Tuned S-Scheme Cu2S_TiO2_WO3 Heterostructure Photocatalyst toward S-Metolachlor (S-MCh) Herbicide Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Photocatalyst Materials
2.1.1. Preparation of Mono-Component Photocatalysts
- (i)
- Cu2S was prepared by mixing 0.2 mol of copper nitrate (Cu(NO3)2, 99.9%, Scharlau, Barcelona, Spain) aqueous solution with 0.5 mol of sodium thiosulfate (Na2S2O3, 99.9%, Scharlau) aqueous solution. After 15 min of stirring the gel was formed and kept 3 h undisturbed to achieve the complete precipitation. The precipitate was centrifuged and thermally treated at 120 °C in a ceramic capsule in sulfured (Sulfur, 99%, Sigma Aldrich, Munich, Germany) atmosphere.
- (ii)
- WO3 was obtained by dissolving tungsten hexachloride (WCl6, 99.4%, Acros Organics, Geel, Belgium) in a mixture of ethanol (100%, Sigma Aldrich) and 2-propanol (100%, Sigma Aldrich). After 120 min of stirring a light yellow alcoholic solution was obtained. Then, 0.15 mol of natrium hydroxide (99.98%, Honeywell, Charlotte, NC, USA) was added drop by drop and the gel was formed. After precipitation and centrifugation the resulting powder was annealed for 8 h at 500 °C.
2.1.2. Preparation of Multi-Component Photocatalysts
- (i)
- Cu2S_TiO2 sample was obtained using the same procedure as described for Cu2S, with the single modification that TiO2 powder was dispersed into copper nitrate solution, considering the Cu:Ti atomic ratio of 1:1. The final powder was thermally treated at 150 °C for 2 h.
- (ii)
- Cu2S_WO3 sample was obtained following the procedure described for Cu2S. The WO3 powder already prepared was added into copper nitrate solution, considering the Cu:W atomic ratio of 1:1. The final powder was thermally treated at 150 °C for 1.5 h.
- (iii)
- TiO2_WO3 sample was prepared as using a similar procedure as presented for WO3, and the TiO2 power was dispersed into tungsten hexachloride solution. The uniform TiO2 distribution was assured by adding polyethylene glycol (99%, Scharlau) and the Ti:W atomic ratio was 1:1. The annealing treatment was done at 500 °C for 5 h.
