Effect of Hydrogen Nanobubbles on the Mechanical Strength and Watertightness of Cement Mixtures
Abstract
1. Introduction
2. Experimental Setup
2.1. Hydrogen Nanobubble Water (HNBW)
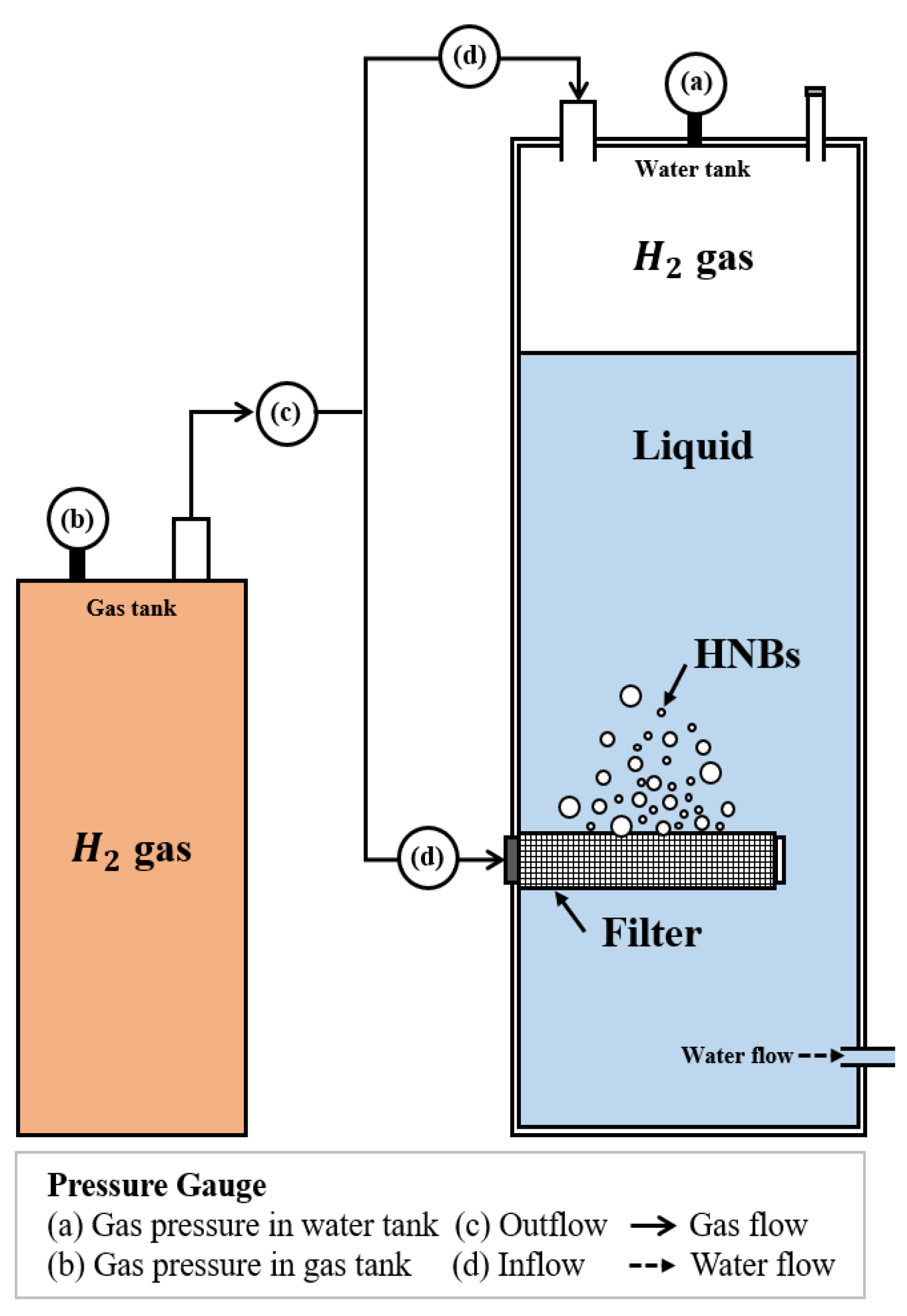
2.1.1. HNBW Production Method
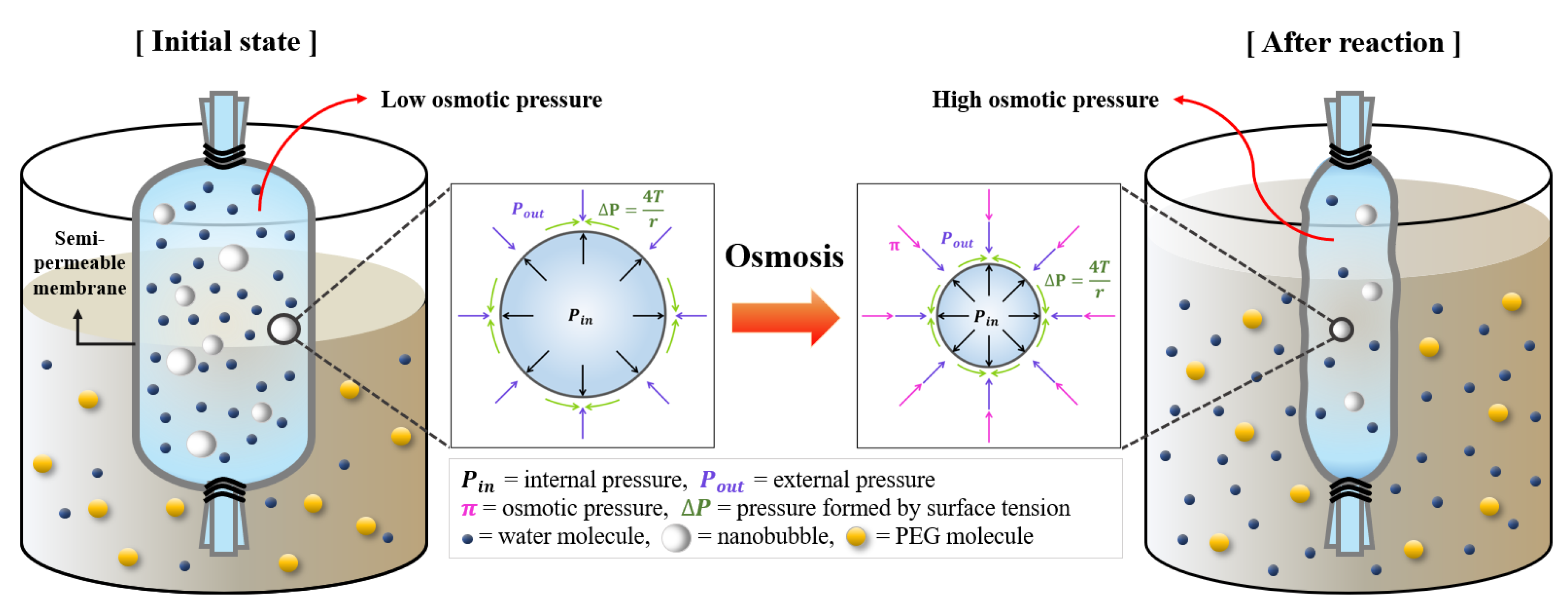
