Performance Evaluation of Modified Rubberized Concrete Exposed to Aggressive Environments
Abstract
1. Introduction
2. Materials and Methods
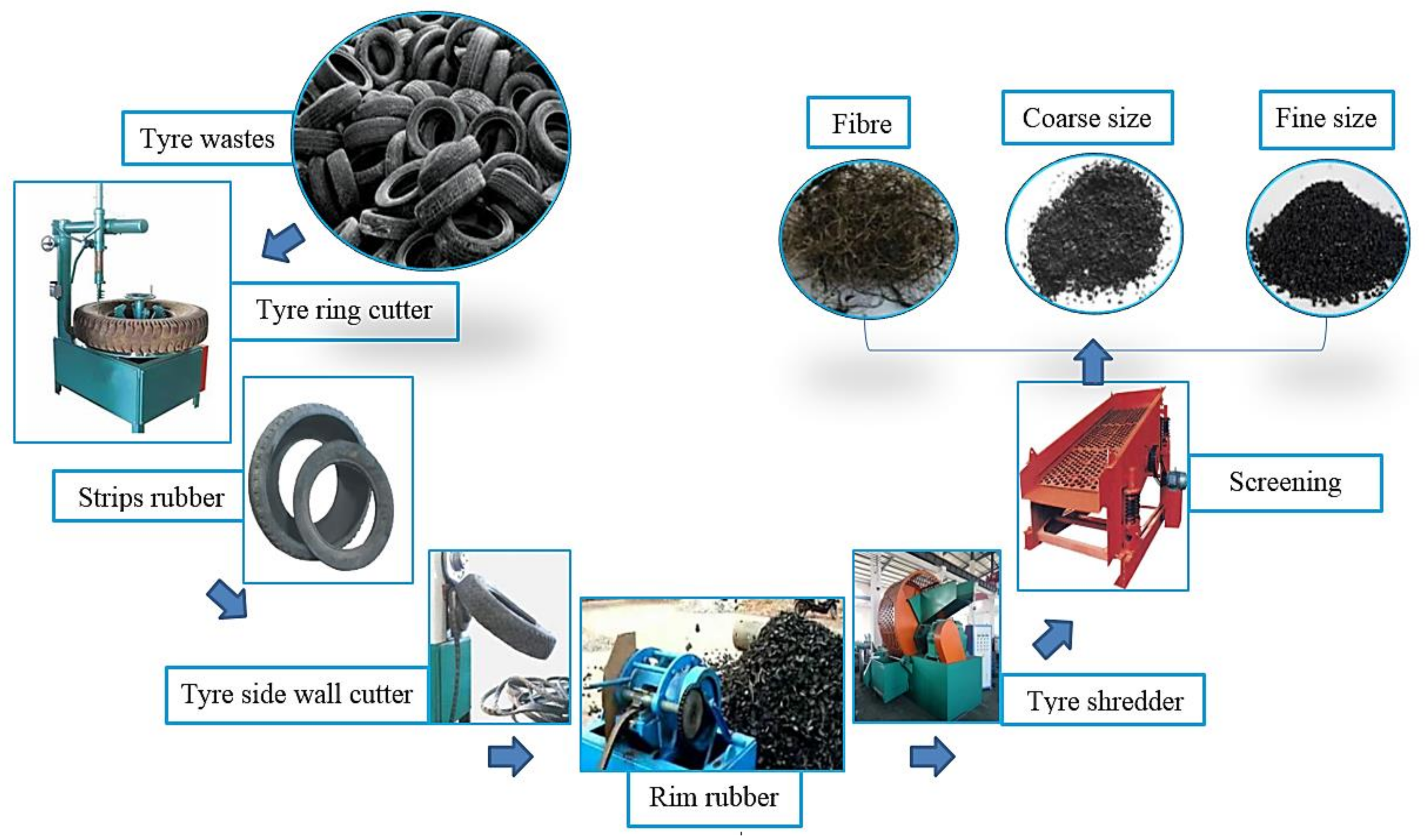
2.1. Materials Properties
2.2. Mix Design and Specimens Preparation
2.3. Tests Program
3. Results and Discussion
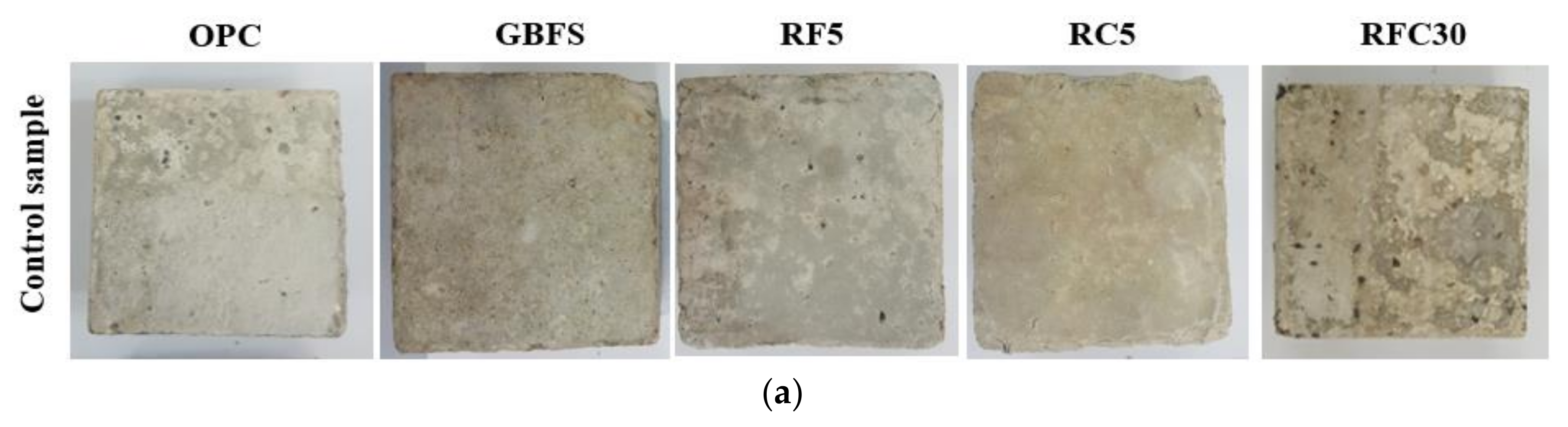
