Abstract
Blend films based on sodium alginate (SA) and citrus pectin (P) reinforced with different concentrations of SiO2 (0–10% w/w) were developed in this study. From the morphological (SEM) and structural (FT-IR) evaluation, it was verified that the incorporation of the reinforcing agent did not drastically modify the microstructure of the films, nor did new chemical bonds form. However, the XRD results suggested a slight reduction in the crystallinities of the blends by the incorporation of SiO2. Among the formulations prepared, the addition of a 5% reinforcing agent was responsible for the simultaneous improvement of mechanical and barrier properties. Comparing the control sample (SA/P) with the SA/P/5.0%SiO2 film, the tensile strength increased from 27.7 ± 3.7 to 40.6 ± 4.5 MPa, and the water-vapor transmission rate decreased from 319.8 ± 38.7 to 288.9 ± 23.5 g m−2 day−1. Therefore, SiO2, as a reinforcing agent in SA/P blends, represents a simple and effective strategy for improving the properties of biopolymer-based films in applications, such as packaging.
1. Introduction
Modern society is increasingly concerned with the use of fossil resources and environmental impact caused by the production and incorrect disposal of these materials. Within this context, packaging from fossil sources is important and widely used by society, as they are responsible for the containment, preservation, and distribution of products. Due to their low cost and favorable properties for the production of packaging, these plastics are consolidated in the globalized world [1,2]. Therefore, whenever possible, the use of natural, biodegradable, and renewable biopolymers should be considered for the production of packaging, as they can be alternatives in the reduction of environmental impact [1,3].
Biopolymers are materials obtained from substances derived from living organisms, and thus, an increasing number of biopolymers, such as polysaccharides and proteins, have been studied [3,4]. Polysaccharides have film-forming capacity due to their structural characteristics and they are being broadly applied in the production of biodegradable films [3]. Despite great promise regarding the application of biopolymers, these materials have high sensitivity to water vapor and poor mechanical properties, making their wide use in packaging difficult [5]. Consequently, ways to improve the properties of biopolymer-based films at a low cost have been created. Among them, the formulation of blends with different biopolymers and the incorporation of reinforcing agents are highlighted [5,6].
Among some of the promising candidates for the production of films based on biopolymers are sodium alginate and pectin. These polysaccharides were selected for this study due to their high availability in Brazil and low cost. Sodium alginate is obtained from brown algae (Phaeophyceae) that have high growth rates and are present on coasts with temperate climates, being greatly available to exploration [3,7,8]. Sodium alginate is an unbranched copolymer composed of mannuronic and glucuronic acid units and is widely used as a stabilizing thickener, gel-forming or film-forming agent in the pharmaceutical, medical, and food fields [9,10,11]. Pectin is present in the primary cell wall as well as the intercellular layer of plants, and is most commonly extracted from citrus peel, apple pomace, and beet pulp [12,13]. Pectin is a homopolymer composed mainly of galacturonic acid linked through α-1,4-glycosidic bonds and, similarly as alginate, is widely used in the food industry as a gelling, stabilizing, and thickening agent in products such as jellies, yogurt, fruity dairy drinks, and ice-cream [12,13,14]. Although sodium alginate and pectin are great candidates for the production of films in food packaging [15], these polysaccharides have poor mechanical properties and high sensitivity to water, making their widespread application in this area difficult [5,12].
The use of biopolymer blends is an alternative to improve properties and reduce the costs of films. Both alginate and pectin are of low cost, having nontoxic properties and demonstrating biodegradability, while showing some potential for application in biopolymer packaging [5,12]. To obtain a blend of 2 biopolymers, it is necessary to mix the compounds, resulting in a material with properties of both biopolymers. In this way, the production of blends is carried out to maximize the performance of a material, decreasing its sensitivity to water and improving its properties for a given application [16]. The combination of sodium alginate and pectin was investigated in other studies, giving rise to continuous and homogeneous films [17]. Furthermore, the inclusion of sodium alginate in pectin-based formulations improved the strength of the biopolymer network [18].
Nevertheless, in this context, the formulation of blends of different biopolymers, associated with the use of reinforcing agents, is an interesting strategy to improve film properties [5,19,20]. The use of reinforcing agents in films can result in improving their physical properties, such as tensile strength, thermal stability, and water-vapor barrier [10]. Some examples of commonly used inorganic reinforcing agents are TiO2, ZnO, and SiO2 [5,21]. Nanosilica (SiO2) has been widely studied in several polymeric systems and is found naturally in quartz sands, rocks, and clays [22]. However, it is necessary to know how these particles influence the organic matrix, and, therefore, research is necessary.
