Study of the Electrical Properties of Aluminate Cement Adhesives for Porcelain Insulators
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing Methods
2.2.1. Compressive Strength
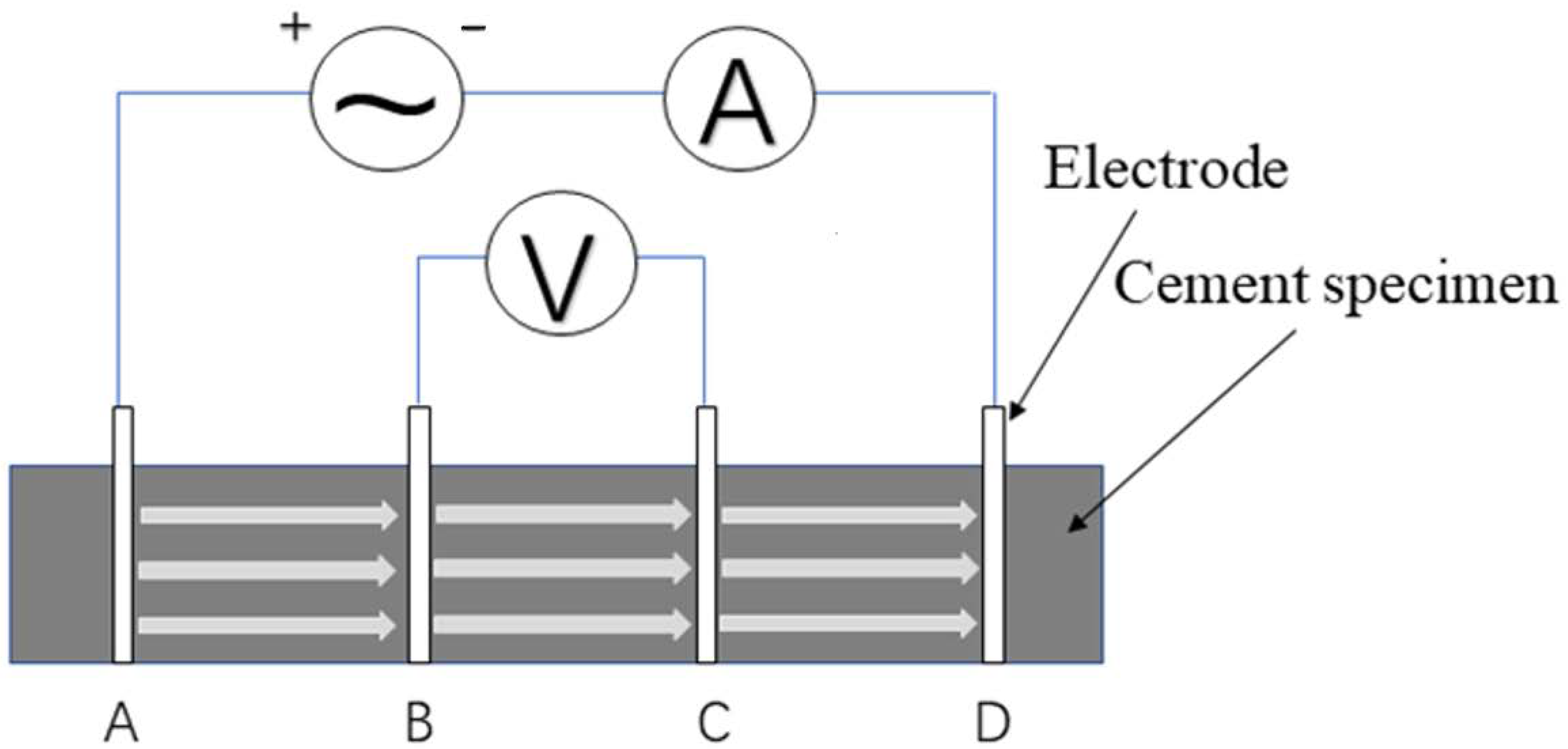
2.2.2. Measurement of the Electrical Resistivity of Cement Pastes
2.2.3. Moisture Content
2.2.4. Low-Field 1H NMR
3. Results and Discussion
3.1. Surface Dry Saturated Conditions
3.1.1. Compressive Strength
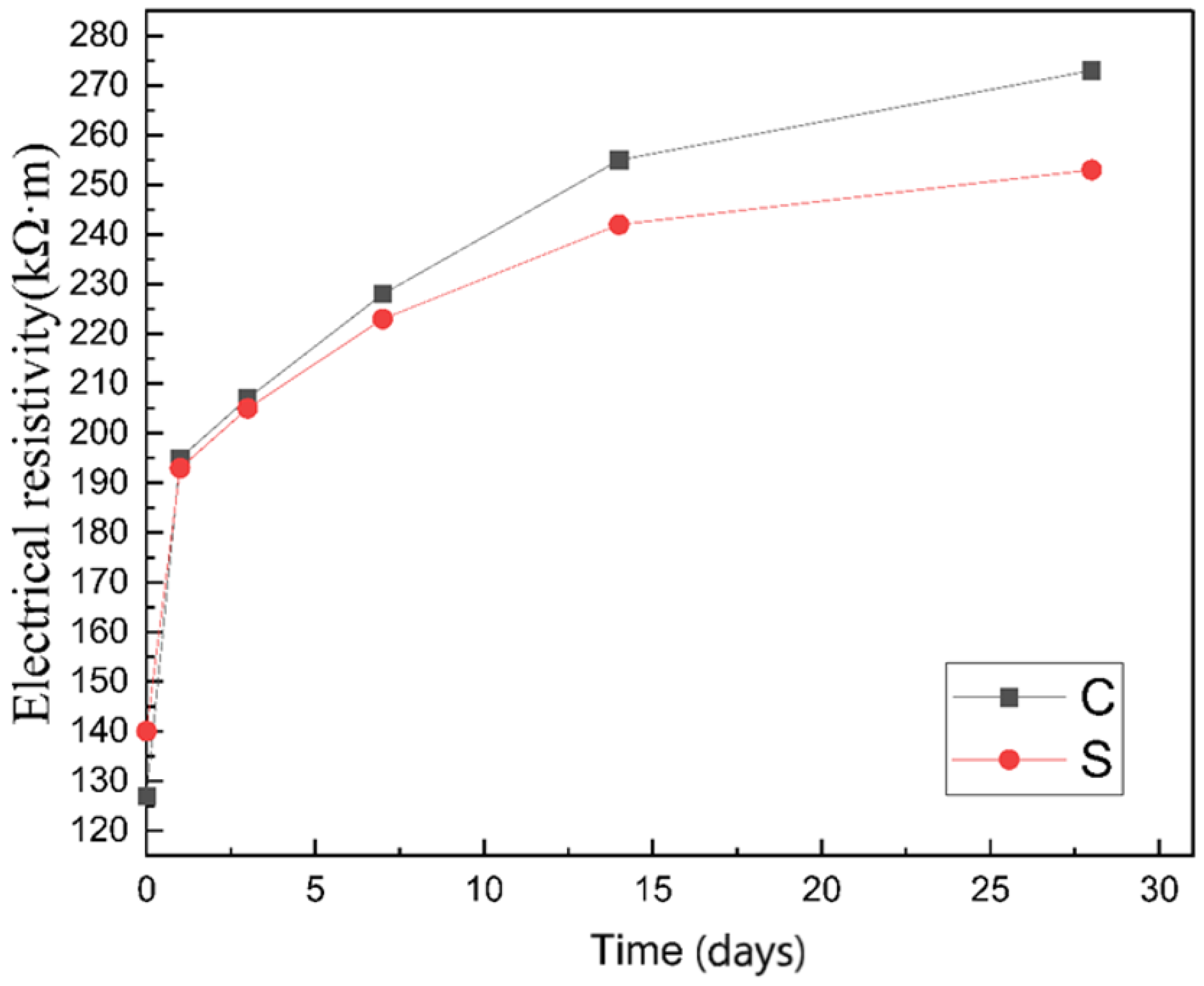
3.1.2. Electrical Resistivity
3.2. Unsaturated State
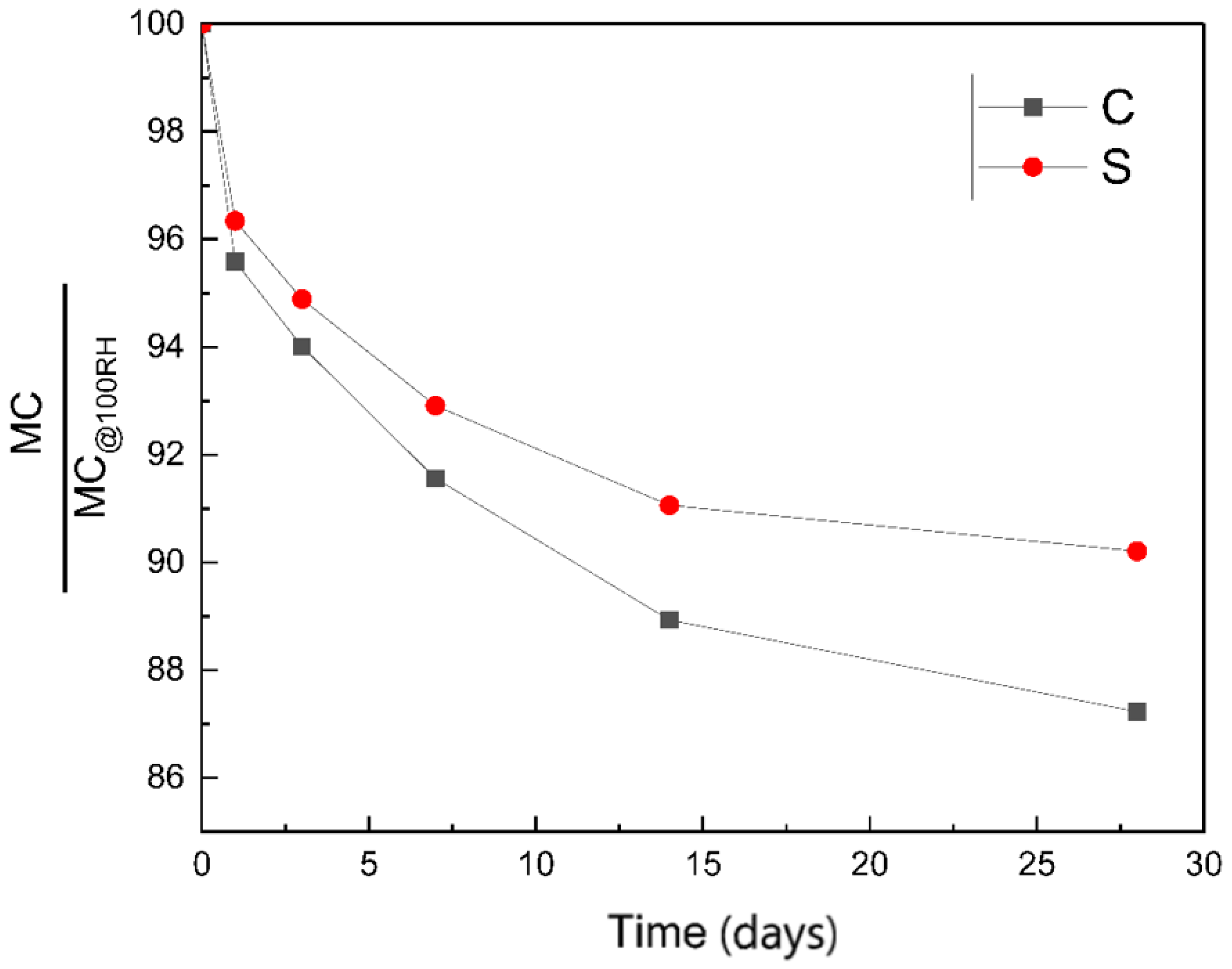
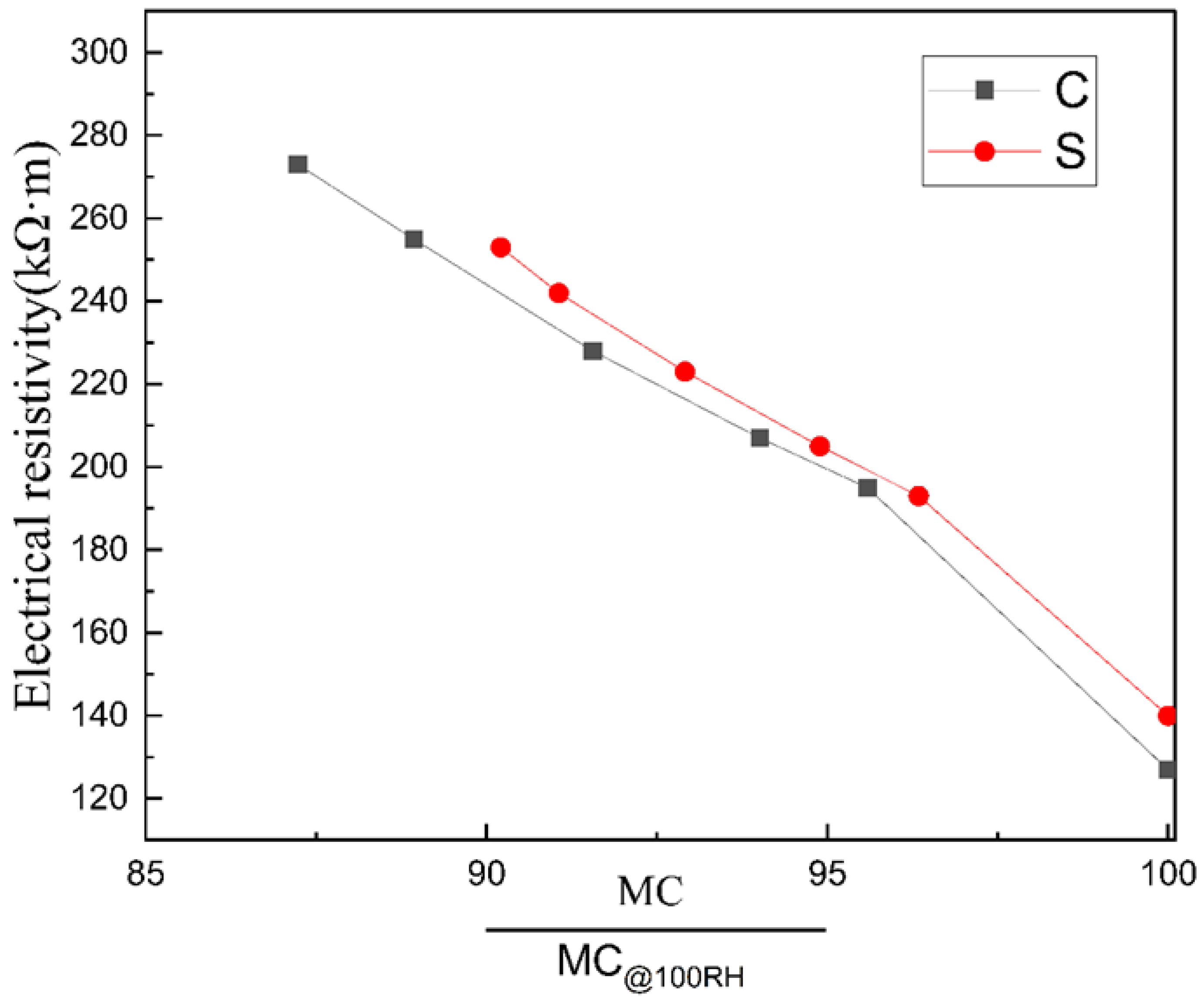
3.2.1. Moisture Content
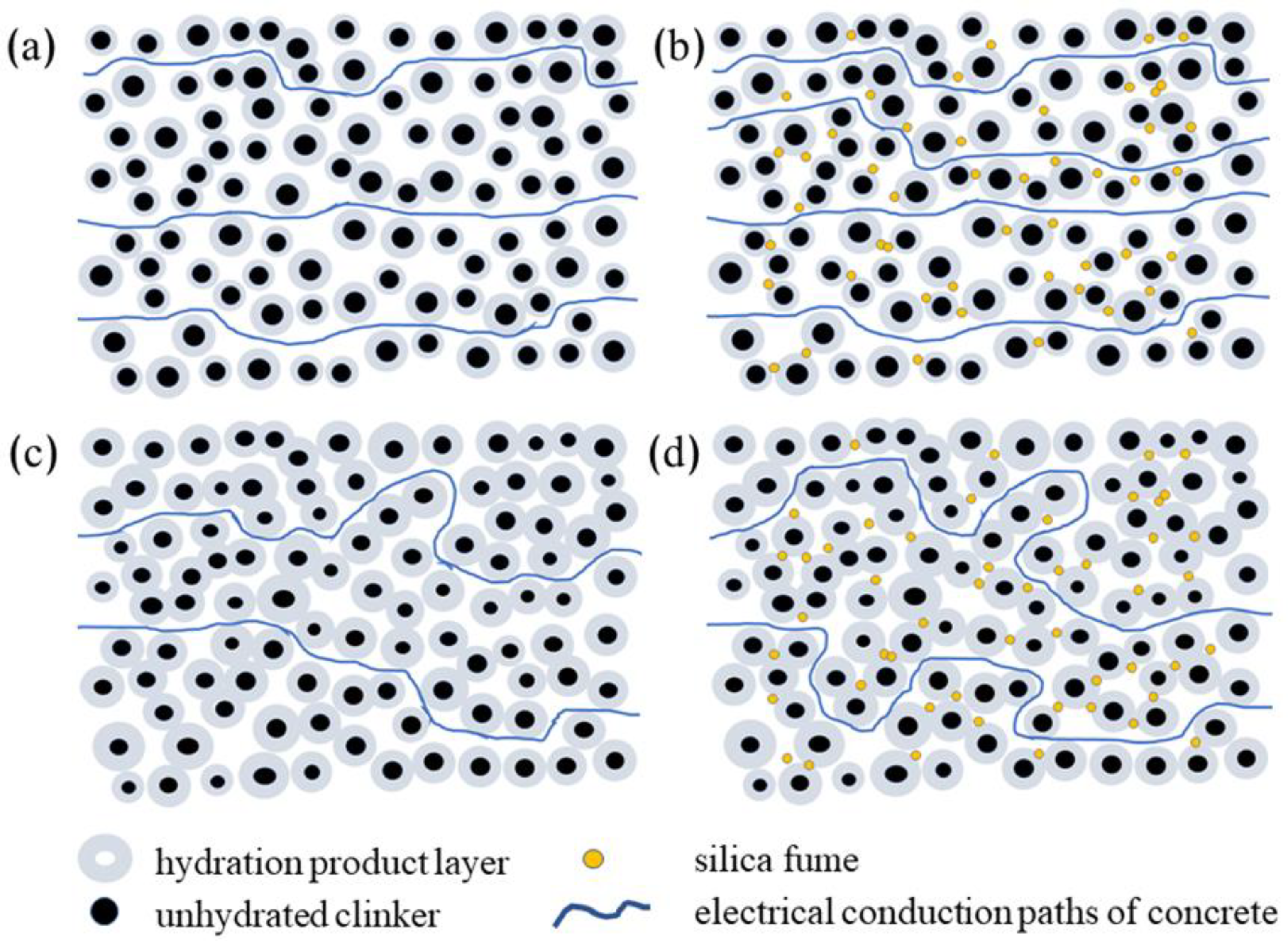
3.2.2. Conductive Model of Cement Adhesive for Porcelain Insulators
3.2.3. The Resistivity of Unsaturated Cement-Based Materials
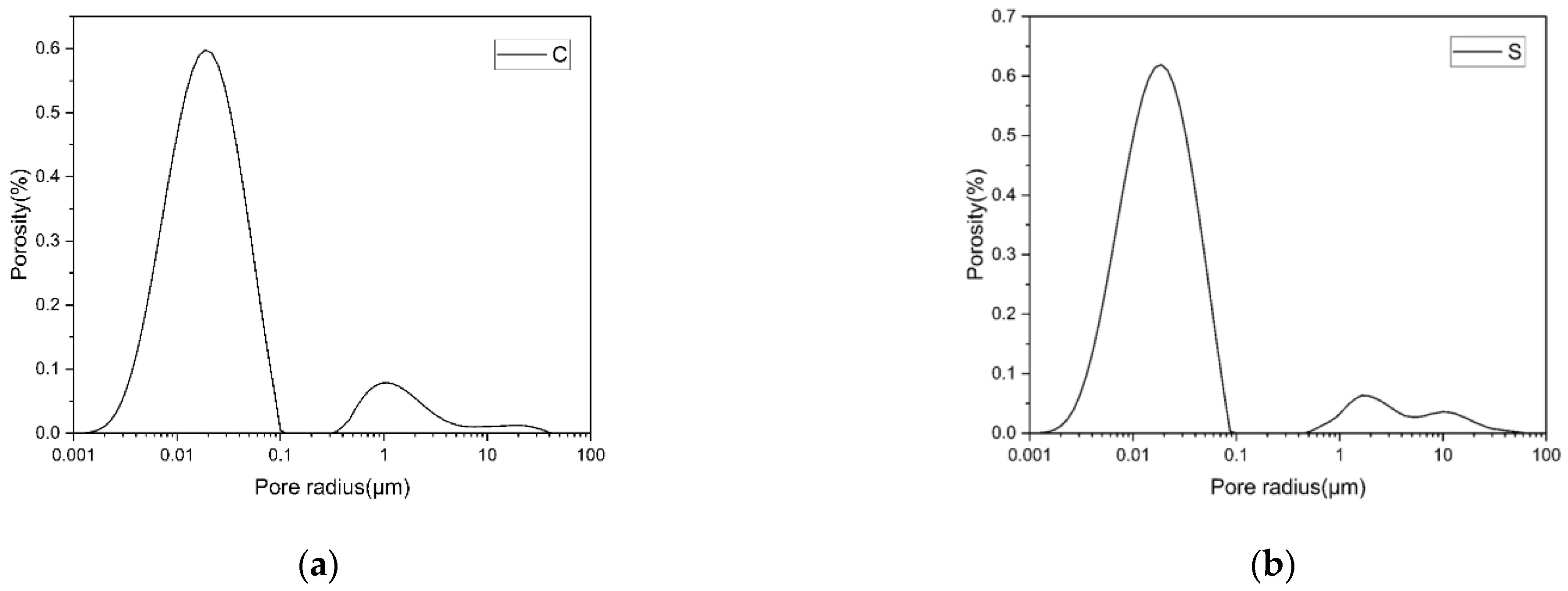
3.3. Pore Diameter Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, K.; Zeng, X.; Zhou, X.; Qu, F.; Wang, P. A new model for the electrical conductivity of cement-based material by considering pore size distribution. Mag. Concr. Res. 2017, 69, 1067–1078. [Google Scholar] [CrossRef]