- (iv)
- Cu2S_TiO2_WO3 sample was synthesized by adding TiO2_WO3 powder, previously obtained, into copper nitrate solution and the mixture was stirred for 2 h. The Cu:Ti:W atomic ratio was 1:1:1. Then, 0.7 mol of sodium thiosulfate (Na2S2O3, 99.9%, Scharlau) was added drop by drop under continuous stirring. The precipitate was centrifuged and thermally treated at 150 °C for 2 h.
2.2. Photocatalytic Activity
2.3. Characterization Instruments
3. Results and Discussions
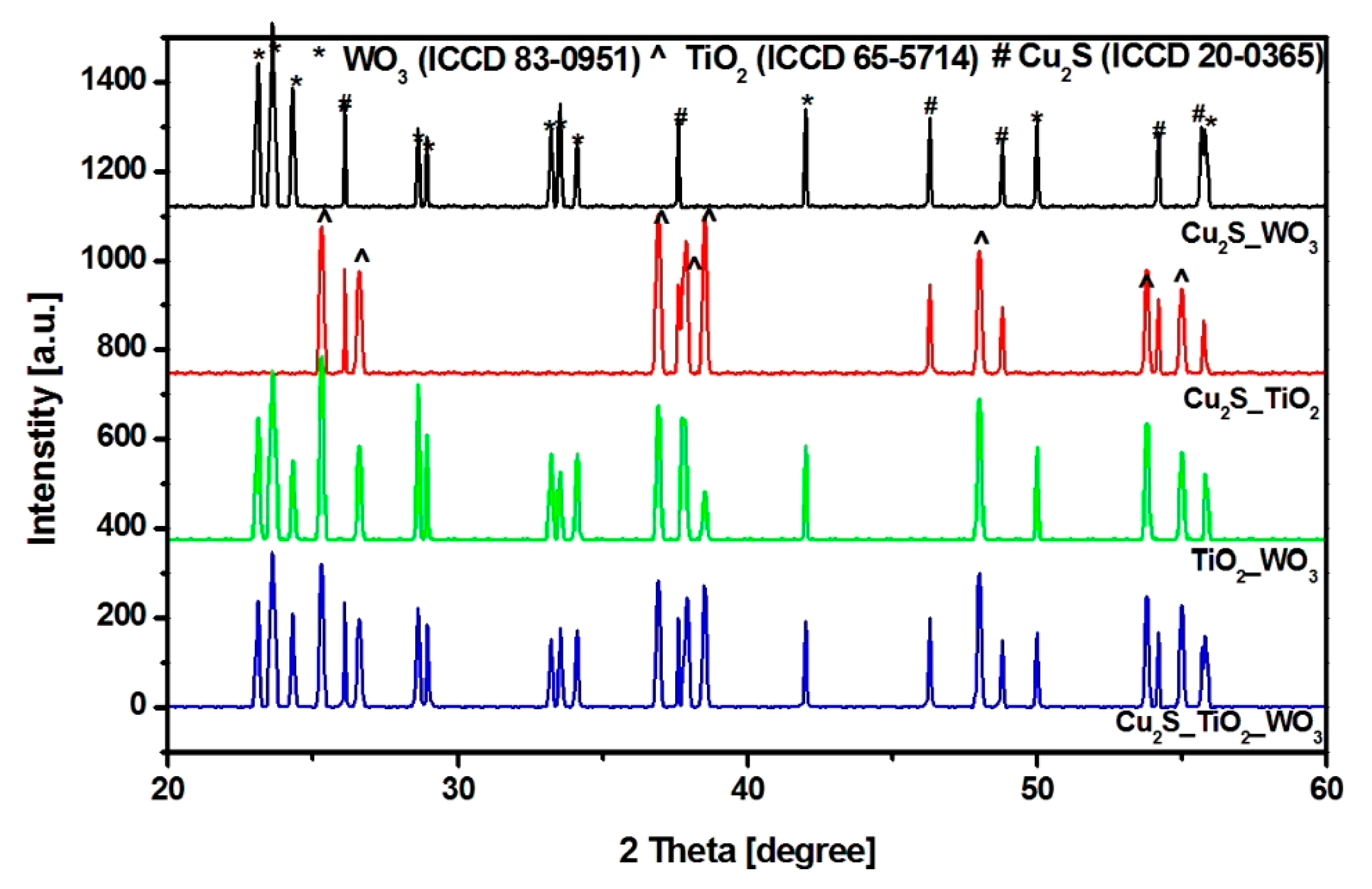
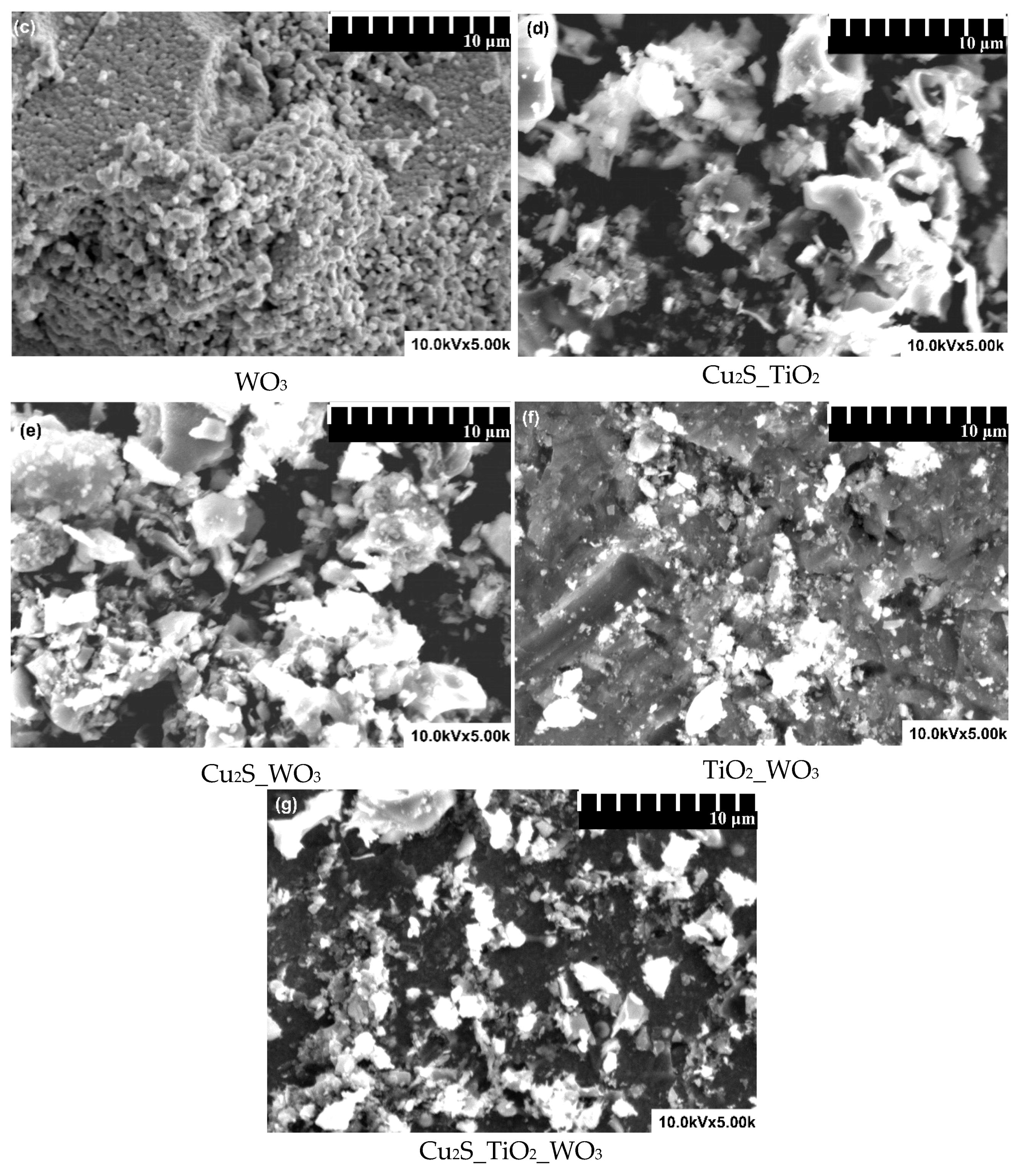
3.1. Composition and Morphology
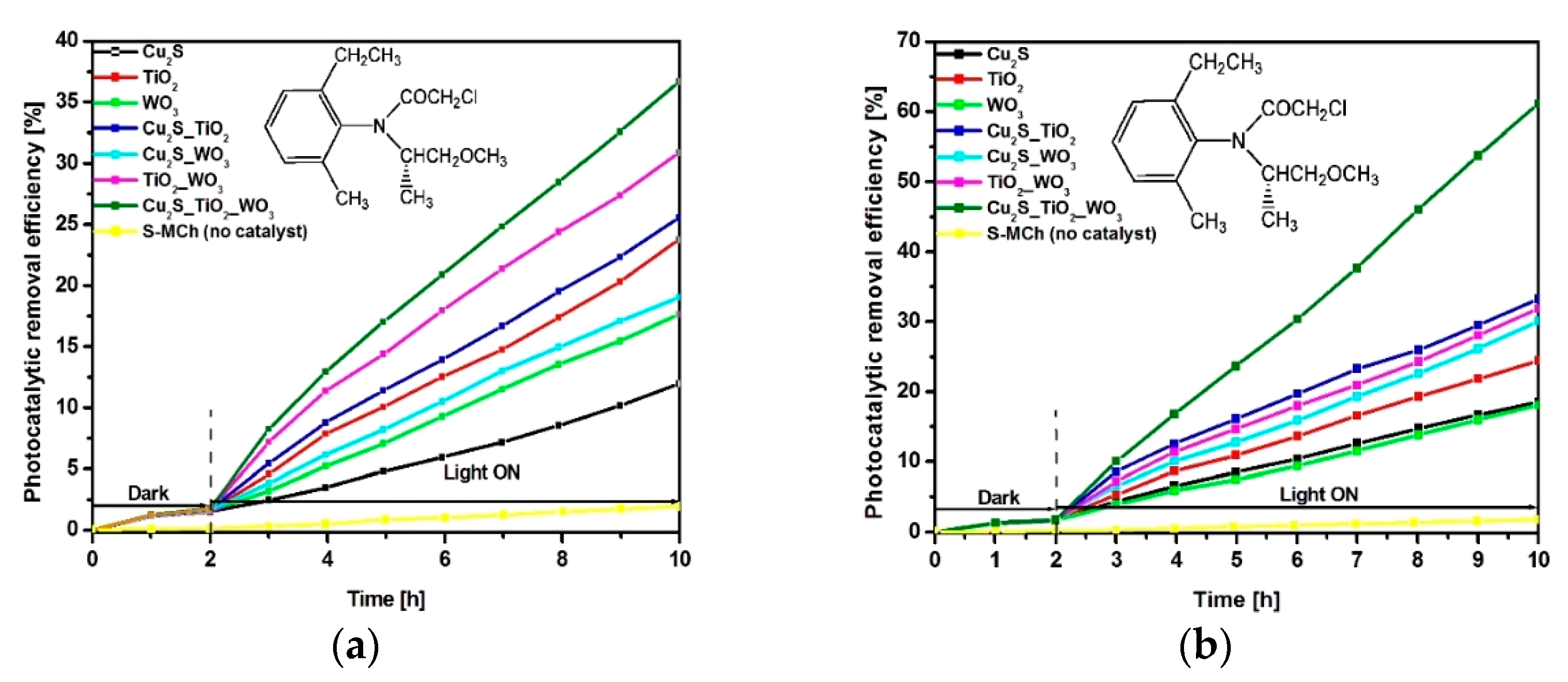
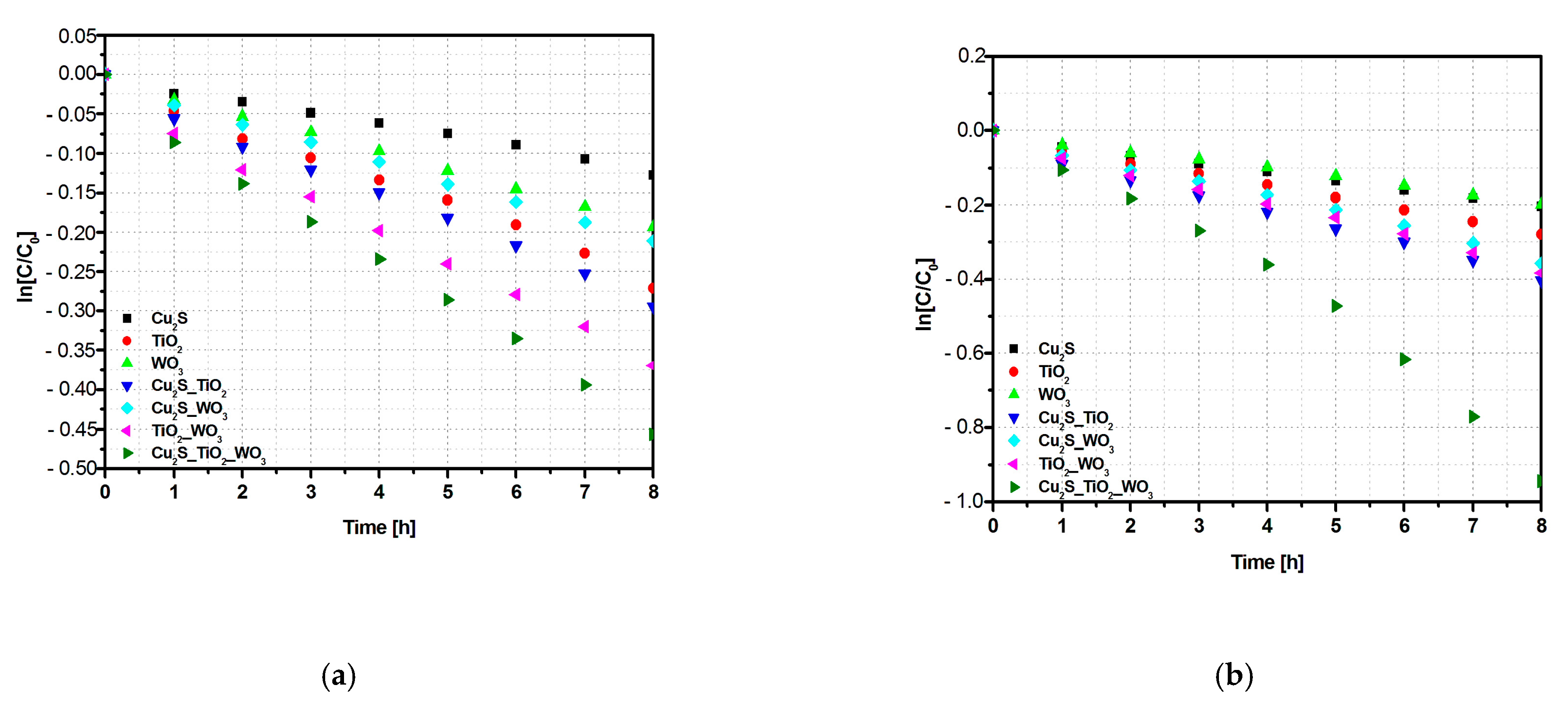
3.2. Photocatalytic Properties and Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matheus, M.C.; Lourenço, G.R.; Solano, B.A.; Dezotti, M.W.C.; Bassin, J.P. Assessing the impact of hydraulic conditions and absence of pretreatment on the treatability of pesticide formulation plant wastewater in a moving bed biofilm reactor. J. Water Proc. Eng. 2020, 36, 101243. [Google Scholar] [CrossRef]
- Alistea, M.; Garridoa, I.; Floresa, P.; Hellína, P.; Velab, N.; Navarroc, S.; Fenoll, J. Reclamation of agro-wastewater polluted with thirteen pesticides by solar photocatalysis to reuse in irrigation of greenhouse lettice grown. J. Environ. Manag. 2020, 266, 110565. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, L.; Zhang, Y.; Wang, Y.; Saleha, A.S.M.; Zhu, M.; Gao, Y.; Xu, C.; Hassana, M.E.; Yang, Q.; et al. Synthesis and characterization of a novel rice bran protein-cerium complex for the removal of organophosphorus pesticide residues from wastewater. Food Chem. 2020, 320, 126604. [Google Scholar]
- Fang, H.; Zhang, H.; Han, L.; Mei, J.; Ge, Q.; Long, Z.; Yu, Y. Exploring bacterial communities and biodegradation genes in activated sludge from pesticide wastewater treatment plants via metagenomic analysis. Environ. Pollut. 2018, 243, 1206–1216. [Google Scholar] [CrossRef]
