Influence of Groundwater pH on Water Absorption and Waterproofness of Polymer Modified Bituminous Thick Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Sample 1—two-component, solvent-free sealing compound based on asphalt, plastics and fillers, with a polystyrene filler, non-volatile components—67%, water in a liquid component—30%, density in a mineral component—(1.10–1.35) g/cm3, bulk density in a liquid component—(0.6–0.75)g/cm3, waterproofness Class W2B (at a pressure of 0.075 N/mm2 for 72 h), no sliding from vertical surface at 70 °C for 2 h.
- -
- Sample 2—one-component, solvent-free waterproofing coating with polystyrene filler, mineral content—19.6%, water content—30%, waterproofness Class W2A (at a pressure of 0.075 N/mm2 for 72 h—with the reinforcement mesh), crack bridging ability Class CB2 (no damage at ≥2 mm wide crack), and compressive strength Class C2A (0.30 MN/m2—with reinforcement mesh),
- -
- Sample 3—two-component, solvent-free waterproofing coating, waterproofness Class W2A (at a pressure of 0.075 N/mm2 for 72 h—with the reinforcement mesh), crack bridging ability Class CB2 (no damage at ≥2 mm wide crack) and compressive strength Class C2A (0.30 MN/m2—with reinforcement mesh),
- -
- Sample 4—two-component, solvent-free waterproofing coating, non-emulsified asphalt—1.47%, water in the liquid component—37%, waterproofness Class W2A (at a pressure of 0.075 N/mm2 for 72 h—with the reinforcement mesh), crack bridging ability Class CB2 (no damage at ≥2 mm wide crack), and compressive strength Class C2A (0.30 MN/m2—with reinforcement mesh).
