. 2016, 2016, 8123769. [Google Scholar]
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Baldassarri, M.; Margolis, H.C.; Beniash, E. Compositional determinants of mechanical properties of enamel. J. Dent. Res. 2008, 87, 645–649. [Google Scholar] [CrossRef]
- An, B.; Wang, R.; Zhang, D. Role of crystal arrangement on the mechanical performance of enamel. Acta Biomater. 2012, 8, 3784–3793. [Google Scholar] [CrossRef]
- Lee, J.J.-W.; Morris, D.J.; Constantino, P.J.; Lucas, P.W.; Smith, T.M.; Lawn, B.R. Properties of tooth enamel in great apes. Acta Biomater. 2010, 6, 4560–4565. [Google Scholar] [CrossRef]
- Fong, H.; Foster, B.L.; Sarikaya, M.; Somerman, M.J. Structure and mechanical properties of Ank/Ank mutant mouse dental tissues—An animal model for studying periodontal regeneration. Arch. Oral Biol. 2009, 54, 570–576. [Google Scholar] [CrossRef]
- Frýdová, B.; Šepitka, J.; Stejskal, V.; Frýda, J.; Lukeš, J. Nanoindentation mapping reveals gradients in the mechanical properties of dental enamel in rat incisors. Comput. Methods Biomech. Biomed. Eng. 2013, 16 (Suppl. 1), 290–291. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, C.; Korach, C.S. Time Dependent Constitutive Behavior and Fracture/Failure Processes. In Measurement of Structural Variations in Enamel Nanomechanical Properties Using Quantitative Atomic Force Acoustic Microscopy; Proulx, T., Ed.; Springer: New York, NY, USA, 2011; Volume 3, pp. 373–381. [Google Scholar]
- Koenigswald, W.V.; Clemens, W.A. Levels of complexity in the microstructure of mammalian enamel and their application in studies of systematics. Scanning Microsc. 1992, 6, 195–217, discussion 217–218. [Google Scholar]
- Bechtle, S.; Özcoban, H.; Lilleodden, E.T.; Huber, N.; Schreyer, A.; Swain, M.V.; Schneider, G.A. Hierarchical flexural strength of enamel: Transition from brittle to damage-tolerant behaviour. J. R. Soc. Interface 2012, 9, 1265–1274. [Google Scholar] [CrossRef]
- Shahmoradi, M.; Bertassoni, L.E.; Elfallah, H.M.; Swain, M. Fundamental structure and properties of enamel, dentin and cementum. In Advances in Calcium Phosphate Biomaterials; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2. [Google Scholar]
- Xu, C.; Reed, R.; Gorski, J.P.; Wang, Y.; Walker, M.P. The distribution of carbonate in enamel and its correlation with structure and mechanical properties. J. Mater. Sci. 2012, 47, 8035–8043. [Google Scholar] [CrossRef] [PubMed]
- Qamar, Z.; Haji Abdul Rahim, Z.B.; Chew, H.P.; Fatima, T. Influence of trace elements on dental enamel properties: A review. JPMA J. Pak. Med. Assoc. 2017, 67, 116–120. [Google Scholar] [PubMed]
- Świetlicka, I.; Tomaszewska, E.; Muszyński, S.; Valverde Piedra, J.L.; Świetlicki, M.; Prószyński, A.; Cieślak, K.; Wiącek, D.; Szymańczyk, S.; Kamiński, D. The effect of cadmium exposition on the structure and mechanical properties of rat incisors. PLoS ONE 2019, 14, e0215370. [Google Scholar] [CrossRef] [PubMed]
- Halse, A. Effect of dietary iron deficiency on the pigmentation and iron content of rat incisor enamel. Eur. J. Oral Sci. 1973, 81, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Thong, Z.; Tan, J.Y.Y.; Loo, E.S.; Phua, Y.W.; Chan, X.L.S.; Syn, C.K.-C. Artificial neural network, predictor variables and sensitivity threshold for DNA methylation-based age prediction using blood samples. Sci. Rep. 2021, 11, 1744. [Google Scholar] [CrossRef]
