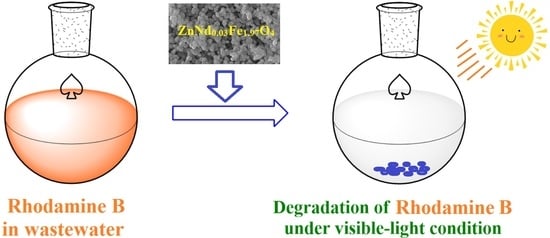
Enhanced Photocatalytic Activity of Spherical Nd3+ Substituted ZnFe2O4 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnNdxFe2−xO4 Nanoparticles
2.2. Characterization
2.3. Photocatalytic Degradation of Rhodamine B
3. Results
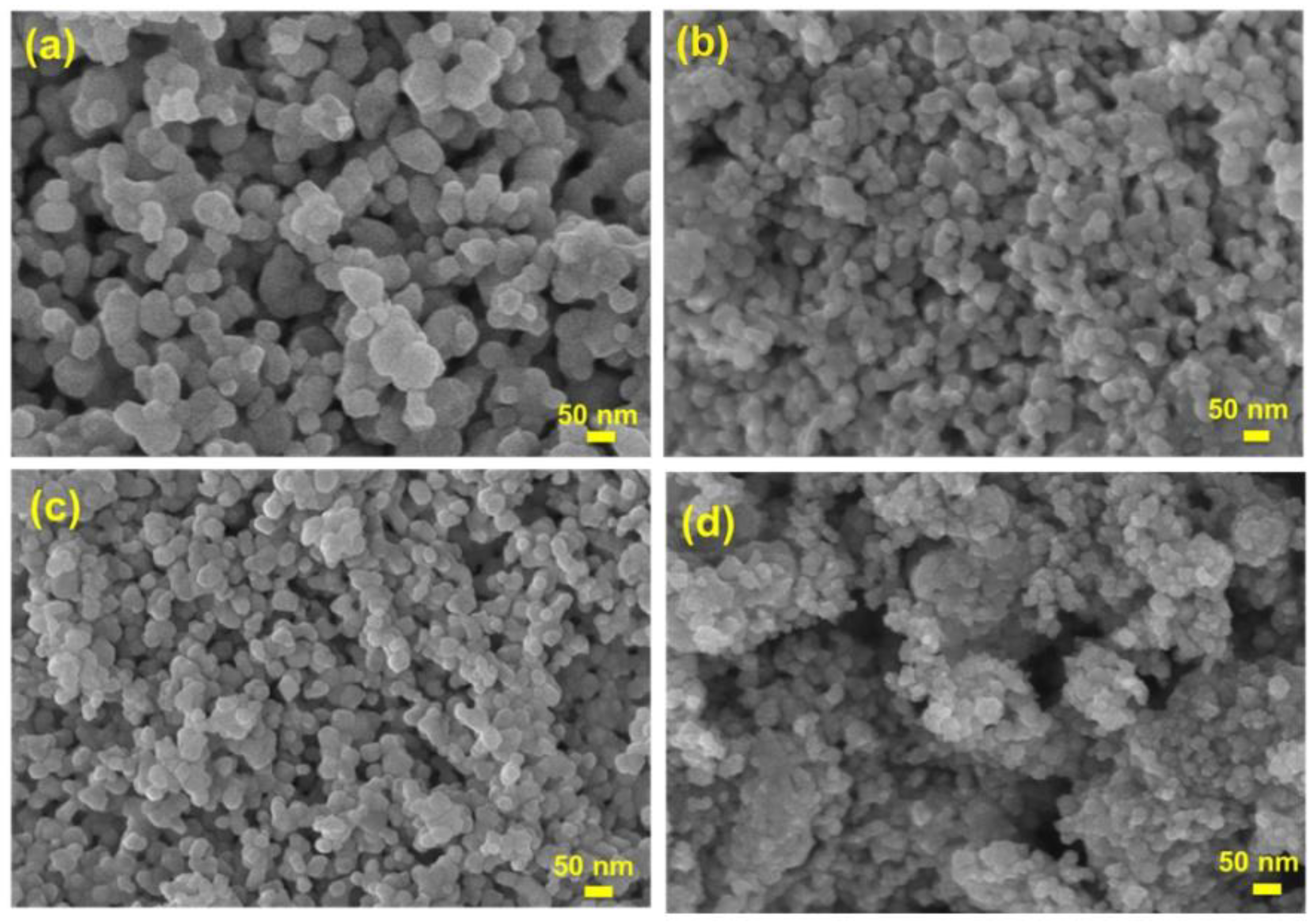
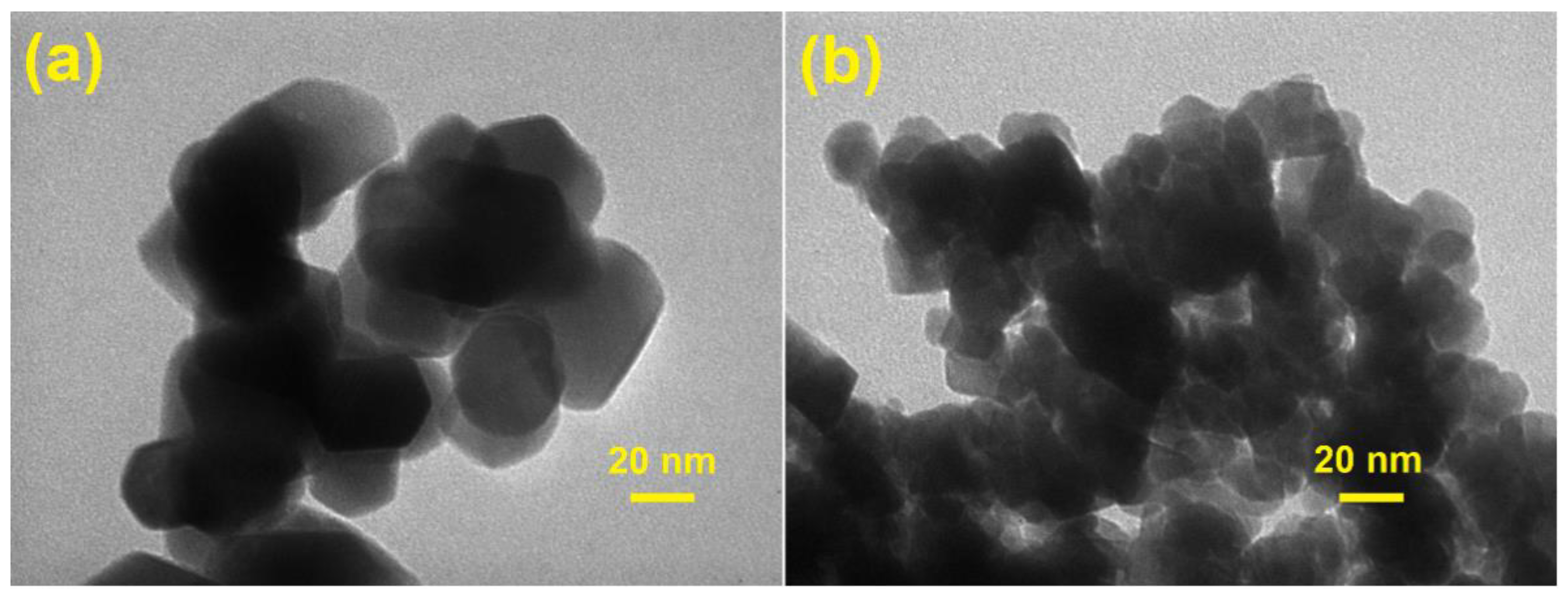
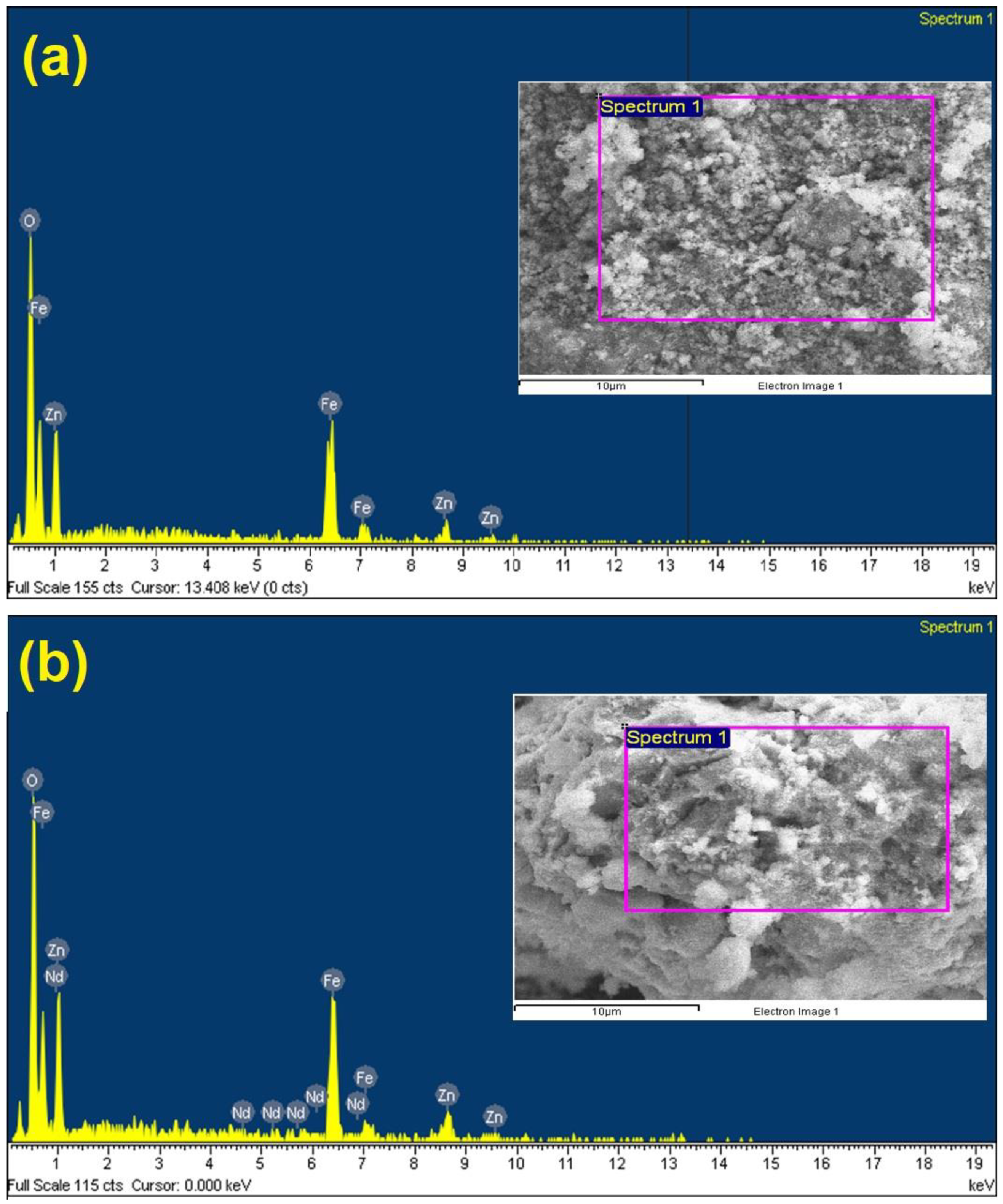
3.1. Characterization
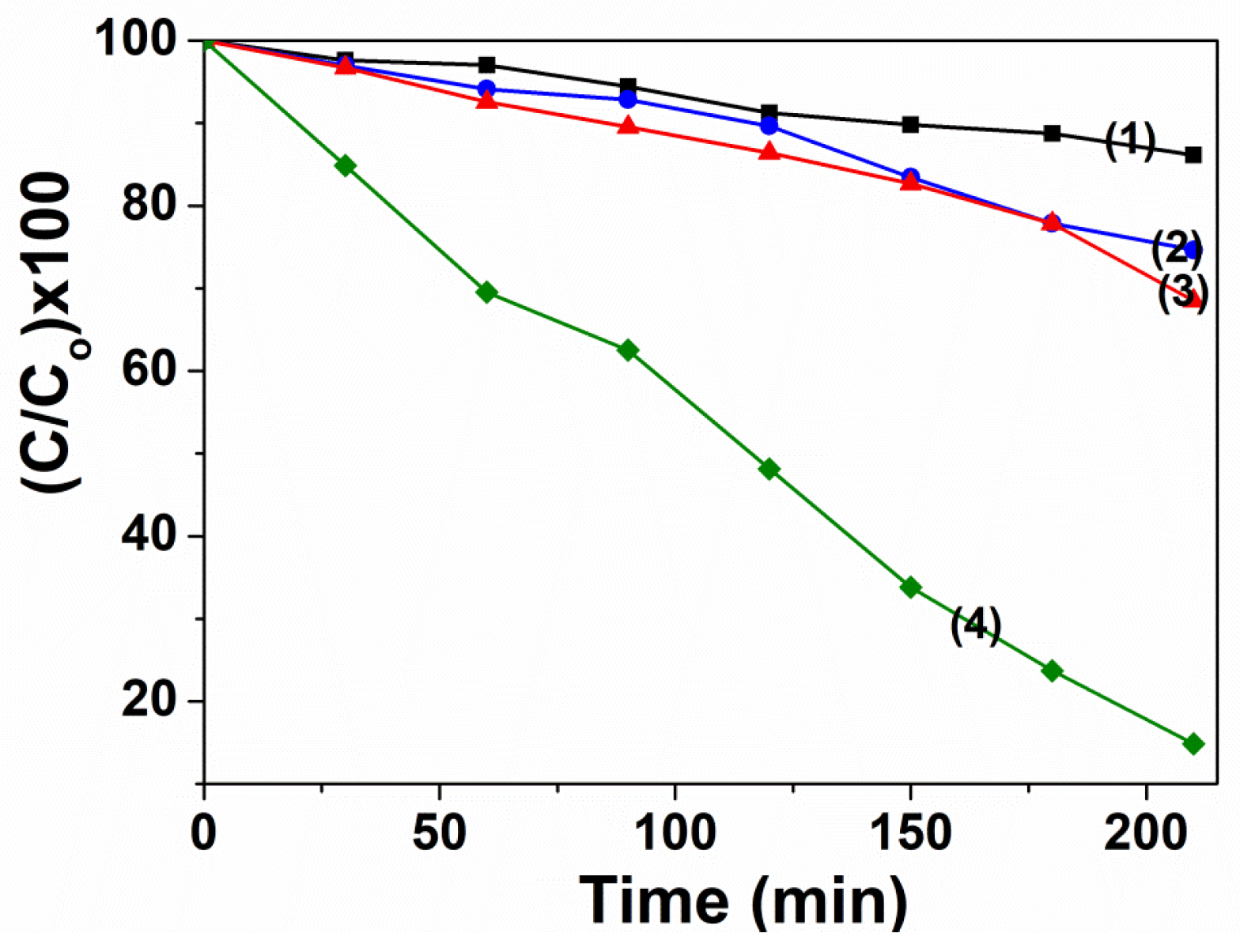
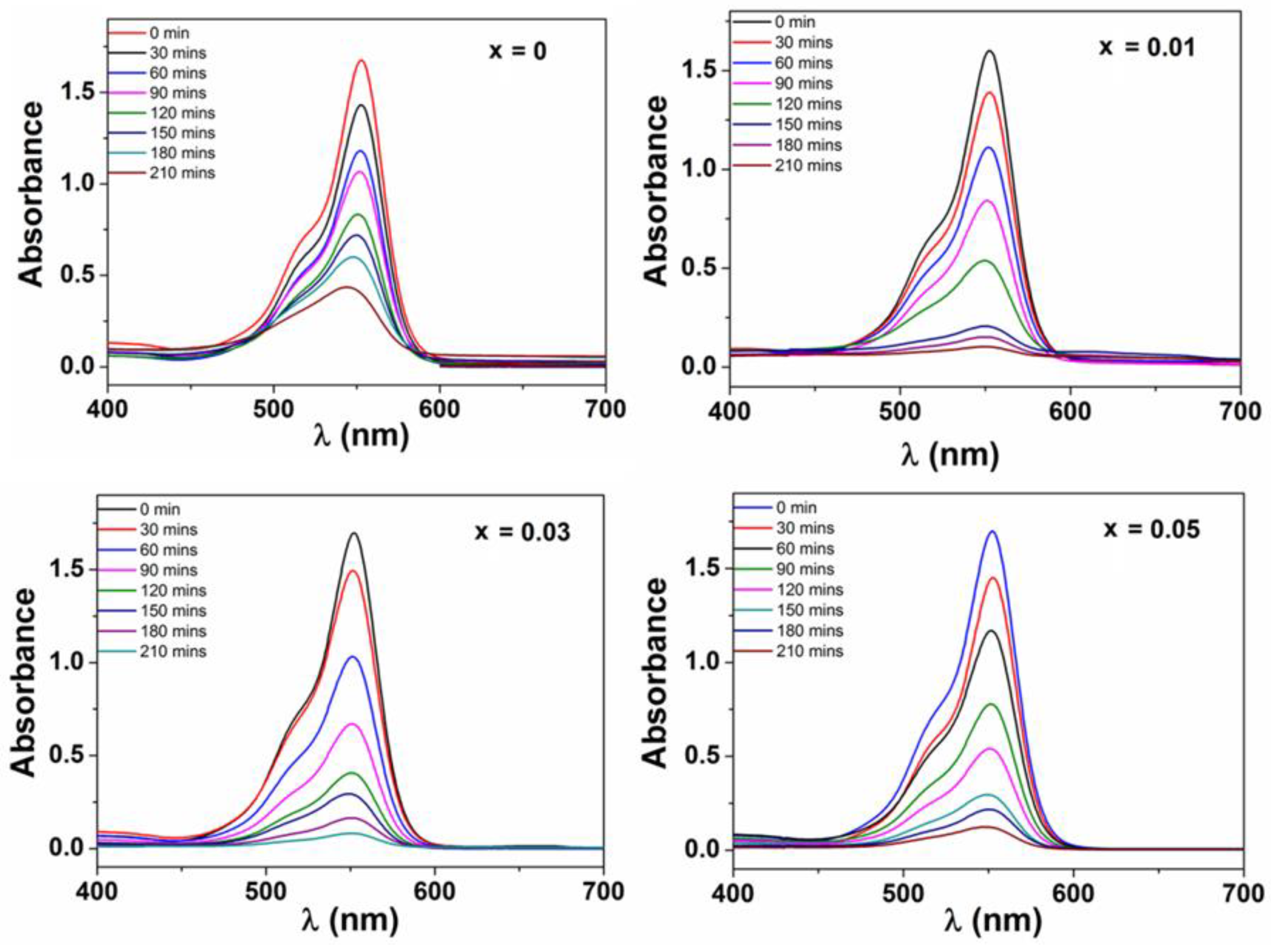
3.2. Photocatalytic Activity
3.2.1. Influence of Experimental Conditions
3.2.2. Influence of H2O2 Concentration
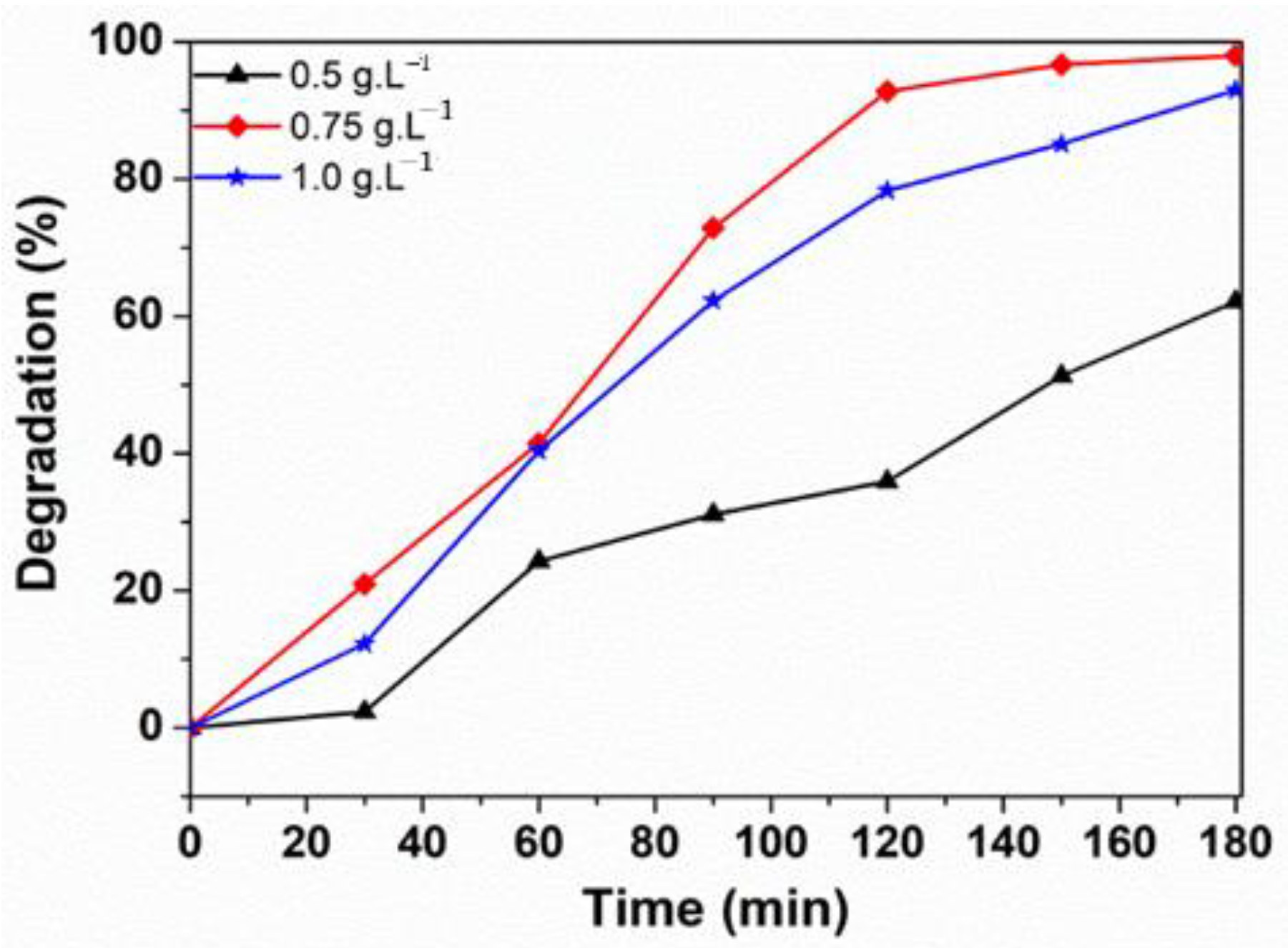
3.2.3. Influence of the Catalyst Loading
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Tran, T.; Phan, T.-Q.T.; Nguyen, D.T.C.; Nguyen, T.T.; Nguyen, D.H.; Vo, D.-V.N.; Bach, L.G.; Nguyen, T.D. Recyclable Fe3O4@C nanocomposite as potential adsorbent for a wide range of organic dyes and simulated hospital effluents. Environ. Technol. Innov. 2020, 20, 101122. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Dang, H.H.; Vo, D.-V.N.; Bach, L.G.; Nguyen, T.D.; Van Tran, T. Biogenic synthesis of MgO nanoparticles from different extracts (flower, bark, leaf) of Tecoma stans (L.) and their utilization in selected organic dyes treatment. J. Hazard. Mater. 2021, 404, 124146. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, H.-T.T.; Dang, H.H.; Nguyen, D.T.C.; Nguyen, D.H.; Van Pham, T.; Van Tan, L. Central composite design for optimizing the organic dyes remediation utilizing novel graphene oxide@CoFe2O4 nanocomposite. Surf. Interfaces 2020, 21, 100687. [Google Scholar] [CrossRef]
