Nondegenerate Polycrystalline Hydrogen-Doped Indium Oxide (InOx:H) Thin Films Formed by Low-Temperature Solid-Phase Crystallization for Thin Film Transistors
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
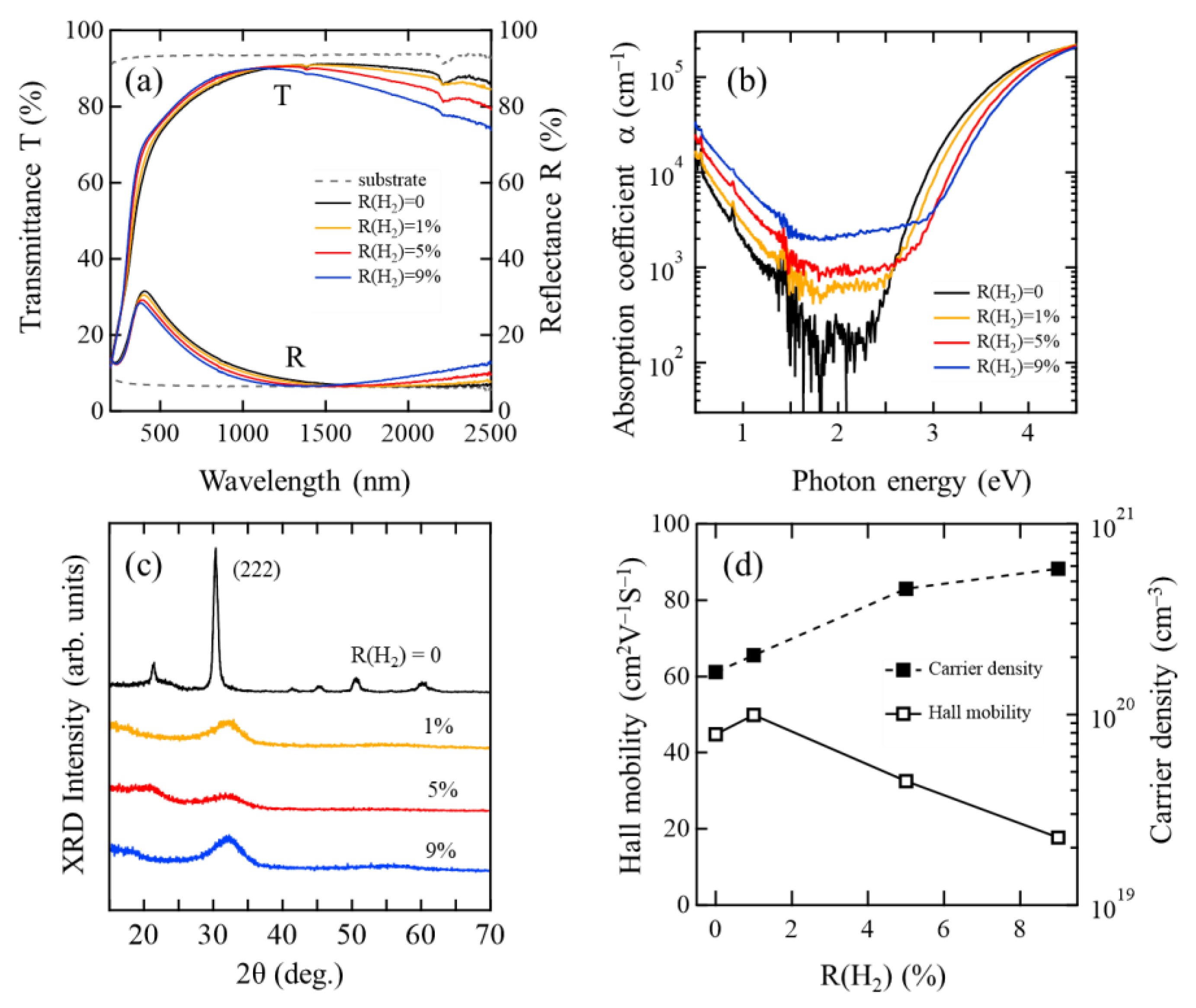
3.1. Optical, Electrical, and Structural Properties of As-Deposited InOx and InOx:H Films
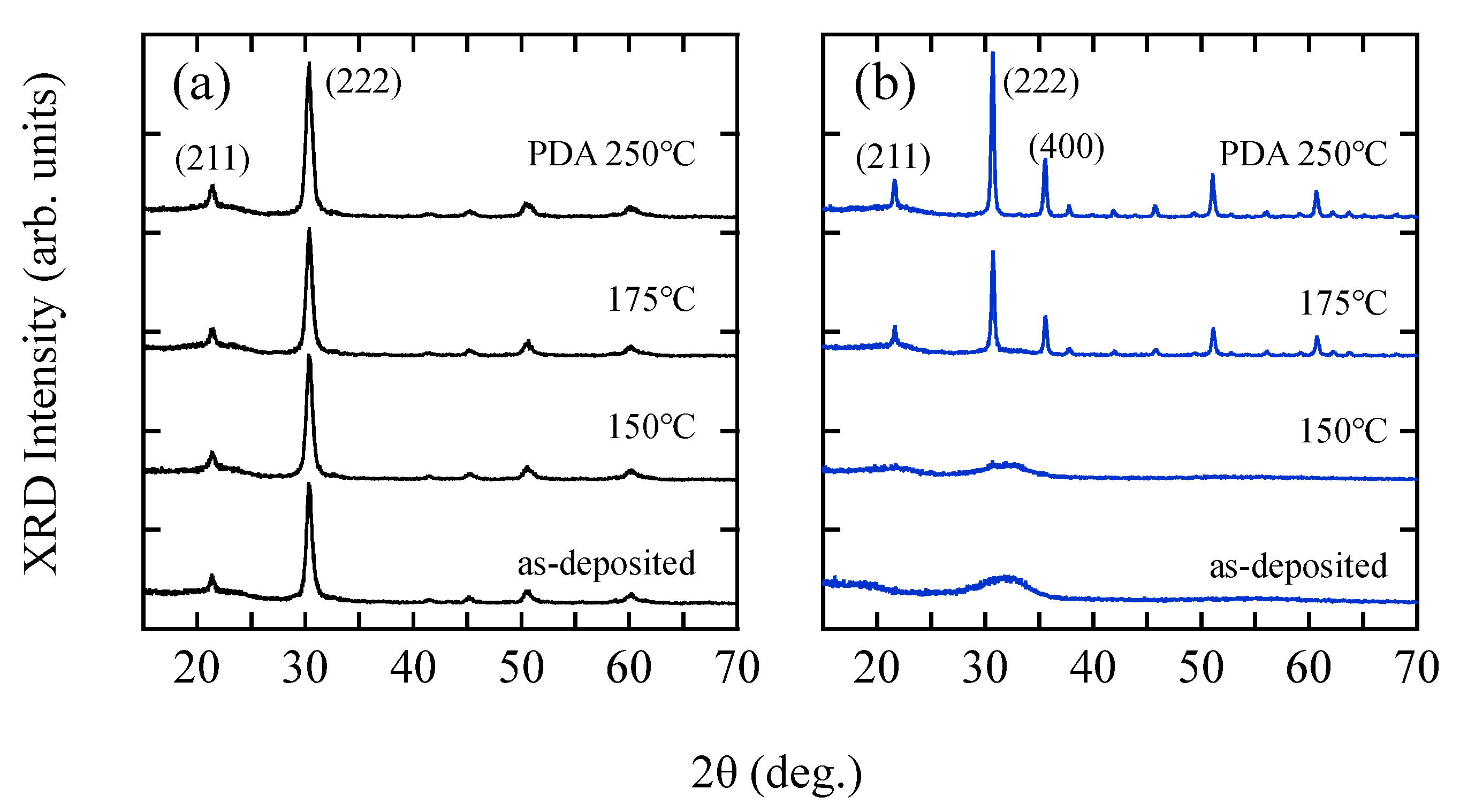
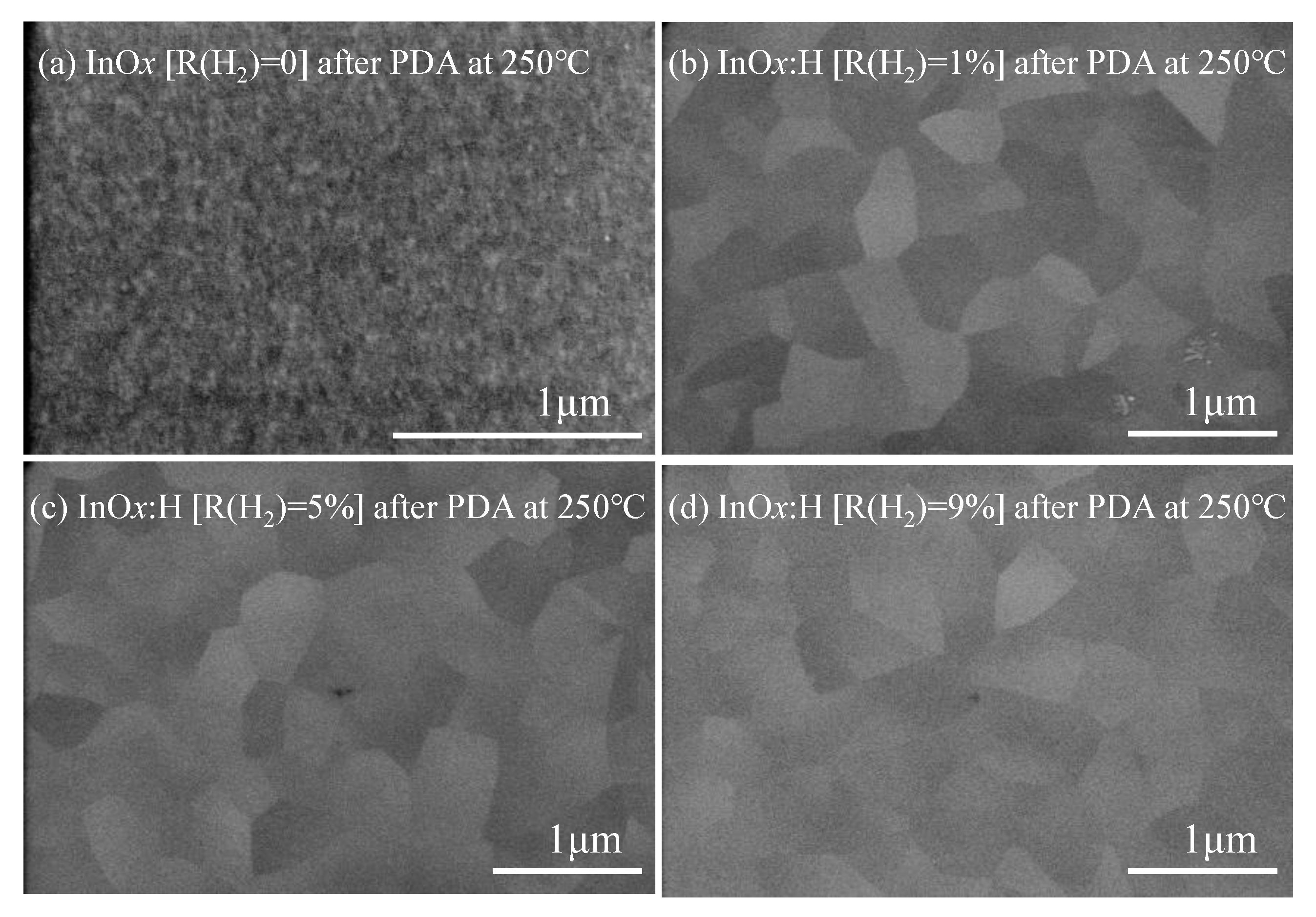
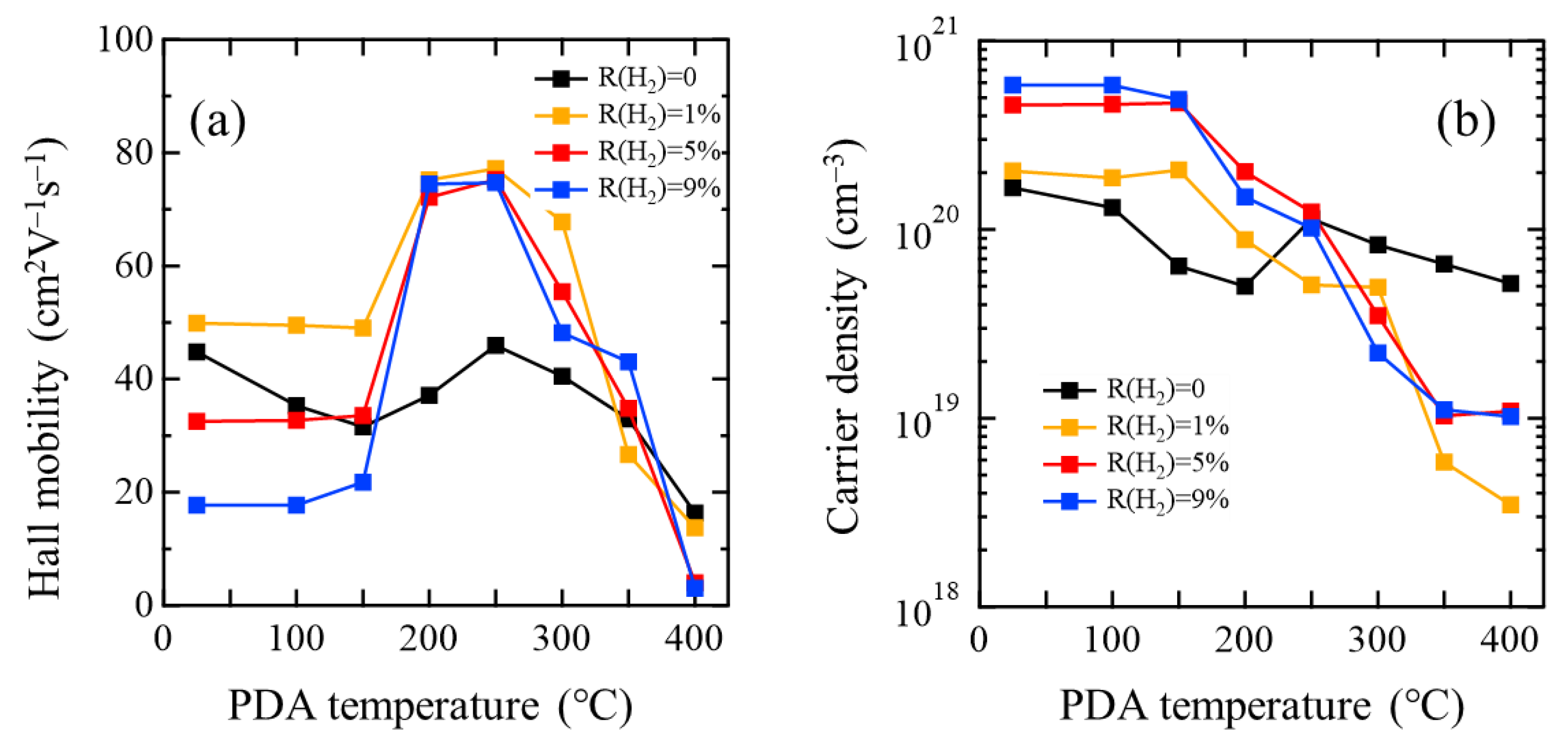
3.2. Changes in Film Properties through PDA
3.3. TDS and HXPS Analysis of InOx and InOx:H Films
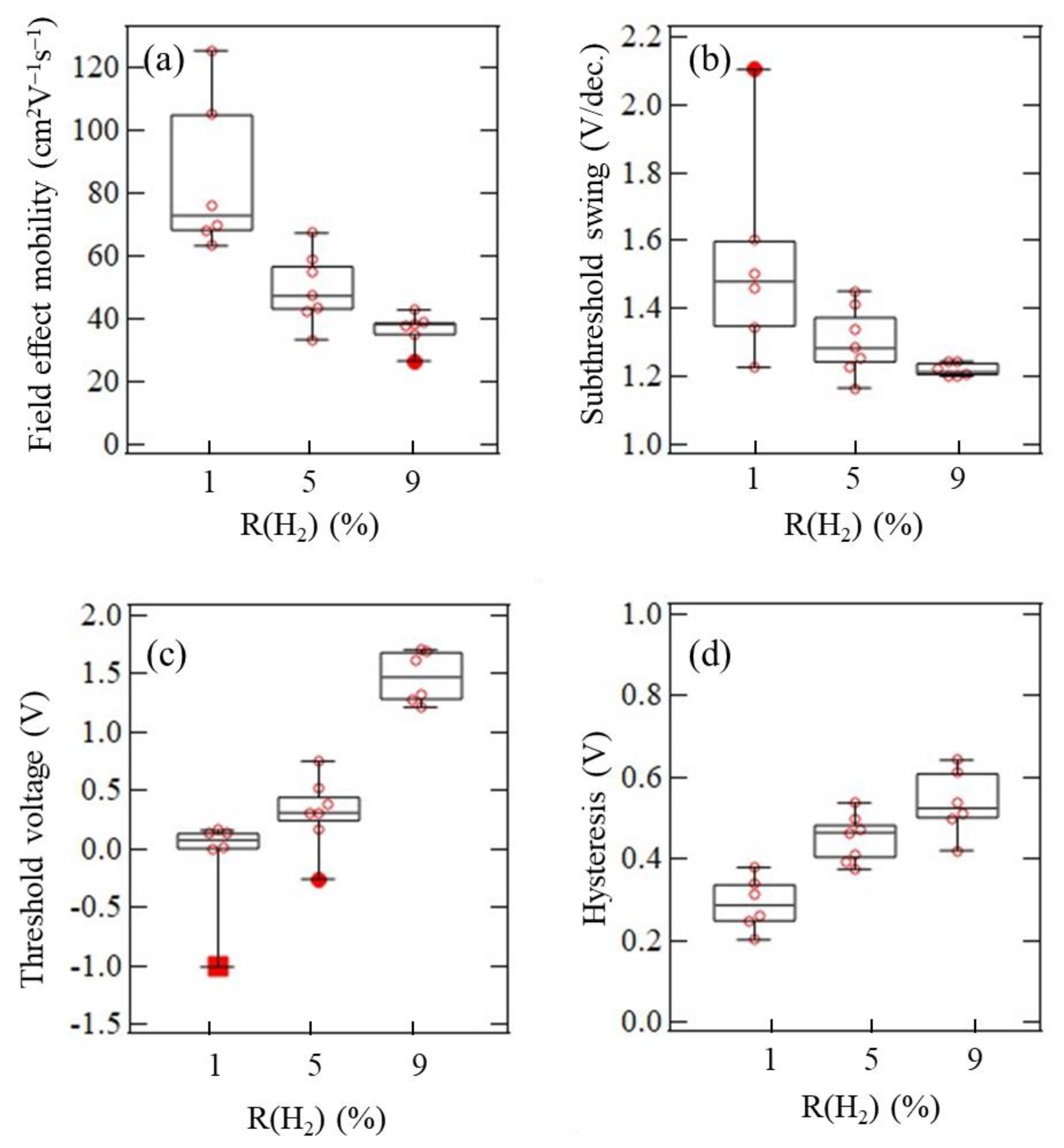
3.4. TFT Application of Polycrystalline InOx and InOx:H Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, M.; Kon, M.; Ishizaki, M.; Sekine, N. Flexible Active-Matrix TFT Array with Amorphous Oxide Semiconductors for Electronic Paper. In Proceedings of the Display Workshops in Conjunction with Asia Display 2005 (IDW/AD’05), Takamatsu, Japan, 6–9 December 2005; p. 845. [Google Scholar]
- Hirao, T.; Furuta, M.; Furuta, H.; Matsuda, T.; Hiramatsu, T.; Hokari, H.; Yoshida, M. 4.1: Distinguished Paper: High Mobility Top-Gate Zinc Oxide Thin-Film Transistors (ZnO-TFTs) for Active-Matrix Liquid Crystal Displays. SID Symp. Dig. Tech. Pap. 2006, 37, 18–20. [Google Scholar] [CrossRef]