- Sharma, L.; Kakkar, R. Magnetically retrievable one-pot fabrication of mesoporous magnesium ferrite (MgFe2O4) for the remediation of chlorpyrifos and real pesticide wastewater. J. Environ. Chem. Eng. 2018, 6, 6891–6903. [Google Scholar] [CrossRef]
- Ghodsi, J.; Rafati, A.A. A voltammetric sensor for diazinon pesticide based on electrode modified with TiO2 nanoparticles covered multi walled carbon nanotube nanocomposite. J. Electroanal. Chem. 2017, 807, 1–9. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; Muñoz-Portero, M.J.; García-Antón, J. Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets. Chem. Eng. J. 2018, 350, 1114–1124. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Sun, Y.; Meng, F.; Liu, J. Quantitative analysis of pesticide residue based on the dynamic response of a single SnO2 gas sensor. Sens. Acuat. B 2004, 99, 330–335. [Google Scholar] [CrossRef]
- Honarnezhad, R.; Fathinia, M.; Khataee, A. Mechanical production and sonocatalytic application of Cu2S nanoparticles for degradation of isopropylxanthic acid: Kinetic modeling via white and black box methods. J. Mol. Liq. 2019, 287, 110899. [Google Scholar] [CrossRef]
- Tunesi, M.M.; Kalwar, N.; Abbas, M.W.; Karakus, S.; Soomro, R.A.; Kilislioglu, A.; Abro, M.I.; Hallam, K.R. Functionalised CuO nanostructures for the detection of organophosphorus pesticides: A non-enzymatic inhibition approach coupled with nano-scale electrode engineering to improve electrode sensitivity. Sens. Acuat. B 2018, 260, 480–489. [Google Scholar] [CrossRef]
- Ramu, A.G.; Arun Kumari, M.L.; Elshikh, M.S.; Alkhamis, H.H.; Alrefaei, A.F.; Choi, D. A facile and green synthesis of CuO/NiO nanoparticles and their removal activity of toxic nitro compounds in aqueous medium. Chemosphere 2020, 271, 129475. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotech. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical 350 properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B Adv. 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Nicholls, T.P.; Bissember, A.C. Developments in visible-light-mediated copper photocatalysis. Tetrahedron Lett. 2019, 60, 150883. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, J.; Chen, S.; Zhang, S.; Huang, W. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Deng, H.; Fei, X.; Yang, Y.; Fan, J.; Yu, J.; Cheng, B.; Zhang, L. S-scheme heterojunction based on p-type ZnMn2O4 and n-type ZnO with improved photocatalytic CO2 reduction activity. Chem. Eng. J. 2021, 409, 127377. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Jin, Z. 0D CdxZn1-xS and amorphous Co9S8 formed S-scheme heterojunction boosting photocatalytic hydrogen evolution. Mol. Catal. 2021, 501, 111378. [Google Scholar] [CrossRef]
- Pham, T.T.; Shin, E.W. Thermal formation effect of g-C3N4 structure on the visible light driven photocatalysis of g-C3N4/NiTiO3 Z-scheme composite photocatalysts. Appl. Surf. Sci. 2018, 447, 757–766. [Google Scholar] [CrossRef]
- Chang, N.; Chen, Y.R.; Xie, F.; Liu, Y.P.; Wang, H.T. A promising Z-scheme heterojunction via loading Ag/AgCl into porous Co3O4 derived from ZIF-67 for visible light driven photocatalysis. Micropor. Mesopor. Mat. 2020, 307, 110530. [Google Scholar] [CrossRef]
- Dursun, S.; Koyuncu, S.N.; Kaya, I.C.; Kaya, G.G.; Kalem, V.; Akyildiz, H. Production of CuO–WO3 hybrids and their dye removal capacity/performance from wastewater by adsorption/photocatalysis. J. Water Proc. Eng. 2020, 36, 101390. [Google Scholar] [CrossRef]
- Ojha, D.P.; Karki, H.P.; Song, J.H.; Kim, H.J. Amine-assisted synthesis of FeWO4 nanorodg-C3N4 for enhanced visible light-driven Z-scheme photocatalysis. Compos. Part B Eng. 2019, 160, 277–284. [Google Scholar] [CrossRef]
- Li, Y.; He, R.; Han, P.; Hou, B.; Peng, S.; Ouyang, C. A new concept: Volume photocatalysis for efficient H2 generation using low polymeric carbon nitride as an example. Appl. Cat. B Environ. 2020, 279, 119379. [Google Scholar] [CrossRef]
- Chen, H.J.; Yang, Y.L.; Hong, M.; Chen, J.G.; Suo, G.Q.; Hou, X.J.; Feng, L.; Chen, Z.G. Separable and recyclable meso-carbon@TiO2/carbon fiber composites for visible-light photocatalysis and photoelectrocatalysis. Sustain. Mater. Technol. 2019, 21, e00105. [Google Scholar] [CrossRef]