2.1.2. High-Concentration Nanobubbles with Osmosis
2.1.3. HNBW Analysis Method
2.2. Experimental Conditions and Method
2.2.1. Cement Mixture Proportions
2.2.2. Mortar Flowability and Strength
2.2.3. Paste Porosity and Microstructure
3. Experimental Results
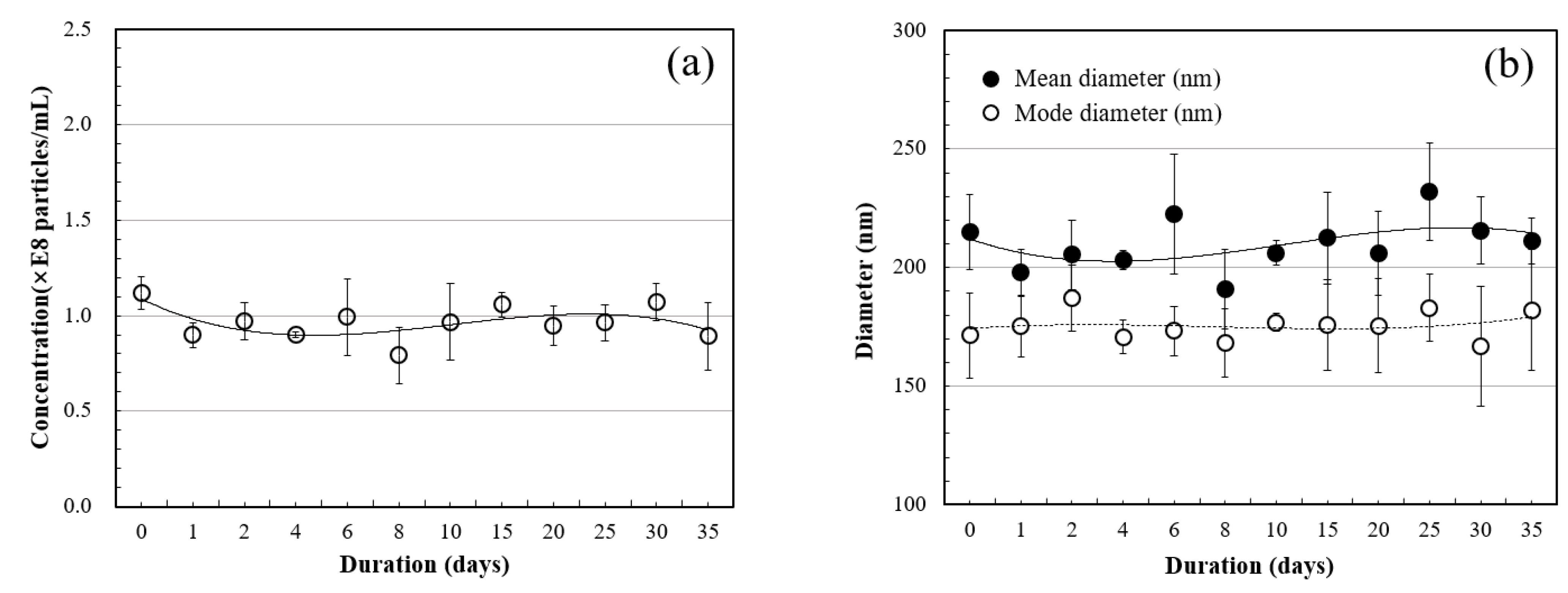