- Park, S.-H.K.; Hwang, C.-S.; Lee, J.-I.; Chung, S.M.; Yang, Y.S.; Do, L.-M.; Chu, H.Y. 4.3: Transparent ZnO Thin Film Transistor Array for the Application of Transparent AM-OLED Display. SID Symp. Dig. Tech. Pap. 2006, 37, 25–28. [Google Scholar] [CrossRef]
- Inoue, H.; Matsuzaki, T.; Nagatsuka, S.; Okazaki, Y.; Sasaki, T.; Noda, K.; Matsubayashi, D.; Ishizu, T.; Onuki, T.; Isobe, A.; et al. Nonvolatile Memory With Extremely Low-Leakage Indium-Gallium-Zinc-Oxide Thin-Film Transistor. IEEE J. Solid State Circuits 2012, 47, 2258–2265. [Google Scholar] [CrossRef]
- Yoon, S.-M.; Kim, S.-J.; Park, M.-J.; Yun, D.-J. (Invited) Flexible Memory Applications Using Oxide Semiconductor Thin-Film Transistors. ECS Trans. 2016, 75, 227–233. [Google Scholar] [CrossRef]
- Aihara, S.; Seo, H.; Namba, M.; Watabe, T.; Ohtake, H.; Kubota, M.; Egami, N.; Hiramatsu, T.; Matsuda, T.; Furuta, M.; et al. Stacked Image Sensor With Green- and Red-Sensitive Organic Photoconductive Films Applying Zinc Oxide Thin-Film Transistors to a Signal Readout Circuit. IEEE Trans. Electron Devices 2009, 56, 2570–2576. [Google Scholar] [CrossRef]
- Sakai, T.; Seo, H.; Takagi, T.; Kubota, M.; Ohtake, H.; Furuta, M. Color image sensor with organic photoconductive films. In Proceedings of the IEEE International Electron Devices Meeting (IEDM), Washington, DC, USA, 7–9 December 2015; Volume 8659. [Google Scholar] [CrossRef]