- Li, X.; Hu, T.; Zhang, J.; Dai, K.; Liang, C. Novel 2D SnNb2O6/Ag3VO4 S-scheme heterojunction with enhanced visible-light photocatalytic activity. Ceram. Int. 2021, 47, 7169–7176. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Zhang, W.; Liu, Q.; Zhang, M.; Tang, H. Build-in electric field induced step-scheme TiO2/W18O49 heterojunction for enhanced photocatalytic activity under visible-light irradiation. Ceram. Int. 2020, 46, 23–30. [Google Scholar] [CrossRef]
- Hu, X.; Wang, G.; Wang, J.; Hu, Z.; Su, Y. Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. Appl. Surf. Sci. 2020, 511, 145499. [Google Scholar] [CrossRef]
- Xu, A.J.; Tu, W.; Shen, S.; Lin, Z.; Gao, N.; Zhong, W. BiVO4@MoS2 core-shell heterojunction with improved photocatalytic activity for discoloration of Rhodamine B. Appl. Surf. Sci. 2020, 528, 146949. [Google Scholar] [CrossRef]
- Pei, C.Y.; Chen, Y.G.; Wang, L.; Chen, W.; Huang, G.B. Step-scheme WO3/CdIn2S4 hybrid system with high visible light activity for tetracycline hydrochloride photodegradation. Appl. Surf. Sci. 2021, 535, 147682. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Zhang, H.; Zhuang, Z.; Yu, Y. Hierarchically porous S-scheme CdS/UiO-66 photocatalyst for efficient 4-nitroaniline reduction. Chin. J. Catal. 2021, 42, 78–86. [Google Scholar] [CrossRef]
- Guan, R.; Zhai, H.; Li, J.; Qi, Y.; Li, M.; Song, M.; Zhao, Z.; Zhang, J.; Wang, D.; Tan, H. Reduced mesoporous TiO2 with Cu2S heterojunction and enhanced hydrogen production without noble metal cocatalyst. Appl. Surf. Sci. 2019, 507, 144772. [Google Scholar] [CrossRef]
- Singh, S.V.; Sharma, A.; Biring, S.; Pal, B.N. Solution processed Cu2S/TiO2 heterojunction for visible-near infrared photodetector. Thin Solid Films 2020, 710, 138275. [Google Scholar] [CrossRef]
- Baviskar, V.; Salunkhe, D.; Tarkas, H.; Wagh, R.; Baviskar, P.; Patil, R. Sensitization of TiO2 by chemically deposited Cu2S for solar cell: Effect of deposition time on photoelectrochemical performance. Optik 2020, 207, 163890. [Google Scholar] [CrossRef]
