Polycyclic Aromatic Hydrocarbons (PAHs) in Fired Clay Bricks Incorporating Cigarette Butts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Brick Manufacturing Methodology
2.2. Carbon Analysis
2.3. Polycyclic Aromatic Hydrocarbon (PAH) Analysis
2.3.1. Extraction Method
2.3.2. Standards
2.3.3. GC–MS Analysis
3. Results and Discussion
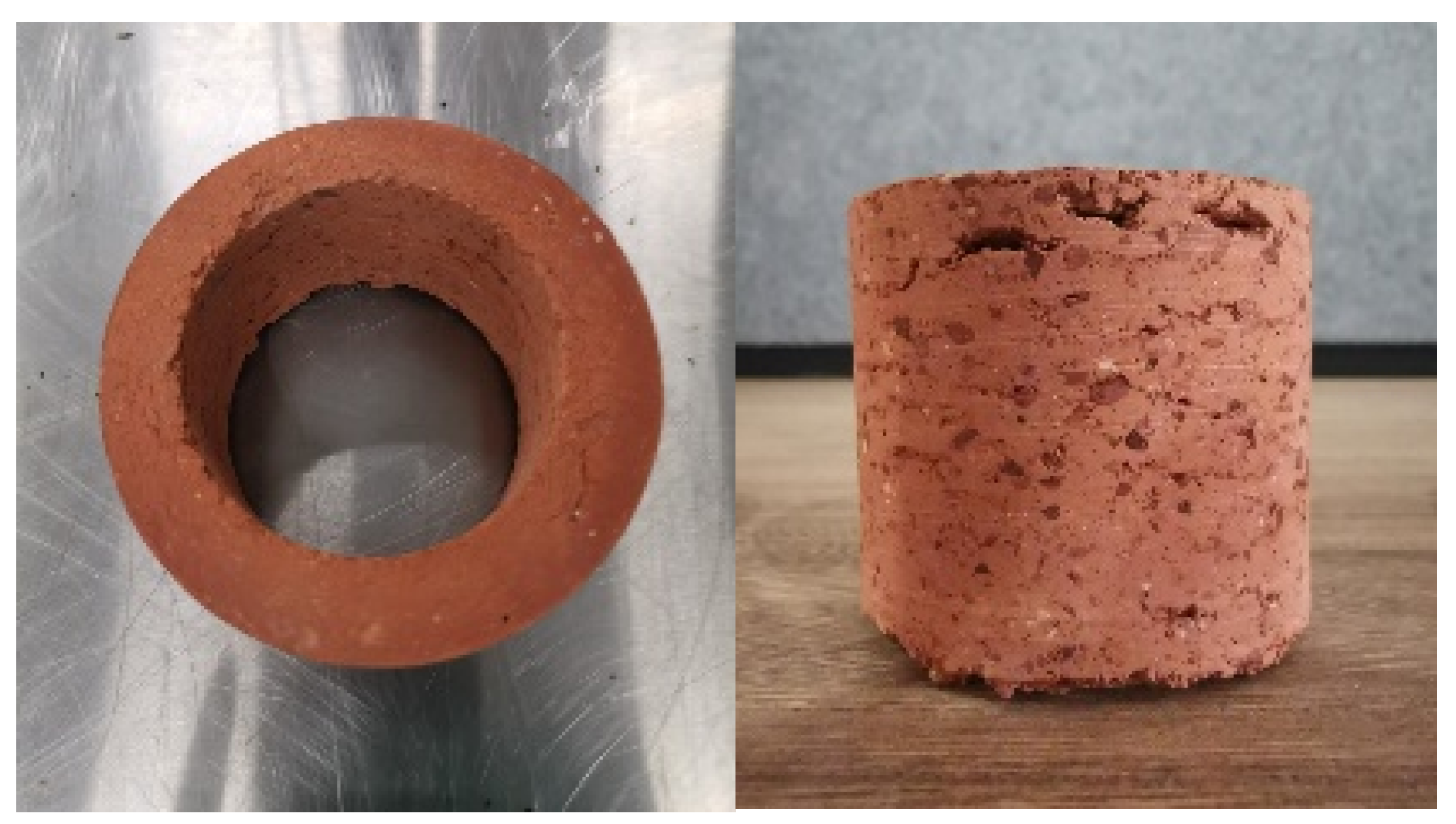