The incorporation of different SiO2 concentrations in sodium alginate or pectin films was investigated by evaluating the physical, chemical, and optical properties of the films [23,24,25]. Pectin films with SiO2 showed that nanoparticles can reduce water-vapor permeability by 30–60%, compared to films without nanoparticles [25]. Films with SiO2 concentrations between 0 and 8% were produced, the tensile strength and elongation at break being higher for the composition of 4.5% SiO2, and the water-vapor barrier of the films reduced with increasing concentrations of SiO2 [23]. The application of low SiO2 concentrations (0 to 1.5%) in sodium alginate films resulted in better thermal stability of the films; nevertheless, this did not affect the morphological or barrier-related properties [24]. The mechanical and barrier properties of SiO2 embedded films depend on the concentration and dispersion of the reinforcing agents in the biopolymer matrix [23]. Thus, it is important to investigate which SiO2 concentration can improve the performance properties of each biopolymer matrix.
To the best of our knowledge, studies incorporating SiO2 as a reinforcing agent in a film based on sodium alginate and pectin have not yet been conducted. Therefore, to obtain materials with improved properties, the objective of this study was to produce biopolymer films composed of sodium alginate and pectin added to different concentrations of SiO2 and to characterize them in terms of their morphological, structural, and physicochemical characteristics.
2. Materials and Methods
2.1. Materials
The materials used in the preparation of the films were:
- -
- Sodium alginate (SA) (Dinâmica Química Contemporânea Ltda., Indaiatuba/SP, Brazil) (purity of 99% viscosity (2% solution at 25 °C): 2.000 cps) as a film-forming compound;
- -
- Pectin (P) powder from citrus (Dinâmica Química Contemporânea Ltda., Indaiatuba/SP, Brazil) (degree of methyl esterification: >75%) as a film-forming compound;
- -
- Glycerol (Dinâmica Química Contemporânea Ltda., Indaiatuba/SP, Brazil) as a plasticizer; and
- -
- Nanosilica (SiO2) (Degussa Brasil Ltda., Americana/SP, Brazil) as a strengthening component, with an average particle size of 12 nm.
2.2. Preparation of SA/P/SiO2 Films
The casting method was employed for the preparation of SA/P/SiO2 films. First, aqueous solutions of sodium alginate (2% w/w), pectin (2% w/w), glycerol plasticizer (30% w/w alginate + pectin) and SiO2 (0; 2.5; 5.0; 7.5 and 10.0% w/w alginate + pectin) were prepared. The compounds were dispersed using the Ultra-Turrax (model T18 basic, IKA®-Werke GmbH and Co. KG, Staufen, Germany) for 5 min at 7000 rpm. After preparation of the solution, it was heated up to 80 °C while stirring, and then maintained at this temperature for 15 min. Following, the solutions were homogenized again using the Ultra-Turrax for another 5 min at 7000 rpm. Subsequently, the final solutions were treated in an ultrasound bath for 15 min to remove bubbles. After cooling, 60 g of each solution was poured into glass Petri dishes (diameter equal to 15 cm) and then placed in an oven (Ethik Technology, Vargem Grande Paulista/SP, Brazil) at 40 °C for 24 h. Finally, the films were removed from the plates and placed in an air-conditioning chamber (Weiss Technik, Reiskirchen, Germany) at 25 °C and a relative humidity of 75% until the films were characterized.
2.3. Characterization of SA/P/SiO2 Films
2.3.1. Film Morphology
The surface and cross-section of the SA/P/SiO2 films were analyzed using the method of scanning electron microscopy (SEM) (Leo 440i, LEO Electron Microscopy/Oxford, Cambridge, UK). The samples were previously fractured with liquid nitrogen, fixed on a metallic support using double-sided carbon tape and coated with gold in a sputter coater (SC7620, VG Microtech, Kent, UK). The visualization was performed with a magnitude of 1000×, voltage of 15 kV and current of 50 pA.
2.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
FT-IR analysis was performed using the Thermo Scientific spectrometer (Nicolet Continuum, Madison, WI, USA). For all composite films, the attenuated total reflectance module (ATR) was used at 4000–675 cm−1, with a resolution of 4 cm−1 and 128 scans.
2.3.3. Thermal Stability
Thermal stability testing was performed on 10 mg film samples heated from 25 to 600 °C at a nitrogen flow of 50 mL min−1 with a heating rate of 20 °C min−1. Analysis was performed using the Mettler Toledo Thermogravimetric Analyzer (TGA), model TGA/DSC1 (Schwerzenbach, Switzerland).
2.3.4. X-ray Diffraction (XRD)
XRD measurements of composite films were performed with Cu Kα radiation (λ = 1.54056 Å) at a scan rate of 0.033333°/s (step = 0.04° and time per step = 1.2 s) with an accelerated voltage of 40 kV and an applied current of 40 mA, varying from 5 to 60°. The analysis was recorded on an X-ray analyzer (X’Pert-MPD, Philips, Almelo, The Netherlands).