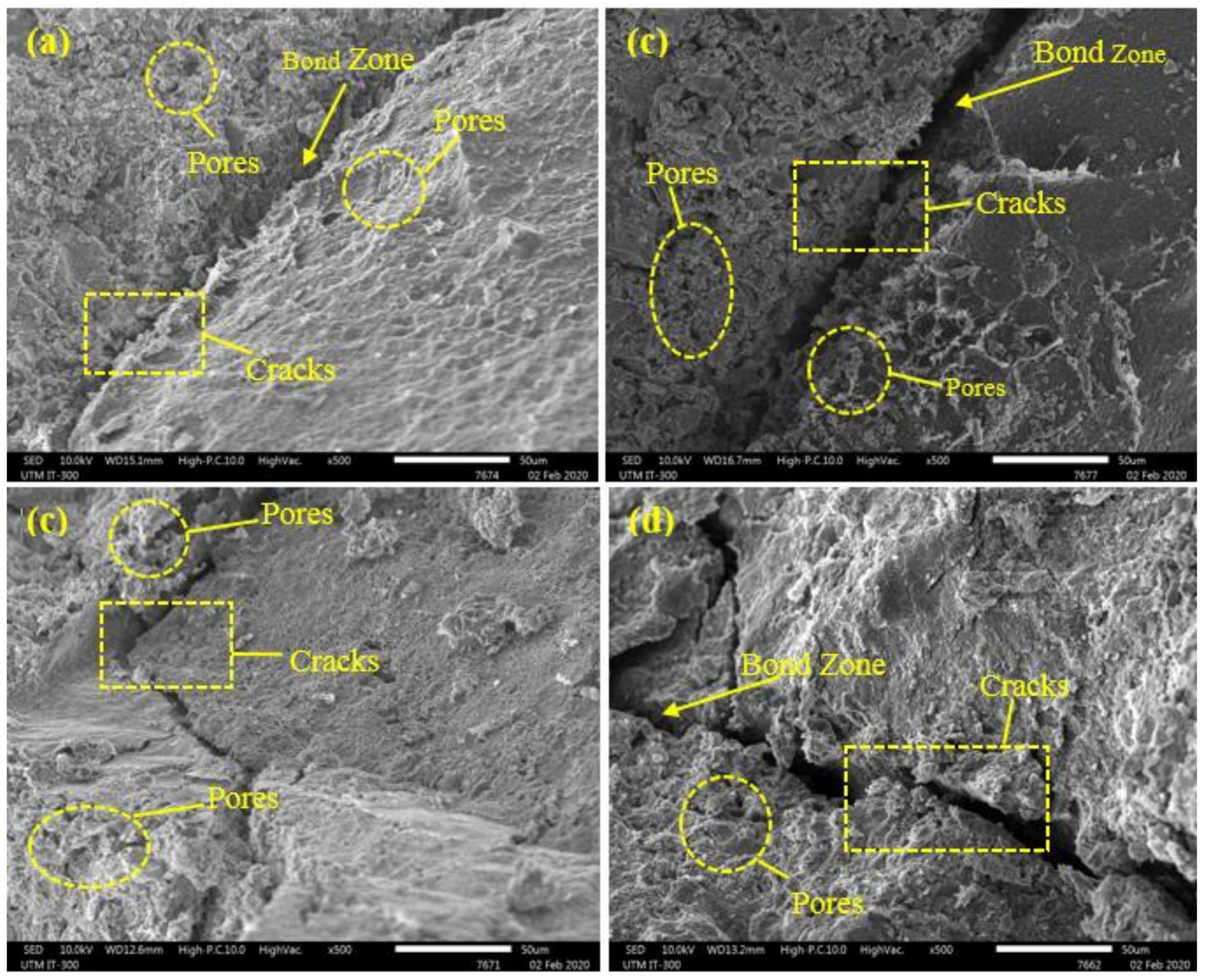
3.1. Resistance Against Sulphate and Acid Attacks
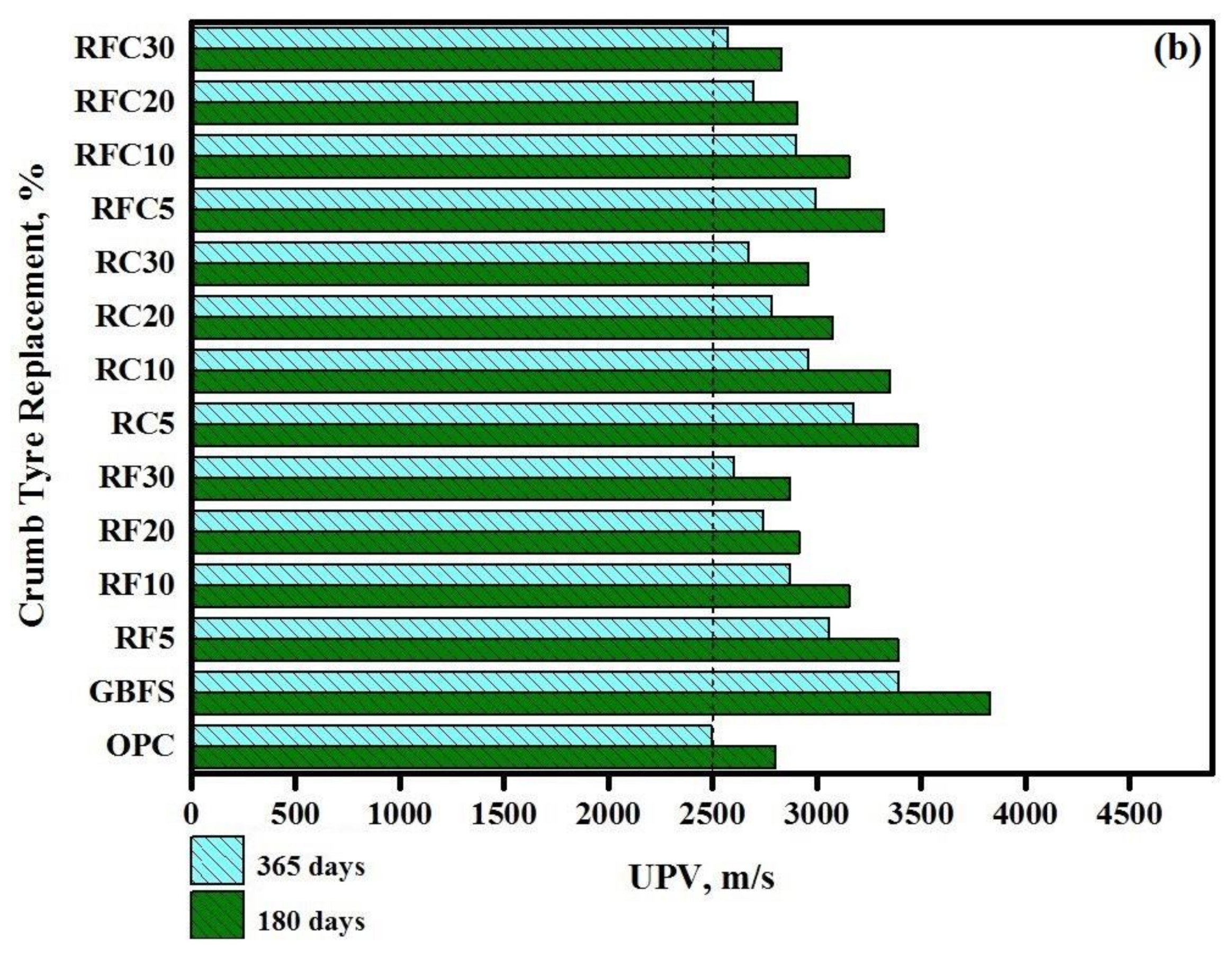
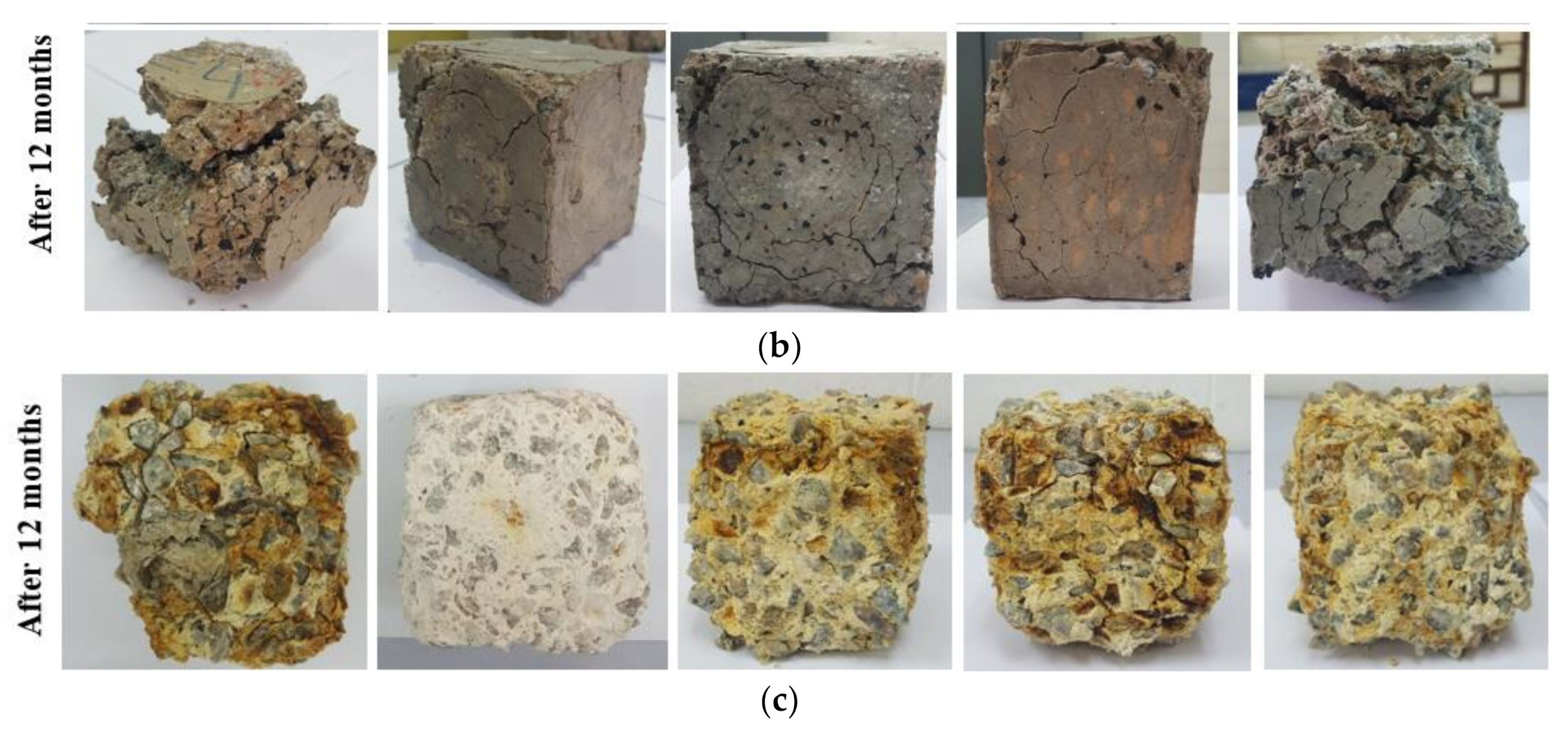
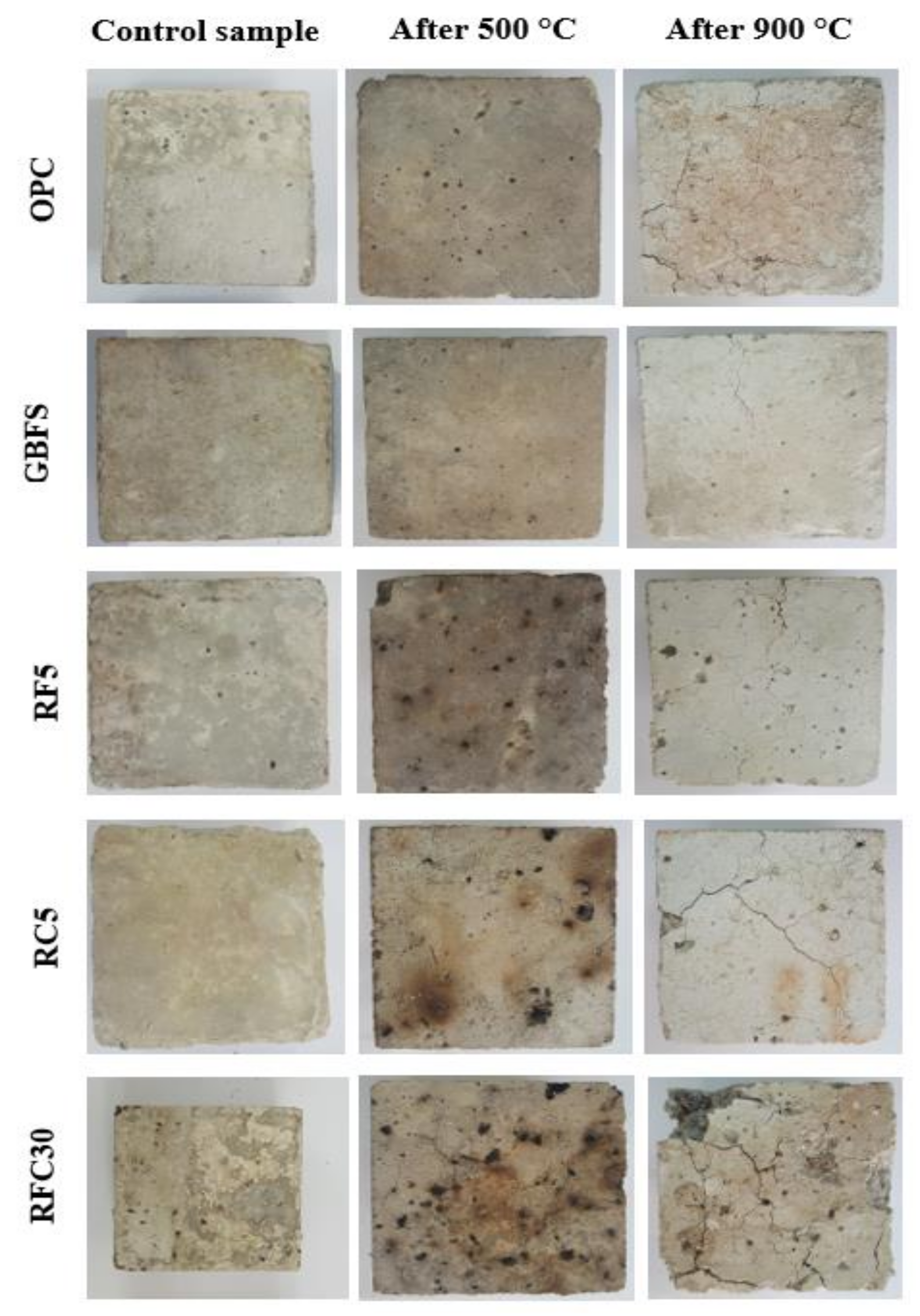
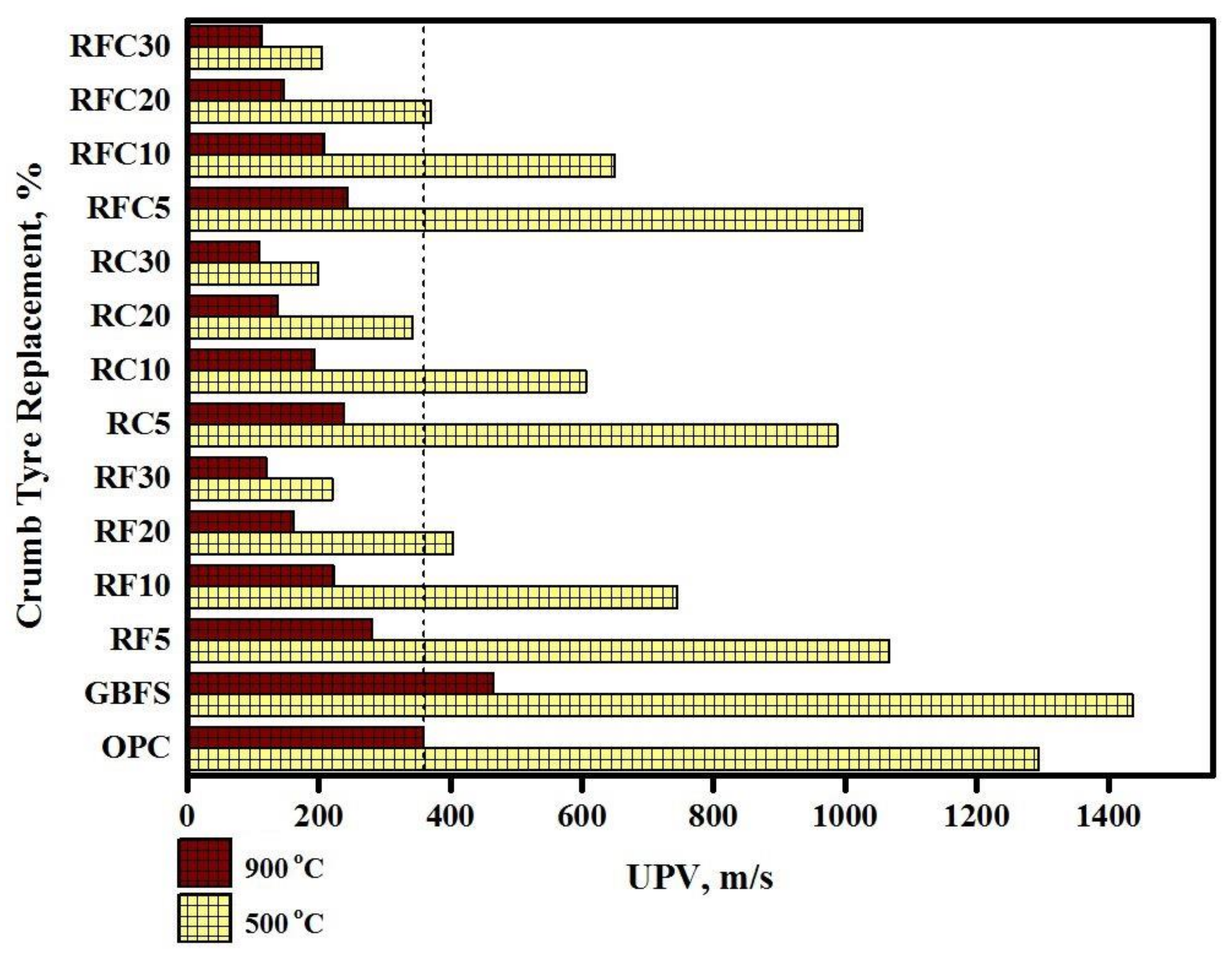
3.2. Resistance to Elevated Temperatures