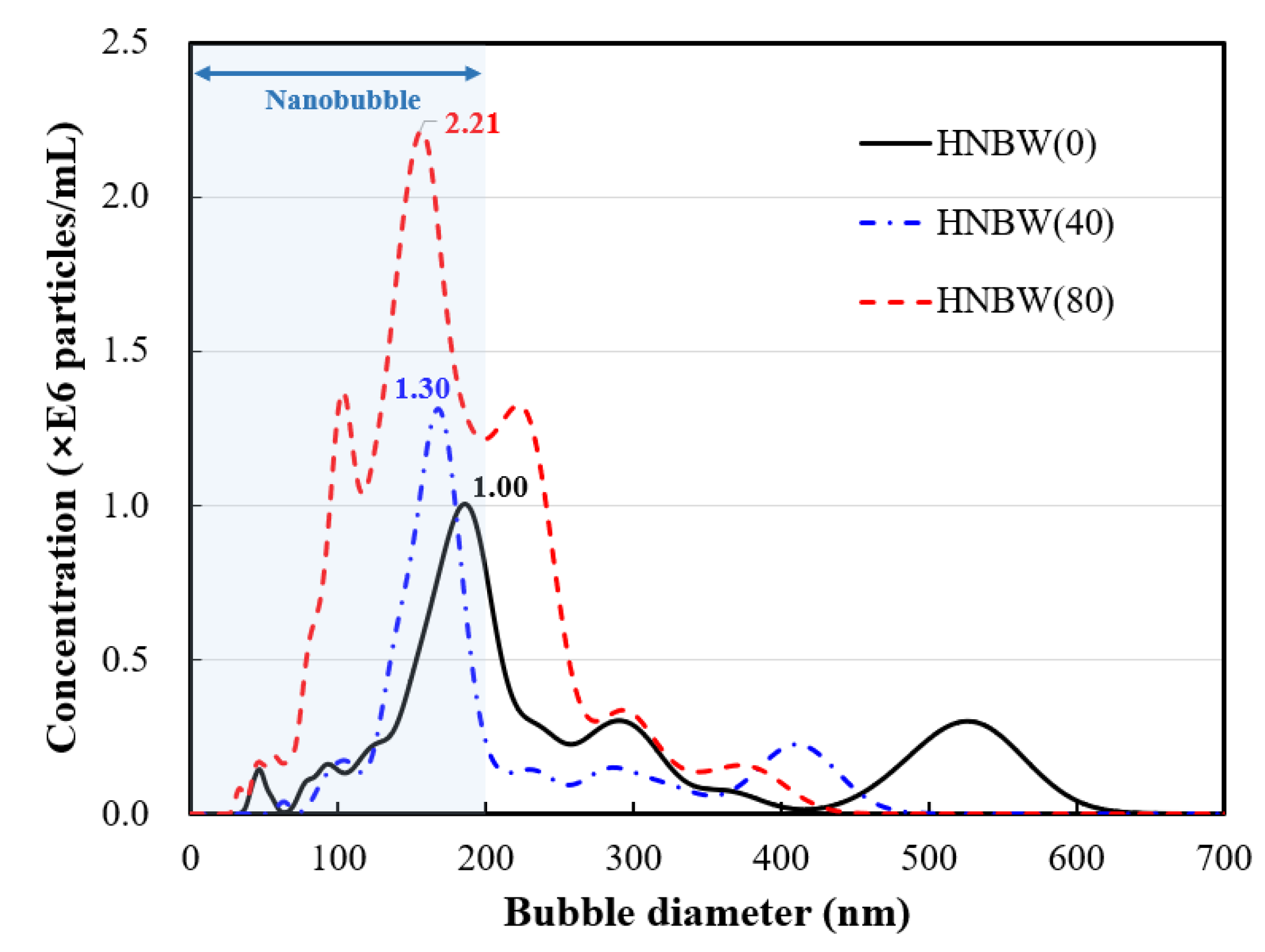
3.1. HNBW
3.2. Cement Mortar
3.2.1. Flowability
3.2.2. Mechanical Properties
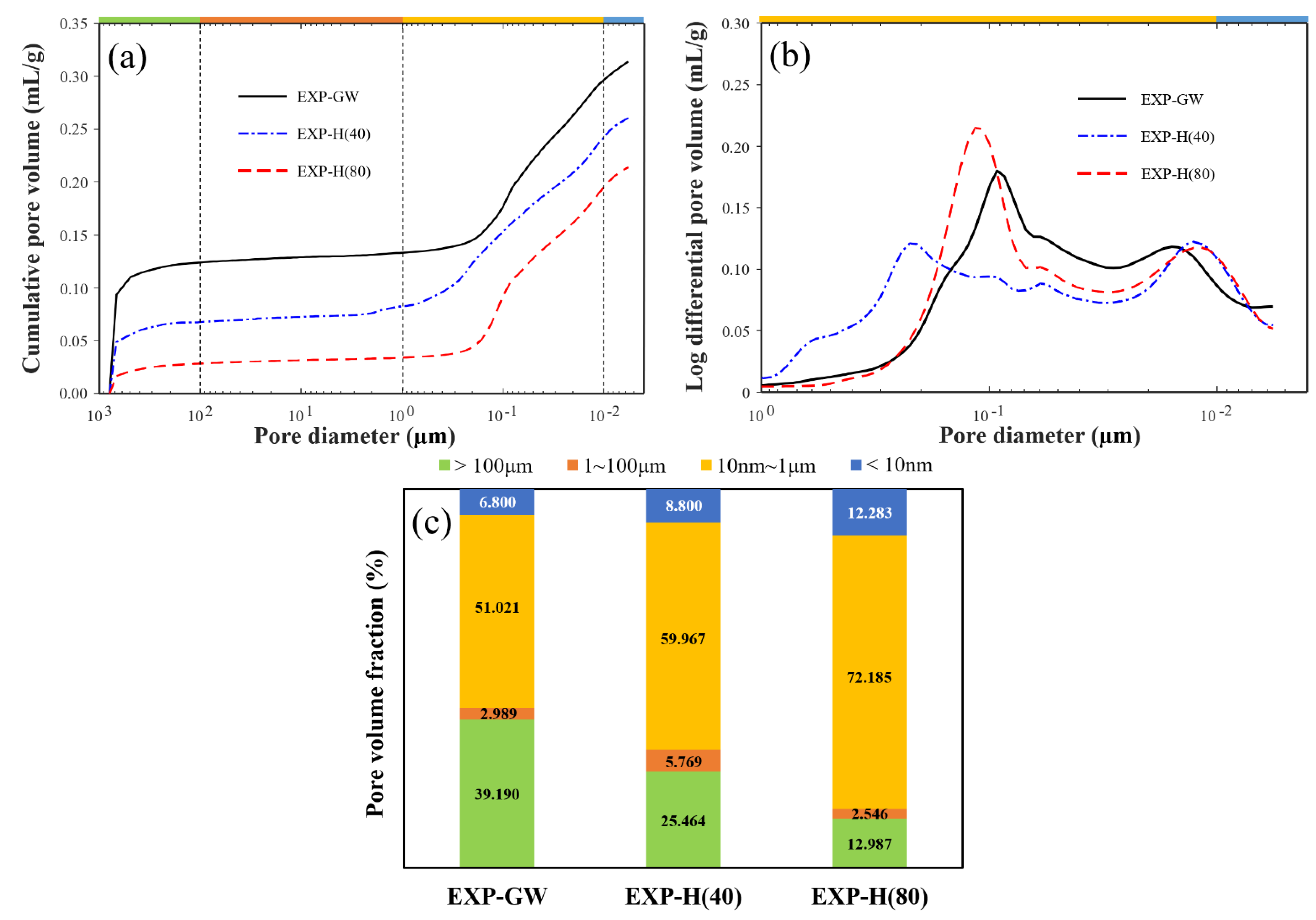
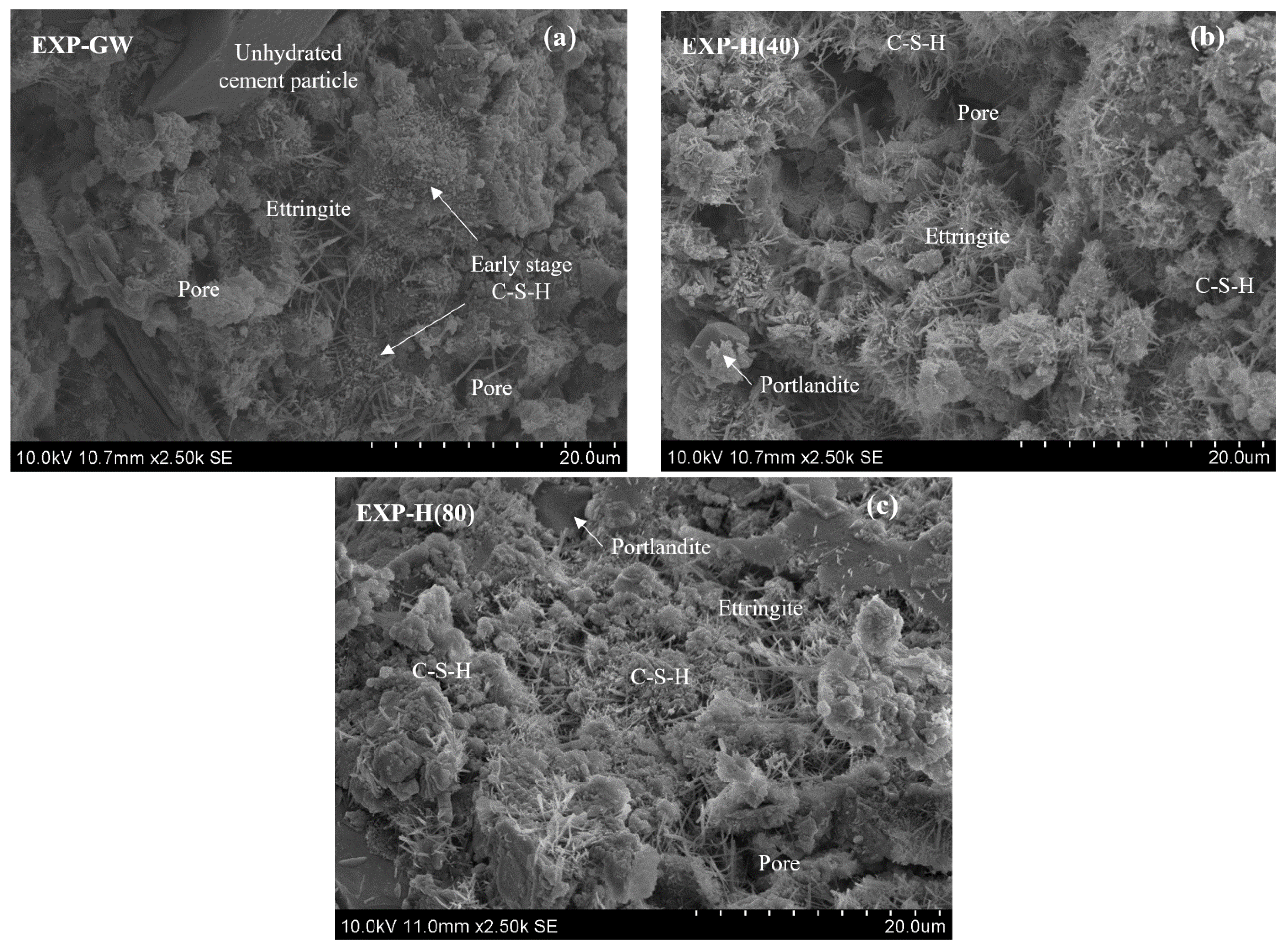
3.2.3. Pore Structure Characteristics
4. Discussion