2.3.5. Thickness
Film thickness was measured using a micrometer (Mitutoyo, MDC-SX, Kawasaki, Japan). Five random locations around each film sample were used for thickness determination [26]. All tests were carried out with 5 repetitions.
2.3.6. Mechanical Properties
The samples were cut to a width of 15 mm using high-precision equipment (RDS-100-C, ChemInstruments, Fairfield, OH, USA). Then, they were conditioned for 48 h at 23 ± 2 °C and 50 ± 5% RH. Tensile strength (TS), elongation at break (EB) and modulus of elasticity (ME) were determined using the curve generated from the software of a universal testing machine (Instron, 5966-E2, Norwood, MA, USA). The tests were performed with a 1 kN load cell at a speed of 12 mm min−1 and distances of 50 mm [27]. All tests were carried out with 5 repetitions.
2.3.7. Water-Vapor Transmission Rate (WVTR)
The water-vapor transmission rate (WVTR) was determined by gravimetric analysis (3 replicates for each sample) using capsules (sealed by the films) with a 50 cm2 permeation area and analytical balance (Mettler Toledo, Columbus, OH, USA) with a resolution of 10−4 g. The tests were performed in an air-conditioned chamber (Weiss Technik, Reiskirchen, Germany) at 25 °C and 75% RH with anhydrous calcium chloride desiccant inside the capsule. The WVTR (g m−2 s−1) was determined from the slope of the curve “mass change vs. time” [28].
2.3.8. Light Barrier
The light transmittance of the film samples was measured over a broad wavelength range (from UV to visible) between 200 and 800 nm using a UV-visible spectrophotometer (Cary 60, Agilent Technologies) with a scanning speed of 300 nm min−1 [29]. The tests were performed in triplicate.
2.3.9. Statistical Analysis
The results were statistically evaluated using analysis of variance (ANOVA) and Tukey’s test to compare the means (p < 0.05).
3. Results and Discussion
3.1. Film Morphology
The SA/P films were transparent and had a dark yellow color. The addition of SiO2 does not significantly affect the color of the film (Figure 1). SEM photos of the tested types of films are shown in Figure 1. SA/P films have an even, smooth surface without any cracks. However, cracks are visible in the cross-section, which may indicate slight compatibility between SA and P. Mallakpour and Mohammadi (2022), who also obtained films based on pectin and sodium alginate, reached similar conclusions [30]. The addition of SiO2 to the SA/P film changed the morphology of the matrix. An increase in the roughness of the film can be observed with a simultaneous increase in the concentration of SiO2. However, no accumulation of SiO2 in the film was noted. The type of biopolymer matrix used for SiO2 incorporation is of great significance. In the case of films based on sodium alginate and hydrolyzed collagen, the addition of SiO2 caused agglomeration of nanoparticles in the matrix, indicating poor dispersion and, consequently, the formation of a composite material instead of a nanocomposite [20]. Chitosan-SiO2 nanoparticles, formed at different times by ultrasonic treatment, were added to the thermoplastic starch and it was observed that the film surface became flatter while the size of the nanoparticles decreased. In addition, improvement in the mechanical properties was demonstrated [31]. The addition of SiO2 to the whey protein isolate/pullulan matrix made the structure compact and smooth [32]. Nonetheless, whether there is a dispersion of SiO2 in the biopolymer matrix or agglomeration of nanoparticles, in both cases, the mechanical properties of the presented films improved due to the presence of SiO2.
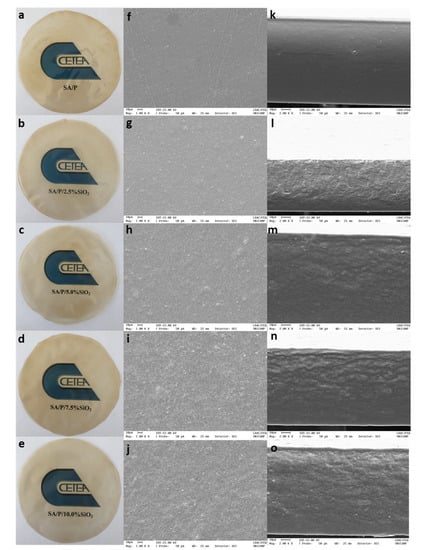
Figure 1.
Photographs and SEM micrographs of the surface and cross-section of SA/P (a,f,k), SA/P/2.5%SiO2 (b,g,l), SA/P/5.0%SiO2 (c,h,m), SA/P/7.5%SiO2 (d,i,n), and SA/P/10.0%SiO2 (e,j,o) films.