2.2. Methods of Tests
2.2.1. Water Absorption of the Coating
2.2.2. Waterproofness Test
3. Results
4. Discussion
5. Conclusions
- ➢
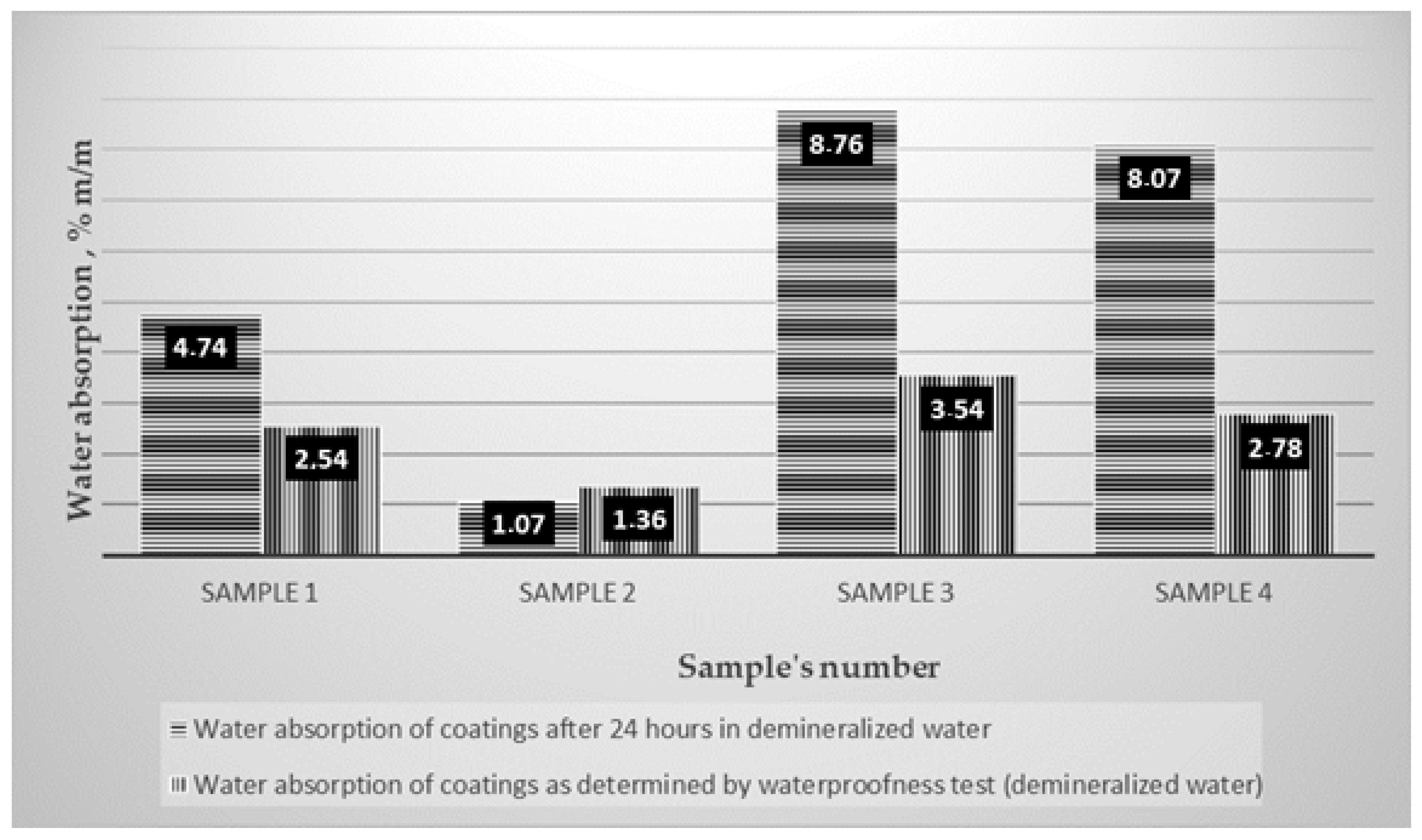
- Water absorbed by the coatings is retained within the layers and is not transferred to concrete substrates on which they are installed, meaning that they provide proper waterproofing,
- ➢
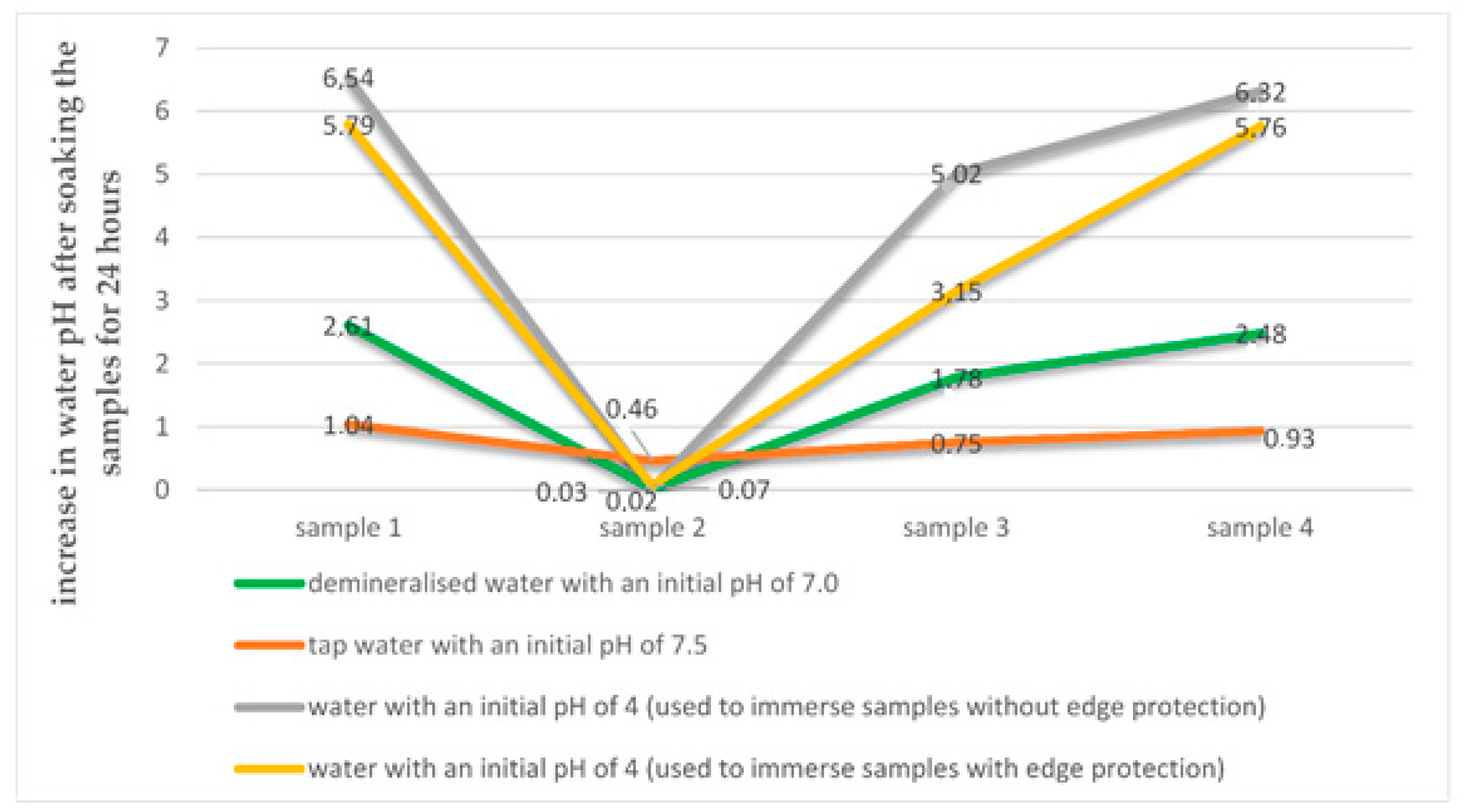
- Water pH has a significant impact on water absorption of polymer modified bituminous thick coatings. The highest water absorption values are observed in the acidic medium, with water pH of about 4, used to assess the resistance of the construction substance to soil and water conditions [18]. Changing water pH towards an alkaline medium (from 7.0 to 7.5) significantly reduces water absorption of polymer modified bituminous thick coatings.
- ➢
- Products with a polystyrene filler show lower susceptibility to water absorption than products with other filler types, which is especially noticeable in one-component products,
- ➢
- Assessment of water absorption of the described products by total water immersion may be used only for comparative purposes and does not fully reflect actual loads acting on coatings under field conditions. For this reason, the article proposes a modification of this test method using one-sided exposure of specimens to water,
- ➢