- Koike, K.; Hashimoto, M.; Tsuji, K.; Seiwa, Y.; Ogata, K.-I.; Sasa, S.; Inoue, M.; Yano, M. Characteristics of Polycrystalline ZnO-Based Electrolyte-Solution-Gate Field-Effect Transistors Fabricated on Glass Substrates. Appl. Phys. Express 2009, 2, 087001. [Google Scholar] [CrossRef]
- Takechi, K.; Iwamatsu, S.; Konno, S.; Yahagi, T.; Abe, Y.; Katoh, M.; Tanabe, H. Gate-to-source voltage response in high-sensitivity amorphous InGaZnO4 thin-film transistor pH sensors. Jpn. J. Appl. Phys. 2015, 54, 078004. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef]
- Kamiya, T.; Nomura, K.; Hosono, H. Present status of amorphous In–Ga–Zn–O thin-film transistors. Sci. Technol. Adv. Mater. 2010, 11, 044305. [Google Scholar] [CrossRef]
- Weiher, R.L. Electrical Properties of Single Crystals of Indium Oxide. J. Appl. Phys. 1962, 33, 2834–2839. [Google Scholar] [CrossRef]
- Aikawa, S.; Nabatame, T.; Tsukagoshi, K. Effects of dopants in InOx-based amorphous oxide semiconductors for thin-film transistor applications. Appl. Phys. Lett. 2013, 103, 172105. [Google Scholar] [CrossRef] [Green Version]
- Aikawa, S.; Nabatame, T.; Tsukagoshi, K.; Shinya, A. Si-incorporated amorphous indium oxide thin-film transistors. Jpn. J. Appl. Phys. 2019, 58, 090506. [Google Scholar] [CrossRef]