- Hsiao, P.-H.; Li, T.-C.; Chen, C.-Y. ZnO/Cu 2 O/Si Nanowire Arrays as Ternary Heterostructure-Based Photocatalysts with Enhanced Photodegradation Performances. Nanoscale Res. Lett. 2019, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.-H.; Timjan, S.; Kuo, K.-Y.; Juan, J.-C.; Chen, C.-Y. Optical Management of CQD/AgNP@ SiNW Arrays with Highly Efficient Capability of Dye Degradation. Catalysts 2021, 11, 399. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Niaz, N.A.; Nabi, G.; Ahmad, M.; Tahir, M.B.; Rafique, M.; Rizwan, M.; Khan, Y. Highly visible light responsive metal loaded N/TiO2 nanoparticles for photocatalytic conversion of CO2 into methane. Ceram. Int. 2017, 43, 6771–6777. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Khalid, N.R.; Liaqat, M.; Tahir, M.B.; Nabi, G.; Iqbal, T.; Niaz, N.A. The role of graphene and europium on TiO2 performance for photocatalytic hydrogen evolution. Ceram. Int. 2018, 44, 546–549. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Hassan, A.; Iqbal, T.; Kiran, H.; Majid, A. Morphology tailored synthesis of C-WO3 nanostructures and its photocatalytic application. J. Inorg. Organomet. Polym. Mater. 2018, 28, 738–745. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Iqbal, T.; Sagir, M.; Rafique, M. Role of MoSe2 on nanostructures WO3-CNT performance for photocatalytic hydrogen evolution. Ceram. Int. 2018, 44, 6686–6690. [Google Scholar] [CrossRef]