3.2. FT-IR Analysis
The FTIR spectra for the SA/P films and their nanocomposites are presented in Figure 2. In general, the peaks related to pure biopolymers reported in previous studies were assigned [24,33]. The characteristic peaks for SA/P films were observed at 1097 and 1030 cm−1, which describe the O-H stretching vibration and glycosidic bonds linking 2 galacturonic sugar units, respectively [34]. Additionally, the peaks between 1400 and 1600 cm−1 are related to the COO− asymmetric and symmetric stretching vibrations [20]. The broad band at approximately 3340 cm−1 corresponds to –OH stretching, which can be associated with the presence of hydroxyl groups from biopolymers and glycerol. The peak at 1740 cm−1 corresponds to the C=O stretching of aliphatic ester groups from P-based films [33]. The addition of SiO2 to the SA/P film did not cause any significant changes in the FTIR spectra, which may indicate that there was no chemical bonding between the components. The lack of changes in the spectrum can be attributed to the overlapping of the characteristic biopolymer and SiO2 bands [20,24].
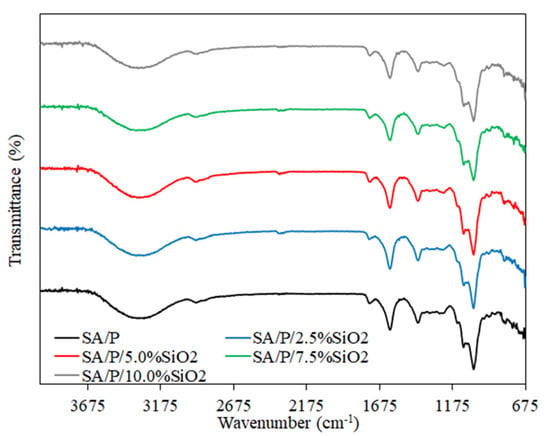
Figure 2.
FT-IR spectra of SA/P and SA/P/SiO2 films.
3.3. XRD Analysis
In Figure 3, XRD patterns are provided for films from all samples evaluated in this study. Analyzing the profile of the SA/P control film, the presence of 2 broad peaks at 2θ ≈ 13° and 2θ ≈ 21° is verified, highlighting their mostly amorphous structure. This poor crystalline nature may also explain the presence of noise in all XRD results, which is commonly found in both SA [24] and P films [33]. As the SiO2 concentration increased, it could be seen that the broad peak at 2θ ≈ 13° reduced in intensity, indicating a slight reduction in the crystallinity of the blend film. Similar behavior was observed in the case of incorporating SiO2 in SA films [23] and adding montmorillonite clay to these films [35]. It is noteworthy that, even with the increase in SiO2 concentration, the typical broad and strong diffraction peak at 2θ ≈ 23° of its amorphous structure was not observed in any of the samples, confirming that there was good filler dispersion along the biopolymer matrix.
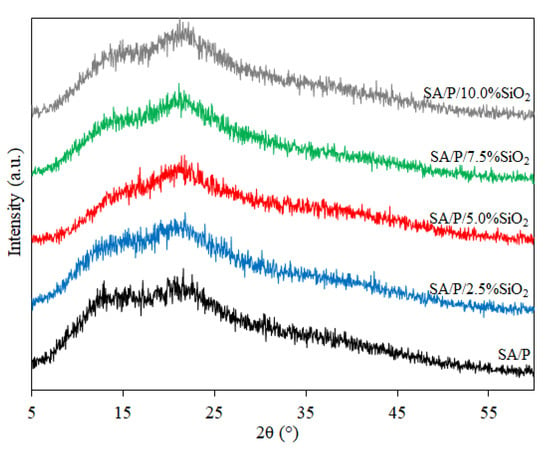
Figure 3.
XRD patterns of SA/P and SA/P/SiO2 films.
3.4. Thermal Stability of the Films
In Figure 4a,b, the TGA curves are presented, as well as their associated derivative (DTG) with 2 main thermal events. Additionally, in Table 1, the initial degradation temperature (Tonset) is presented, as well as the temperature of maximum decomposition rate (Tmax), and mass loss (%) of these thermal events. The first thermal event, up to approximately 200 °C, is related to water evaporation. Water exists in 3 states within hydrophilic polymer matrices: (i) free water, which presents the same phase transition temperature as bulk water and becomes crystallized at 0 °C; (ii) freezable bound water, which crystallizes at a temperature lower than 0 °C due to the weak intermolecular forces between water molecules and polymeric chains, such as van der Waals interactions; and (iii) non-freezable bound water, which does not crystallize even when the sample is cooled to −100 °C. This last behavior is due to the strong hydrogen bonds between the polar moieties of hydrophilic polymers and water molecules [5,18,36,37]. In the thermal behavior profile of the present study, the mass loss at low temperatures can be ascribed to the elimination of free water, while above 100 °C, the release of freezable and non-freezable bound water may occur.