4. Developing an Artificial Neural Network to Estimated CS Lose after Sulphate and Acid Immersion
4.1. Particle Swarm Optimization
4.2. Generation of Training and Testing Data Sets
4.3. Results
5. Conclusions
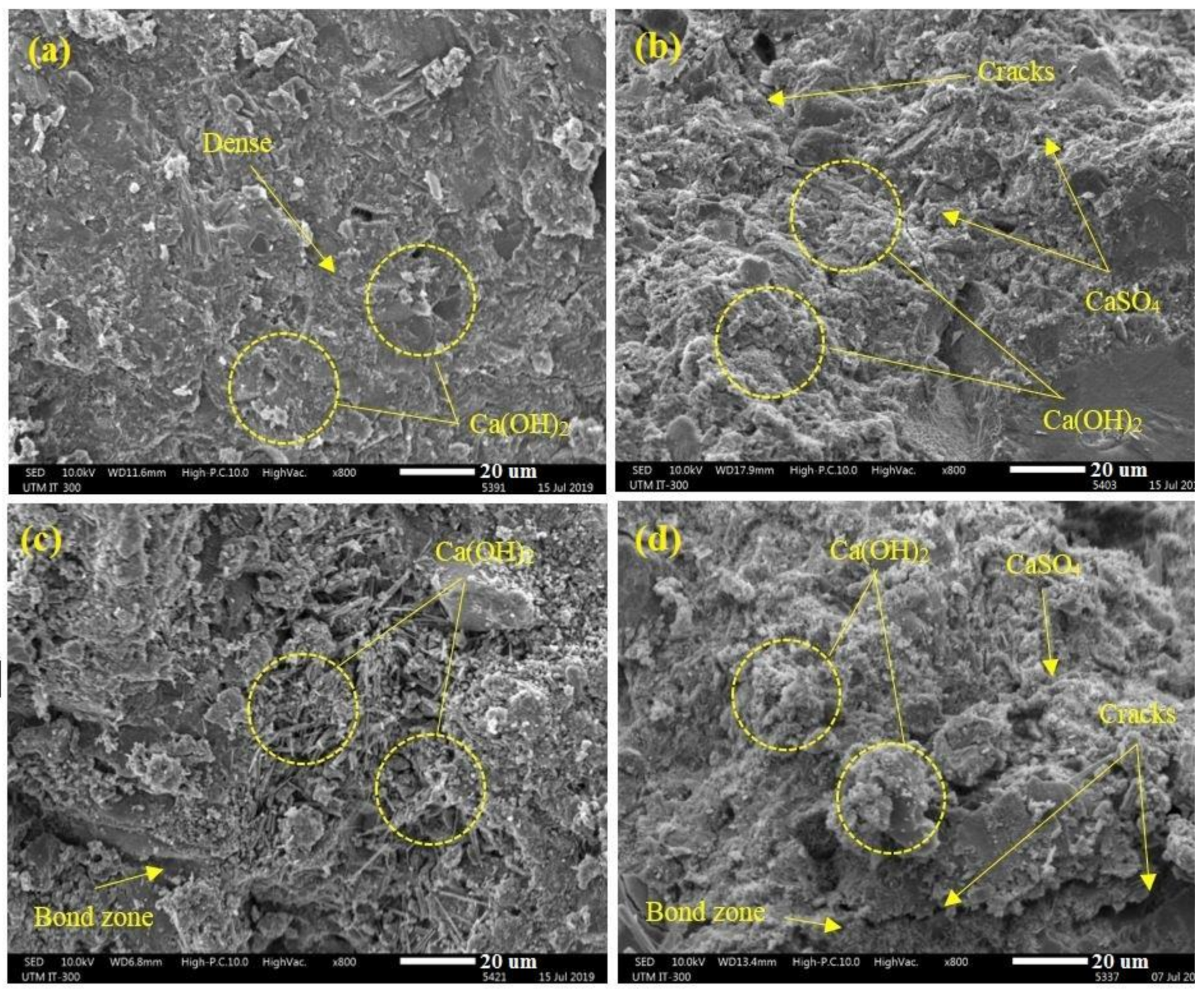
- The results indicate that the CS in modified rubberized concrete experienced a major reduction after immersion in acid solution (by average 95%) compared to sulphate solution (by average 53%). Such a significant reduction can explain by SO4−2 reaction with the Ca(OH)2 that produces CaSO4·2H2O, leading to an expansion of the paste matrix and extra cracks generation in the concrete.
