Reuse of Industrial and Agricultural Waste in the Fabrication of Geopolymeric Binders: Mechanical and Microstructural Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
3. Results and Discussion
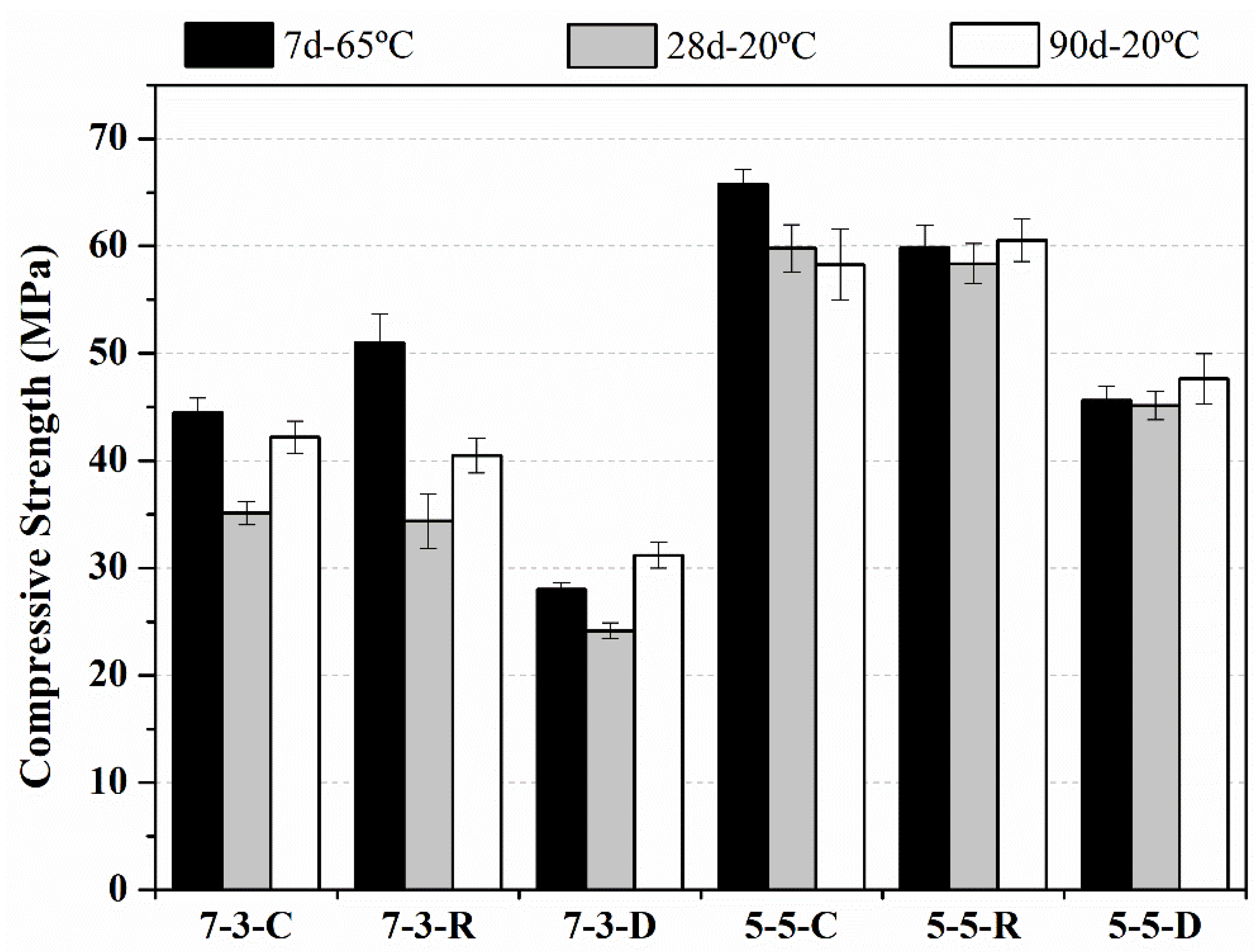
3.1. Compressive Strength
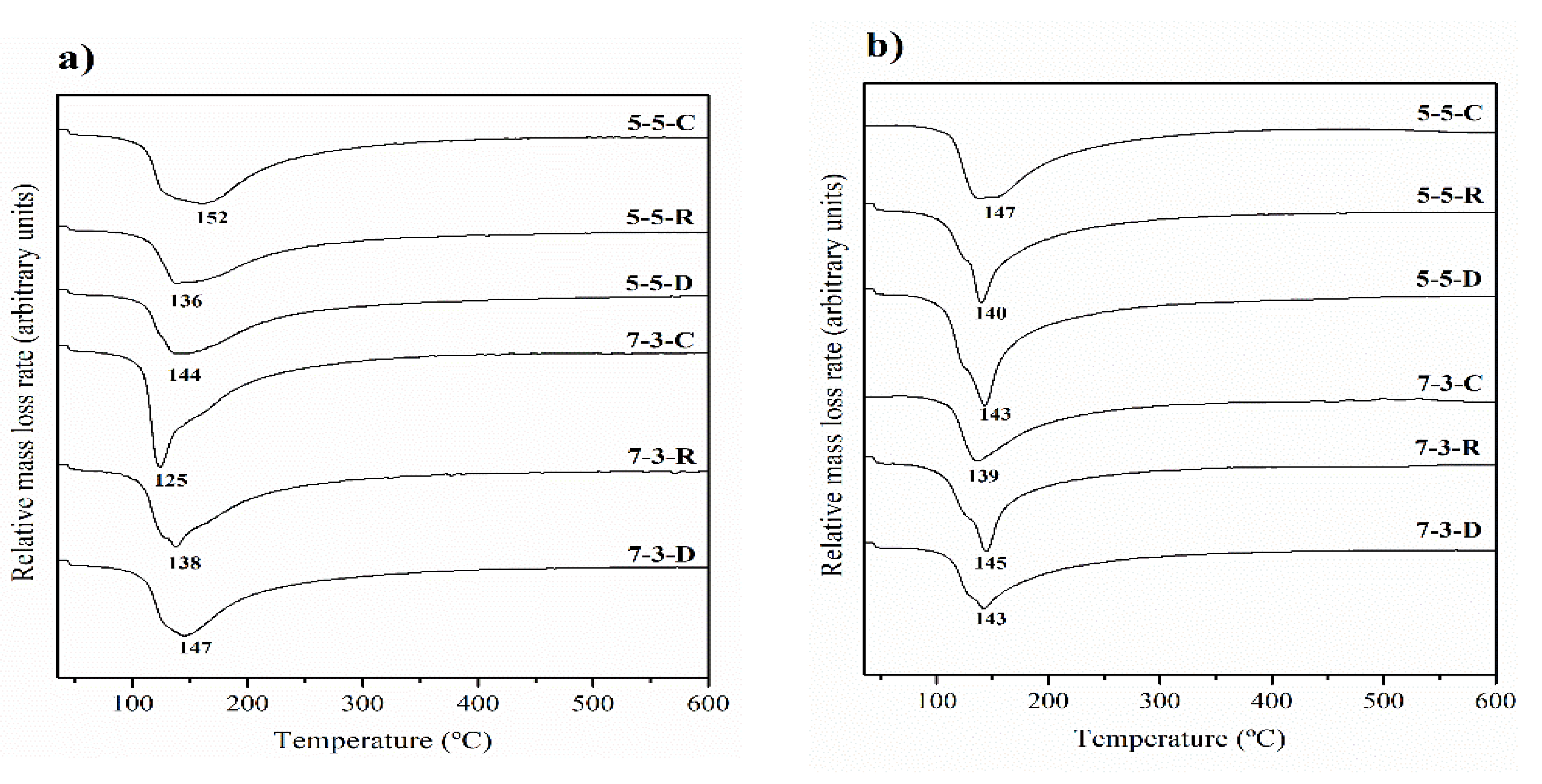
3.2. Thermogravimetric Analysis
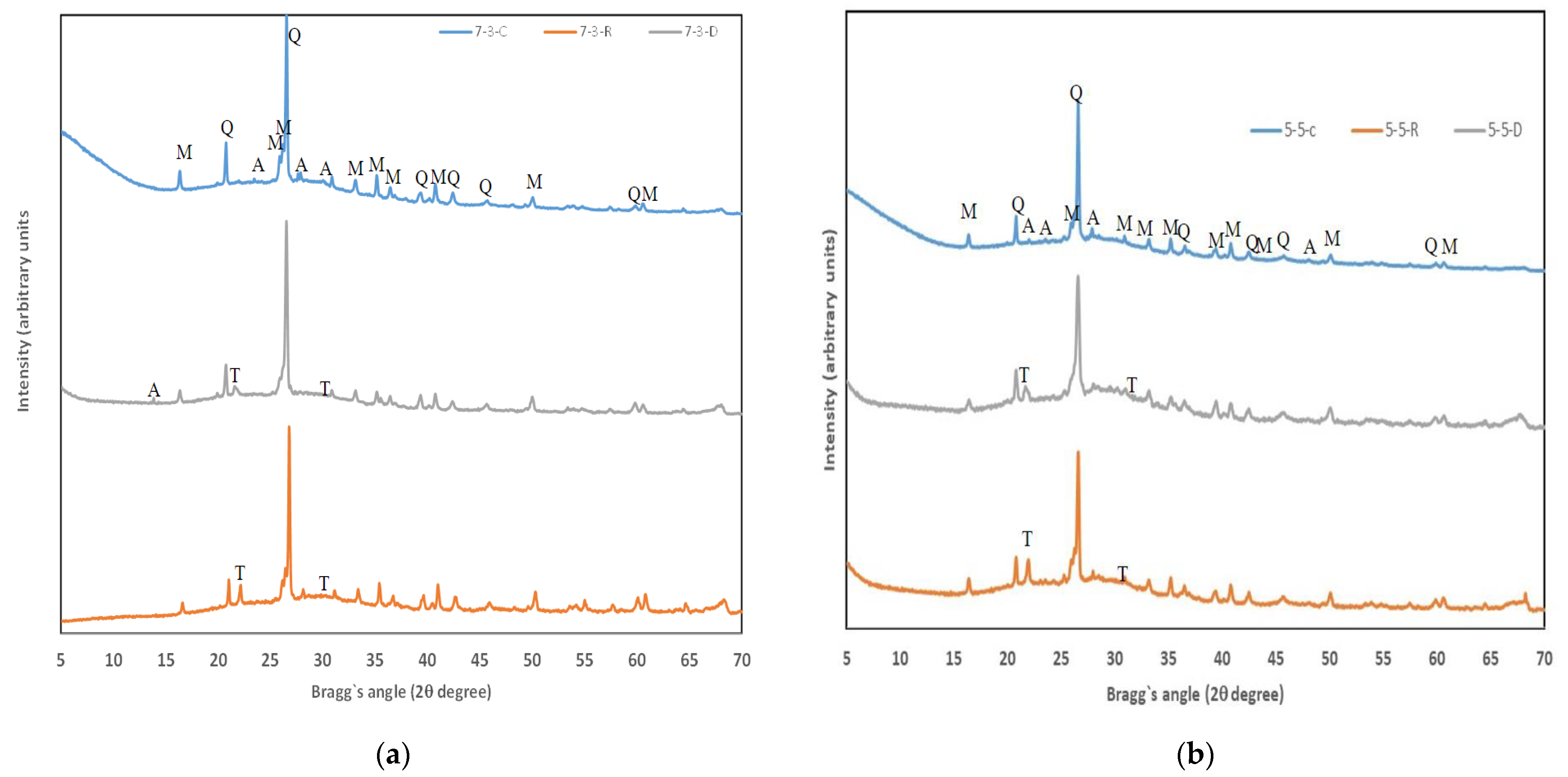