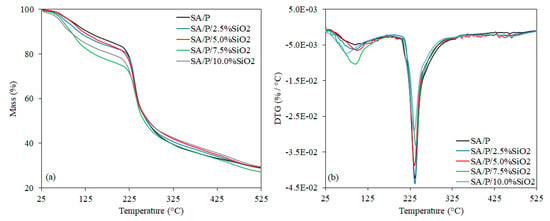
Figure 4.
TGA (a) and DTG (b) of SA/P and SA/P/SiO2 films.

Table 1.
Onset temperature (Tonset), temperature of maximum degradation rate (Tmax) and mass loss of SA/P and SA/P/SiO2 films.
From Table 1, it is also possible to note that the incorporation of SiO2 apparently contributed to the higher moisture content of the films, which is associated with the higher percentages of mass loss in this first thermal event. This result apparently suggests a reduction in the thermal stability of the films. In the second thermal event, polysaccharide decomposition starts, involving random rupture of glycosidic bonds, vaporization, and elimination of volatile products [18]. In previous research, it has been demonstrated that the 2 polymers (SA and P) present similar depolymerization patterns, displaying very close thermal stability [18,33,38]. Furthermore, it was also shown that the incorporation of SiO2 increased the thermal stability of pure sodium alginate films [23,24,39] and sodium alginate/hydrolyzed collagen blend films [20].
However, when the second thermal event is evaluated (Figure 4a), it can be observed that the incorporation of SiO2 displayed different profiles. Apparently, there were no significant differences at the beginning of Tonset when the SA/P, SA/P/2.5%SiO2, SA/P/7.5%SiO2 and SA/P/10.0%SiO2 samples were compared. However, there was noted improvement of Tonset from 222.27 °C (SA/P film) to 241.47 °C for the SA/P/5.0%SiO2 film sample. The activation energies, determined using Broido’s method [40], were 85 kJ mol−1 (R2 = 0.993) and 74 kJ mol−1 (R2 = 0.991) for these samples, respectively, being within the range of 225–250 °C. The lower activation energy for SA/P/5.0%SiO2 indicates that less energy is needed to trigger its degradation process. Thus, despite having identified a larger Tonset, it is not possible to state that the incorporation of SiO2 improved thermal stability during the second thermal event.
3.5. Thickness and Mechanical Properties
The control film (SA/P) had a thickness of 115.5 ± 13.0 µm (Table 2). The addition of SiO2 between 2.5% and 7.5% did not result in a significant difference in thickness with regard to the control film (p < 0.05). However, the addition of the highest concentration of SiO2 (10%) resulted in a significant increase in film thickness in relation to the other films. The increase in SA/P/10.0%SiO2 film thickness is due to the increase in dry matter from SiO2 in the polymer matrix, which is able to increase the free volume between macromolecular chains. Similar results were found for alginate/CuS films [3] and sodium alginate/hydrolyzed collagen/SiO2 films [20].

Table 2.
Thickness and water-vapor transmission rate (WVTR) of SA/P and SA/P/SiO2 films.
The control film (SA/P) showed a tensile strength (TS) of 27.7 ± 3.7 MPa (Figure 5a). The addition of SiO2 concentrations higher than 5% resulted in a significant increase in TS (p < 0.05). The highest TS was observed for the SA/P/5.0%SiO2 film, presenting a value of 40.6 ± 4.5 MPa. The increase in TS with the addition of SiO2 is related to the possible intermolecular interactions between SiO2 and the carboxylic groups of the polymer matrix, as previously observed for sodium alginate/hydrolyzed collagen/SiO2 films [20]. Despite the increase in TS, an increasing trend was not observed as a function of the rise in SiO2 content. This behavior may be related to the aggregation of SiO2 particles in the films with higher SiO2 loads, as observed in the SEM images (Figure 1).
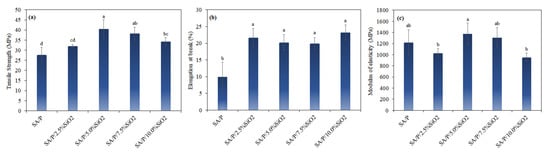
Figure 5.
Mechanical properties of SA/P and SA/P/SiO2 films—(a) tensile strength, (b) elongation at break, and (c) modulus of elasticity. a, b, c, d The values followed by the same letter do not differ at the 95% confidence level (p > 0.05).