- Vaillancourt, G.; Bellerive, J.; St-Jean, M.; Jean, C. New live line tester for porcelain suspension insulators on high-voltage power lines. IEEE Trans. Power Deliv. 1994, 9, 208–219. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Shi, F.; Liu, J.; Cao, P.; Du, T.; Gu, H. Development of relationships between permeability coefficient and electrical and thermal conductivity of recycled aggregates permeable cement concrete. Constr. Build. Mater. 2020, 254, 119247. [Google Scholar] [CrossRef]
- Ma, H.; Hou, D.; Li, Z. Two-scale modeling of transport properties of cement paste: Formation factor, electrical conductivity and chloride diffusivity. Comput. Mater. Sci. 2015, 110, 270–280. [Google Scholar] [CrossRef]
- Honorio, T.; Carasek, H.; Cascudo, O. Electrical properties of cement-based materials: Multiscale modeling and quantification of the variability. Constr. Build. Mater. 2020, 245, 118461. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Zeng, Q. The effect of water saturation degree on the electrical properties of cement-based porous material. Cem. Concr. Compos. 2016, 70, 35–47. [Google Scholar] [CrossRef]
- Liu, K.; Cheng, X.; Li, J.; Gao, X.; Cao, Y.; Guo, X.; Zhuang, J.; Zhang, C. Effects of microstructure and pore water on electrical conductivity of cement slurry during early hydration. Compos. Part B Eng. 2019, 177, 107435. [Google Scholar] [CrossRef]
- Yousuf, F.; Xiaosheng, W. Investigation of the early-age microstructural development of hydrating cement pastes through electrical resistivity measurements. Case Stud. Constr. Mater. 2020, 13, e00391. [Google Scholar]
- Yousuf, F.; Wei, X.; Zhou, J. Monitoring the setting and hardening behaviour of cement paste by electrical resistivity measurement. Constr. Build. Mater. 2020, 252, 118941. [Google Scholar] [CrossRef]
- Scrivener, K.; Capmas, A. Calcium Aluminate Cements. In Advanced Concrete Technology; Scrivener, K., Ed.; Elviser: Amsterdam, The Netherlands, 2003; pp. 1–31. [Google Scholar]
