Preparation and Characterization of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution Obtained by Microwave-Assisted Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
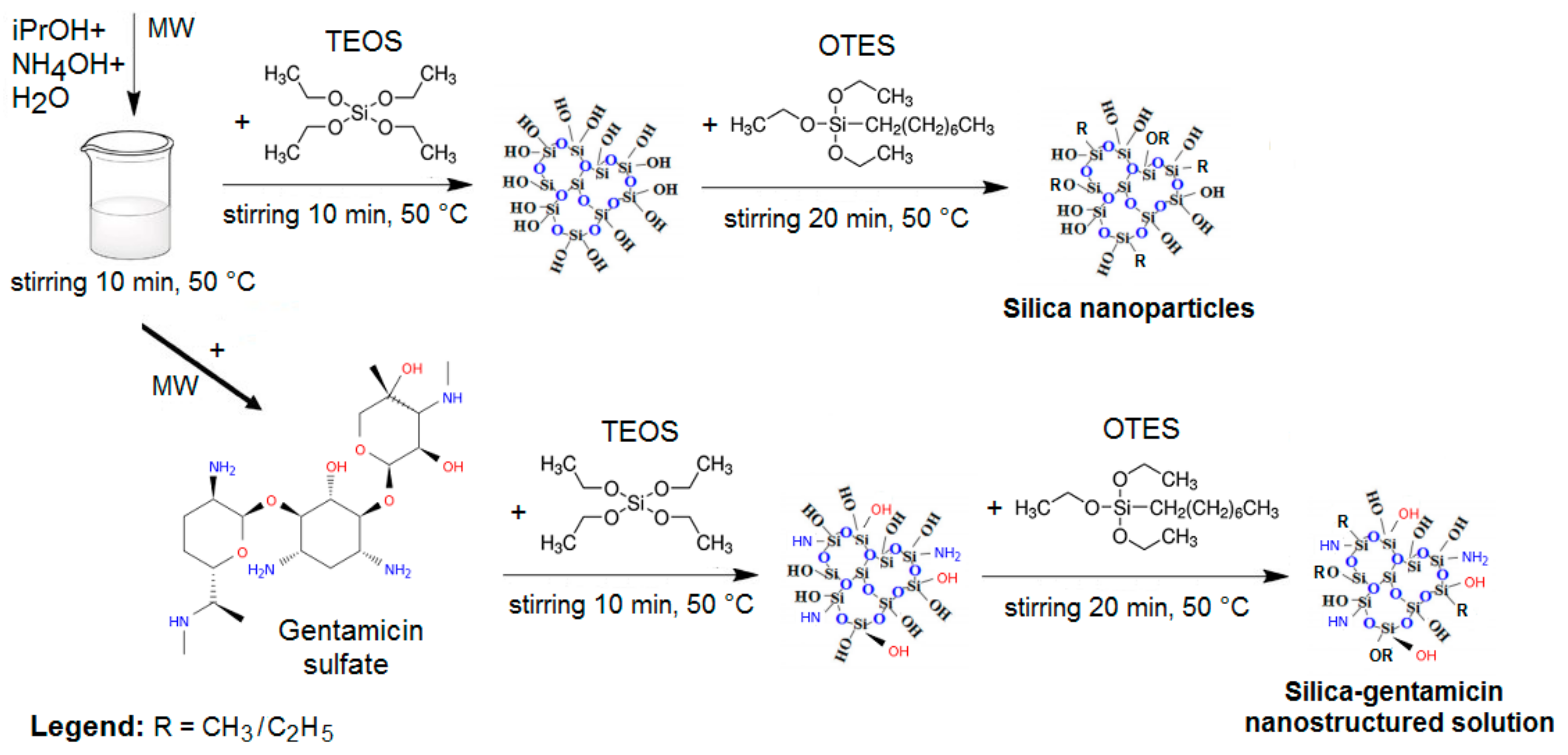
2.2. Preparation of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution by Microwave-Assisted Synthesis
2.3. Characterization of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution
3. Results
3.1. FTIR Spectroscopy
3.2. TGA Analysis
3.3. Microscopic Studies
3.4. Antimicrobial Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devi, M.G.; Balachandran, S. A Review on synthesis, characterization and applications of silica particles. Int. J. Eng. Res. Technol. 2016, 4, 249–255. [Google Scholar]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Mebert, A.M.; Camporotondi, D.E.; Foglia, M.L.; Alvarez, G.S.; Orihuela, P.L.S.; Diaz, L.E.; DeSimone, M.F. Controlling the Interaction Between Cells and Silica Nanoparticles. J. Biomater. Tissue Eng. 2013, 3, 108–121. [Google Scholar] [CrossRef]
- Pedraza, D.; Díez, J.; Isabel-Izquierdo-Barba, N.; Colilla, M.; Vallet-Regí, M. Amine-Functionalized Mesoporous Silica Nanoparticles: A New Nanoantibiotic for Bone Infection Treatment. Biomed. Glas. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Pathak, R.; Jha, D.; Roy, I.; Gautam, H.K.; Sharma, A.K.; Kumar, P. Synthesis and antimicrobial activity of aminoglycoside-conjugated silica nanoparticles against clinical and resistant bacteria. New J. Chem. 2015, 39, 6746–6755. [Google Scholar] [CrossRef]
- Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Dye-doped silica nanoparticles as luminescent organized systems for nanomedicine. Chem. Soc. Rev. 2014, 43, 4243–4268. [Google Scholar] [CrossRef]
- Gounani, Z.; Asadollahi, M.A.; Meyer, R.L.; Arpanaei, A. Loading of polymyxin B onto anionic mesoporous silica nanoparticles retains antibacterial activity and enhances biocompatibility. Int. J. Pharm. 2018, 537, 148–161. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Perni, S.; Martini-Gilching, K.; Prokopovich, P. Controlling release kinetics of gentamicin from silica nano-carriers. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 212–221. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; He, W.; Hynönen, U.; Meng, Y.; Mohammadi, P.; Palva, A.; Feng, Q.; Hannula, S.-P.; Nordström, K.; Linder, M.B. Silica–gentamicin nanohybrids: Combating antibiotic resistance, bacterial biofilms, and in vivo toxicity. Int. J. Nanomed. 2018, 13, 7939–7957. [Google Scholar] [CrossRef] [PubMed]
- Barbé, C.J.; Bartlett, J.R.; Kong, L.; Finnie, K.S.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.J. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Corrêa, G.; Morais, E.; Brambilla, R.; Bernardes, A.; Radtke, C.; Dezen, D.; Júnior, A.; Fronza, N.; Dos Santos, J. Effects of the sol–gel route on the structural characteristics and antibacterial activity of silica-encapsulated gentamicin. Colloids Surf. B Biointerfaces 2014, 116, 510–517. [Google Scholar] [CrossRef]