3.3. XRD Analysis
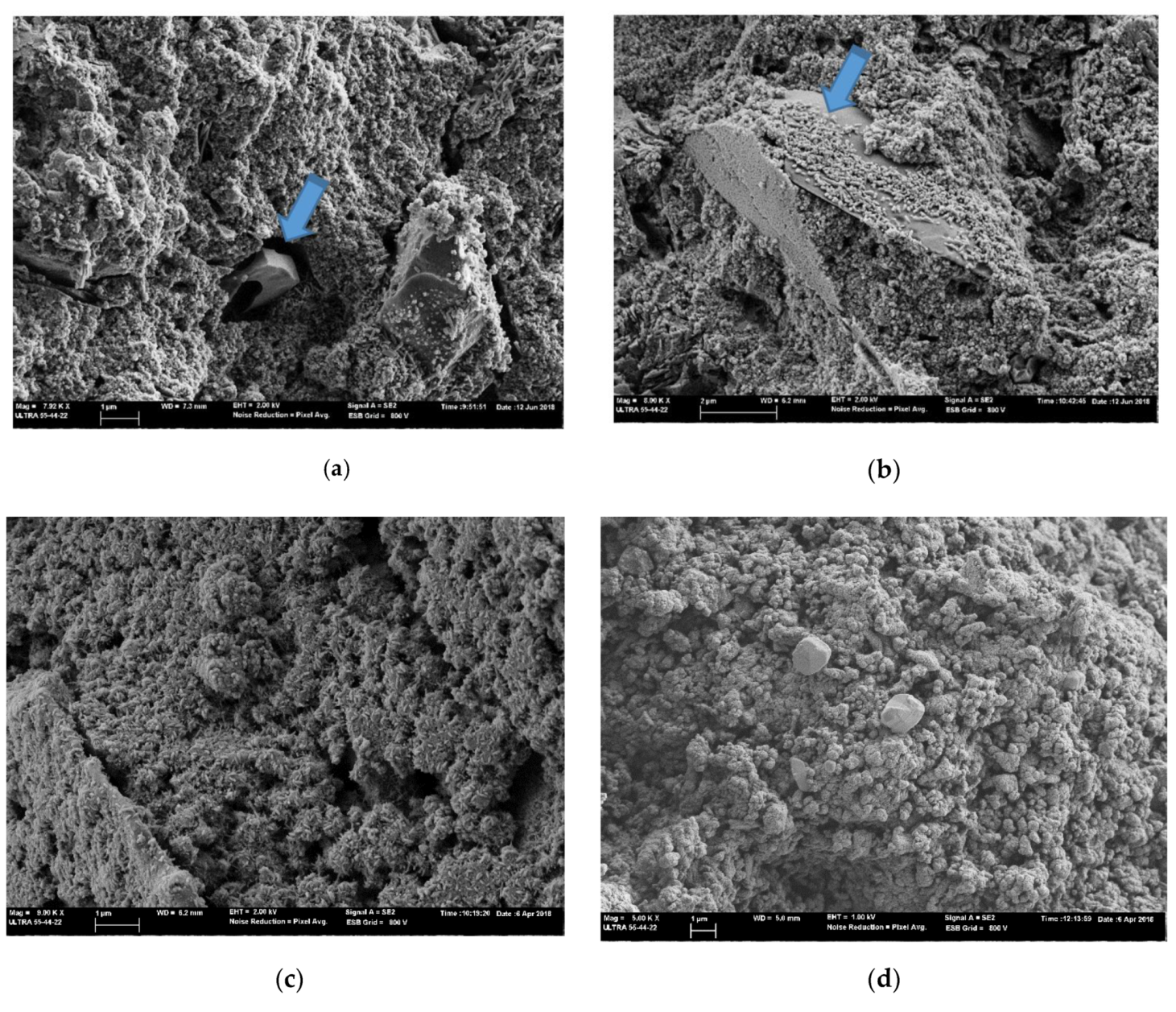
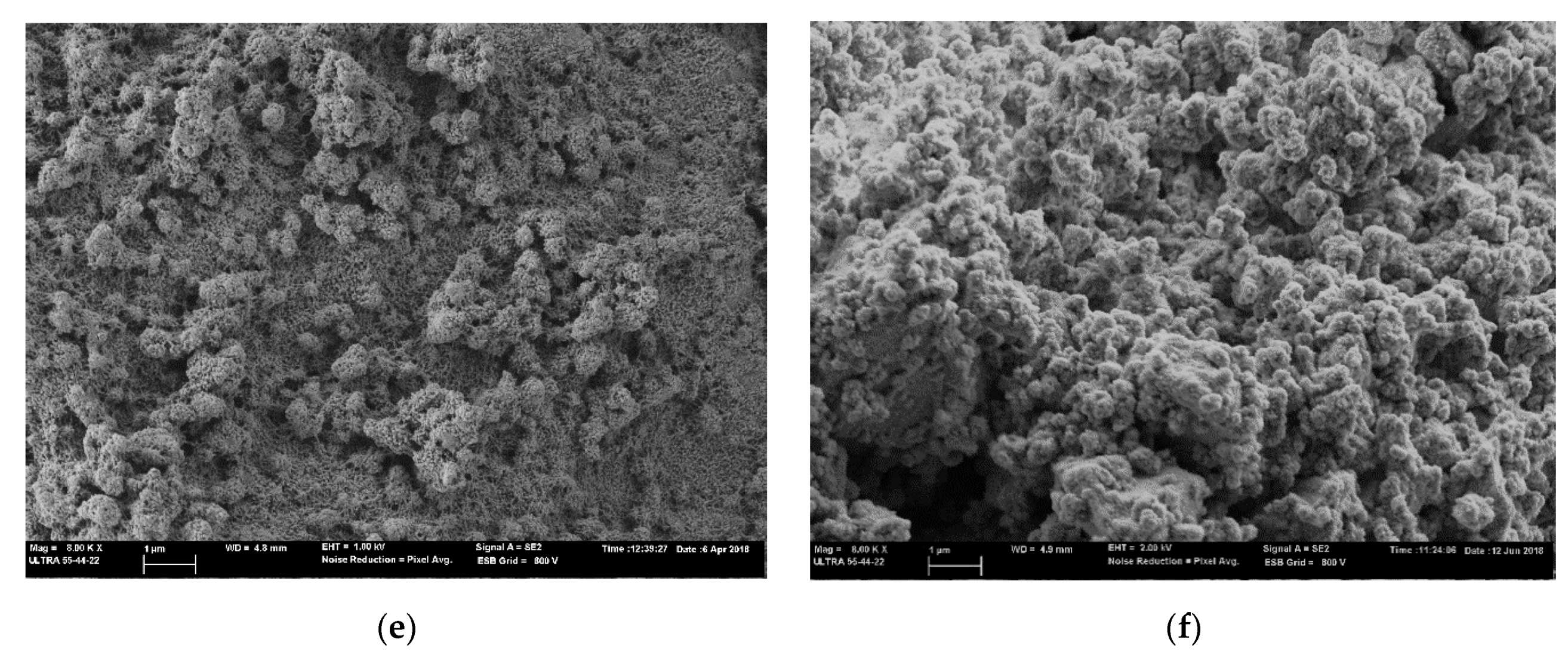
3.4. FESEM Analysis
4. Conclusions
- The mortars activated with RHA were comparable to the behavior of the mixtures activated with commercial reagents. The best option for reaching high compressive strength was to use the precursor with the 5-5 FCC/CSW ratio.