- Yeom, H.-I.; Ko, J.B.; Mun, G.; Park, S.-H.K. High mobility polycrystalline indium oxide thin-film transistors by means of plasma-enhanced atomic layer deposition. J. Mater. Chem. C 2016, 4, 6873–6880. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Moon, J.; Pi, J.-E.; Ahn, S.-D.; Oh, H.; Kang, S.-Y.; Kwon, K.-H. High mobility ultra-thin crystalline indium oxide thin film transistor using atomic layer deposition. Appl. Phys. Lett. 2018, 113, 112102. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, H.-M.; Shao, Y.; Zhu, B.; Liu, W.-J.; Ding, S.-J.; Zhang, D.W. Atomic-Layer-Deposition of Indium Oxide Nano-films for Thin-Film Transistors. Nanoscale Res. Lett. 2018, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, R.; Nabatame, T.; Onaya, T.; Ohi, A.; Ikeda, N.; Nagata, T.; Tsukagoshi, K.; Ogura, A. Comparison of characteristics of thin-film transistor with In2O3 and carbon-doped In2O3 channels by atomic layer deposition and post-metallization annealing in O3. Jpn. J. Appl. Phys. 2021, 60, 030903. [Google Scholar] [CrossRef]
- Noh, J.H.; Ryu, S.Y.; Jo, S.J.; Kim, C.S.; Sohn, S.-W.; Rack, P.D.; Kim, D.-J.; Baik, H.K. Indium Oxide Thin-Film Transistors Fabricated by RF Sputtering at Room Temperature. IEEE Electron Device Lett. 2010, 31, 567–569. [Google Scholar] [CrossRef]