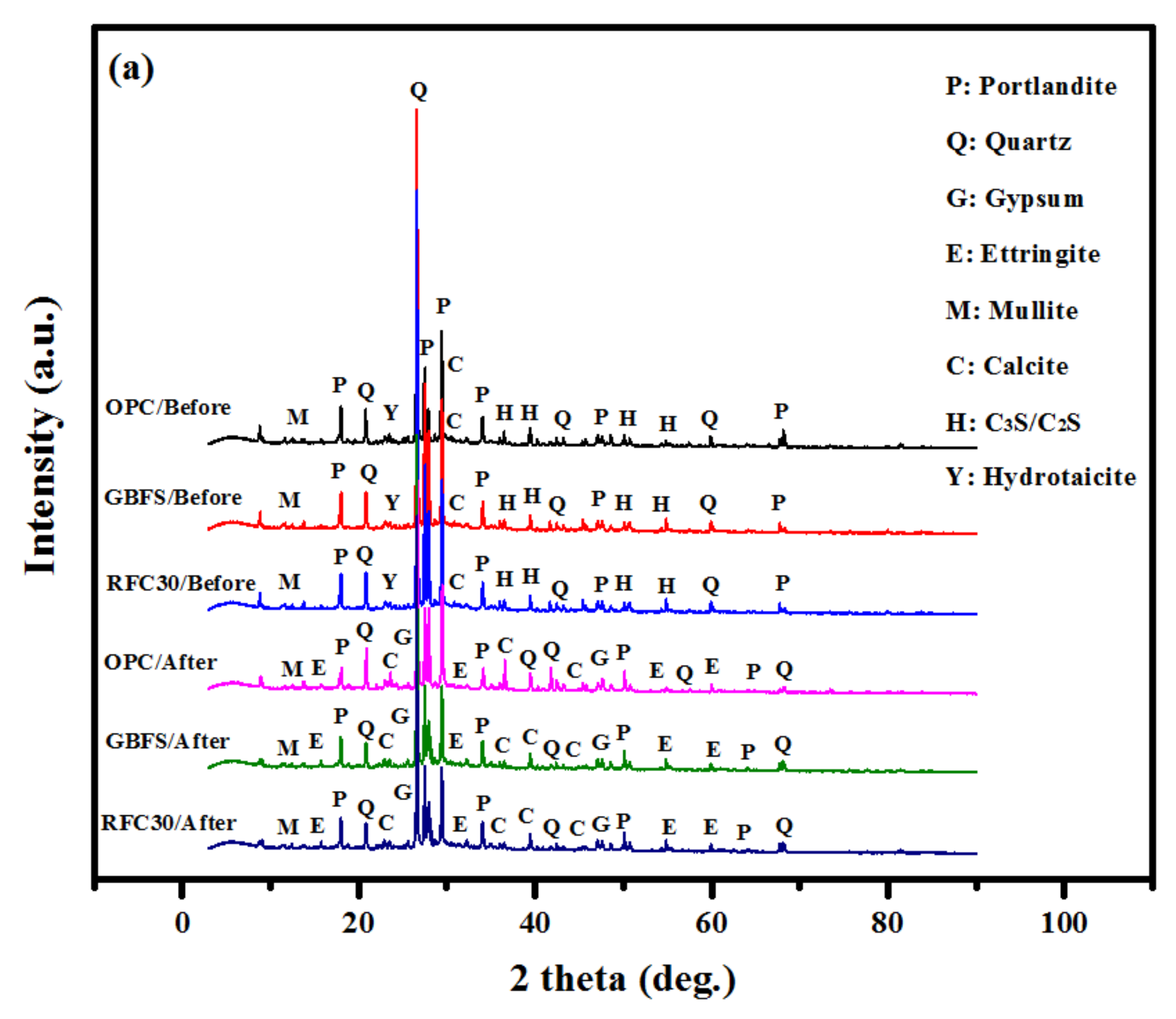
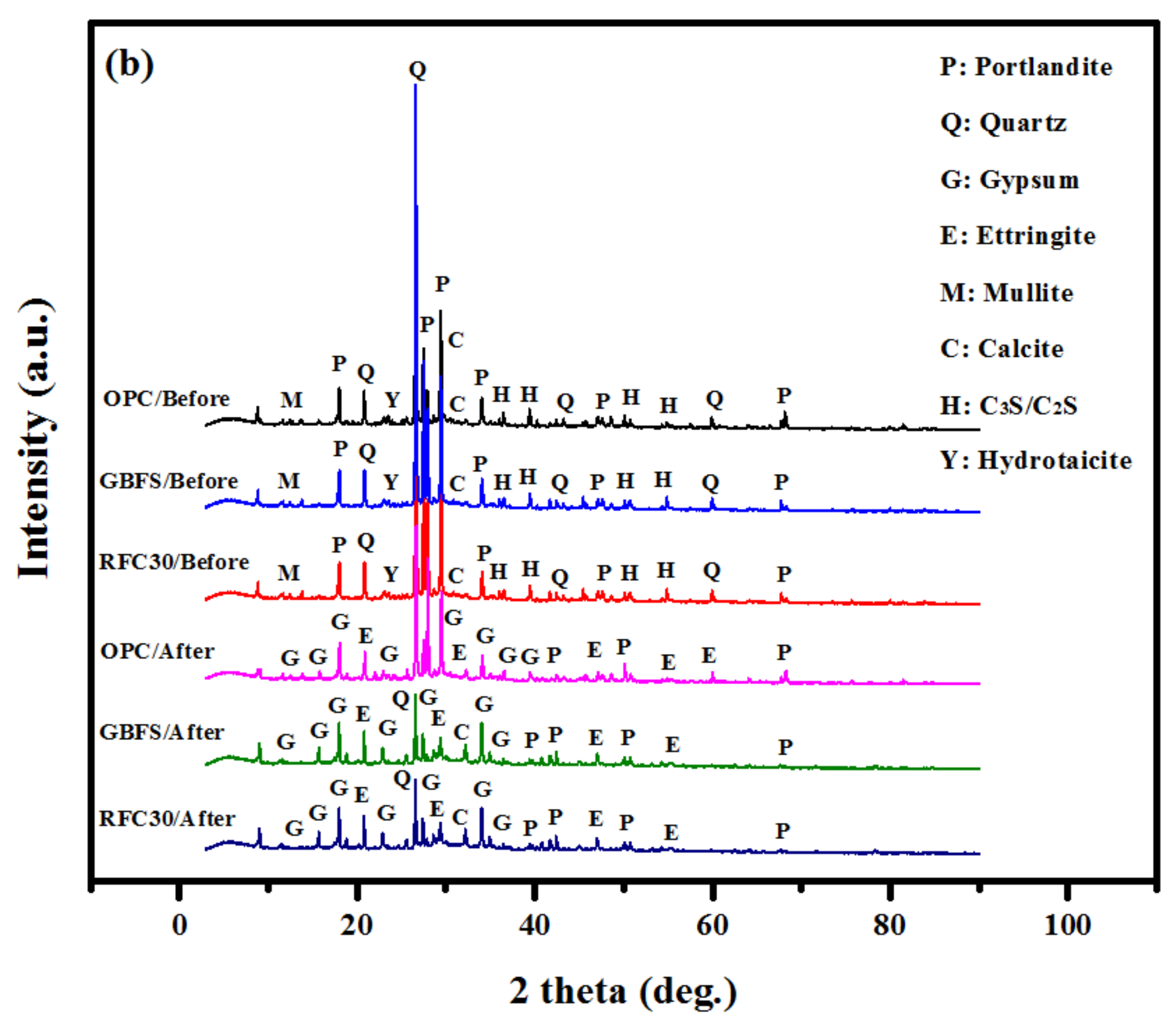
- The XRD patterns showed an increase in ettringite and gypsum in GBFS modified concrete after immersion in MgSO4 and H2SO4 solutions, indicating a high durability performance in such aggressive environment.
