Fabrication of a Novel Protein Sponge with Dual-Scale Porosity and Mixed Wettability Using a Clean and Versatile Microwave-Based Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Foam Preparation and Drying
2.3. In-Line Temperature Measurement
2.4. In-Line Expansion Determination
2.5. Scanning Electron Microscopy
2.6. Absorption Test
2.7. Synchrotron Computed Microtomography (μ-CT)
2.8. Mechanical Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fabrication Process of the Protein Sponge
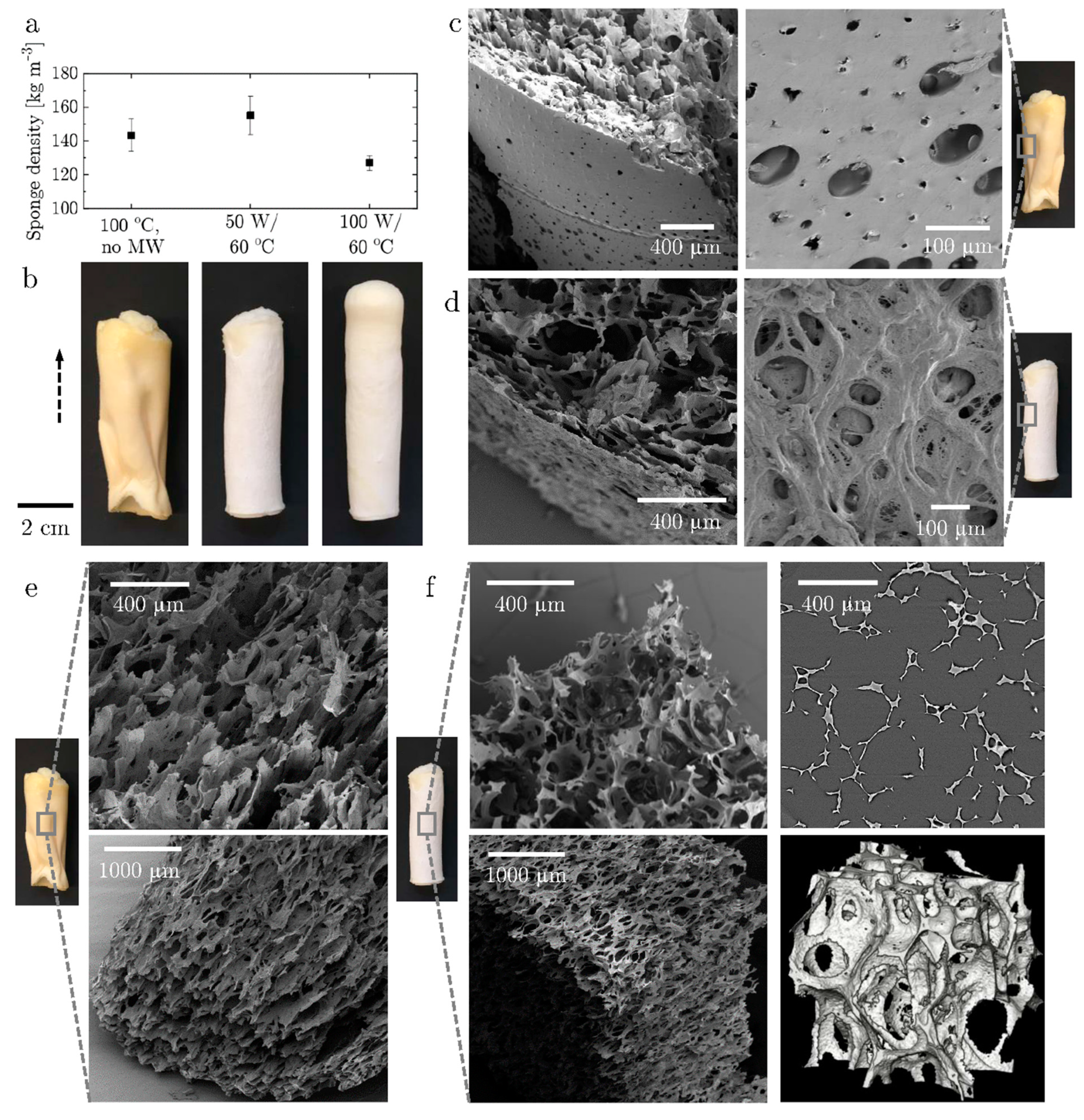
3.2. Structure of the Dried Sponge
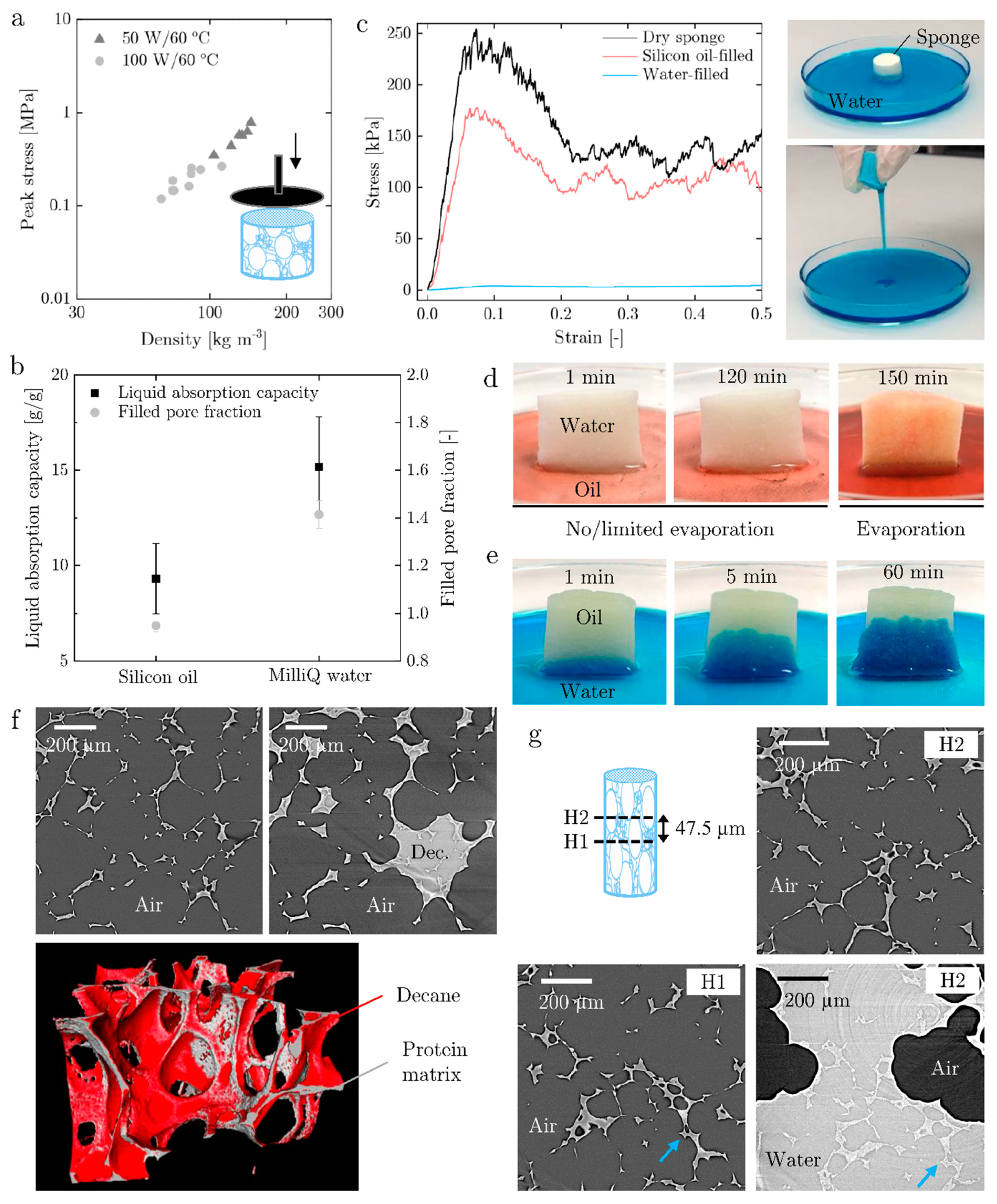
3.3. Mechanical Properties and Absorption Performance of the Dried Sponge
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.; Le, A.T.T.; Hoang, N.; Tan, V.B.C.; Duong, H.M. Cellulose aerogel from paper waste for crude oil spill cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Barbetta, A.; Dentini, M.; De Vecchis, M.S.; Filippini, P.; Formisano, G.; Caiazza, S. Scaffolds based on biopolymeric foams. Adv. Funct. Mater. 2005, 15, 118–124. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Nyström, G.; Fernández-Ronco, M.P.; Bolisetty, S.; Mazzotti, M.; Mezzenga, R. Amyloid Templated Gold Aerogels. Adv. Mater. 2016, 28, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef]
- Muhammad, A.; Lee, D.; Shin, Y.; Park, J. Recent Progress in Polysaccharide Aerogels: Their Synthesis, Application, and Future Outlook. Polymers 2021, 13, 1347. [Google Scholar] [CrossRef]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Rahman, N.A.; Liou, N.S. Recent advances in celluloses and their hybrids for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2020, 158, 670–688. [Google Scholar] [CrossRef]
- Franco, P.; Aliakbarian, B.; Perego, P.; Reverchon, E.; De Marco, I. Supercritical Adsorption of Quercetin on Aerogels for Active Packaging Applications. Ind. Eng. Chem. Res. 2018, 57, 15105–15113. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Starch aerogel loaded with poorly water-soluble vitamins through supercritical CO2 adsorption. Chem. Eng. Res. Des. 2017, 119, 221–230. [Google Scholar] [CrossRef]