The elongation at break (EB) of the control film (SA/P) was 9.9 ± 4.4%, while the films with the addition of SiO2 presented significantly higher values (p < 0.05), i.e., EB between 19.9 ± 1.9% and 23.2 ± 2.3% (Figure 5b). Regardless of the concentration, the films with SiO2 did not show significant differences between them concerning EB (p < 0.05). This behavior can be explained by the dispersion of SiO2 particles in the polymer matrix, causing mutual rearrangements of the chain units and resulting in better flexibility of the polymeric chains [41]. This result is in agreement with that obtained for sodium alginate/hydrolyzed collagen/SiO2 [20] and sodium alginate/agar/SiO2 films [41]. The modulus of elasticity (ME) of the control film (SA/P) showed an ME of 1215.3 ± 232.1 MPa, and the films with SiO2 addition did not present significantly different values (p < 0.05) (Figure 5c). Thus, the incorporation of SiO2 particles did not influence film stiffness.
In order to compare the obtained results regarding mechanical strength of the obtained films, in Table 3, the results are shown for the mechanical strength of films from other biopolymers. It may be noted that the obtained films have, in many cases, higher TS values than films obtained from other biopolymers, which also confirms the correct choice of material components.

Table 3.
Influence of SiO2 additive on mechanical properties (tensile strength- TS and elongation at break-EAB) of selected biopolymer films.
3.6. WVTR
The results obtained from the water-vapor transmission rate (WVTR) of the SA/P/SiO2 films are presented in Table 2. The WVTR of the SA/P/2.5%SiO2 and SA/P/10.0%SiO2 films did not show any significant differences in relation to the control film (SA/P), with values varying between 319.8 ± 38.7 and 369.8 ± 30.0 g m−2 day−1. In other studies, low concentrations of SiO2 were also not sufficient to reduce the WVTR of K-carrageenan [44], sodium alginate [24] or sodium alginate/hydrolyzed collagen films [5]. On the other hand, films with excess SiO2 tend not to show a reduction in WVTR, as the high SiO2 charge can cause greater interaction with water molecules [41], as noted for the SA/P/10.0%SiO2 film.
The films with amounts of SiO2 fillers totaling 5.0 and 7.5% were effective in reducing the WVTR of the films, presenting values significantly lower than those for the control, with the lowest value equal to 276.5 ± 6.5 g m−2 day−1 obtained for the SA/P/7.5%SiO2 film. This reduction in the WVTR value may be associated with the formation of hydrogen bonds between the biopolymer matrix and the hydroxyls from the particles [32]. The strong interaction formed between SiO2 and the polymer matrix can be conducive to the formation of a denser matrix, preventing the passage of water molecules through the film [43]. In addition, the good dispersion of SiO2 particles contributes to the reduction of void spaces in the polymer matrix, consequently, reducing the WVTR of the SA/P films, as observed in the SEM images (Figure 1).
3.7. Light Barrier Properties
The transmittance results as a function concerning the wavelength of the SA/P/SiO2 films are shown in Figure 6. The control film (SA/P) demonstrated higher transmittance within the visible light wavelength range (400–800 nm) than films incorporated with SiO2. The reduction in visible light transmission as the concentration of SiO2 increased may be related to light scattering caused by the incorporation of particles at the nanoscale [23]. Considering the ultraviolet (UV) region, particularly between 200 and 300 nm, the transmittance was very close to 0 for all samples. The biopolymer molecules that make up the films themselves comprise chromophore groups with high UV-light absorption capacity. For example, P-based films presented a UV light-barrier effect up to 300 nm related to n→π∗ transitions in carbonyl groups of carboxylate or ester moieties [33], which may also be present in the SA behavior. Hence, the addition of SiO2 had no apparent, significant influence on this result. Furthermore, for all films, regardless of SiO2 concentration, the UV-light barrier profiles were similar even at wavelengths between 300 and 400 nm. The reduction of transmittance occurred, but at a very little pronounced intensity.
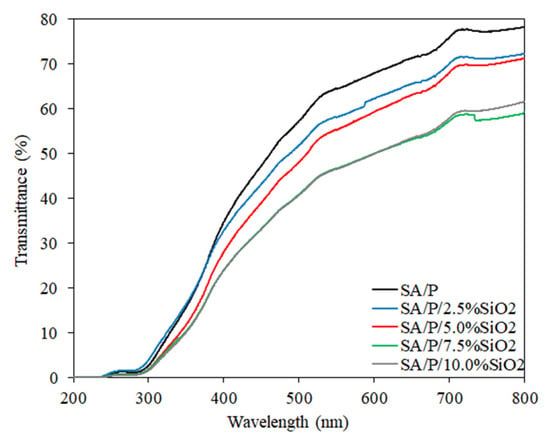
Figure 6.
Light transmission profile (%) of SA/P and SA/P/SiO2 films.