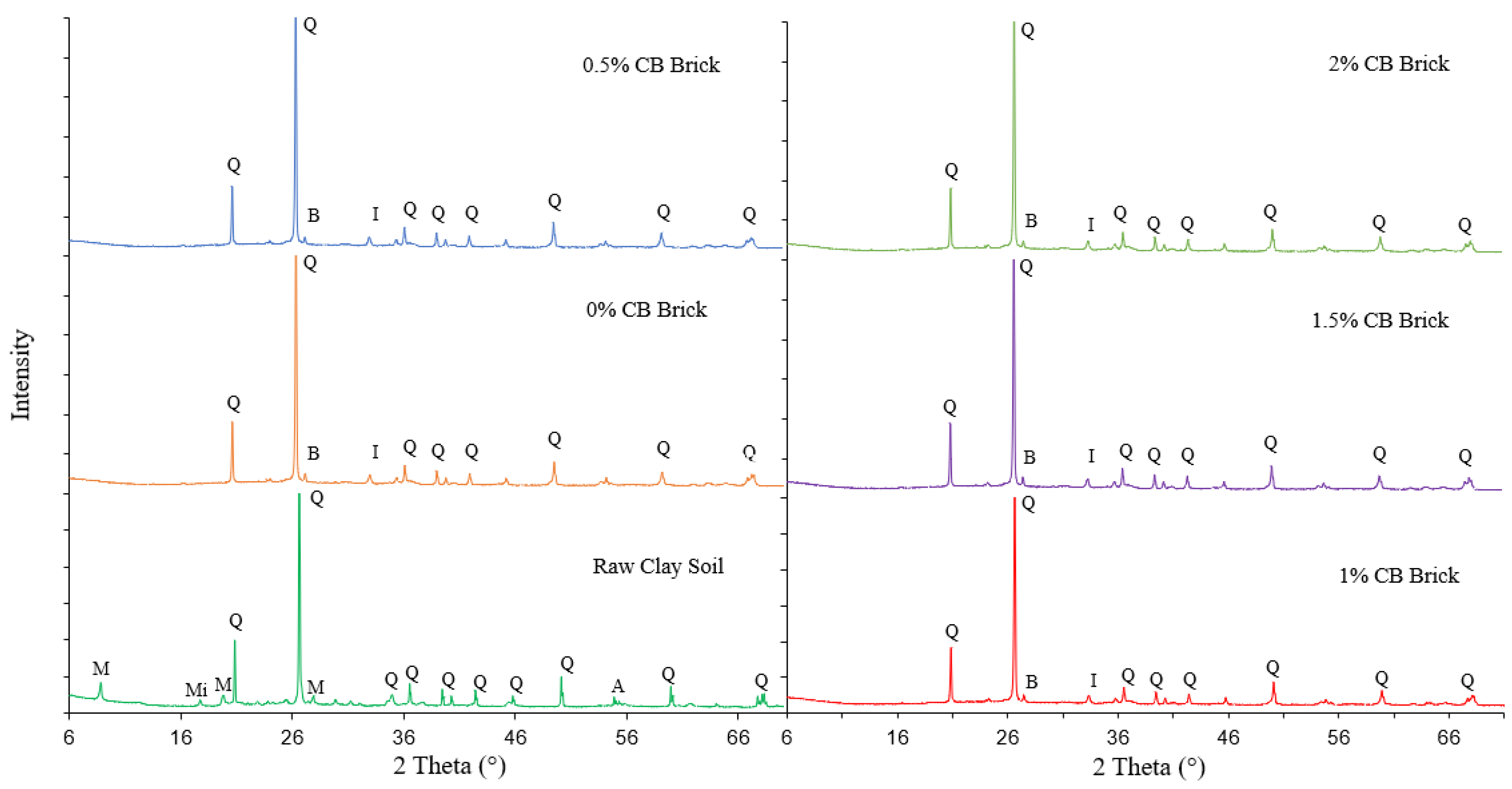
3.1. Characterization of Brick Samples
3.2. Carbon Analysis
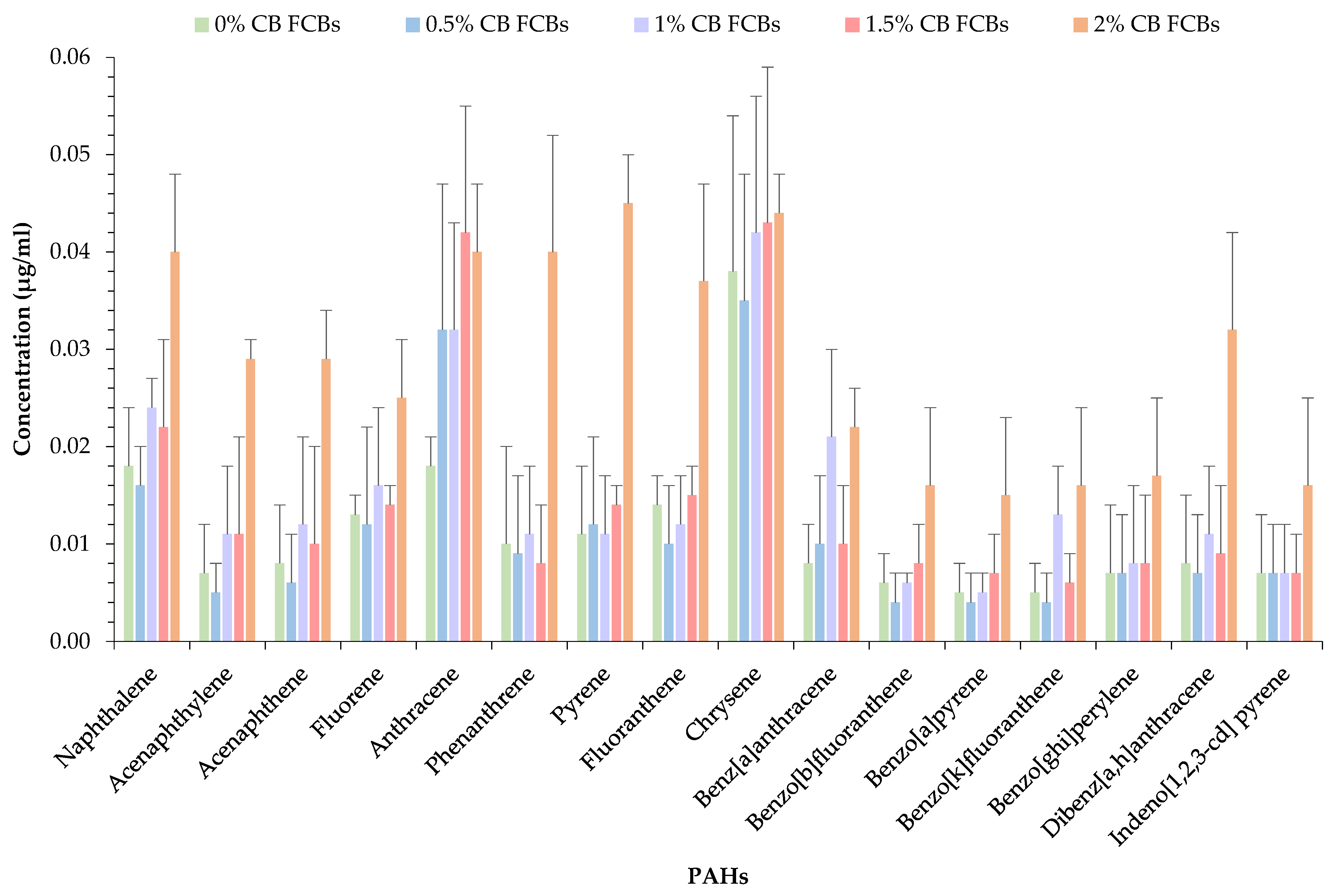
3.3. Polycyclic Aromatic Hydrocarbon (PAH) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Euromonitor International. Cigarettes, Euromonitor International. 2018. Available online: https://www.euromonitor.com/cigarettes (accessed on 24 December 2020).
- WHO Report on the Global Tobacco Epidemic: Monitoring Tobacco Use and Prevention Policies; World Health Organization: Geneva, Switzerland, 2017. Available online: http://apps.who.int/iris/bitstream/handle/10665/255874/9789241512824-eng.pdf;jsessionid=C8F97F8009293F9E2655830842B70178?sequence=1 (accessed on 9 December 2020).