- Rao, V.N.; Reddy, N.L.; Kumari, M.M.; Ravi, P.; Sathish, M.; Kuruvilla, K.M.; Preethi, V.; Reddy, K.R.; Shettie, N.P.; Aminabhavi, T.M.; et al. Photocatalytic recovery of H2 from H2S containing wastewater: Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures. Appl. Catal. B Environ. 2019, 254, 174–185. [Google Scholar]
- Baneto, M.; Enesca, A.; Mihoreanu, C.; Lare, Y.; Jondo, K.; Napo, K.; Duta, A. Effects of the growth temperature on the properties of spray deposited CuInS2 thin films for photovoltaic applications. Ceram. Int. 2015, 41, 4742–4749. [Google Scholar] [CrossRef]
- Dudita, M.; Bogatu, C.; Enesca, A.; Duta, A. The influence of the additives composition and concentration on the properties of SnOx thin films used in photocatalysis. Mater. Lett. 2011, 65, 2185–2189. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Zhong, L.; Wang, Y.; Chai, S.; Yang, T.; Han, X. Cu2S-Cu-TiO2 mesoporous carbon composites for the degradation of high concentration of methyl orange under visible light. Appl. Surf. Sci. 2017, 422, 1093–1101. [Google Scholar] [CrossRef]
- Tang, L.; Deng, Y.; Zeng, G.; Hu, W.; Wang, J.; Zhou, Y.; Wang, J.; Tang, J.; Fang, W. CdS/Cu2S co-sensitized TiO2 branched nanorod arrays of enhanced photoelectrochemical properties by forming nanoscale heterostructure. J. Alloy. Compound. 2016, 662, 516–527. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Ordaxa, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. The role of two organic amendments to modify the environmental fate of S-metolachlor in agricultural soils. Environ. Res. 2021, 195, 110871. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ivantsova, E.; Souders, C.L., II; Martyniuk, C.J. The agrochemical S-metolachlor disrupts molecular mediators and morphology of the swim bladder: Implications for locomotor activity in zebrafish (Danio rerio). Ecotox. Environ. Safe 2021, 208, 111641. [Google Scholar] [CrossRef]