- Reaction of leachates formed during total immersion of polymer modified bituminous thick coating samples in an aqueous solution with different initial pH values, i.e., 4.0, 7.0 and 7.5, changes significantly towards alkaline, which may indicate a leaching of the coatings which contributes to contamination of groundwater.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henshell, J. The Manual of Below-Grade Waterproofing, 2nd ed.; CRC Press: New York, NY, USA, 2016; ISBN 9781317211891. [Google Scholar]
- Klem, P.; Chwieduk, D.; Francke, B.; Grabarczyk, S.; Kosiorek, M.; Kupik, J.; Pogorzelski, J.A.; Szudrowicz, B.; Ściślewski, Z.; Śliwowski, L.; et al. General Construction, Volume 2-Building Physics, 1st ed.; Arkady: Warsaw, Poland, 2005; ISBN 83-213-4408-9. (In Polish) [Google Scholar]
- Dong Soo, A.; Kyu Hwan, O.; Jin Sang, P.; Sang Keun, O. Viscosity and waterproofing performance evaluation of synthetic polymerized rubber gel (SPRG) after screw mixing. Appl. Sci. 2018, 8, 1989. [Google Scholar] [CrossRef] [Green Version]
- Lyapidevskaya, O. Waterproofing material for protection of underground structures. E3S Web Conf. 2019, 97, 02008. [Google Scholar] [CrossRef]
- Alfano, G.; Chiancarella, C.; Cirillo, E.; Fato, I.; Martellotta, F. Long-term performance of chemical damp-proof courses: Twelve years of laboratory testing. Build. Environ. 2006, 41, 1060–1069. [Google Scholar] [CrossRef]
- Polymer Modified Bituminous Thick Coatings for Waterproofing-Definitions and Requirements 2011 + 2014; EN 15814 +A2; European Committee for Standardization (CEN): Belgium, Brussels, 2011.