- Al Thaher, Y. Tailored gentamicin release from silica nanocarriers coated with polyelectrolyte multilayers. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126210. [Google Scholar] [CrossRef]
- Tariq, S.; Rahim, A.; Muhammad, N.; Rahman, S.U.; Azhar, U.; Sultana, K.; Sharif, F.; Siddiqi, S.A.; Zaman, M.; Rehman, F. Controllable delivery from gentamicin loaded polycaprolactone/grafted silica nanoparticles composite mats. J. Mol. Liq. 2019, 290, 111205. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lu, P.-S.; Chen, W.-C.; Chiang, Y.-W.; Chien, C.-S. Evaluation of gentamicin encapsulated hierarchically meso-macroporous silica-based calcium phosphates glass powders. Ceram. Int. 2014, 40, 15019–15025. [Google Scholar] [CrossRef]
- Nampi, P.P.; Mohan, V.S.; Sinha, A.K.; Varma, H. High surface area sol–gel nano silica as a novel drug carrier substrate for sustained drug release. Mater. Res. Bull. 2012, 47, 1379–1384. [Google Scholar] [CrossRef]
- Tamanna, T.; Landersdorfer, C.B.; Ng, H.J.; Bulitta, J.B.; Wood, P.; Yu, A. Prolonged and continuous antibacterial and anti-biofilm activities of thin films embedded with gentamicin-loaded mesoporous silica nanoparticles. Appl. Nanosci. 2018, 8, 1471–1482. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, J. Study on Gentamicin Loading and Release from Mesoporous Bioactive Glasses (MBGs) with Different Shapes, Chemical Composition and Surface Property. J. Biomater. Tissue Eng. 2012, 2, 177–183. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Tsuji, M. Microwave-Assisted Synthesis of Metallic Nanomaterials in Liquid Phase. ChemistrySelect 2017, 2, 805–819. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Ebner, C.; Bodner, T.; Stelzer, F.; Wiesbrock, F. One Decade of Microwave-Assisted Polymerizations: Quo vadis? Macromol. Rapid Commun. 2011, 32, 254–288. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.R.; Hosamani, K.M. Microwave-assisted synthesis, computational studies and antibacterial/anti-inflammatory activities of compounds based on coumarin-pyrazole hybrid. R. Soc. Open Sci. 2018, 5, 172435. [Google Scholar] [CrossRef] [PubMed]
- Newalkar, B.L.; Olanrewaju, A.J.; Komarneni, S. Microwave-Hydrothermal Synthesis and Characterization of Zirconium Substituted SBA-15 Mesoporous Silica. J. Phys. Chem. B 2001, 105, 8356–8360. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Chang, J.-S.; Kwon, Y.-U.; Park, S.-E. Microwave synthesis of cubic mesoporous silica SBA-16. Microporous Mesoporous Mater. 2004, 68, 21–27. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. Gentamicin-Releasing Mesoporous ZnO Structures. Materials 2018, 11, 314. [Google Scholar] [CrossRef]