- Sithole, R.K.; Machogo, L.F.E.; Moloto, M.J.; Gqoba, S.S.; Mubiayi, K.P.; Wyk, J.V.; Moloto, N. One-step synthesis of Cu3N, Cu2S and Cu9S5 and photocatalytic degradation of methyl orange and methylene blue. J. Photochem. Photobiol. A Chem. 2020, 397, 112577. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Xu, F.; Costa, M.F.; Sun, S.; Teixeira, V.; Wang, Y.; Bian, F.; Wang, H.; Cui, H. Structure Optimization on the Photoelectric and Photocatalytic Properties of Cu2S and ZnO Complex Films. Mater. Today Proc. 2015, 2, 253–260. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Guillard, C.; Pichat, P. Heterogeneous photocatalysis: An emerging technology for water treatment. Catal. Today 1993, 17, 7–20. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Shan, Z.; Huang, F.; Shen, H. Preparation and visible-light photocatalytic activity of In2S3/TiO2 composite. Mater. Chem. Phys. 2010, 122, 183–187. [Google Scholar] [CrossRef]
- Mise, T.; Nakada, T. Low temperature growth and properties of Cu–In–Te based 433 thin films for narrow bandgap solar cells. Thin Solid Films 2010, 518, 5604–5609. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Z.; Lv, H.; Ren, H.; Xing, X. Rational design of Au decorated Mn0.5Cd0.5S/WO3 step-scheme heterostructure with multichannel charge transfer and efficient H2 generation. Appl. Surf. Sci. 2020, 526, 146734. [Google Scholar] [CrossRef]
- Jia, X.; Han, Q.; Liu, H.; Li, S.; Bi, H. A dual strategy to construct flowerlike S-scheme BiOBr/BiOAc1−xBrx heterojunction with enhanced visible-light photocatalytic activity. Chem. Eng. J. 2020, 399, 125701. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Liu, H.; Xu, D.; Zhang, L. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. Appl. Catal. B. 2015, 162, 352–363. [Google Scholar] [CrossRef]


| Irradiation Sources | UV Radiation Sources (310–390 Nm) | Vis Radiation Sources (400–800 Nm) | Total Irradiance (W/M2) |
|---|---|---|---|
| UVa light, 18 W | 8 | 0 | 16.3 |
| UVa–Vis light, 18 W | 4 | 4 | 24.9 |
| Samples Code | Crystallite Size (Å) | ||
|---|---|---|---|
| Cu2S | TiO2 | WO3 | |
| Cu2S_TiO2 | 64 | 82 | - |
| Cu2S_WO3 | 79 | - | 103 |
| TiO2_WO3 | - | 81 | 96 |
| Cu2S_TiO2_WO3 | 65 | 83 | 97 |
| Scheme | Elemental Composition (% at) | ||||||