- Francke, B.; Athanasopoulou, A.; Bezuijen, A.; Bogusz, W.; Bournas, D.; Brandtner, M.; Breunese, A.; Burbaum, D.; Dimova, S.; Frank, R.; et al. Defining the Criteria of Construction Utility in the Field of Waterproofing of Underground Parts of Buildings-Research Work; Building Research Institute: Warsaw, Poland, 2011. (In Polish) [Google Scholar]
- Rokiel, M. Foundation waterproofing using KMB masses. Part I. Design and production according to German guidelines. Izolacje 2011, 4, 38–40. (In Polish) [Google Scholar]
- Francke, B. Technical requirements for technical properties of polymer-modified asphalt products for insulation of underground parts of buildings in accordance with PN-EN 15814 + A1: 2013-04. Mater. Budowl. 2014, 3, 2–3. (In Polish) [Google Scholar]
- Gasewicz, J. Thick-layered bituminous waterproofing coatings. Izolacje 2010, 6, 18–21. (In Polish) [Google Scholar]
- Keher, H.; Denzel, H. Coatings Compositions for a Polimer-Modified Roofing and Waterproofing Sheet. U.S. Patent 4,707,413, 17 November 1987. [Google Scholar]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S. Bitumen and bitumen modification: A review on latest advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Boutevin, B.; Pietrasanta, Y.; Robin, J.J. Bitumen-polymer blends for coatings applied to roads and public constructions. Prog. Org. Coat. 1989, 17, 221–249. [Google Scholar] [CrossRef]
- Polacco, G.; Stastna, J.; Biondi, D.; Zanzotto, L. Relation between polymer architecture and nonlinear viscoelastic behaviour of modified asphalts. Curr. Opin. Colloid Interface Sci. 2006, 11, 230–245. [Google Scholar] [CrossRef]
- Walkering, C.P.; Vonk, W.C.; Whiteoak, C.D. Improved asphalt properties using SBS modified bitumens. Shell Bitum. Rev. 1992, 66, 9–11. [Google Scholar]
- Lu, X.; Isacsson, U.; Ekblad, J. Phase separation of SBS polymer modified bitumens. J. Mater. Civ. Eng. 1999, 11, 51–57. [Google Scholar] [CrossRef]
- Rodrigues, C.; Hanumanthgari, R. Polymer modified bitumens and other modified binders. In The Shell Bitumen Handbook, 6th ed.; Hunter, R.N., Self, A., Read, J., Eds.; ICE Publishing: London, UK, 2015; ISBN 978-0727758378. [Google Scholar]
- Yu, R.; Zhu, X.; Zhang, M.; Fang, C. Investigation on the short-term aging-resistance of thermoplastic polyurethane-modified asphalt binders. Polymers 2018, 10, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, K.D.; Lee, S.J.; Amirkhanian, S.N.; Kim, K.W. Interaction effects of crumb rubber modified asphalt binders. Constr. Build. Mater. 2010, 24, 824–831. [Google Scholar] [CrossRef]
- Pang, L.; Liu, K.Y.; Wu, S.P.; Lei, M.; Chen, Z.W. Effect of LDHs on the aging resistance of crumb rubber modified asphalt. Constr. Build. Mater. 2014, 67, 239–243. [Google Scholar] [CrossRef]
- Modarres, A. Investigating the toughness and fatigue behavior of conventional and SBS modified asphalt mixes. Constr. Build. Mater. 2013, 47, 218–222. [Google Scholar] [CrossRef]
- Bai, M. Investigation of low-temperature properties of recycling of aged SBS modified asphalt binder. Constr. Build. Mater. 2017, 150, 766–773. [Google Scholar] [CrossRef]