- Tahir, M.; Nabi, G.; Rafique, M.; Khalid, N. Nanostructured-based WO photocatalysts: Recent development, activity enhancement, perspectives and applications for wastewater treatment. Int. J. Environ. Sci. Technol. 2017, 14, 2519–2542. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Khalid, N.R. Enhanced photocatalytic performance of visible-light active graphene-WO3 nanostructures for hydrogen production. Mater. Sci. Semicond. Process. 2018, 84, 36–41. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Khalid, N.R.; Rafique, M. Role of europium on WO3 performance under visible-light for photocatalytic activity. Ceram. Int. 2018, 44, 5705–5709. [Google Scholar] [CrossRef]
- Tahir, M.B.; Iqbal, T.; Kiran, H.; Hasan, A. Insighting role of reduced graphene oxide in BiVO4 nanoparticles for improved photocatalytic hydrogen evolution and dyes degradation. Int. J. Energy Res. 2019, 43, 2410–2417. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, V.-H.; Nanda, S.; Vo, D.-V.N.; Nguyen, V.H.; Van Tran, T.; Nong, L.X.; Nguyen, T.T.; Bach, L.-G.; Abdullah, B. BiVO4 photocatalysis design and applications to oxygen production and degradation of organic compounds: A review. Environ. Chem. Lett. 2020, 18, 1779–1801. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.-Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Guo, X.; Wang, D. Photo-Fenton degradation of methylene blue by synergistic action of oxalic acid and hydrogen peroxide with NiFe2O4 hollow nanospheres catalyst. J. Environ. Chem. Eng. 2019, 7, 102814. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Z.; Gao, M.; Gu, M.; Wang, J.; Zeng, W.; Lv, Y.; Ren, Y.; Fan, Z. Effect of the solvents on the photocatalytic properties of ZnFe2O4 fabricated by solvothermal method. Mater. Chem. Phys. 2019, 223, 758–761. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, C.; Bi, H.; Liu, Y.; Yan, Q. Magnetically separable CuFe2O4/AgBr composite photocatalysts: Preparation, characterization, photocatalytic activity and photocatalytic mechanism under visible light. Appl. Surf. Sci. 2017, 392, 701–707. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Xavier, B.; Anceila, D.; Das, S.J. Nanocrystalline ferrite (MFe2O4, M = Ni, Cu, Mn and Sr) photocatalysts synthesized by homogeneous Co-precipitation technique. Optik 2018, 157, 441–448. [Google Scholar] [CrossRef]
- To Loan, N.T.; Hien Lan, N.T.; Thuy Hang, N.T.; Quang Hai, N.; Tu Anh, D.T.; Thi Hau, V.; Van Tan, L.; Van Tran, T. CoFe2O4 nanomaterials: Effect of annealing temperature on characterization, magnetic, photocatalytic, and photo-Fenton properties. Processes 2019, 7, 885. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Liu, J.; Shan, N.; Zhang, H.; Dionysiou, D.D. Visible light-assisted heterogeneous Fenton with ZnFe2O4 for the degradation of Orange II in water. Appl. Catal. B Environ. 2016, 182, 456–468. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, Y.; Xiao, B.; Wu, X.; Zhang, G. Magnetic yolk-shell structure of ZnFe2O4 nanoparticles for enhanced visible light photo-Fenton degradation towards antibiotics and mechanism study. Appl. Surf. Sci. 2020, 513, 145820. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Fan, S.; Yang, T. Enhanced visible light assisted Fenton-like degradation of dye via metal-doped zinc ferrite nanosphere prepared from metal-rich industrial wastewater. J. Taiwan Inst. Chem. Eng. 2019, 96, 185–192. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, J.; Zhou, J.; Ruan, X.; Chen, D.; Liu, J.; Liu, Q.; Qian, G. Electroplating sludge derived zinc-ferrite catalyst for the efficient photo-Fenton degradation of dye. J. Environ. Manag. 2017, 193, 146–153. [Google Scholar] [CrossRef]