- Koida, T.; Fujiwara, H.; Kondo, M. Hydrogen-doped In2O3 as High-mobility Transparent Conductive Oxide. Jpn. J. Appl. Phys. 2007, 46, L685–L687. [Google Scholar] [CrossRef]
- Koida, T.; Kondo, M.; Tsutsumi, K.; Sakaguchi, A.; Suzuki, M.; Fujiwara, H. Hydrogen-doped In2O3 transparent conducting oxide films prepared by solid-phase crystallization method. J. Appl. Phys. 2010, 107, 033514. [Google Scholar] [CrossRef]
- Li, S.; Shi, Z.; Tang, Z.; Li, X. Study on the hydrogen doped indium oxide for silicon heterojunction solar cell application. J. Alloy. Compd. 2017, 705, 198–204. [Google Scholar] [CrossRef]
- Erfurt, D.; Heinemann, M.D.; Körner, S.; Szyszka, B.; Klenk, R.; Schlatmann, R. Improved electrical properties of pulsed DC magnetron sputtered hydrogen doped indium oxide after annealing in air. Mater. Sci. Semicond. Process. 2018, 89, 170–175. [Google Scholar] [CrossRef]
- Husein, S.; Stuckelberger, M.; West, B.; Ding, L.; Dauzou, F.; Morales-Masis, M.; Duchamp, M.; Holman, Z.; Bertoni, M.I. Carrier scattering mechanisms limiting mobility in hydrogen-doped indium oxide. J. Appl. Phys. 2018, 123, 245102. [Google Scholar] [CrossRef]