- Scrivener, K.L.; Cabiron, J.-L.; Letourneux, R. High-performance concretes from calcium aluminate cements. Cem. Concr. Res. 1999, 29, 1215–1223. [Google Scholar] [CrossRef]
- Son, H.M.; Park, S.M.; Jang, J.G.; Lee, H.K. Effect of nano-silica on hydration and conversion of calcium aluminate cement. Constr. Build. Mater. 2018, 169, 819–825. [Google Scholar] [CrossRef]
- Son, H.M.; Park, S.; Kim, H.Y.; Seo, J.H.; Lee, H.K. Effect of CaSO4 on hydration and phase conversion of calcium aluminate cement. Constr. Build. Mater. 2019, 224, 40–47. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Vázquez, T.; Palomo, A. Effect of sodium silicate on calcium aluminate cement hydration in highly alkaline media: A microstructural characterization. J. Am. Ceram. Soc. 2011, 94, 1297–1303. [Google Scholar] [CrossRef]
- Kirca, N.; Yaman, Z.; Tokyay, M. Compressive strength development of calcium aluminate cement-GGBFS blends. Cem. Concr. Compos. 2013, 35, 163–170. [Google Scholar] [CrossRef]
- ASTM C39. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens USA; ASTM: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Lakshminarayanan, V.; Ramesh, P.S.; Rajagopalan, S.R. A new technique for the measurement of the electrical resistivity of concrete. Mag. Concr. Res. 1992, 44, 47–52. [Google Scholar] [CrossRef]
- Forde, M.C.; Mccarter, W.J.; Whittington, H.W. Resistivity characteristics of concrete. Proc. Inst. Civil Eng. 1982, 73, 223–224. [Google Scholar]
- Mostafa, N.Y.; Zaki, Z.I.; Elkader, O.H.A. Chemical activation of calcium aluminate cement composites cured at elevated temperature. Cem. Concr. Compos. 2012, 34, 1187–1193. [Google Scholar] [CrossRef]
- Hidalgo, A.; García, J.L.; Alonso, M.C.; Fernández, L.; Andrade, C. Microstructure development in mixes of calcium aluminate cement with silica fume or fly ash. J. Therm. Anal. Calorim. 2009, 96, 335–345. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, J.G.; Son, H.M.; Lee, H.K. Stable conversion of metastable hydrates in calcium aluminate cement by early carbonation curing. J. CO2 Util. 2017, 21, 224–226. [Google Scholar] [CrossRef]