- Özen, H. Rutting evaluation of hydrated lime and SBS modified asphalt mixtures for laboratory and field compacted samples. Constr. Build. Mater. 2011, 25, 756–765. [Google Scholar] [CrossRef]
- Behnood, A.; Olek, J. Rheological properties of asphalt binders modified with styrene-butadiene-styrene (SBS), ground tire Rubber (GTR), or polyphosphoric acid (PPA). Constr. Build. Mater. 2017, 151, 464–478. [Google Scholar] [CrossRef]
- Yu, R.E.; Liu, X.L.; Zhang, M.R.; Zhu, X.J.; Fang, C.Q. Dynamic stability of ethylene-vinyl acetate copolymer/crumb rubber modified asphalt. Constr. Build. Mater. 2017, 156, 284–292. [Google Scholar] [CrossRef]
- Liang, M.; Xin, X.; Fan, W.Y.; Ren, S.S.; Shi, J.T.; Luo, H. Thermo-stability and aging performance of modified asphalt with crumb rubber activated by microwave and TOR. Mater. Des. 2017, 127, 84–96. [Google Scholar] [CrossRef]
- Rodríguez-Alloza, A.M.; Gallego, J.; Pérez, I.; Bonati, A.; Giuliani, F. High and low temperature properties of crumb rubber modified binders containing warm mix asphalt additives. Constr. Build. Mater. 2014, 53, 460–466. [Google Scholar] [CrossRef]
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer Interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef]
- Zhang, H.L.; Yu, J.Y.; Wang, H.C.; Xue, L.H. Investigation of microstructures and ultraviolet aging properties of organo-montmorillonite/SBS modified bitumen. Mater. Chem. Phys. 2011, 129, 769–776. [Google Scholar] [CrossRef]
- Zhang, H.L.; Yu, J.Y.; Wu, S.P. Effect of montmorillonite organic modification on ultraviolet aging properties of SBS modified bitumen. Constr. Build. Mater. 2012, 27, 553–559. [Google Scholar] [CrossRef]
- Vollpracht, A.; Brameshuber, W. Environmental compatibility of bitumen waterproofing. Mater. Struct. 2013, 46, 1257–1264. [Google Scholar] [CrossRef]
- Adebiyi, F.M.; Asubiojo, O.I.; Ajayi, T.R. Multi elemental analysis of Nigerian bitumen by TXRF spectrometry and the physical constants characterization of its hydrocarbon content. Fuel 2006, 85, 396–400. [Google Scholar] [CrossRef]
- Fagbote, E.O.; Olanipekun, E. Levels of poly-cyclic aromatic hydrocarbons and polychlorinated biphenyls in sediments of bitumen deposit impacted area. Int. J. Environ. Sci. Technol. 2010, 7, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Olajire, A.A.; Alade, O.A.; Adeniyi, A.A.; Olabemiwo, O.M. Distribution of polycyclic aromatic hydrocarbons in surface soils and water from the vicinity of Agbabu bitumen field of Southwestern Nigeria. J. Environ. Sci. Health Part A 2007, 42, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.N.; Schindler, D.W.; Hodson, P.V.; Short, J.W.; Radmanovich, R. Oil sands development contributes elements toxic at low concentrations to the Athabasca River and its tributaries. Proc. Natl. Acad. Sci. USA 2010, 107, 16178–16183. [Google Scholar] [CrossRef] [Green Version]
- Olabemiwo, O.M.; Adediran, G.O.; Adekola, F.A.; Olajire, A.A.; Adedeji, O.S. Impacts of simulated agbabu bitumen leachate on heamatological and biochemical parameters of wistar albino rats. Res. J. Environ. Toxicol. 2011, 5, 213–221. [Google Scholar] [CrossRef]
- Concrete Requirements, Properties, Production and Compliance 2013 + 2016; EN 206+A1; European Committee for Standardization (CEN): Belgium, Brussels, 2013.