- Muydinov, R.; Steigert, A.; Wollgarten, M.; Michałowski, P.P.; Bloeck, U.; Pflug, A.; Erfurt, D.; Klenk, R.; Körner, S.; Lauermann, I.; et al. Crystallisation Phenomena of In2O3:H Films. Materials 2019, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Aman, S.G.M.; Magari, Y.; Shimpo, K.; Hirota, Y.; Makino, H.; Koretomo, D.; Furuta, M. Low-temperature (150 °C) activation of Ar+O2+H2-sputtered In–Ga–Zn–O for thin-film transistors. Appl. Phys. Express 2018, 11, 081101. [Google Scholar] [CrossRef]
- Koretomo, D.; Hamada, S.; Mori, M.; Magari, Y.; Furuta, M. Marked improvement in reliability of 150 °C processed IGZO thin-film transistors by applying hydrogenated IGZO as a channel material. Appl. Phys. Express 2020, 13, 076501. [Google Scholar] [CrossRef]
- Magari, Y.; Aman, S.G.M.; Koretomo, D.; Masuda, K.; Shimpo, K.; Makino, H.; Kimura, M.; Furuta, M. Record-High-Performance Hydrogenated In–Ga–Zn–O Flexible Schottky Diodes. ACS Appl. Mater. Interfaces 2020, 12, 47739–47746. [Google Scholar] [CrossRef]
- Magari, Y.; Furura, M. Effects of water and hydrogen introduction during In–Ga–Zn–O sputtering on the performance of low-temperature processed thin-film transistors. Jpn. J. Appl. Phys. 2021, 60, SBBM04. [Google Scholar] [CrossRef]
- Mott, N. Review lecture: Metal–insulator transitions. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1982, 382, 1–24. [Google Scholar] [CrossRef]