- Kamarudin, N.H.N.A.; Jalil, A.; Triwahyono, S.; Timmiati, S.N. Microwave-assisted synthesis of mesoporous silica nanoparticles as a drug delivery vehicle. Malays. J. Anal. Sci. 2016, 20, 1382–1389. [Google Scholar] [CrossRef]
- Purcar, V.; Nichita, C.; Alexandrescu, E.; Şomoghi, R.; Rădiţoiu, V.; Căprărescu, S. Modified silica nanoparticles obtained by microwave synthesis. In Proceedings of the 4th International Conference Geography, Environment and GIS for Students and Young Researchers, Targoviste, Romania, 18–20 May 2017. [Google Scholar]
- Lazar, V.; Balotescu, C.; Moldovan, L.; Alexandru, V.; Bulai, D.; Cernat, R.; Ditu, L.-M. Comparative eval-uation of qualitative and quantitative methods used in the study of antibacterial and antifungal activity of hydroalcoholic vegetal extracts. Rom. Biotech. Lett. 2005, 10, 2225–2232. [Google Scholar]
- Mosselhy, D.A.; Ge, Y.; Gasik, M.; Nordström, K.; Natri, O.; Hannula, S.-P. Silica-Gentamicin Nanohybrids: Synthesis and Antimicrobial Action. Materials 2016, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Parler, C.M.; Ritter, J.A.; Amiridis, M.D. Infrared spectroscopic study of sol–gel derived mixed-metal oxides. J. Non-Cryst. Solids 2001, 279, 119–125. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Rădiţoiu, A.; Raduly, F.M.; Manea, R.; Frone, A.; Anastasescu, M.; Ispas, G.C.; Căprărescu, S. Bilayer coatings based on silica materials and iron (III) phthalocyanine—Sensitized TiO2 photocatalyst. Mater. Res. Bull. 2021, 138, 111222. [Google Scholar] [CrossRef]
- He, W.; Mosselhy, D.A.; Zheng, Y.; Feng, Q.; Li, X.; Yang, X.; Yue, L.; Hannula, S.-P. Effects of silica–gentamicin nanohybrids on osteogenic differentiation of human osteoblast-like SaOS-2 cells. Int. J. Nanomed. 2018, 13, 877–893. [Google Scholar] [CrossRef]
- Mebert, A.M.; Aimé, C.; Alvarez, G.S.; Shi, Y.; Flor, S.A.; Lucangioli, S.E.; DeSimone, M.F.; Coradin, T. Silica core–shell particles for the dual delivery of gentamicin and rifamycin antibiotics. J. Mater. Chem. B 2016, 4, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Van de Belt, H.; Neut, D.; Uges, D.; Schenk, W.; van Horn, J.; van der Mei, H.; Busscher, H. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000, 21, 1981–1987. [Google Scholar] [CrossRef]
- Inada, M.; Nishinosono, A.; Kamada, K.; Enomoto, N.; Hojo, J. Microwave-assisted sol–gel process for production of spherical mesoporous silica materials. J. Mater. Sci. 2007, 43, 2362–2366. [Google Scholar] [CrossRef]
- Ballarre, J.; Aydemir, T.; Liverani, L.; Roether, J.; Goldmann, W.; Boccaccini, A. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: Improving early stage performance of titanium implants. Surf. Coat. Technol. 2020, 381, 125138. [Google Scholar] [CrossRef]
- Anand, A.; Das, P.; Nandi, S.K.; Kundu, B. Development of antibiotic loaded mesoporous bioactive glass and its drug release kinetics. Ceram. Int. 2020, 46, 5477–5483. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ordered Mesoporous Materials in the Context of Drug Delivery Systems and Bone Tissue Engineering. Chem. A Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef]