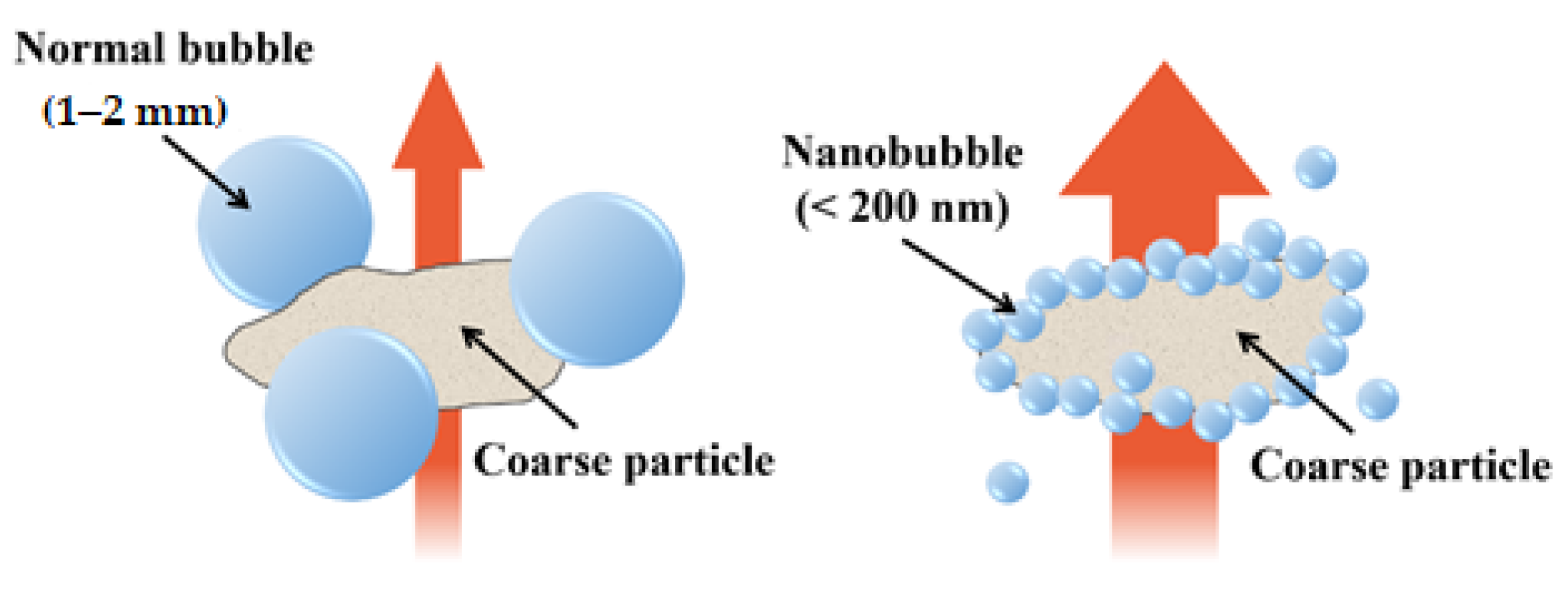
4.1. Collision Impact of HNBs and Cement Particles
4.2. Effect of Bubble Surface Tension and Zeta Potential
5. Conclusions
- The number of hydrogen nanobubbles was concentrated by more than twice, and the diameter of the concentrated bubbles was stabilized to a uniform size of about 100–150 nm by applying osmosis. When hydrogen nanobubble water was applied as a mixing water, the compressive strength of the cement mortar improved by about 3.7–15.79% compared to the control group that used general water. Moreover, the compressive strength increased as the concentration of hydrogen nanobubbles increased.
- The compressive strength increased because the hydrogen nanobubbles induced more contact between the mixing water and the cement particles through stabilization. Further, the mixing water reached the microstructures constituting the cement-hydration reactant because the zeta potential and surface tension of the HNBs increased. This led to the activation of the hydration reaction and the acceleration of the pozzolanic reaction, and the cement mortar’s compressive strength and watertightness were improved.