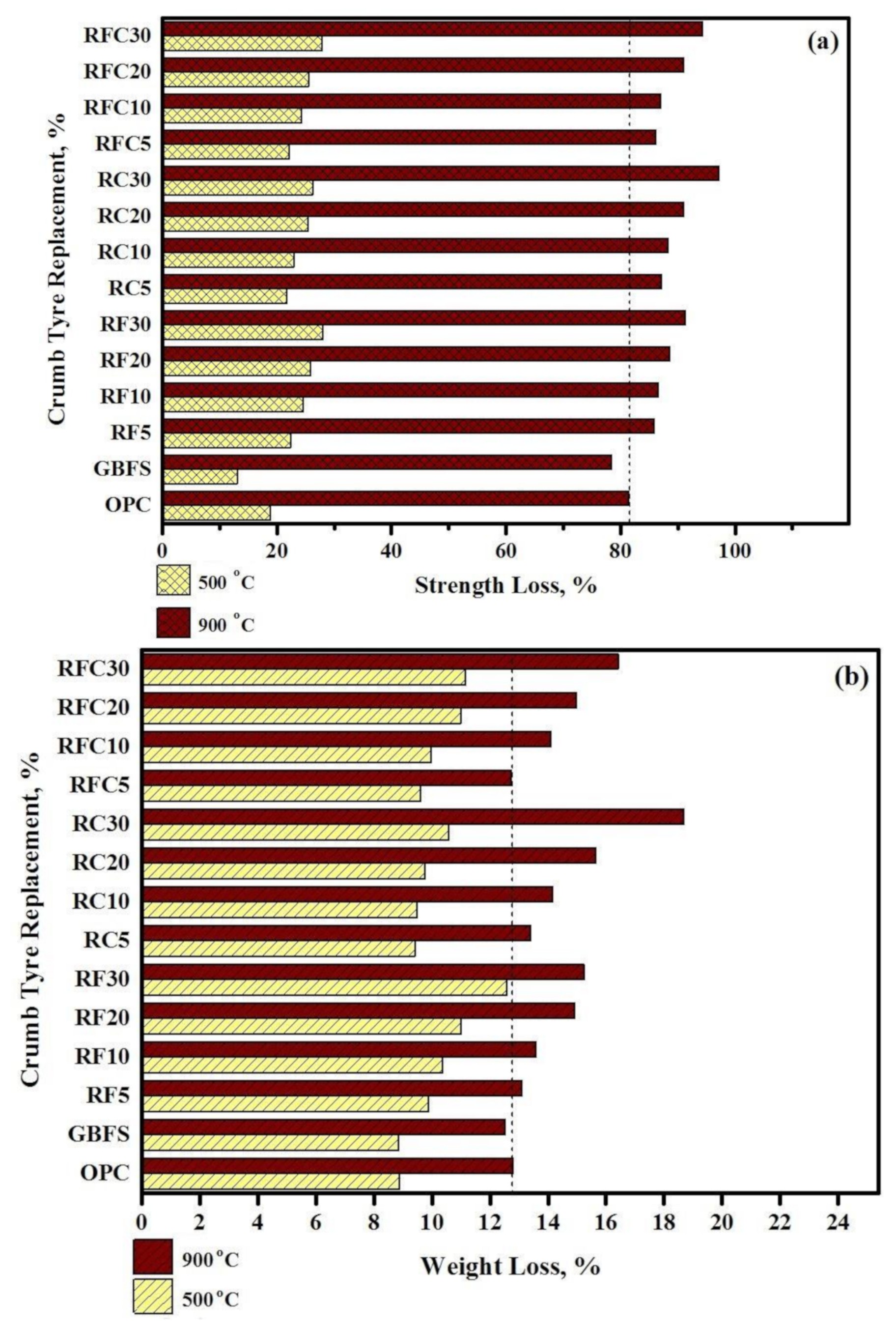
- GBFS modified concrete provides the lowest CS loss 15 and 80 MPa in 500 and 900 °C elevated temperatures, respectively. However, by increasing the WRTCs content in modified rubberized concrete from 5 to 30%, the residual CS and weight decreased by 9 and 29% that related to melting of the WRTCs, sluggish evaporation of the water, dehydration of the C-A-S-H gels, and decay of Ca (OH)2 that occurred above 400 °C.
- The ANN combined with particle swarm optimization algorithm provided satisfactorily results to estimate the residual CS of modified rubberized concrete after immersion in MgSO4 and H2SO4 solutions. In addition, the particle swarm optimization algorithm can also be used as a powerful tool in optimizing ANN weights. By using the optimized weight and bias of PSO-ANN, it is possible to design modified rubberized concrete with targeted mechanical properties and simultaneously manage the consumption of waste materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, B.S.; Kumar, S.; Mehra, P.; Gupta, R.C.; Joseph, M.; Csetenyi, L.J. Abrasion resistance of sustainable green concrete containing waste tire rubber particles. Constr. Build. Mater. 2016, 124, 906–909. [Google Scholar] [CrossRef]
- Youssf, O.; Mills, J.E.; Hassanli, R. Assessment of the mechanical performance of crumb rubber concrete. Constr. Build. Mater. 2016, 125, 175–183. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e002005. [Google Scholar] [CrossRef]
- Gupta, P.K.; Khaudhair, Z.A.; Ahuja, A.K. A new method for proportioning recycled concrete. Struct. Concr. 2016, 17, 677–687. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.P.; Rogkala, A.; Lampropoulou, P.; Tsikouras, B.; Rigopoulos, I.; Hatzipanagiotou, K. Petrographic and mechanical characteristics of concrete produced by different type of recycled materials. Geosciences 2019, 9, 264. [Google Scholar] [CrossRef]
- Abdallah, S.; Fan, M. Characteristics of concrete with waste glass as fine aggregate replacement. Int. J. Eng. Technol. Res. (IJETR) 2014, 2, 11–17. [Google Scholar]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Mhaya, A.M.; Abidin, A.R.Z.; Sarbini, N.N.; Ismail, M. Role of crumb tyre aggregates in rubberised concrete contained granulated blast-furnace slag. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 220, p. 012029. [Google Scholar]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high volume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Murugan, R.B.; Natarajan, C. Investigation of the Behaviour of Concrete Containing Waste Tire Crumb Rubber. In Advances in Structural Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1795–1802. [Google Scholar]
- Elchalakani, M. High strength rubberized concrete containing silica fume for the construction of sustainable road side barriers. In Structures; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Khatib, Z.K.; Bayomy, F.M. Rubberized Portland cement concrete. J. Mater. Civ. Eng. 1999, 11, 206–213. [Google Scholar] [CrossRef]
- Fu, C.; Ye, H.; Wang, K.; Zhu, K.; He, C. Evolution of mechanical properties of steel fiber-reinforced rubberized concrete (FR-RC). Compos. Part B Eng. 2019, 160, 158–166. [Google Scholar] [CrossRef]
- Huang, B.; Li, G.; Pang, S.-S.; Eggers, J. Investigation into Waste Tire Rubber-Filled Concrete. J. Mater. Civ. Eng. 2004, 16, 187–194. [Google Scholar] [CrossRef]
- Turatsinze, A.; Bonnet, S.; Granju, J.-L. Mechanical characterisation of cement-based mortar incorporating rubber aggregates from recycled worn tyres. Build. Environ. 2005, 40, 221–226. [Google Scholar] [CrossRef]
- Si, R.; Guo, S.; Dai, Q. Durability performance of rubberized mortar and concrete with NaOH-Solution treated rubber particles. Constr. Build. Mater. 2017, 153, 496–505. [Google Scholar] [CrossRef]
- Hossain, F.Z.; Shahjalal, M.; Islam, K.; Tiznobaik, M.; Alam, M.S. Mechanical properties of recycled aggregate concrete containing crumb rubber and polypropylene fiber. Constr. Build. Mater. 2019, 225, 983–996. [Google Scholar] [CrossRef]
- Hesami, S.; Hikouei, I.S.; Emadi, S.A.A. Mechanical behavior of self-compacting concrete pavements incorporating recycled tire rubber crumb and reinforced with polypropylene fiber. J. Clean. Prod. 2016, 133, 228–234. [Google Scholar] [CrossRef]
- Turki, M.; Zarrad, I.; Bretagne, E.; Queneudec, M. Influence of Filler Addition on Mechanical Behavior of Cementitious Mortar-Rubber Aggregates: Experimental Study and Modeling. J. Mater. Civ. Eng. 2012, 24, 1350–1358. [Google Scholar] [CrossRef]
- Siddique, R.; Kaur, D. Properties of concrete containing ground granulated blast furnace slag (GGBFS) at elevated temperatures. J. Adv. Res. 2012, 3, 45–51. [Google Scholar] [CrossRef]
- Chowdhury, S.; Maniar, A.; Suganya, O. Strength development in concrete with wood ash blended cement and use of soft computing models to predict strength parameters. J. Adv. Res. 2015, 6, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Abolpour, B.; Afsahi, M.M.; Hosseini, S.G. Statistical analysis of the effective factors on the 28 days compressive strength and setting time of the concrete. J. Adv. Res. 2015, 6, 699–709. [Google Scholar] [CrossRef]
- Osborne, G. Durability of Portland blast-furnace slag cement concrete. Cem. Concr. Compos. 1999, 21, 11–21. [Google Scholar] [CrossRef]