- Kurmus, H.; Mohajerani, A. The toxicity and valorization options of cigarette butts. Waste Manag. 2020, 104, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Clean Up Australia. Clean Up Australia Rubbish Report. 2017. Available online: https://www.cleanup.org.au/rubbish-report (accessed on 1 August 2018).
- Robertson, R.M.; Thomas, W.C.; Suthar, J.N.; Brown, D.M. Accelerated degradation of cellulose acetate cigarette filters using controlled-release acid catalysis. Green Chem. 2012, 14, 2266–2272. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Cesarano, G.; Gaglione, S.A.; Lanzotti, V. Cigarette butt decomposition and associated chemical changes assessed by 13 C CPMAS NMR. PLoS ONE 2015, 10, e0117393. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, D.; Hoffmann, I.; El-Bayoumy, K. The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001, 14, 767–790. [Google Scholar] [CrossRef]
- Hoffmann, D.H.I. The changing cigarette, 1950-1995. J. Toxicol. Environ. Health Part A 1997, 50, 307–364. [Google Scholar] [CrossRef]
- Micevska, T.; Warne, M.S.J.; Pablo, F.; Patra, R. Variation in, and causes of, toxicity of cigarette butts to a cladoceran and microtox. Arch. Environ. Contam. Toxicol. 2006, 50, 205–212. [Google Scholar] [CrossRef]
- Novotny, T.E.; Lum, K.; Smith, E.; Wang, V.; Barnes, R. Cigarettes butts and the case for an environmental policy on hazardous cigarette waste. Int. J. Environ. Res. Public Health 2009, 6, 1691–1705. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Nabipour, I.; Saeedi, R.; Ostovar, A.; Khorsand, M.; Khajeahmadi, N.; Hayati, R.; Keshtkar, M. Association of metals (Cd, Fe, As, Ni, Cu, Zn and Mn) with cigarette butts in northern part of the Persian Gulf. Tob. Control 2017, 26, 461–463. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Schmidt, T.C.; Lorenzo-Parodi, N.; Jochmann, M.A.; Nabipour, I.; Raeisi, A.; Stojanović, N.; Mahmoodi, M. Cigarette butts: An overlooked source of PAHs in the environment? Environ. Pollut. 2019, 249, 932–939. [Google Scholar] [CrossRef]
- Slaughter, E.; Gersberg, R.M.; Watanabe, K.; Rudolph, J.; Stransky, C.; Novotny, T.E. Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob. Control 2011, 20, 25–29. [Google Scholar] [CrossRef]
- Rebischung, F.; Chabot, L.; Biaudet, H.; Pandard, P. Cigarette butts: A small but hazardous waste, according to European regulation. Waste Manag. 2018, 82, 9–14. [Google Scholar] [CrossRef]
- Mohajerani, A.; Kadir, A.A.; Larobina, L. A practical proposal for solving the world’s cigarette butt problem: Recycling in fired clay bricks. Waste Manag. 2016, 52, 228–244. [Google Scholar] [CrossRef]
- Kadir, A.A.; Mohajerani, A. Recycling cigarette butts in lightweight fired clay bricks. Constr. Mater. 2011, 164, 219–229. [Google Scholar] [CrossRef]
- Kadir, A.A.; Mohajerani, A. Possible utilization of cigarette butts in light-weight fired clay bricks. Int. J. Civ. Environ. Eng. 2008, 2, 137–141. [Google Scholar]
- Kadir, A.A.; Mohajerani, A.; Roddick, F.; Buckeridge, J. Density, strength, thermal conductivity and leachate characteristics of light-weight fired clay bricks incorporating cigarette butts. Proc. World Acad. Sci. Eng. Technol. 2009, 53, 1035–1040. [Google Scholar] [CrossRef]