- Pantić, M.; Knez, Ž.; Novak, Z. Supercritical impregnation as a feasible technique for entrapment of fat-soluble vitamins into alginate aerogels. J. Non. Cryst. Solids 2016, 432, 519–526. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk fibroin aerogels: Potential scaffolds for tissue engineering applications. Biomed. Mater. 2015, 10, 35002. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, J.C.; Hughes, M.; Lucia, L.A.; Laine, J.; Ekman, K.; Rojas, O.J. Soy protein-nanocellulose composite aerogels. Cellulose 2013, 20, 2417–2426. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Selmer, I.; Karnetzke, J.; Kleemann, C.; Lehtonen, M.; Mikkonen, K.S.; Kulozik, U.; Smirnova, I. Encapsulation of fish oil in protein aerogel micro-particles. J. Food Eng. 2019, 260, 1–11. [Google Scholar] [CrossRef]
- Duan, B.; Gao, H.; He, M.; Zhang, L. Hydrophobic modification on surface of chitin sponges for highly effective separation of oil. ACS Appl. Mater. Interfaces 2014, 6, 19933–19942. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Lo Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef]
- Wang, R.; Wang, F.; Wang, J.; He, Y.; Wang, Y. Preparation Method for corn Protein-Sodium Alginate Composite Porous Hydrophobic Oil-Absorbing Sponge Material; CN108273476; Northwest Normal University: Lanzhou, China, 2018. [Google Scholar]
- Meena, R.; Sanandiya, N.D.; Chaudhary, J.P.; Mondal, D.; Kotrappanavar, N.S. Seaweed Polysaccharide Based Superhydrophilic Foam Membrane for Energy-Efficient Oil-Water Separation. WO2015056273A4, 17 October 2013. [Google Scholar]
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of egg white protein aerogels as new matrix material for microencapsulation in food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Bornkeßel, A.C.; Jung, I.; Schröter, B.; Smirnova, I.; Kulozik, U. Microstructures of potato protein hydrogels and aerogels produced by thermal crosslinking and supercritical drying. Food Hydrocoll. 2021, 112. [Google Scholar] [CrossRef]