- Gong, K.; White, C.E. Impact of chemical variability of ground granulated blast-furnace slag on the phase formation in alkali-activated slag pastes. Cem. Concr. Res. 2016, 89, 310–319. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozbakkaloglu, T. Performance of sustainable concretes containing very high volume Class-F fly ash and ground granulated blast furnace slag. J. Clean. Prod. 2017, 162, 1407–1417. [Google Scholar] [CrossRef]
- Huseien, G.; Ismail, M.; Tahir, M.; Mirza, J.; Hussein, A.; Khalid, N.; Sarbini, N. Performance of sustainable alkali activated mortars containing solid waste ceramic powder. Chem. Eng. Trans. 2018, 63, 673–678. [Google Scholar]
- Shetty, M. Concrete technology; S. Chand & Company LTD: New Delhi, India, 2005; pp. 420–453. [Google Scholar]
- ASTM C33/C33M. Standard Specification for Concrete Aggregates; American Society for Testing and Materials: Philadelphia, PA, USA, 2003. [Google Scholar]
- Scott, H., IV; Gress, D. Recycling Concrete and Other Materials for Sustainable Development, ACI SP-219; American Concrete Institute: Indianapolis, IN, USA, 2004. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longman: London, UK, 1995; Volume 4. [Google Scholar]
- ASTM C192/C192M. Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM C267-97. Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacing and Polymer Concretes; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM C597-09. Standard Test Method for Pulse Velocity Through Concrete; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Mhaya, A.M.; Huseien, G.F.; Abidin, A.R.Z.; Ismail, M. Long-term mechanical and durable properties of waste tires rubber crumbs replaced GBFS modified concretes. Constr. Build. Mater. 2020, 256, 119505. [Google Scholar] [CrossRef]
- Moghaddam, S.C.; Madandoust, R.; Jamshidi, M.; Nikbin, I.M. Mechanical properties of fly ash-based geopolymer concrete with crumb rubber and steel fiber under ambient and sulfuric acid conditions. Constr. Build. Mater. 2021, 281, 122571. [Google Scholar]
- Aslani, F.; Khan, M. Properties of High-Performance Self-Compacting Rubberized Concrete Exposed to High Temperatures. J. Mater. Civ. Eng. 2019, 31, 04019040. [Google Scholar] [CrossRef]
- Sofi, A. Effect of waste tyre rubber on mechanical and durability properties of concrete—A review. Ain Shams Eng. J. 2018, 9, 2691–2700. [Google Scholar] [CrossRef]
- Karri, K.S.; Rao, G.R.; Raju, P.M. Strength and Durability Studies on GGBS Concrete. Int. J. Civ. Eng. 2015, 2, 34–41. [Google Scholar] [CrossRef]
- Huseien, G.F.; Tahir, M.M.; Mirza, J.; Ismail, M.; Shah, K.W.; Asaad, M.A. Effects of POFA replaced with FA on durability properties of GBFS included alkali activated mortars. Constr. Build. Mater. 2018, 175, 174–186. [Google Scholar] [CrossRef]
- Noruzman, A.; Ismail, M.; Bhutta MA, R.; Yusuf, T.O.; Shehu, I.A.; Hassan, I.O. Strength and durability characteristics of polymer modified concrete incorporating Vinyl acetate effluent. In Advanced Materials Research; Trans Tech Publ: Stafa-Zurich, Switzerland, 2013. [Google Scholar]
- Mohammadhosseini, H.; Yatim, J.M.; Sam, A.R.M.; Awal, A.A. Durability performance of green concrete composites containing waste carpet fibers and palm oil fuel ash. J. Clean. Prod. 2017, 144, 448–458. [Google Scholar] [CrossRef]
- Trtnik, G.; Kavčič, F.; Turk, G. Prediction of concrete strength using ultrasonic pulse velocity and artificial neural networks. Ultrasonics 2009, 49, 53–60. [Google Scholar] [CrossRef]
- Jeon, I.K.; Qudoos, A.; Jakhrani, S.H.; Kim, H.G.; Ryou, J.-S. Investigation of sulfuric acid attack upon cement mortars containing silicon carbide powder. Powder Technol. 2020, 359, 181–189. [Google Scholar] [CrossRef]
- García-Vera, V.E.; Tenza-Abril, A.J.; Saval, J.M.; Lanzón, M. Influence of crystalline admixtures on the short-term behaviour of mortars exposed to sulphuric acid. Materials 2018, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Ganjian, E.; Khorami, M.; Maghsoudi, A.A. Scrap-tyre-rubber replacement for aggregate and filler in concrete. Constr. Build. Mater. 2009, 23, 1828–1836. [Google Scholar] [CrossRef]
- Matschei, T.; Bellmann, F.; Stark, J. Hydration behaviour of sulphate-activated slag cements. Adv. Cem. Res. 2005, 17, 167–178. [Google Scholar] [CrossRef]
- Rashad, A.M.; Sadek, D.M.; Hassan, H.A. An investigation on blast-furnace stag as fine aggregate in alkali-activated slag mortars subjected to elevated temperatures. J. Clean. Prod. 2016, 112, 1086–1096. [Google Scholar] [CrossRef]
- Noumowe, A.N.; Clastres, P.; Debicki, G.; Bolvin, M. High temperature effect on high performance concrete (70-600 C) strength and porosity. Spec. Publ. 1994, 145, 157–172. [Google Scholar]
- Sancak, E.; Sari, Y.D.; Simsek, O. Effects of elevated temperature on compressive strength and weight loss of the light-weight concrete with silica fume and superplasticizer. Cem. Concr. Compos. 2008, 30, 715–721. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- Liew, K.; Sojobi, A.; Zhang, L. Green concrete: Prospects and challenges. Constr. Build. Mater. 2017, 156, 1063–1095. [Google Scholar] [CrossRef]
- Basheer, P. 40 Designs of Concrete to Resist Carbonation; NRC Research Press: Ottawa, ON, Canada, 1999; Volume 1. [Google Scholar]
- Nikoo, M.; Hadzima-Nyarko, M.; Karlo Nyarko, E.; Nikoo, M. Determining the natural frequency of cantilever beams using ANN and heuristic search. Appl. Artif. Intell. 2018, 32, 309–334. [Google Scholar] [CrossRef]
- Haykin, S. Neural Networks: A Comprehensive Foundation; Prentice-Hall, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Zambrano-Bigiarini, M.; Clerc, M.; Rojas, R. Standard particle swarm optimisation 2011 at cec-2013: A baseline for future pso improvements. In Proceedings of the 2013 IEEE Congress on Evolutionary Computation, Cancun, Mexico, 20–23 June 2013; IEEE: Piscataway, NJ, USA, 2013. [Google Scholar]