- Kurmus, H.; Mohajerani, A. Recycling of Cigarette Butts in Fired Clay Bricks: A New Laboratory Investigation. Materials 2020, 13, 790. [Google Scholar] [CrossRef] [Green Version]
- Kurmus, H.; Mohajerani, A. Energy savings, thermal conductivity, micro and macro structural analysis of fired clay bricks incorporating cigarette butts. Constr. Build. Mater. 2021, 283, 122755. [Google Scholar] [CrossRef]
- Mohajerani, A.; Qun Hui, S.; Shen, C.; Suntovski, J.; Rodwell, G.; Kurmus, H.; Hana, M.; Rahman, M.T. Implementation of Recycling Cigarette Butts in Lightweight Bricks and a Proposal for Ending the Littering of Cigarette Butts in Our Cities. Materials 2020, 13, 4023. [Google Scholar] [CrossRef] [PubMed]
- Kurmus, H.; Mohajerani, A. Leachate Analysis of Heavy Metals in Cigarette Butts and Bricks Incorporated with Cigarette Butts. Materials 2020, 13, 2843. [Google Scholar] [CrossRef] [PubMed]
- Dobaradaran, S.; Schmidt, T.C.; Lorenzo-Parodi, N.; Kaziur-Cegla, W.; Jochmann, M.A.; Nabipour, I.; Lutze, H.V.; Telgheder, U. Polycyclic aromatic hydrocarbons (PAHs) leachates from cigarette butts into water. Environ. Pollut. 2020, 259, 113916. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Manzano, C.; Hentschel, B.T.; Simonich, S.L.M.; Hoh, E. Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environ. Sci. Technol. 2013, 47, 13976–13984. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Polycyclic Aromatic Hydrocarbons (PAHs); U.S. Environmental Protection Agency: Washington, DC, USA, 2008. Available online: https://archive.epa.gov/epawaste/hazard/wastemin/web/pdf/pahs.pdf (accessed on 6 September 2020).
- Hatzinger, P.B.; Alexander, M. Biodegradation of organic compounds sequestered in organic solids or in nanopores within silica particles. Environ. Toxicol. Chem. 1997, 16, 2215–2221. [Google Scholar] [CrossRef]
- Saim, N.; Dean, J.R.; Abdullah, M.P.; Zakaria, Z. Extraction of polycyclic aromatic hydrocarbons from contaminated soil using Soxhlet extraction, pressurised and atmospheric microwave-assisted extraction, supercritical fluid extraction and accelerated solvent extraction. J. Chromatogr. A 1997, 791, 361–366. [Google Scholar] [CrossRef]
- USEPA. Method 3540C: Soxhlet Extraction; USEPA: Washington, DC, USA, 1996. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3540c.pdf (accessed on 7 September 2020).
- Industrial Waste Resource Guidelines. Solid Industrial Waste Hazard Categorization and Management; E.P.A.: Carlton, Australia, 2009. Available online: https://www.epa.vic.gov.au/about-epa/publications/iwrg631 (accessed on 12 April 2020).
- Standards Australia 2009. Methods for Testing Soils for Engineering Purposes. In Soil Classification Tests—Determination of the Particle Size Distribution of a Soil—Standard Method of Analysis by Sieving (AS1289.3.6.1); SAI Global Limited: Chicago, IL, USA, 2009. [Google Scholar]
- Lau, E.; Gan, S.; Ng, H. Extraction techniques for polycyclic aromatic hydrocarbons in soils. Int. J. Anal. Chem. 2010, 2010. [Google Scholar] [CrossRef]
- Provin, T.; Hossner, L. What Happens to Nitrogen in Soils? 2001. Available online: https://agrilifeextension.tamu.edu/library/gardening/what-happens-to-nitrogen-in-soils/ (accessed on 12 December 2020).