- Surendra, B.S.; Shekhar, T.R.S.; Veerabhadraswamy, M.; Nagaswarupa, H.P.; Prashantha, S.C.; Geethanjali, G.C.; Likitha, C. Probe sonication synthesis of ZnFe2O4 NPs for the photocatalytic degradation of dyes and effect of treated wastewater on growth of plants. Chem. Phys. Lett. 2020, 745, 137286. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A. Impact of surfactant ratios on mesostructured MnFe2O4 nanocomposites and their photocatalytic performance. Ceram. Int. 2020, 46, 10925–10933. [Google Scholar] [CrossRef]
- Kalam, A.; Al-Sehemi, A.G.; Assiri, M.; Du, G.; Ahmad, T.; Ahmad, I.; Pannipara, M. Modified solvothermal synthesis of cobalt ferrite (CoFe2O4) magnetic nanoparticles photocatalysts for degradation of methylene blue with H2O2/visible light. Results Phys. 2018, 8, 1046–1053. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Nadumane, A.; Shetty, K.; Anantharaju, K.S.; Nagaswarupa, H.P.; Rangappa, D.; Vidya, Y.S.; Nagabhushana, H.; Prashantha, S.C. Sunlight photocatalytic performance of Mg-doped nickel ferrite synthesized by a green sol-gel route. J. Sci. Adv. Mater. Devices 2019, 4, 89–100. [Google Scholar] [CrossRef]
- Bhukal, S.; Bansal, S.; Singhal, S. Magnetic Mn substituted cobalt zinc ferrite systems: Structural, electrical and magnetic properties and their role in photo-catalytic degradation of methyl orange azo dye. Phys. B Condens. Matter 2014, 445, 48–55. [Google Scholar] [CrossRef]