- Zhu, H.G.; Yang, S.; Chen, D.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. A Robust Absorbent Material Based on Light-Responsive Superhydrophobic Melamine Sponge for Oil Recovery. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Ansari, F.; Berglund, L.A. Towards semi-structural cellulose nanocomposites—The need for scalable processing and interface tailoring. Biomacromolecules 2018, 19, 2341–2350. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Development of eco-friendly biodegradable superabsorbent materials obtained by injection moulding. J. Clean. Prod. 2018, 198, 312–319. [Google Scholar] [CrossRef]
- Alhammadi, A.M.; Alratrout, A.; Singh, K.; Bijeljic, B.; Blunt, M.J. In situ characterization of mixed-wettability in a reservoir rock at subsurface conditions. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- AlRatrout, A.; Blunt, M.J.; Bijeljic, B. Wettability in complex porous materials, the mixed-wet state, and its relationship to surface roughness. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef]
- Marone, F.; Stampanoni, M. Regridding reconstruction algorithm for real-time tomographic imaging. J. Synchrotron Radiat. 2012, 19, 1029–1037. [Google Scholar] [CrossRef]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, L. Dynamic Membrane Aeration Processing of Novel Micro-Structure in Water-and Fat-Continuous Multiphase Food Systems; ETH: Zurich, Switzerland, 2017. [Google Scholar]
- Mayor, L.; Sereno, A.M. Modelling shrinkage during convective drying of food materials: A review. J. Food Eng. 2004, 61, 373–386. [Google Scholar] [CrossRef]
- Schiffmann, R.F. Microwave and dielectric drying. In Handbook of Industrial Drying, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 286–307. [Google Scholar] [CrossRef]
- Turner, I.W.; Jolly, P.G. Combined microwave and convective drying of a porous material. Dry. Technol. 1991, 9, 1209–1269. [Google Scholar] [CrossRef]
- de Carvalho-Silva, L.B.; Vissotto, F.Z.; Amaya-Farfan, J. Physico-Chemical Properties of Milk Whey Protein Agglomerates for Use in Oral Nutritional Therapy. Food Nutr. Sci. 2013. [Google Scholar] [CrossRef]