- Bitumen and Bituminous Binders-Determination of the pH Value of Bitumen Emulsions; EN 12850; European Committee for Standardization (CEN): Belgium, Brussels, 2011.
- Plastics-Determination of Water Absorption; EN ISO 62; European Committee for Standardization (CEN): Belgium, Brussels, 2008.



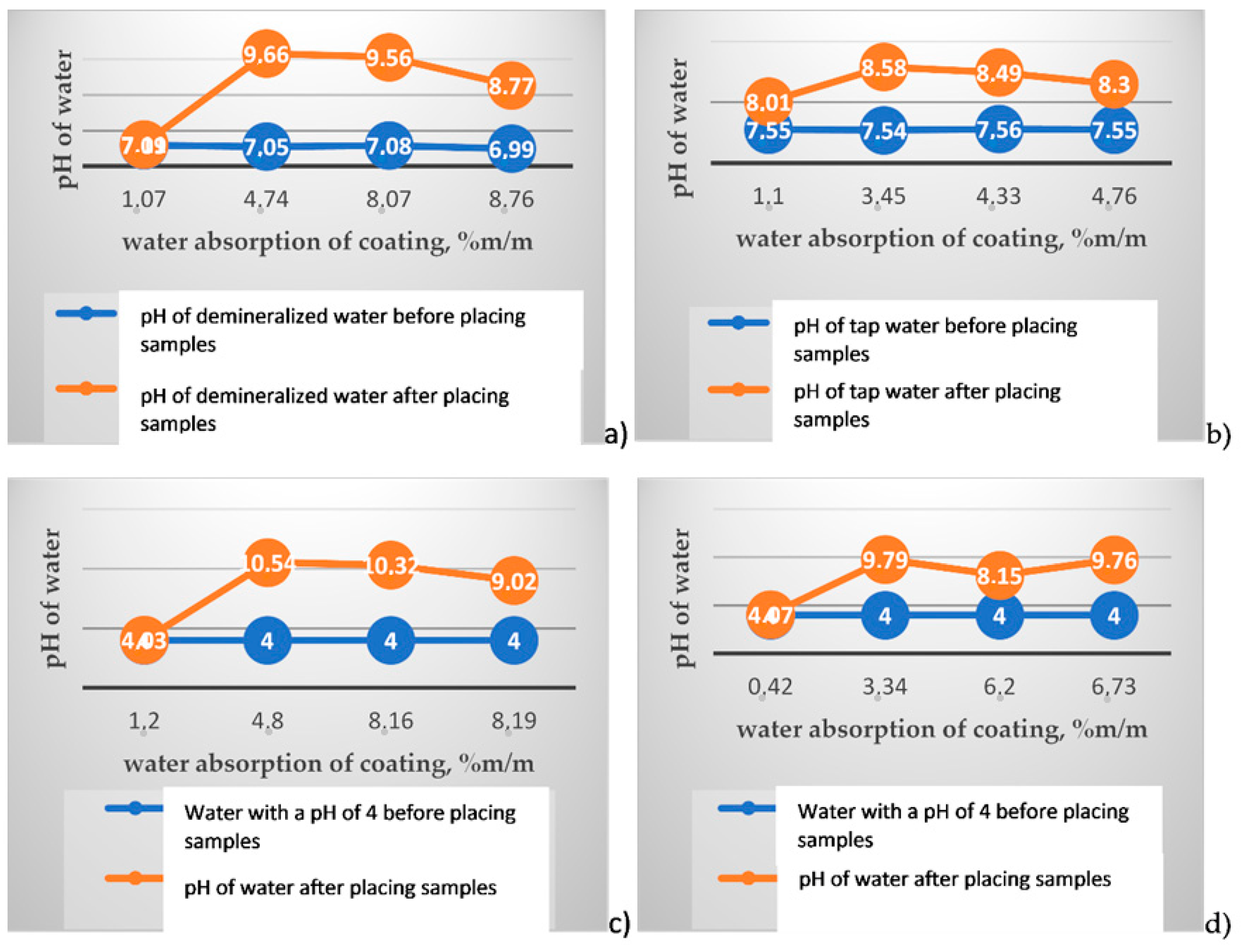
| Test Sample Number | Type of Product | Mixture/ Component *) Density (g/cm3) | pH of a Liquid Component | Mixing Ratio (by Weight)/ Bitumen Emulsion:Powder Component | Average Coating Thickness (mm) |
|---|---|---|---|---|---|
| 1 | two-component polymer modified bituminous coating | 1.0 | 8.64 | 5:1 | 4.5 |
| 2 | one-component polymer modified bituminous coating | 0.75 | 7.50 | - | 4.6 |
| 3 | two-component polymer modified bituminous coating | 1.07 | 9.62 | 3:1 | 3.6 |
| 4 | two-component polymer modified bituminous coating | 1.15 | 9.52 | 3:1 | 3.7 |
| Test Sample Number | Water Absorption When Using Demineralized Water, % m/m/Coefficient of Variation, % | Water pH | |
|---|---|---|---|
| Before the Test | After the Test | ||
| 1 | 4.74/8.09 | 7.05 | 9.66 |
| 2 | 1.07/5.20 | 7.09 | 7.11 |
| 3 | 8.76/2.56 | 6.99 | 8.77 |
| 4 | 8.07/2.88 | 7.08 | 9.56 |
| Test Sample Number | Water Absorption When Using Tap Water, % m/m/Coefficient of Variation, % | Water pH | |
|---|---|---|---|
| Before the Test | After the Test | ||
| 1 | 3.45/5.57 | 7.54 | 8.58 |
| 2 | 1.10/8.32 | 7.55 | 8.01 |
| 3 | 4.76/5.58 | 7.55 | 8.30 |
| 4 | 4.33/2.76 | 7.56 | 8.49 |
| Test Sample Number | Water with a pH of 4 Absorption % m/m/Coefficient of Variation, % | pH of Water after the Test | ||
|---|---|---|---|---|