- Abraham, A.G.; Manikandan, A.; Manikandan, E.; Vadivel, S.; Jaganathan, S.K.; Baykal, A.; Renganathan, P.S. Enhanced magneto-optical and photo-catalytic properties of transition metal cobalt (Co2+ ions) doped spinel MgFe2O4 ferrite nanocomposites. J. Magn. Magn. Mater. 2018, 452, 380–388. [Google Scholar] [CrossRef]
- Sharma, R.; Singhal, S. Structural, magnetic and electrical properties of zinc doped nickel ferrite and their application in photo catalytic degradation of methylene blue. Phys. B Condens. Matter 2013, 414, 83–90. [Google Scholar] [CrossRef]
- Joshi, S.; Kumar, M.; Pandey, H.; Singh, M.; Pal, P. Structural, magnetic and dielectric properties of Gd3+ substituted NiFe2O4 nanoparticles. J. Alloys Compd. 2018, 768, 287–297. [Google Scholar] [CrossRef]
- Chauhan, L.; Singh, N.; Dhar, A.; Kumar, H.; Kumar, S.; Sreenivas, K. Structural and electrical properties of Dy3+ substituted NiFe2O4 ceramics prepared from powders derived by combustion method. Ceram. Int. 2017, 43, 8378–8390. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Kou, Q.; Wang, Z.; Han, D.; Sun, Y.; Yang, J.; Liu, Y.; Yang, L. Effects of Nd concentration on structural and magnetic properties of ZnFe 2 O 4 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 3665–3671. [Google Scholar] [CrossRef]
- Masoudpanah, S.M.; Ebrahimi, S.A.S.; Derakhshani, M.; Mirkazemi, S.M. Structure and magnetic properties of La substituted ZnFe2O4 nanoparticles synthesized by sol–gel autocombustion method. J. Magn. Magn. Mater. 2014, 370, 122–126. [Google Scholar] [CrossRef]