- Zhang, Y.; Jia, L.; Mei, H.; Cui, Q.; Zhang, P.; Sun, Z. Fabrication, microstructure and properties of bricks fired from lake sediment, cinder and sewage sludge. Constr. Build. Mater. 2016, 121, 154–160. [Google Scholar] [CrossRef]
- Pena, M.; Casais, M.; Mejuto, M.; Cela, R. Optimization of the matrix solid-phase dispersion sample preparation procedure for analysis of polycyclic aromatic hydrocarbons in soils: Comparison with microwave-assisted extraction. J. Chromatogr. A 2007, 1165, 32–38. [Google Scholar] [CrossRef]
- Shu, Y.Y.; Lai, T.L. Effect of moisture on the extraction efficiency of polycyclic aromatic hydrocarbons from soils under atmospheric pressure by focused microwave-assisted extraction. J. Chromatogr. A 2001, 927, 131–141. [Google Scholar] [CrossRef]
- Yu, X.; Gao, Y.; Wu, S.; Zhang, H.; Cheung, K.; Wong, M.H. Distribution of polycyclic aromatic hydrocarbons in soils at Guiyu area of China, affected by recycling of electronic waste using primitive technologies. Chemosphere 2006, 65, 1500–1509. [Google Scholar] [CrossRef]
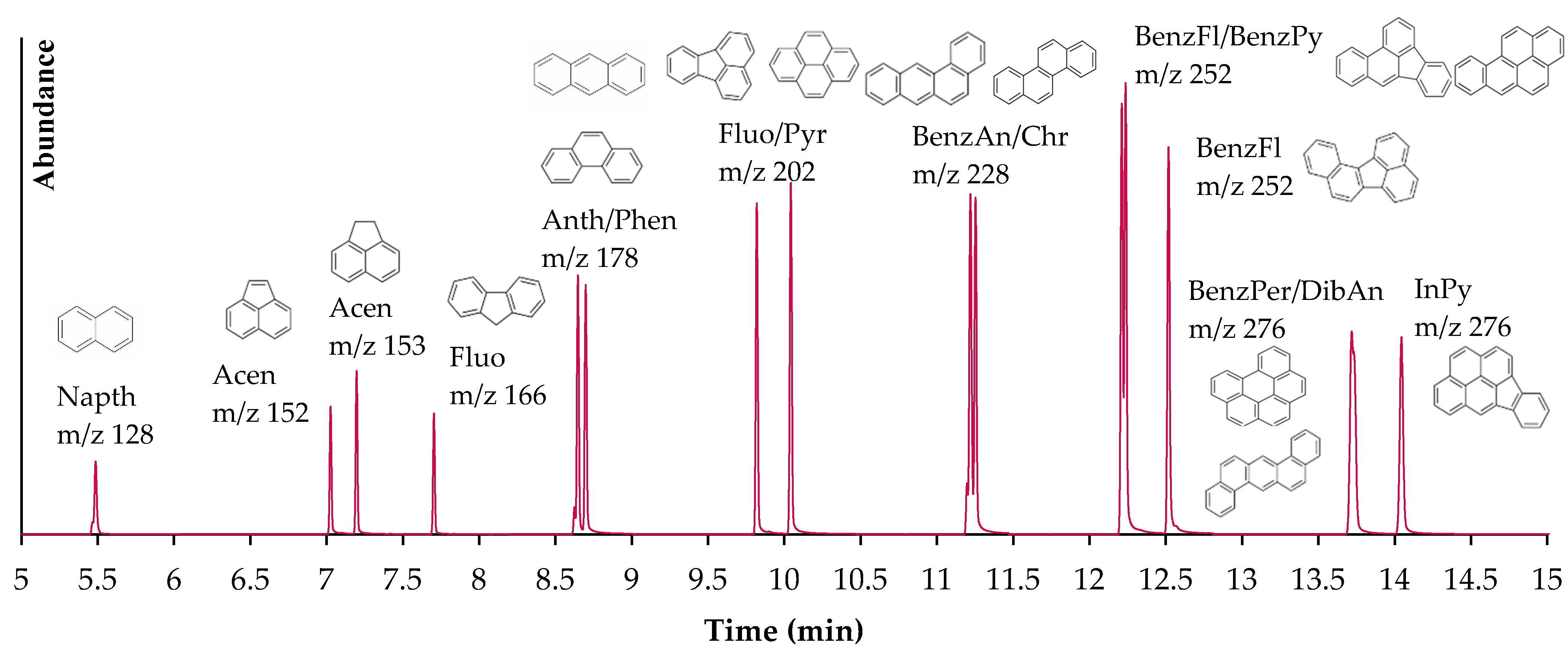

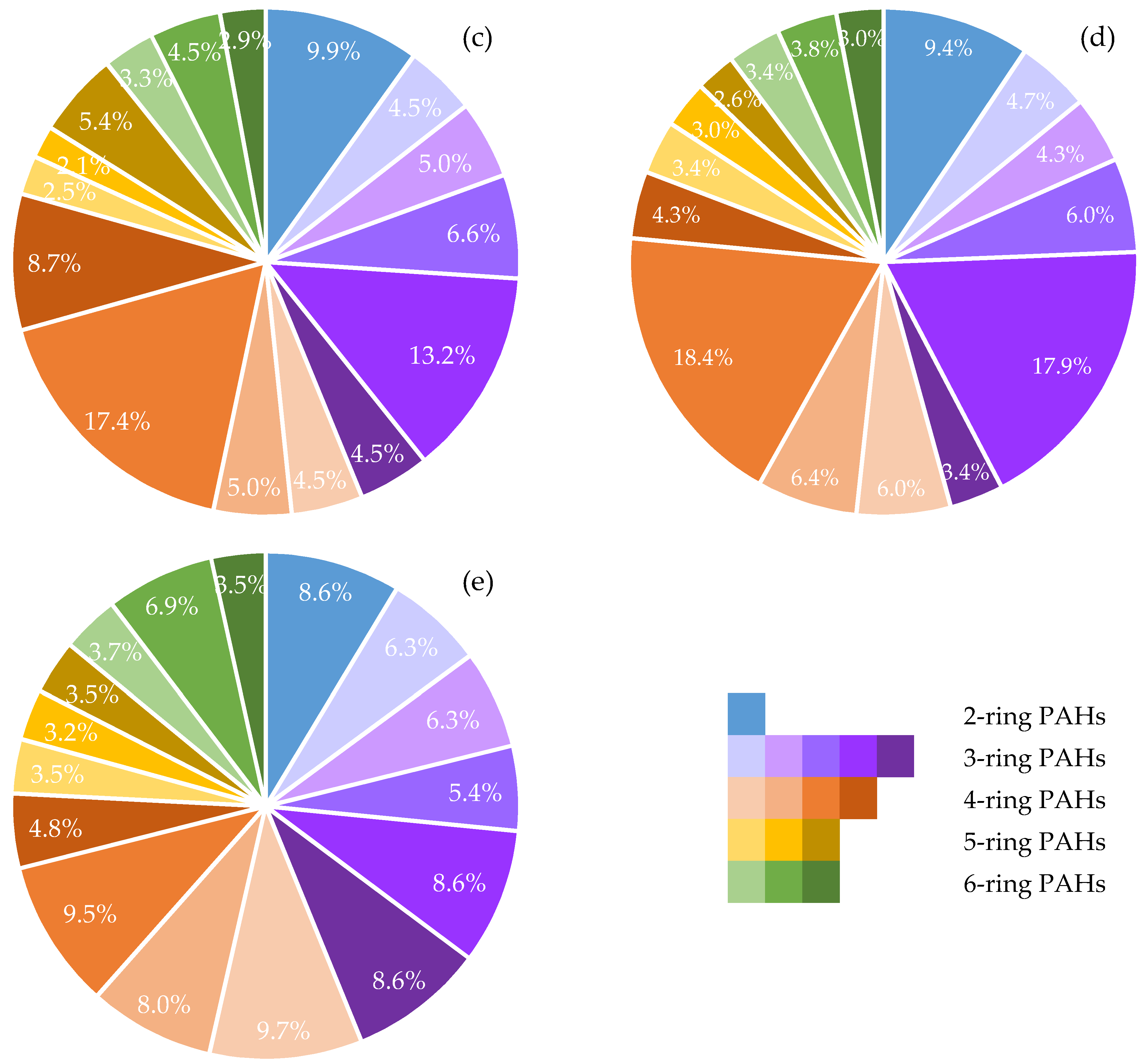
| Compound | Retention Time (min) | Precursor Ion (m/z) | Product Ion (m/z) | No. of Aromatic Rings | Water Solubility (mg/L) |
|---|---|---|---|---|---|
| Naphthalene | 5.51 | 128 | 128/128 | 2 | 31.69 |
| Naphthalene-d8 (IS) | 5.49 | 136 | 108/136 | - | |
| Acenaphthylene | 7.07 | 152 | 152/152 | 3 | 3.93 |
| Acenaphthene | 7.24 | 153 | 153/153 | 3 | 3.93 |