- Faridmehr, I.; Huseien, G.F.; Baghban, M.H. Evaluation of Mechanical and Environmental Properties of Engineered Alkali-Activated Green Mortar. Materials 2020, 13, 4098. [Google Scholar] [CrossRef] [PubMed]
- Huseien, F.G.; Sam, A.R.; Faridmehr, I.; Baghban, H.M. Performance of Epoxy Resin Polymer as Self-Healing Cementitious Materials Agent in Mortar. Materials 2021, 14, 1255. [Google Scholar] [CrossRef] [PubMed]









| Materials | Elements (Mass %) | ||||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | LOI | |
| OPC | 67.8 | 17.6 | 4.5 | 3.4 | 2.2 | 0.3 | 1.7 |
| GBFS | 51.8 | 30.8 | 10.9 | 0.6 | 4.6 | 0.4 | 0.2 |
| Material | Maximum Size (mm) | Specific Gravity | Water Absorption (%) | |
|---|---|---|---|---|
| Natural aggregates | Fine | 4.7 | 2.7 | 2.2 |
| Coarse | 10 | 2.8 | 1.2 | |
| Rubber aggregates | Fine | 4 | 1.3 | - |
| coarse | 8 | 1.4 | - | |
| Chemical Compositions Mass % | |
|---|---|
| Acetone extract | 10 |
| Ash content | 24 |
| Carbon black | 14 |
| Rubber Hydrocarbon (RHC) | 52 |
| Physical properties | |
| Size of fine rubber, mm | 1–4 |
| Size of coarse rubber, mm | 5–8 |
| Heat loss, kgf/cm2 | <1 |
| Metal content, % | <0.5 |
| Fibre content, % | <1 |
| Mixes | Binder | WRTCs Aggregate | Natural Aggregates | ||||
|---|---|---|---|---|---|---|---|
| OPC (kg/m3) | GBFS (kg/m3) | Fine Rubber (kg/m3) | Coarse Rubber (kg/m3) | River Sand (kg/m3) | Crushed Stone (kg/m3) | ||
| Batch A | OPC | 419 | - | 0 | 0 | 721 | 995 |
| GBFS | 335.2 | 83.8 | |||||
| Batch B | RF5 | 335.2 | 83.8 | 18.2 | 0 | 684.95 | 995 |
| RF10 | 36.5 | 648.90 | |||||
| RF20 | 72.9 | 576.80 | |||||
| RF30 | 109.4 | 504.70 | |||||
| Batch C | RC5 | 335.2 | 83.8 | 0 | 25.5 | 721 | 945.25 |
| RC10 | 51.1 | 895.5 | |||||
| RC20 | 102.1 | 796.17 | |||||
| RC30 | 153.2 | 696.5 | |||||
| Batch D | RFC5 | 335.2 | 83.8 | 9.1 | 12.8 | 702.77 | 970.12 |
| RFC10 | 18.2 | 25.5 | 684.95 | 945.25 | |||
| RFC20 | 36.5 | 51.1 | 648.90 | 895.5 | |||
| RFC30 | 63.8 | 89.3 | 612.85 | 845.75 | |||
| No. | Test | Age, Day | Number of Samples | Notes |
|---|---|---|---|---|
| 1 | Acid | 28 | 84 (6 for each mixture) | Evaluated after 365 days |
| 2 | Sulphate | 28 | 84 (6 for each mixture) | Evaluated after 365 days |
| 3 | Elevated temperatures | 28 | 126 (9 for each mixture) | Evaluated at 500 and 900 °C |
| Mix Design | Weight (%) | ||||
|---|---|---|---|---|---|
| Gypsum | Ettringite | Portland | Quartz | Others | |
| OPC based concrete | 19.7 | 13.9 | 12.3 | 34.5 | 1.6 |
| GBFS modified concrete | 13.8 | 29.7 | 22.9 | 31.8 | 1.8 |
| Network Topology | Training | Testing | ||||||
|---|---|---|---|---|---|---|---|---|
| MSE | ME | MAE | RMSE | MSE | ME | MAE | RMSE | |
| ANN-PSO (7-5-6-2) | 2.689 | 0.002 | 1.193 | 1.640 | 5.471 | −0.740 | 1.846 | 2.339 |
| ANN-PSO (7-8-6-2) | 0.000 | 0.000 | 0.000 | 0.000 | 96.745 | −3.688 | 6.559 | 9.836 |
| ANN-PSO (7-4-7-2) | 0.043 | 0.000 | 0.101 | 0.207 | 189.692 | 3.052 | 8.139 | 13.773 |
| ANN-PSO (7-7-5-2) | 0.000 | 0.000 | 0.000 | 0.000 | 537.116 | −6.380 | 12.987 | 23.176 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Lower Bound | −1 | Swarm Size | 200 |
| Upper Bound | 1 | Maximum Iteration | 100 |
| Cognition Coefficient (C1) | 2 | Social Coefficient (C2) | 2 |
| IW | b1 | ||||||
|---|---|---|---|---|---|---|---|
| −0.5274 | 1.0940 | 0.1130 | 0.2984 | −0.5184 | −0.1860 | −0.1587 | −0.3396 |
| 0.0714 | 0.1071 | 0.1291 | 0.2456 | −0.5523 | 0.1663 | −0.5016 | −0.0201 |
| 0.3507 | 0.1851 | 0.1027 | 0.4281 | 0.1688 | 0.4901 | 0.5762 | 0.7749 |
| −0.0951 | 0.0294 | −0.1359 | −0.1078 | −0.6578 | −0.2505 | 0.2984 | −0.7203 |
| −0.1100 | 0.6376 | 0.1747 | 0.6571 | −0.1104 | 0.2225 | −0.0218 | 0.0496 |
| LW1 | - | - | b2 | ||||
| −1.0485 | 0.0214 | 0.0112 | −0.5400 | 0.5249 | 0.4195 | ||
| 0.4383 | −0.0296 | 0.8801 | −0.7924 | −0.8425 | 0.0452 | ||
| 0.2861 | −0.1557 | −0.5375 | −0.2095 | 0.5081 | −0.1088 | ||
| −0.0628 | 0.1352 | −0.2958 | 0.3030 | 0.2954 | 0.6771 | ||
| 0.0133 | 0.1122 | 0.5471 | −0.4729 | −0.0676 | 0.2375 | ||
| 0.0730 | 1.0582 | 0.6763 | 0.6170 | 0.2833 | 0.3055 | ||
| LW2 | b3 | ||||||
| 0.7316 | −0.0601 | −0.4191 | −1.0144 | 0.7436 | 0.2015 | −0.0182 | |
| 0.0103 | 0.9972 | 0.0474 | −0.3476 | 0.3334 | −0.1799 | −0.3690 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Mhaya, A.; Baghban, M.H.; Faridmehr, I.; Huseien, G.F.; Abidin, A.R.Z.; Ismail, M. Performance Evaluation of Modified Rubberized Concrete Exposed to Aggressive Environments. Materials 2021, 14, 1900. https://doi.org/10.3390/ma14081900
M. Mhaya A, Baghban MH, Faridmehr I, Huseien GF, Abidin ARZ, Ismail M. Performance Evaluation of Modified Rubberized Concrete Exposed to Aggressive Environments. Materials. 2021; 14(8):1900. https://doi.org/10.3390/ma14081900
Chicago/Turabian StyleM. Mhaya, Akram, Mohammad Hajmohammadian Baghban, Iman Faridmehr, Ghasan Fahim Huseien, Ahmad Razin Zainal Abidin, and Mohammad Ismail. 2021. "Performance Evaluation of Modified Rubberized Concrete Exposed to Aggressive Environments" Materials 14, no. 8: 1900. https://doi.org/10.3390/ma14081900
APA StyleM. Mhaya, A., Baghban, M. H., Faridmehr, I., Huseien, G. F., Abidin, A. R. Z., & Ismail, M. (2021). Performance Evaluation of Modified Rubberized Concrete Exposed to Aggressive Environments. Materials, 14(8), 1900. https://doi.org/10.3390/ma14081900