- Rajabipour, F.; Weiss, J. Electrical conductivity of drying cement paste. Mater. Struct. 2006, 40, 1143–1160. [Google Scholar] [CrossRef]
- Parlange, J.Y. Porous media: Fluid transport and pore structure. Soil Sci. 1981, 132, 316. [Google Scholar] [CrossRef]
- Archie, G.E. The electrical resistivity log as an aid in determining some reservoir characteristics. Trans. AIME 1942, 146, 54–62. [Google Scholar] [CrossRef]
- Ewing, R.P.; Hunt, A.G. Dependence of the electrical conductivity on saturation in real porous media. Vadose Zone J. 2006, 5, 731–741. [Google Scholar] [CrossRef]
- Johnson, D.L.; Sen, P.N. Dependence of the conductivity of a porous medium on electrolyte conductivity. Phys. Rev. B Condens. Matter. 1988, 37, 3502–3510. [Google Scholar] [CrossRef]
- Kenyon, W.; Howard, J. Pore-Size Distribution and NMR in Microporous Cherty Sandstones. In Proceedings of the SPWLA Annual Logging Symposium, Denver, CO, USA, 11–14 June 1989. [Google Scholar]
- Bowers, M.C.; Ehrlich, R.; Howard, J.J.; Kenyon, W.E. Determination of porosity types from NMR data and their relationship to porosity types derived from thin section. Int. J. Multiph. Flow 1996, 13, 1–14. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, Z.; Yang, X.; Li, C.; Tang, X. Assessment and mechanism study of bleeding process in cement paste by 1H low-field NMR. Constr. Build. Mater. 2015, 100, 255–261. [Google Scholar] [CrossRef]
- Zhou, C.; Ren, F.; Zeng, Q.; Xiao, L.; Wang, W. Pore-size resolved water vapor adsorption kinetics of white cement mortars as viewed from proton NMR relaxation. Cem. Concr. Res. 2018, 105, 31–43. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Q.; Deng, D.; Ye, T.; Fang, L. Measuring the pore structure of cement asphalt mortar by nuclear magnetic resonance. Constr. Build. Mater. 2017, 137, 450–458. [Google Scholar] [CrossRef]








| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | I.L |
|---|---|---|---|---|---|---|---|---|---|