- Alvarez, G.S.; Hélary, C.; Mebert, A.M.; Wang, X.; Coradin, T.; DeSimone, M.F. Antibiotic-loaded silica nanoparticle–collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. J. Mater. Chem. B 2014, 2, 4660–4670. [Google Scholar] [CrossRef] [PubMed]






| Sample | Silane Precursors | Gentamicin Sulfate (Solubilized in Water, 3%) (mg) | Resulted Materials | |
|---|---|---|---|---|
| TEOS (Moles) | OTES (Moles) | |||
| G0 | 1.34 × 10−4 | 1.34 × 10−4 | - | Silica nanoparticles (SiO2 NPs) |
| G1 | 1.34 × 10−4 | 1.34 × 10−4 | 3 | Silica-gentamicin nanostructured solution |
| Sample | 40–156 °C | 156–262 °C | 262–700 °C | Residue | |||
|---|---|---|---|---|---|---|---|
| Wt. Loss | Tmax * | Wt. Loss | Tmax | Wt. Loss | Tmax | 700 °C | |
| % | °C | % | °C | % | °C | % | |
| GS | 11.73 | 55.0 | 16.93 | 293.1 | 39.37 | 289.1 | 31.97 |
| G0 | 2.87 | 115.5 | 1.76 | - | 29.23 | 492.5 | 66.14 |
| G1 | 2.39 | 95.2 | 1.15 | - | 48.63 | 475.8 | 47.83 |
| Bacterial Strains | Diameter of Inhibition Zone (mm) | ||
|---|---|---|---|
| G0 | GS | G1 | |
| Pseudomonas aeruginosa ATCC 27853 | - | 30 | 18 |
| Stapylococcus aureus ATCC 29213 | - | 27 | 18 |
| Escherichia coli 25322 | - | 29 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcar, V.; Rădiţoiu, V.; Nichita, C.; Bălan, A.; Rădiţoiu, A.; Căprărescu, S.; Raduly, F.M.; Manea, R.; Şomoghi, R.; Nicolae, C.-A.; et al. Preparation and Characterization of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution Obtained by Microwave-Assisted Synthesis. Materials 2021, 14, 2086. https://doi.org/10.3390/ma14082086
Purcar V, Rădiţoiu V, Nichita C, Bălan A, Rădiţoiu A, Căprărescu S, Raduly FM, Manea R, Şomoghi R, Nicolae C-A, et al. Preparation and Characterization of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution Obtained by Microwave-Assisted Synthesis. Materials. 2021; 14(8):2086. https://doi.org/10.3390/ma14082086
Chicago/Turabian StylePurcar, Violeta, Valentin Rădiţoiu, Cornelia Nichita, Adriana Bălan, Alina Rădiţoiu, Simona Căprărescu, Florentina Monica Raduly, Raluca Manea, Raluca Şomoghi, Cristian-Andi Nicolae, and et al. 2021. "Preparation and Characterization of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution Obtained by Microwave-Assisted Synthesis" Materials 14, no. 8: 2086. https://doi.org/10.3390/ma14082086
APA StylePurcar, V., Rădiţoiu, V., Nichita, C., Bălan, A., Rădiţoiu, A., Căprărescu, S., Raduly, F. M., Manea, R., Şomoghi, R., Nicolae, C.-A., Raut, I., & Jecu, L. (2021). Preparation and Characterization of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution Obtained by Microwave-Assisted Synthesis. Materials, 14(8), 2086. https://doi.org/10.3390/ma14082086