4. Conclusions
The aim of this study was to investigate the effects of incorporating different concentrations (0–10%) of SiO2 on the physicochemical properties of blend films based on natural polymers: sodium alginate (SA) and citrus pectin (P). Overall, it was concluded that the most suitable formulation contained 5% SiO2, as it enabled simultaneous improvement of mechanical, barrier-related and thermal properties. Comparing the control sample (SA/P) with the SA/P/5.0%SiO2 one, tensile strength increased from 27.7 ± 3.7 to 40.6 ± 4.5 MPa, elongation at break increased from 9.9 ± 4.4 to 19.9 ± 1.9%, while modulus of elasticity did not present significant alteration. Thus, it was possible to improve the strength of the film and its plasticity without drastically modifying its rigidity. In parallel, the WVTR decreased from 319.8 ± 38.7 to 288.9 ± 23.5 g m−2 day−1 for the same formulation with 5% SiO2, indicating an increased moisture barrier. It is also important to highlight that there was an increase in the light barrier of the films as a function of SiO2 concentration, suggesting applicability in the protection of foods subject to deterioration caused by this variable. Therefore, SiO2 as a reinforcing agent in SA/P blends, represents a simple, inexpensive and effective strategy for improving the properties of biopolymer-based films.
Author Contributions
Data curation, L.M.J., C.R.F., E.J. and R.P.V.; Formal analysis, L.M.J., C.R.F. and R.M.V.A.; Funding acquisition, R.P.V.; Investigation, L.M.J. and R.M.V.A.; Methodology, L.M.J., R.P.V. and R.M.V.A.; Project administration, R.P.V. and R.M.V.A.; Supervision, R.M.V.A.; Writing – original draft, L.M.J., C.R.F., E.J. and R.P.V.; Writing – review & editing, R.P.V., E.J. and R.M.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the National Council for Scientific and Technological Development (CNPq), grant number [403595/2021]; and the São Paulo Research Foundation (FAPESP) for the postdoctoral fellowship of L. Marangoni Júnior [2021/04043-2]. This study was partly financed by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Financial Code [001].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Júnior, L.M.; Vieira, R.P.; Anjos, C.A.R. Kefiran-based films: Fundamental concepts, formulation strategies and properties. Carbohydr. Polym. 2020, 246, 116609. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Solving the plastic problem: From cradle to grave, to reincarnation. Sci. Prog. 2019, 102, 218–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Rhim, J.-W. Effect of CuS reinforcement on the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of alginate-based composite films. Int. J. Biol. Macromol. 2020, 164, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Shamsuri, A.A.; Abdan, K.; Kaneko, T. A Concise Review on the Physicochemical Properties of Biopolymer Blends Prepared in Ionic Liquids. Molecules 2021, 26, 216. [Google Scholar] [CrossRef] [PubMed]
- Júnior, L.M.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Water vapor sorption and permeability of sustainable alginate/collagen/SiO2 composite films. LWT 2021, 152, 112261. [Google Scholar] [CrossRef]
- Júnior, L.M.; Rodrigues, P.R.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Sustainable Packaging Films Composed of Sodium Alginate and Hydrolyzed Collagen: Preparation and Characterization. Food Bioprocess Technol. 2021, 14, 2336–2346. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Biomethane production from various segments of brown seaweed. Energy Convers. Manag. 2018, 174, 855–862. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the brown seaweed Sargassum latifolium in the design of alginate-fucoidan based films with natural antioxidant properties and kinetic modeling of moisture sorption and polyphenolic release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Babu, R.P.; Oconnor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Chen, B. Transparent, UV-proof and mechanically strong montmorillonite/alginate/Ca2+ nanocomposite hydrogel films with solvent sensitivity. Appl. Clay Sci. 2018, 165, 223. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Hu, Z.; Hu, L.; Li, G.; Yao, Q.; Hu, Y. Pectin-based active packaging: A critical review on preparation, physical properties and novel application in food preservation. Trends Food Sci. Technol. 2021, 118, 167–178. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.-X.; Avena-Bustillos, R.D.J.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Moreno, L.; Wosiacki, G.; de Scheer, A.P. Pectin: From raw material to the final product. Polímeros 2012, 22, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Kurczewska, J.; Ratajczak, M.; Gajecka, M. Alginate and pectin films covering halloysite with encapsulated salicylic acid as food packaging components. Appl. Clay Sci. 2021, 214, 106270. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, E.J.S.; Pius, A. Biopolymer Blends and Composites; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2012, 115, 459–465. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidovic, S.; Dimitrijevic-Brankovic, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.; Wicklein, B.; Alcântara, A.C.; Aranda, P. Fibrous clays based bionanocomposites. Prog. Polym. Sci. 2013, 38, 1392–1414. [Google Scholar] [CrossRef] [Green Version]
- Júnior, L.M.; Rodrigues, P.R.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Improving the mechanical properties and thermal stability of sodium alginate/hydrolyzed collagen films through the incorporation of SiO2. Curr. Res. Food Sci. 2021, 5, 96–101. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, L.; Sun, J.; Shen, W. The change of the properties of acrylic-based polyurethane via addition of nano-silica. Prog. Org. Coat. 2002, 45, 33–42. [Google Scholar] [CrossRef]
- Barik, T.K.; Sahu, B.; Swain, V. Nanosilica—from medicine to pest control. Parasitol. Res. 2008, 103, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xia, Y. Preparation and characterization of nano-SiO2 reinforced alginate-based nanocomposite films (II). J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Júnior, L.M.; da Silva, R.G.; Anjos, C.A.R.; Vieira, R.P.; Alves, R.M.V. Effect of low concentrations of SiO2 nanoparticles on the physical and chemical properties of sodium alginate-based films. Carbohydr. Polym. 2021, 269, 118286. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.S.S.; Cavazos, P.A.S.; Paz, H.M.; Fragoso, A.V. External factors and nanoparticles effect on water vapor permeability of pectin-based films. J. Food Eng. 2018, 245, 73–79. [Google Scholar] [CrossRef]
- ISO-4593; Plastics: Film and Sheeting Determination of Thickness by Mechanical Scanning. ISO: Geneva, Switzerland, 1993; 2p.