- Activation with DB obtained lower compressive strength values than the other matrices, but this strength sufficed for applications, which require strength below 50 MPa for the mixtures with the 5-5 ratio of precursors, and around 30 MPa with the 7-3 ratio.
- The main reaction product was a mixed gel, N-A-S-H and N-(C)-A-S-H, as corroborated by the TG, XRD, and FESEM analyses.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronyms
| FCC | Fluid catalytic cracking residue |
| CSW | Ceramic sanitary ware |
| RHA | Rice husk ash |
| DB | Diatomaceous waste from beer filtration |
| SDG | Sustainable Development Goals |
| N-A-S-H | Hydrated sodium aluminosilicate gel |
| FESEM | Field emission scanning electron microscopy |
| N(C)ASH | Hydrated sodium(calcium)aluminosilicate gel |
| PC | Portland cement |
| DE | Diatomaceaous earth (bibliography) |
| FA | Fly ash |
| MK | Metakaolin |
| XRF | X-Ray fluorescence |
| XRD | X-Ray Diffraction |
| DTG | Derivative thermogravimetric curve |
References
- Available online: https://www.un.org/sustainabledevelopment/es/ (accessed on 18 April 2021).
- Available online: https://www.un.org/sustainabledevelopment/climate-change/ (accessed on 18 April 2021).
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Robayo, R.A.; Mulford, A.; Munera, J.; Mejía de Gutiérrez, R. Alternative cements based on alkali-activated red clay brick waste. Constr. Build. Mater. 2019, 128, 163–169. [Google Scholar] [CrossRef]
- Belmokhtar, N.; Ammari, M.; Brigui, J.; Ben, L. Comparison of the microstructure and the compressive strength of two geopolymers derived from metakaolin and an industrial sludge. Const. Build. Mater. 2017, 146, 621–629. [Google Scholar] [CrossRef]
- Thakur, A.K.; Pappu, A.; Thakur, V.K. Synthesis and characterization of new class of geopolymer hybrid composite materials from industrial wastes. J. Clean. Prod. 2019, 230, 11–20. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.K.; Huang, B.S.; Shu, X.; He, Q. Mechanical and microstructural characterization of geopolymers derived from red mud and fly ashes. J. Clean. Prod. 2018, 186, 799–806. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mat. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, A. Sustainable alkali activated materials: Precursor and activator derived from industrial wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Bilginer, A.; Canbek, O.; Erdoğan, S.T. Activation of blast furnace slag with soda production waste. J. Mater. Civ. Eng. 2020, 32, 04019316. [Google Scholar] [CrossRef]
- El-Naggar, M.R.; El-Dessouky, M.I. Re-use of wastes glass in improving properties of metakaolin-based geopolymers:mechanical and microstructure examination. Constr. Build. Mater. 2017, 132, 543–555. [Google Scholar] [CrossRef]
- Jamieson, E.; Kealley, C.S.; van Riessen, A.; Hart., R.D. Optimising ambient setting Bayer derived fly ash geopolymers. Materials 2016, 9, 392. [Google Scholar] [CrossRef]
- Bouzón, N.; Payá, J.; Borrachero, M.V.; Soriano, L.; Tashima, M.M.; Monzó, J. Refluxed rice husk ash/NaOH suspension for preparing alkali activated binders. Mater. Lett. 2014, 115, 72–74. [Google Scholar] [CrossRef]
- Mejía, J.M.; Mejía de Gutiérrez, R.; Puertas, F. Rice husk ash as source of silica in alkali-activated fly ash and granulated blast furnae slag systems. Mater. Constr. 2013, 311, 361–375. [Google Scholar] [CrossRef]