| Fluorene | 7.73 | 166 | 165/166 | 3 | 1.68–1.98 |
| Anthracene | 8.66 | 178 | 178/178 | 3 | 0.0446 |
| Phenanthrene | 8.71 | 178 | 178/178 | 3 | 1–1.6 |
| Phenanthrene-d10 (IS) | 8.64 | 188 | 160/188 | - | |
| Pyrene | 9.83 | 202 | 202/202 | 4 | 0.129–0.165 |
| Fluoranthene | 10.05 | 202 | 202/202 | 4 | 0.206 |
| Chrysene | 11.22 | 228 | 228/228 | 4 | 0.0015–0.0022 |
| Chrysene-d12 (IS) | 11.20 | 240 | 236/240 | - | |
| Benz[a]anthracene | 11.26 | 228 | 228/228 | 4 | 0.011 |
| Benz[a]anthracene-d12 (IS) | 11.23 | 240 | 236/240 | - | |
| Perylene-d12 (IS) | 11.75 | 241 | 241/242 | - | |
| Benzo[b]fluoranthene | 12.21 | 252 | 252/252 | 5 | 0.0012 |
| Benzo[a]pyrene | 12.24 | 252 | 252/252 | 5 | 0.0038 |
| Benzo[k]fluoranthene | 12.52 | 252 | 252/252 | 5 | 0.0008 |
| Benzo[ghi]perylene | 13.72 | 276 | 276/276 | 6 | 0.00026 |
| Dibenz[a,h]anthracene | 13.74 | 278 | 278/278 | 6 | 0.0005 |
| Indeno[1,2,3-cd] pyrene | 14.05 | 276 | 276/276 | 6 | 0.062 |
| Component | Raw Clay Soil (%) | Percentages of CBs by wt. | ||||
|---|---|---|---|---|---|---|
| 0% | 0.50% | 1% | 1.50% | 2% | ||
| Na2O | 2.46 | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 |
| MgO | 1.62 | 1.1 | 1.1 | 1.2 | 1.1 | 1.2 |
| Al2O3 | 19.95 | 16.8 | 16 | 16.5 | 16.3 | 16.8 |
| SiO2 | 62.84 | 53.8 | 52.6 | 54.8 | 53.2 | 55.3 |
| P2O5 | - | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 |
| K2O | 4.87 | 3.5 | 3.4 | 3.4 | 3.5 | 3.5 |
| CaO | 0.27 | 0.2 | 0.2 | 0.3 | 0.3 | 0.4 |