|---|---|---|---|---|---|---|---|
| Cu | Ti | W | O | Oth 1 | S | Sth 1 | |
| Cu2S_TiO2 | 23.7 | 22.6 | - | 45.4 | 45.2 | 8.3 | 11.8 |
| Cu2S_WO3 | 14.2 | - | 16.7 | 62.5 | 50.1 | 6.6 | 7.1 |
| TiO2_WO3 | - | 13.8 | 12.1 | 74.1 | 63.9 | - | - |
| Cu2S_TiO2_WO3 | 14.3 | 9.8 | 9.6 | 59.9 | 48.4 | 6.4 | 7.1 |
| Kinetic Data | Cu2S | TiO2 | WO3 | Cu2S_TiO2 | Cu2S_WO3 | TiO2_WO3 | Cu2S_TiO2_WO3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K [S−1] | R2 | K [S−1] | R2 | K [S−1] | R2 | K [S−1] | R2 | K [S−1] | R2 | K [S−1] | R2 | K [S−1] | R2 | |
| UV | 0.0148 | 0.9958 | 0.0316 | 0.9964 | 0.0235 | 0.9992 | 0.0346 | 0.9971 | 0.0256 | 0.9984 | 0.0435 | 0.9966 | 0.0540 | 0.9977 |
| UV–Vis | 0.0243 | 0.9962 | 0.0334 | 0.9961 | 0.0237 | 0.9972 | 0.0469 | 0.9949 | 0.0419 | 0.9967 | 0.0448 | 0.9964 | 0.1140 | 0.9899 |
| Photocatalyst, Pollutant | kr·108 (Mol·L−1·Min−1) | Ks·102 (Mol·L−1) |
|---|---|---|
| Cu2S_TiO2, UVa | 1.63 | 624 |
| Cu2S_TiO2, UVa–Vis | 2.94 | 1682 |
| Cu2S_WO3, UVa | 1.42 | 556 |
| Cu2S_WO3, UVa–Vis | 2.98 | 1625 |
| TiO2_WO3, UVa | 2.68 | 1044 |
| TiO2_WO3,UVa–Vis | 2.73 | 1592 |
| Cu2S_TiO2_WO3, UVa | 3.19 | 1788 |
| Cu2S_TiO2_WO3, UVa–Vis | 5.26 | 2389 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Isac, L. Tuned S-Scheme Cu2S_TiO2_WO3 Heterostructure Photocatalyst toward S-Metolachlor (S-MCh) Herbicide Removal. Materials 2021, 14, 2231. https://doi.org/10.3390/ma14092231
Enesca A, Isac L. Tuned S-Scheme Cu2S_TiO2_WO3 Heterostructure Photocatalyst toward S-Metolachlor (S-MCh) Herbicide Removal. Materials. 2021; 14(9):2231. https://doi.org/10.3390/ma14092231
Chicago/Turabian StyleEnesca, Alexandru, and Luminita Isac. 2021. "Tuned S-Scheme Cu2S_TiO2_WO3 Heterostructure Photocatalyst toward S-Metolachlor (S-MCh) Herbicide Removal" Materials 14, no. 9: 2231. https://doi.org/10.3390/ma14092231
APA StyleEnesca, A., & Isac, L. (2021). Tuned S-Scheme Cu2S_TiO2_WO3 Heterostructure Photocatalyst toward S-Metolachlor (S-MCh) Herbicide Removal. Materials, 14(9), 2231. https://doi.org/10.3390/ma14092231