- Kamseu, E.; Beluk à Moungam, L.M.; Cannio, M.; Billong, N.; Chaysuwan, D.; Chinje Melo, U.; Leonelli, C. Substitution of sodium silcate with rice husk ash-NaOH solution in metakaolin based geopolymer cement concerning reduction in global warming. J. Clean. Prod. 2017, 142, 3050–3060. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejab, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Comparison of alkali-activated and silica sources in one-part alkali-activated blast furnace slag mortar. J. Clean. Prod. 2018, 187, 171–179. [Google Scholar] [CrossRef]
- Geraldo, R.H.; Fernandes, L.F.R.; Camarini, G. Water treatment sludge and rice husk ash to sustainable geopolymer production. J. Clean. Prod. 2017, 149, 146–155. [Google Scholar] [CrossRef]
- Mellado, A.; Catalán, C.; Bouzón, N.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Carbon footprint of geopolymeric mortar: Study of the contribution of the alkaline activating solution and assessment of an alternative route. RSC. Adv. 2014, 4, 23846–23853. [Google Scholar] [CrossRef]
- Passuello, A.; Rodríguez, E.D.; Hirt, E.; Longhi, M.; Bernal, S.A.; Provis, J.L.; Kirchheim, A.P. Evaluation of the potential improvement in the environmental footprint of geopolymer using waste-derived activators. J. Clean. Prod. 2017, 166, 680–689. [Google Scholar] [CrossRef]
- Mejía, J.M.; Mejía de Gutiérrez, R.; Montes, C. Rice husk ash and diatomaceus earth as a source of silica to fabricate a geopolymeric binary binder. J. Clean. Prod. 2016, 118, 133–139. [Google Scholar] [CrossRef]
- Bagci, C.; Kutyla, G.P.; Kriven, W.M. Fully reacted high strength geopolymer made with diatomite as a fumed silica alternative. Ceram. Int. 2017, 43, 14784–14790. [Google Scholar] [CrossRef]
- Font, A.; Soriano, L.; Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Payá, J. Use of residual diatomaceus earth as a silica source in geopolymer production. Mater. Lett. 2018, 223, 10–13. [Google Scholar] [CrossRef]
- Vogt, E.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Payá, J.; Borrachero, M.V.; Monzó, J.; Soriano, L. Studies on the behaviour of different spent fluidized-be catalytic cracking catalysts on Portland cement. Mater. Construcción 2009, 59, 37–52. [Google Scholar] [CrossRef]
- Lv, J.; Gu, F.; Zhang, W.; Guo, J. Life cycle assessment and life cycle costing of sanitary ware manufacturing a case study in China. J. Clean. Prod. 2019, 238, 117938. [Google Scholar] [CrossRef]
- Cuviella, C.; Colmenar, A.; Borge, D.; López, A. Management tool to optimize energy and water consumption in the sanitary-ware industry. J. Clean. Prod. 2018, 197, 280–296. [Google Scholar] [CrossRef]
- Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Microstructure and properties of recycled concretes using ceramic sanitary ware industry waste as coarse aggregate. Constr. Build. Mater. 2012, 48, 112–118. [Google Scholar] [CrossRef]
- Moaeydi, H.; Aghel, B.; Abdullahi, M.M.; Nguyen, H.; Rashid, A.A.S. Application of rice husk ash as green and sustainable biomass. J. Clean. Prod. 2019, 237, 117851. [Google Scholar] [CrossRef]
- Aprianti, E. A huge number of artificial waste material can be supplementary cementitious material (SCM) for concrete production-a review part II. J. Clean. Prod. 2017, 142, 4178–4194. [Google Scholar] [CrossRef]
- Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/cultivos-herbaceos/arroz/default.aspx (accessed on 18 April 2021).
- Dessalew, G.; Beyene, A.; Nebiyu, A.; Ruelle, M.L. Use of industrial diatomite wastes from beer production to improve soil fertility and cereal yields. J. Clean. Prod. 2017, 157, 22–29. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/-/EDN-20180803-1 (accessed on 18 April 2021).