- Mariosi, F.R.; Venturini, J.; da Cas Viegas, A.; Bergmann, C.P. Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram. Int. 2020, 46, 2772–2779. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y. Sonochemical synthesis of Eu3+ substituted CoFe2O4 nanoparticles and their structural, optical and magnetic properties. Ultrason. Sonochem. 2019, 58, 104621. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bansal, S.; Singhal, S. Augmenting the catalytic activity of CoFe2O4 by substituting rare earth cations into the spinel structure. RSC Adv. 2016, 6, 71676–71691. [Google Scholar] [CrossRef]
- Rashmi, S.K.; Naik, H.S.B.; Jayadevappa, H.; Sudhamani, C.N.; Patil, S.B.; Naik, M.M. Influence of Sm3+ ions on structural, optical and solar light driven photocatalytic activity of spinel MnFe2O4 nanoparticles. J. Solid State Chem. 2017, 255, 178–192. [Google Scholar] [CrossRef]
- Patil, S.B.; Naik, H.S.B.; Nagaraju, G.; Viswanath, R.; Rashmi, S.K. Synthesis of visible light active Gd3+-substituted ZnFe2O4 nanoparticles for photocatalytic and antibacterial activities. Eur. Phys. J. Plus 2017, 132, 1–12. [Google Scholar] [CrossRef]
- Harish, K.N.; Bhojya Naik, H.S.; Prashanth Kumar, P.N.; Viswanath, R. Optical and photocatalytic properties of solar light active Nd-substituted Ni ferrite catalysts: For environmental protection. ACS Sustain. Chem. Eng. 2013, 1, 1143–1153. [Google Scholar] [CrossRef]

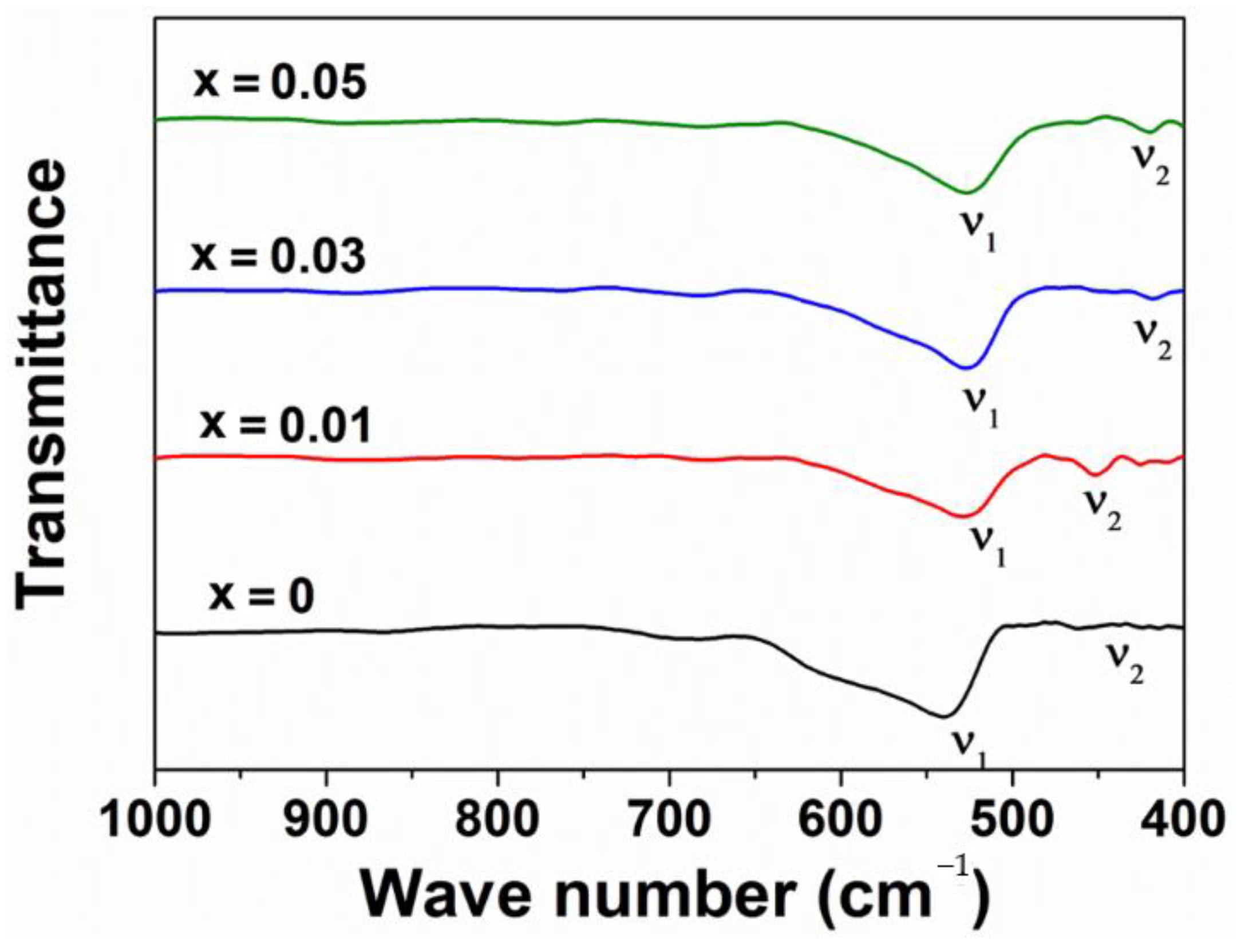



| Samples | DXRD (nm) | a (Å) | V (Å3) | ν1 (cm−1) | ν2 (cm−1) |
|---|---|---|---|---|---|
| ZnFe2O4 | 22 | 8.43 | 599.08 | 522.7 | 447.5 |
| ZnNd0.01Fe1.99O4 | 21 | 8.44 | 601.21 | 528.5 | 451.3 |