| aluminate cement | 4.45 | 52.03 | 1.71 | 37.53 | 0.76 | 0.25 | 0.07 | 2.54 | 0.20 |
| SF | 98.57 | 0.35 | 0.12 | 0.21 | 0.17 | 0.16 | 0.06 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.; Hu, Z.; Liu, G.; Xiao, J.; Wang, Y. Study of the Electrical Properties of Aluminate Cement Adhesives for Porcelain Insulators. Materials 2021, 14, 2232. https://doi.org/10.3390/ma14092232
Wan H, Hu Z, Liu G, Xiao J, Wang Y. Study of the Electrical Properties of Aluminate Cement Adhesives for Porcelain Insulators. Materials. 2021; 14(9):2232. https://doi.org/10.3390/ma14092232
Chicago/Turabian StyleWan, Huiwen, Zhangyin Hu, Gang Liu, Jiadong Xiao, and Yong Wang. 2021. "Study of the Electrical Properties of Aluminate Cement Adhesives for Porcelain Insulators" Materials 14, no. 9: 2232. https://doi.org/10.3390/ma14092232
APA StyleWan, H., Hu, Z., Liu, G., Xiao, J., & Wang, Y. (2021). Study of the Electrical Properties of Aluminate Cement Adhesives for Porcelain Insulators. Materials, 14(9), 2232. https://doi.org/10.3390/ma14092232