- ASTM-D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2018; 12p.
- ASTM-E96/E96M; Standard Test Methods for Water Vapor Transmission of materials. ASTM: West Conshohocken, PA, USA, 2016; 14p.
- ASTM-E-1348; Standard Test Method for Transmittance and Color by Spectrophotometry Using Hemispherical Geometry. ASTM: West Conshohocken, PA, USA, 2015; 3p.
- Mallakpour, S.; Mohammadi, N. Development of sodium alginate-pectin/TiO2 nanocomposites: Antibacterial and bioactivity investigations. Carbohydr. Polym. 2022, 285, 119226. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yan, X. Preparation of chitosan-SiO2 nanoparticles by ultrasonic treatment and its effect on the properties of starch film. Int. J. Biol. Macromol. 2021, 189, 271–278. [Google Scholar] [CrossRef]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Shahabi-Ghahfarrokhi, I. Development of ecofriendly bionanocomposite: Whey protein isolate/pullulan films with nano-SiO2. Int. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef]
- Júnior, L.M.; Gonçalves, S.D.; da Silva, R.G.; Martins, J.T.; Vicente, A.A.; Alves, R.M.V.; Vieira, R.P. Effect of green propolis extract on functional properties of active pectin-based films. Food Hydrocoll. 2022, 131, 1077. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Cui, J.; Yang, L.; Su, H.; Yang, P.; Pan, J. Colorimetric films based on pectin/sodium alginate/xanthan gum incorporated with raspberry pomace extract for monitoring protein-rich food freshness. Int. J. Biol. Macromol. 2021, 185, 959–965. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Effect of montmorillonite clay and biopolymer concentration on the physical and mechanical properties of alginate nanocomposite films. J. Food Eng. 2013, 117, 26–33. [Google Scholar] [CrossRef]
- Higuchi, A.; Iijima, T. D.s.c. investigation of the states of water in poly(vinyl alcohol) membranes. Polymer 1985, 26, 1207–1211. [Google Scholar] [CrossRef]
- Russo, R.; Abbate, M.; Malinconico, M.; Santagata, G. Effect of polyglycerol and the crosslinking on the physical properties of a blend alginate-hydroxyethylcellulose. Carbohydr. Polym. 2010, 82, 1061–1067. [Google Scholar] [CrossRef]
- Makaremi, M.; Yousefi, H.; Cavallaro, G.; Lazzara, G.; Goh, C.B.S.; Lee, S.M.; Solouk, A.; Pasbakhsh, P. Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin. Polymers 2019, 11, 1594. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Xia, Y.; Wang, Y.; Zhao, X.; Xue, Z.; Quan, F.; Geng, C.; Zhao, Z. Preparation and property investigation of crosslinked alginate/silicon dioxide nanocomposite films. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Broido, A. A simple, sensitive graphical method of treating thermogravimetric analysis data. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 1761–1773. [Google Scholar] [CrossRef]
- Hou, X.; Xue, Z.; Xia, Y.; Qin, Y.; Zhang, G.; Liu, H.; Li, K. Effect of SiO2 nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. Int. J. Biol. Macromol. 2018, 125, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-T.; Chen, C.-L.; Huang, K.-S. Synthesis and properties of chitosan/SiO2 hybrid materials. Mater. Lett. 2007, 61, 1292–1295. [Google Scholar] [CrossRef]
- Tabatabaei, R.H.; Jafari, S.M.; Mirzaei, H.; Nafchi, A.M.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-k-carrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef]
- Rane, L.R.; Savadekar, N.R.; Kadam, P.G.; Mhaske, S.T. Preparation and Characterization of K-Carrageenan/Nanosilica Biocomposite Film. J. Mater. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).