| Samples without Protected Edges | Samples with Wax Protected Edges | Samples without Protected Edges | Samples with Wax Protected Edges | |
| 1 | 4.80/23.11 | 3.34/10.59 | 10.54 | 9.79 |
| 2 | 1.20/13.56 | 0.42/12.55 | 4.03 | 4.07 |
| 3 | 8.19/7.07 | 6.20/4.92 | 9.02 | 8.15 |
| 4 | 8.16/10.04 | 6.73/16.21 | 10.32 | 9.76 |
| Test Sample Number | Waterproofness, No Leakage at Pressure, MPa | The Concrete Substrate under the Coating after the Test | Water Absorption of Coatings after Waterproofness Test, % m/m/Variation Coefficient, % |
|---|---|---|---|
| 1 | 0.5 | No moisture | 2.54/4.17 |
| 2 | 0.5 | No moisture | 1.36/9.71 |
| 3 | 0.5 | No moisture | 3.54/2.53 |
| 4 | 0.5 | No moisture | 2.78/3.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francke, B.; Wichowska, M. Influence of Groundwater pH on Water Absorption and Waterproofness of Polymer Modified Bituminous Thick Coatings. Materials 2021, 14, 2272. https://doi.org/10.3390/ma14092272
Francke B, Wichowska M. Influence of Groundwater pH on Water Absorption and Waterproofness of Polymer Modified Bituminous Thick Coatings. Materials. 2021; 14(9):2272. https://doi.org/10.3390/ma14092272
Chicago/Turabian StyleFrancke, Barbara, and Maria Wichowska. 2021. "Influence of Groundwater pH on Water Absorption and Waterproofness of Polymer Modified Bituminous Thick Coatings" Materials 14, no. 9: 2272. https://doi.org/10.3390/ma14092272
APA StyleFrancke, B., & Wichowska, M. (2021). Influence of Groundwater pH on Water Absorption and Waterproofness of Polymer Modified Bituminous Thick Coatings. Materials, 14(9), 2272. https://doi.org/10.3390/ma14092272