- Bierwagen, O.; Speck, J.S. High electron mobility In2O3 (001) and (111) thin films with nondegenerate electron concentration. Appl. Phys. Lett. 2010, 97, 072103. [Google Scholar] [CrossRef]
- Nistor, M.; Gherendi, F.; Perrière, J. Degenerate and non-degenerate In2O3 thin films by pulsed electron beam deposition. Mater. Sci. Semicond. Process. 2018, 88, 45–50. [Google Scholar] [CrossRef]
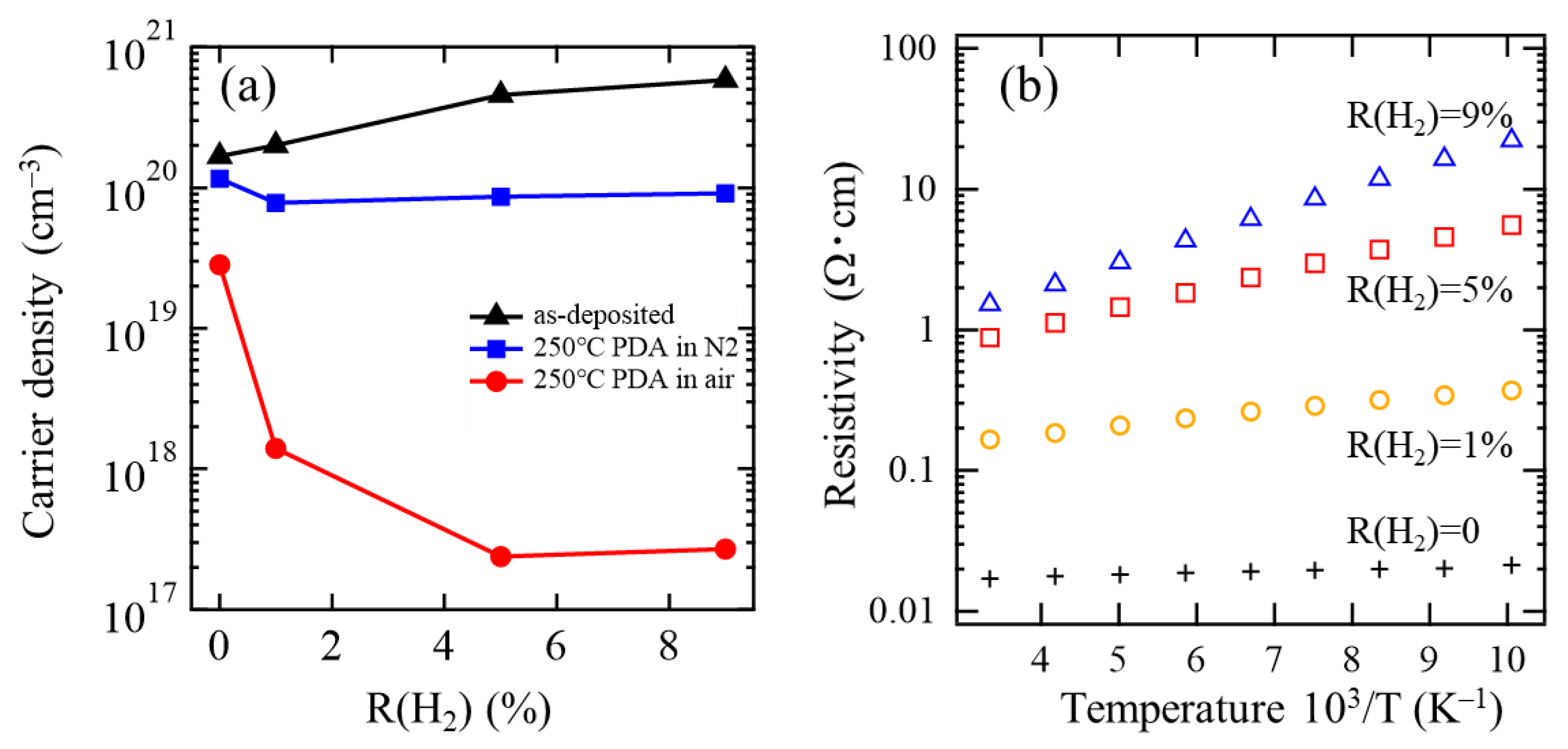
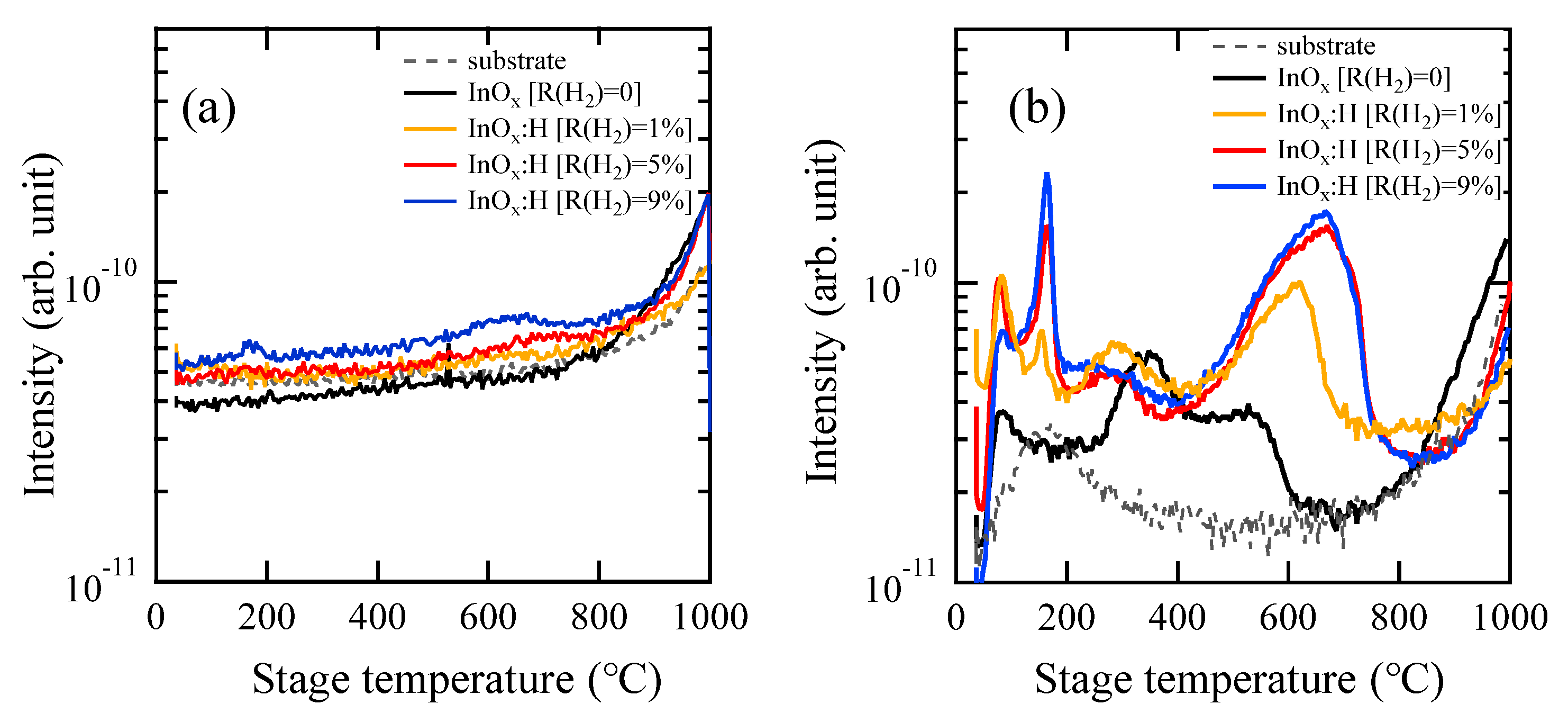
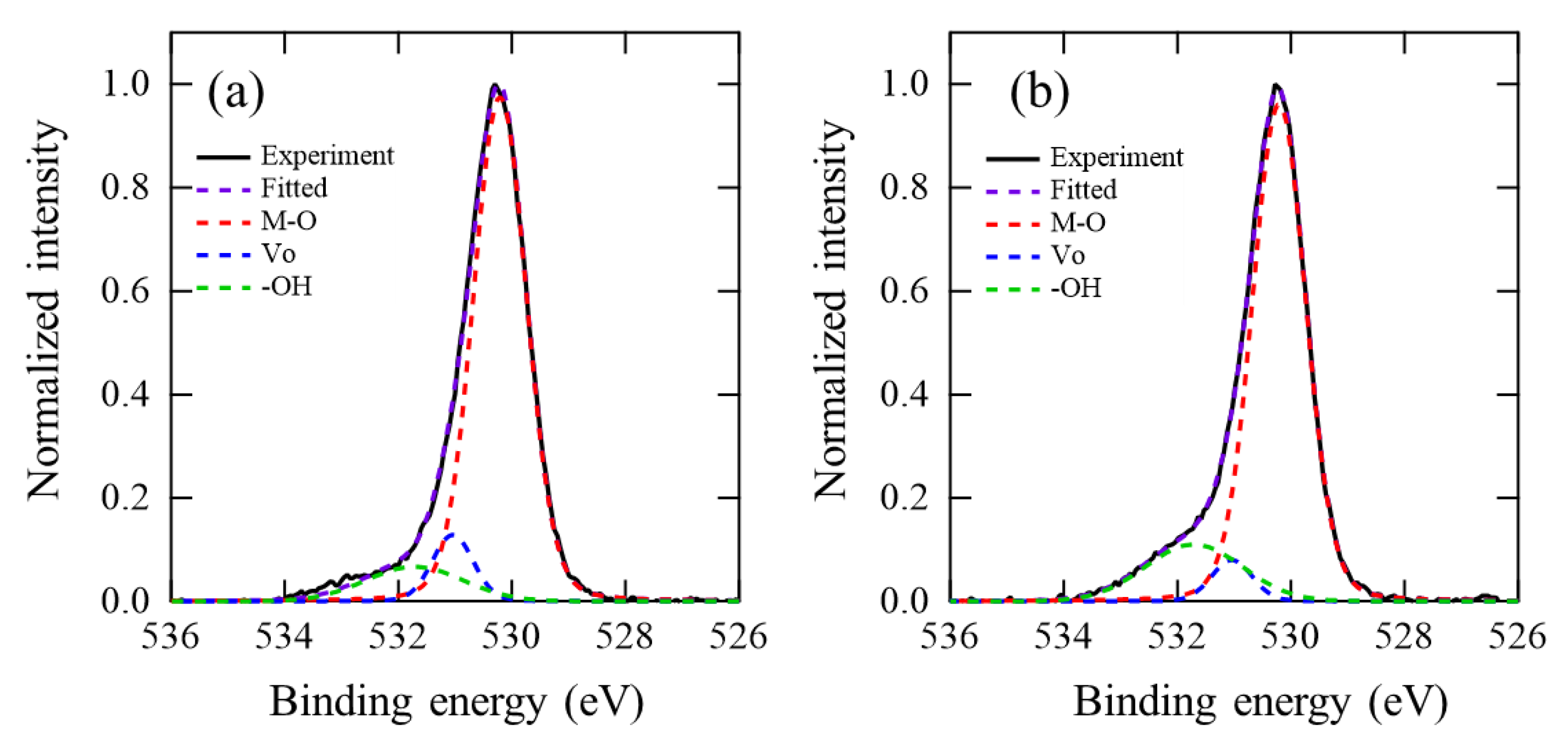
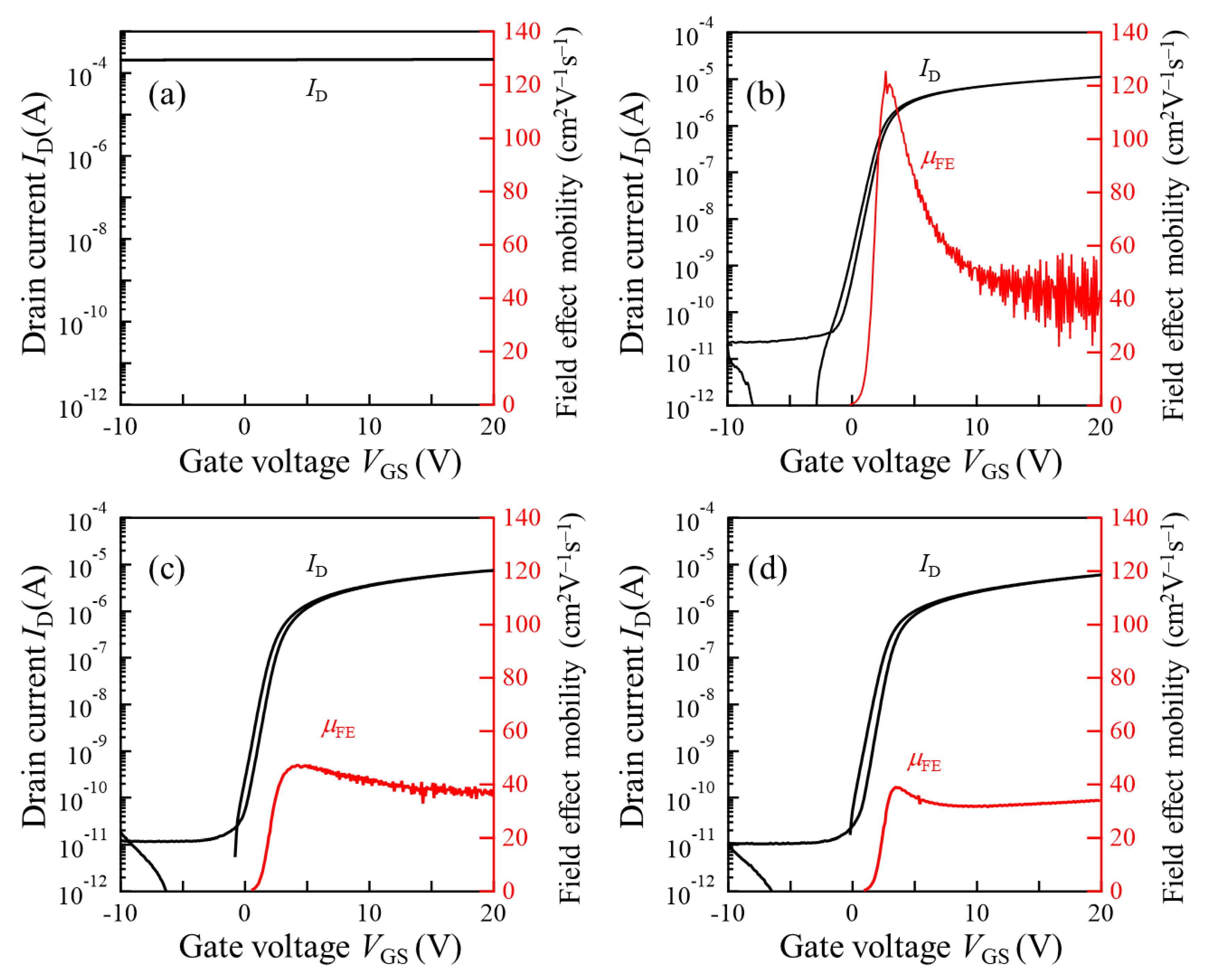
| As-Deposited | 250 °C PDA in N2 | 250 °C PDA in Air | |||||
|---|---|---|---|---|---|---|---|
| R(H2) (%) | μH (cm2V−1s−1) | Ne (cm−3) | μH (cm2V−1s−1) | Ne (cm−3) | μH (cm2V−1s−1) | Ne (cm−3) | |
| InOx | 0 | 44.8 | 1.7 × 1020 | 46.0 | 1.1 × 1020 | 26.5 | 2.8 × 1019 |
| InOx:H | 1 | 49.9 | 2.1 × 1020 | 77.2 | 5.1 × 1019 | 27.0 | 1.4 × 1018 |
| 5 | 32.6 | 4.6 × 1020 | 75.3 | 1.2 × 1020 | 13.8 | 2.4 × 1017 | |
| 9 | 17.7 | 5.8 × 1020 | 74.8 | 1.0 × 1020 | 20.0 | 2.7 × 1017 | |
| μFE (cm2V−1s−1) | Vth (V) | S.S. (V/dec.) | ΔVH (V) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R(H2) (%) | Ave. | Max. | Min. | Ave. | Max. | Min. | Ave. | Max. | Min. | Ave | |
| InOx | 0 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| InOx:H | 1 | 84.7 | 125.7 | 63.4 | −0.10 | 0.16 | −1.01 | 1.54 | 2.10 | 1.22 | 0.29 |