| TiO2 | 1.19 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Fe2O3 | 6.15 | 6.9 | 6.9 | 6.8 | 6.8 | 6.7 |
| Mixture Identification (%) | CHNS Results | TGA Results | |||
|---|---|---|---|---|---|
| Carbon (%) | Hydrogen (%) | Nitrogen (%) | Sulfur (%) | Mass Loss (%) | |
| CB (0) | 0.02 | Not detected | 3.74 | Not detected | 0.41 |
| CB (0.5) | 0.03 | Not detected | 4.74 | Not detected | 0.47 |
| CB (1.0) | 0.02 | Not detected | 6.01 | Not detected | 0.54 |
| CB (1.5) | 0.01 | Not detected | 6.16 | Not detected | 0.65 |
| CB (2.0) | 0.04 | Not detected | 4.88 | Not detected | 0.68 |
| CB (5.0) | 0.29 | Not detected | 6.36 | Not detected | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurmus, H.; Mohajerani, A.; Grist, S. Polycyclic Aromatic Hydrocarbons (PAHs) in Fired Clay Bricks Incorporating Cigarette Butts. Materials 2021, 14, 2032. https://doi.org/10.3390/ma14082032
Kurmus H, Mohajerani A, Grist S. Polycyclic Aromatic Hydrocarbons (PAHs) in Fired Clay Bricks Incorporating Cigarette Butts. Materials. 2021; 14(8):2032. https://doi.org/10.3390/ma14082032
Chicago/Turabian StyleKurmus, Halenur, Abbas Mohajerani, and Stephen Grist. 2021. "Polycyclic Aromatic Hydrocarbons (PAHs) in Fired Clay Bricks Incorporating Cigarette Butts" Materials 14, no. 8: 2032. https://doi.org/10.3390/ma14082032
APA StyleKurmus, H., Mohajerani, A., & Grist, S. (2021). Polycyclic Aromatic Hydrocarbons (PAHs) in Fired Clay Bricks Incorporating Cigarette Butts. Materials, 14(8), 2032. https://doi.org/10.3390/ma14082032