- Chen, H.B.; Wang, Y.Z.; Schiraldi, D.A. Foam-like materials based on whey protein isolate. Eur. Polym. J. 2013, 49, 3387–3391. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Functional biopolymer particles: Design, fabrication, and applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Masoodi, R.; Pillai, K.M. Darcy’s law-based model for wicking in paper-like swelling porous media. AIChE J. 2010, 56, 2257–2267. [Google Scholar] [CrossRef]
- Gilbert, V.; Rouabhia, M.; Wang, H.; Arnould, A.L.; Remondetto, G.; Subirade, M. Characterization and evaluation of whey protein-based biofilms as substrates for in vitro cell cultures. Biomaterials 2005, 26, 7471–7480. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wemmer, J.; Malafronte, L.; Foschini, S.; Schneider, A.; Schlepütz, C.M.; Leser, M.E.; Michel, M.; Burbigde, A.; Windhab, E.J. Fabrication of a Novel Protein Sponge with Dual-Scale Porosity and Mixed Wettability Using a Clean and Versatile Microwave-Based Process. Materials 2021, 14, 2298. https://doi.org/10.3390/ma14092298
Wemmer J, Malafronte L, Foschini S, Schneider A, Schlepütz CM, Leser ME, Michel M, Burbigde A, Windhab EJ. Fabrication of a Novel Protein Sponge with Dual-Scale Porosity and Mixed Wettability Using a Clean and Versatile Microwave-Based Process. Materials. 2021; 14(9):2298. https://doi.org/10.3390/ma14092298
Chicago/Turabian StyleWemmer, Judith, Loredana Malafronte, Socrates Foschini, Aline Schneider, Christian M. Schlepütz, Martin E. Leser, Martin Michel, Adam Burbigde, and Erich J. Windhab. 2021. "Fabrication of a Novel Protein Sponge with Dual-Scale Porosity and Mixed Wettability Using a Clean and Versatile Microwave-Based Process" Materials 14, no. 9: 2298. https://doi.org/10.3390/ma14092298
APA StyleWemmer, J., Malafronte, L., Foschini, S., Schneider, A., Schlepütz, C. M., Leser, M. E., Michel, M., Burbigde, A., & Windhab, E. J. (2021). Fabrication of a Novel Protein Sponge with Dual-Scale Porosity and Mixed Wettability Using a Clean and Versatile Microwave-Based Process. Materials, 14(9), 2298. https://doi.org/10.3390/ma14092298