| 5 | 49.7 | 67.4 | 33.4 | 0.31 | 0.75 | −0.26 | 1.30 | 1.45 | 1.16 | 0.45 | |
| 9 | 36.6 | 42.9 | 26.6 | 1.47 | 1.70 | 1.21 | 1.22 | 1.25 | 1.20 | 0.54 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, T.; Magari, Y.; Makino, H.; Furuta, M. Nondegenerate Polycrystalline Hydrogen-Doped Indium Oxide (InOx:H) Thin Films Formed by Low-Temperature Solid-Phase Crystallization for Thin Film Transistors. Materials 2022, 15, 187. https://doi.org/10.3390/ma15010187
Kataoka T, Magari Y, Makino H, Furuta M. Nondegenerate Polycrystalline Hydrogen-Doped Indium Oxide (InOx:H) Thin Films Formed by Low-Temperature Solid-Phase Crystallization for Thin Film Transistors. Materials. 2022; 15(1):187. https://doi.org/10.3390/ma15010187
Chicago/Turabian StyleKataoka, Taiki, Yusaku Magari, Hisao Makino, and Mamoru Furuta. 2022. "Nondegenerate Polycrystalline Hydrogen-Doped Indium Oxide (InOx:H) Thin Films Formed by Low-Temperature Solid-Phase Crystallization for Thin Film Transistors" Materials 15, no. 1: 187. https://doi.org/10.3390/ma15010187
APA StyleKataoka, T., Magari, Y., Makino, H., & Furuta, M. (2022). Nondegenerate Polycrystalline Hydrogen-Doped Indium Oxide (InOx:H) Thin Films Formed by Low-Temperature Solid-Phase Crystallization for Thin Film Transistors. Materials, 15(1), 187. https://doi.org/10.3390/ma15010187