- Cosa, J.; Soriano, L.; Borrachero, M.V.; Reig, L.; Payá, J.; Monzó, J.M. Influence of addition of fluid catalytic cracking residue (FCC) and the SiO2 concentration in alkali-activated ceramic sanitary ware (CSW) binders. Minerals 2018, 8, 123. [Google Scholar] [CrossRef]
- Reig, L.; Borrachero, M.V.; Monzó, J.M.; Savastano, H.; Tashima, M.M.; Payá, J. Use of ceramic sanitaryware as an alternative for the development of new sustainable binder. Key Eng. Mater. 2015, 668, 172–180. [Google Scholar] [CrossRef]
- UNE-EN 196-1. Methods of Testing Cement-Part 1: Determination of Strength; AENOR: Madrid, Spain, 2005. [Google Scholar]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Nath, S.K. Kumer, Role of particle fineness on engineering properties and microstructural of fly ash derived geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Rodriguez, E.D.; Bernal, S.A.; Provis, J.l.; Payá, J.; Monzó, J.M.; Borrachero, M.V. Effect of nanosilica-based activators on the performance of an alkali-activated fly ash binder. Cem. Concr. Compos. 2013, 35, 1–11. [Google Scholar] [CrossRef]
- Moraes, J.C.B.; Font, A.; Soriano, L.; Akasali, J.L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. New use of sugar cane straw ash in alkali-activated materials: A silica source for the preparation of the alkaline activator. Constr. Build. Mater. 2018, 171, 611–621. [Google Scholar] [CrossRef]
- Tashima, M.M.; Akasaki, J.L.; Melges, J.L.P.; Soriano, L.; Monzó, J.; Payá, J.; Borrachero, M.V. Alkali activated materials based on fluid catalytic cracking catalyst residue (FCC): Influence of SiO2/Na2O and H2O/FCC ration on mechanical strength and microstructure. Fuel 2013, 108, 833. [Google Scholar] [CrossRef]
- Rodriguez, E.D.; Bernal, S.A.; Provis, J.L.; Gehman, J.D.; Monzó, J.; Payá, J.; Borrachero, M.V. Geopolimers based on spent catalyst residue from a fluid catalytic cracking (FCC) process. Fuel. 2013, 109, 493. [Google Scholar] [CrossRef]
- Trochez, J.J.; Mejía de Gutiérrez, R.; Rivera, J.; Bernal, S.A. Synthesis of geopolymer from spent FCC: Effect of SiO2/Al2O3 and Na2O/SiO2 molar ratios. Mater. Constr. 2015, 65, 65. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Tchadjié, L.N.; Tchakoute, H.K.; Kenne, B.B.D.; Elimbi, A.; Njopwouo, D. Synthesis of geopolymer composites from a mixture of volcanic scoria and metakaolin. J. Asian Ceram. Soc. 2014, 2, 387. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of the activator concentration and calcium hydroxide addition on the properties of alkali-activated porcelain stoneware. Constr. Build. Mater. 2014, 63, 214–222. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of calcium additions on the compressive strength and microstructure of alkali-activated ceramic sanitary-ware. J. Am. Ceram. Soc. 2018, 101, 3094–3104. [Google Scholar] [CrossRef]