- Peddada, S.D.; Haseman, J.K. Analysis of nonlinear regression models: A cautionary note. Dose-Response 2005, 3. [Google Scholar] [CrossRef]
- Murugesan, S.; Bhuvaneswaran, R.S.; Khanna Nehemiah, H.; Keerthana Sankari, S.; Nancy Jane, Y. Feature selection and classification of clinical datasets using bioinspired algorithms and super learner. Comput. Math. Methods Med. 2021, 2021, 6662420. [Google Scholar] [CrossRef]
- Lee, E.K. Large-scale optimization-based classification models in medicine and biology. Ann. Biomed. Eng. 2007, 35, 1095–1109. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.-H.; Noh, Y.-K.; Park, F.C.; Auh, Q.S. Age-group determination of living individuals using first molar images based on artificial intelligence. Sci. Rep. 2021, 11, 1073. [Google Scholar] [CrossRef]
- Farhadian, M.; Salemi, F.; Saati, S.; Nafisi, N. Dental age estimation using the pulp-to-tooth ratio in canines by neural networks. Imaging Sci. Dent. 2019, 49, 19–26. [Google Scholar] [CrossRef]
- Yan, W.; Renteria, C.; Huang, Y.; Arola, D.D. A machine learning approach to investigate the materials science of enamel aging. Dent. Mater. 2021, 37, 1761–1771. [Google Scholar] [CrossRef]




| Network MLP 13-5-1 | Training | Validation | Testing |
|---|---|---|---|
| R2 | 0.995 | 0.997 | 0.984 |
| SSE | 11.973 | 6.228 | 21.916 |
| RMSE | 0.959 | 0.692 | 1.298 |
| Independent Variable | Sensitivity Ratio | Independent Variable | Sensitivity Ratio |
|---|---|---|---|
| DMTII | 7.22 | Fe | 1.35 |
| Ca/P | 5.46 | P | 1.31 |
| FWHM002 | 2.63 | CIFTIR | 1.07 |
| DMTI | 1.76 | C | 1.06 |
| A lattice | 1.57 | Ca | 0.996 |
| (C/P)FTIR | 1.53 | CIRaman | 0.994 |
| (C/P)Raman | 1.49 |
| Network MLP 9-5-1 | Training | Validation | Testing |
|---|---|---|---|
| R2 | 0.999 | 0.999 | 0.995 |
| SSE | 4.821 | 2.604 | 7.497 |
| RMSE | 0.731 | 0.538 | 0.912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świetlicka, I.; Tomaszewska, E.; Muszyński, S.; Świetlicki, M.; Skrzypek, T.; Grudziński, W.; Gruszecki, W.I.; Kamiński, D.; Hułas-Stasiak, M.; Arczewska, M. Surface and Structural Studies of Age-Related Changes in Dental Enamel: An Animal Model. Materials 2022, 15, 3993. https://doi.org/10.3390/ma15113993
Świetlicka I, Tomaszewska E, Muszyński S, Świetlicki M, Skrzypek T, Grudziński W, Gruszecki WI, Kamiński D, Hułas-Stasiak M, Arczewska M. Surface and Structural Studies of Age-Related Changes in Dental Enamel: An Animal Model. Materials. 2022; 15(11):3993. https://doi.org/10.3390/ma15113993
Chicago/Turabian StyleŚwietlicka, Izabela, Ewa Tomaszewska, Siemowit Muszyński, Michał Świetlicki, Tomasz Skrzypek, Wojciech Grudziński, Wiesław I. Gruszecki, Daniel Kamiński, Monika Hułas-Stasiak, and Marta Arczewska. 2022. "Surface and Structural Studies of Age-Related Changes in Dental Enamel: An Animal Model" Materials 15, no. 11: 3993. https://doi.org/10.3390/ma15113993
APA StyleŚwietlicka, I., Tomaszewska, E., Muszyński, S., Świetlicki, M., Skrzypek, T., Grudziński, W., Gruszecki, W. I., Kamiński, D., Hułas-Stasiak, M., & Arczewska, M. (2022). Surface and Structural Studies of Age-Related Changes in Dental Enamel: An Animal Model. Materials, 15(11), 3993. https://doi.org/10.3390/ma15113993