| ZnNd0.03Fe1.97O4 | 18 | 8.45 | 603.35 | 526.0 | 418.5 |
| ZnNd0.05Fe1.95O4 | 12 | 8.45 | 603.35 | 526.6 | 420.5 |
| Samples | H (%) | k (min−1) | R2 |
|---|---|---|---|
| ZnFe2O4 | 85.14 ± 0.99 | 0.0095 | 0.952 |
| ZnNd0.01Fe1.99O4 | 96.53 ± 0.95 | 0.0189 | 0.951 |
| ZnNd0.03Fe1.97O4 | 98.00 ± 0.44 | 0.0190 | 0.964 |
| ZnNd0.05Fe1.95O4 | 95.46 ± 0.91 | 0.0163 | 0.972 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.T.; Nguyen, H.T.T.; Le, T.H.; Nguyen, L.T.H.; Nguyen, H.Q.; Pham, T.T.H.; Bui, N.D.; Tran, N.T.K.; Nguyen, D.T.C.; Lam, T.V.; et al. Enhanced Photocatalytic Activity of Spherical Nd3+ Substituted ZnFe2O4 Nanoparticles. Materials 2021, 14, 2054. https://doi.org/10.3390/ma14082054
Nguyen LTT, Nguyen HTT, Le TH, Nguyen LTH, Nguyen HQ, Pham TTH, Bui ND, Tran NTK, Nguyen DTC, Lam TV, et al. Enhanced Photocatalytic Activity of Spherical Nd3+ Substituted ZnFe2O4 Nanoparticles. Materials. 2021; 14(8):2054. https://doi.org/10.3390/ma14082054
Chicago/Turabian StyleNguyen, Loan T. T., Hang T. T. Nguyen, Thieng H. Le, Lan T. H. Nguyen, Hai Q. Nguyen, Thanh T. H. Pham, Nguyen D. Bui, Ngan T. K. Tran, Duyen Thi Cam Nguyen, Tan Van Lam, and et al. 2021. "Enhanced Photocatalytic Activity of Spherical Nd3+ Substituted ZnFe2O4 Nanoparticles" Materials 14, no. 8: 2054. https://doi.org/10.3390/ma14082054
APA StyleNguyen, L. T. T., Nguyen, H. T. T., Le, T. H., Nguyen, L. T. H., Nguyen, H. Q., Pham, T. T. H., Bui, N. D., Tran, N. T. K., Nguyen, D. T. C., Lam, T. V., & Tran, T. V. (2021). Enhanced Photocatalytic Activity of Spherical Nd3+ Substituted ZnFe2O4 Nanoparticles. Materials, 14(8), 2054. https://doi.org/10.3390/ma14082054