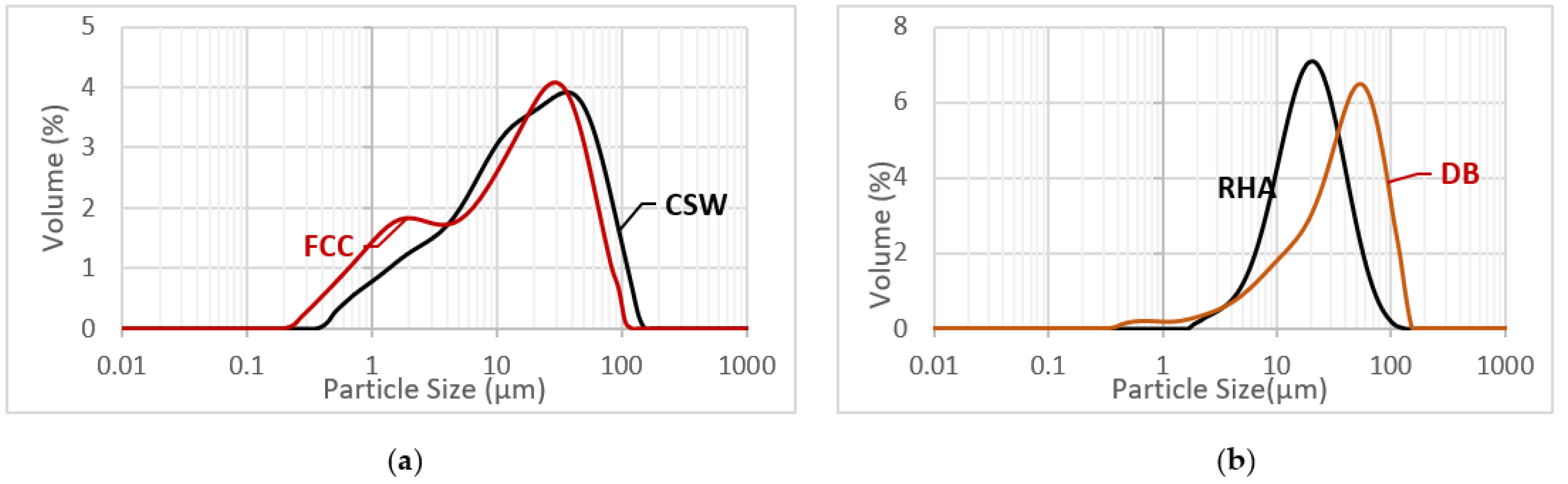


| Al2O3 | SiO2 | CaO | Fe2O3 | K2O | Na2O | P2O5 | Other | LOI * | |
|---|---|---|---|---|---|---|---|---|---|
| FCC | 49.26 | 47.76 | 0.11 | 0.60 | 0.02 | 0.31 | 0.01 | 1.42 | 0.51 |
| CSW | 23.60 | 66.00 | 1.20 | 1.30 | 2.80 | 2.40 | 0.50 | 2.00 | 0.20 |
| RHA | 0.25 | 85.58 | 1.83 | 0.21 | 3.39 | _ | 0.67 | 1.08 | 6.99 |
| DB | 5.67 | 81.70 | 1.28 | 3.71 | 0.86 | 1.30 | 0.36 | 1.78 | 3.34 |
| CSW (g) | FCC (g) | Na2SiO3 (g) | NaOH (g) | H2O (g) | RHA (g) | DB (g) | Ca(OH)2 (g) | Sand (g) | |
|---|---|---|---|---|---|---|---|---|---|
| m-7-3-C | 315.0 | 135.0 | 189.8 | 41.2 | 81.0 | _ | _ | 18.0 | 1350.0 |
| m-7-3-R | 315.0 | 135.0 | _ | 60.8 | 202.5 | 62.1 | _ | 18.0 | 1350.0 |
| m-7-3-D | 315.0 | 135.0 | _ | 60.8 | 202.5 | _ | 65.1 | 18.0 | 1350.0 |
| m-5-5-C | 225.0 | 225.0 | 189.8 | 41.2 | 81.0 | _ | _ | 18.0 | 1350.0 |
| m-5-5-R | 225.0 | 225.0 | _ | 60.8 | 202.5 | 62.1 | _ | 18.0 | 1350.0 |
| m-5-5-D | 225.0 | 225.0 | _ | 60.8 | 202.5 | _ | 65.1 | 18.0 | 1350.0 |
| p-7-3-C | 14.0 | 6.0 | 8.4 | 1.8 | 3.6 | _ | _ | 0.8 | _ |
| p-7-3-R | 14.0 | 6.0 | _ | 2.7 | 9.0 | 2.8 | _ | 0.8 | _ |
| p-7-3-D | 14.0 | 6.0 | _ | 2.7 | 9.0 | _ | 2.9 | 0.8 | _ |
| p-5-5-C | 10.0 | 10.0 | 8.4 | 1.8 | 3.6 | _ | _ | 0.8 | _ |
| p-5-5-R | 10.0 | 10.0 | _ | 2.7 | 9.0 | 2.8 | _ | 0.8 | _ |
| p-5-5-D | 10.0 | 10.0 | _ | 2.7 | 9.0 | _ | 2.9 | 0.8 | _ |
| Paste | 7 d, 65 °C | 28 d, 20 °C |
|---|---|---|
| 7-3-C | 11.95 | 8.77 |
| 7-3-R | 9.61 | 8.03 |
| 7-3-D | 9.72 | 7.44 |
| 5-5-C | 11.24 | 10.23 |
| 5-5-R | 8.55 | 9.00 |
| 5-5-D | 9.83 | 10.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payá, J.; Soriano, L.; Font, A.; Borrachero Rosado, M.V.; Nande, J.A.; Monzo Balbuena, J.M. Reuse of Industrial and Agricultural Waste in the Fabrication of Geopolymeric Binders: Mechanical and Microstructural Behavior. Materials 2021, 14, 2089. https://doi.org/10.3390/ma14092089
Payá J, Soriano L, Font A, Borrachero Rosado MV, Nande JA, Monzo Balbuena JM. Reuse of Industrial and Agricultural Waste in the Fabrication of Geopolymeric Binders: Mechanical and Microstructural Behavior. Materials. 2021; 14(9):2089. https://doi.org/10.3390/ma14092089
Chicago/Turabian StylePayá, Jordi, Lourdes Soriano, Alba Font, Maria Victoria Borrachero Rosado, Javier Alejandro Nande, and Jose María Monzo Balbuena. 2021. "Reuse of Industrial and Agricultural Waste in the Fabrication of Geopolymeric Binders: Mechanical and Microstructural Behavior" Materials 14, no. 9: 2089. https://doi.org/10.3390/ma14092089
APA StylePayá, J., Soriano, L., Font, A., Borrachero Rosado, M. V., Nande, J. A., & Monzo Balbuena, J. M. (2021). Reuse of Industrial and Agricultural Waste in the Fabrication of Geopolymeric Binders: Mechanical and Microstructural Behavior. Materials, 14(9), 2089. https://doi.org/10.3390/ma14092089