- The results of the MIP and SEM analyses indicated that the highly concentrated nanobubble water formed the cement mixture into a tighter structure by reducing the development and porosity of the hydration reactant. The pore volume was reduced by up to 75% when highly concentrated HNBW was used as the mixing water. This is because fewer bubbles were introduced when the cement mixture was mixed as the HNB concentration increased, thereby forming a denser pore structure. As a result, the concentration of HNBs increased, the hydration of the cement was actively induced due to the change in the physicochemical properties of the mixing water. Therefore, the tightness of the internal hydrate structure increased the strength and durability of the cement mixtures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed Ali, K.; Ahmad, M.I.; Yusup, Y. Issues, impacts, and mitigations of carbon dioxide emissions in the building sector. Sustainability 2020, 12, 7427. [Google Scholar] [CrossRef]
- Anwar, A.; Sabih, A.; Husain, S.M.A.; Ahmad, S.A. Salvage of ceramic waste and marble dust for the refinement of sustainable concrete. Int. J. Civ. Eng. Technol. 2015, 6, 79–92. [Google Scholar]
- Olivier, J.G.J.; Schure, K.M.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions: 2017 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017; p. 5. [Google Scholar]
- Kewalramani, M.A.; Syed, Z.I. Application of nanomaterials to enhance microstructure and mechanical properties of concrete3. Int. J. Civ. Eng. Technol. 2018, 10, 98–104. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Cho, G.C. Global CO2 emission-related geotechnical engineering hazards and the mission for sustainable geotechnical engineering. Energies 2019, 12, 2567. [Google Scholar] [CrossRef]
- World Meteorological Association. WMO Statement on the State of the Global Climate in 2019; World Meteorological Organization (WMO): Geneva, Switzerland, 2020. [Google Scholar]
- Wang, X.; Stewart, M.G.; Nguyen, M. Impact of climate change on corrosion and damage to concrete infrastructure in Australia. Clim. Chang. 2012, 110, 941–957. [Google Scholar] [CrossRef]
- Hooton, R.D. Canadian use of ground granulated blast-furnace slag as a supplementary cementing material for enhanced performance of concrete. Can. J. Civ. Eng. 2000, 27, 754–760. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Q.; Chen, Q.; Wang, D.; Guo, H.; Liu, L.; Yang, Q. Hydration and strength development in blended cement with ultrafine granulated copper slag. PLoS ONE 2019, 14, e0215677. [Google Scholar] [CrossRef]
- Han, J.G.; Cho, J.W.; Kim, S.W.; Park, Y.S.; Lee, J.Y. Characteristics of CO2 and energy-saving concrete with porous feldspar. Materials 2020, 13, 4204. [Google Scholar] [CrossRef]
- Anwar, A.; Mohammed, B.S.; Wahab, M.A.; Liew, M.S. Enhanced properties of cementitious composite tailored with grapheme oxide nanomaterial—A review. Dev. Built Environ. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Zhou, Q. Regulation of GO on cement hydration crystals and its toughening effect. Mag. Concr. Res. 2013, 65, 1246–1254. [Google Scholar] [CrossRef]
- Lv, S.; Ting, S.; Liu, J.; Zhou, Q. Use of graphene oxide nanosheets to regulate the microstructure of hardened cement paste to increase its strength and toughness. CrystEngComm 2014, 16, 8508–8516. [Google Scholar] [CrossRef]
- Arefi, A.; Saghravani, S.F.; Naeeni, R.M. Mechanical behavior of concrete, made with micro-nano air bubbles. Civ. Eng. Infrastruct. J. 2016, 49, 139–147. [Google Scholar]
- Khoshroo, M.; Javid, A.A.S.; Katebi, A. Effects of micro-nano bubble water and binary mineral admixtures on the mechanical and durability properties of concrete. Constr. Build. Mater. 2018, 164, 371–385. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, Y.; Bae, S.; Kim, S.Y.; Han, J.G. Compressive strength evaluation of ordinary Portland cement mortar blended with hydrogen nano-bubble water and grapheme. J. Nanosci. Nanotechnol. 2020, 20, 647–652. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Coll. Surf. A Phys. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice. PLoS ONE 2013, 8, e65339. [Google Scholar] [CrossRef]
- Ushida, A.; Hasegawa, T.; Takahashi, N.; Nakajima, T.; Murao, S.; Narumi, T.; Uchiyama, H. Effect of mixed nanobubble and microbubble liquids on the washing rate of cloth in an alternating flow. J. Surfactants Deterg. 2012, 15, 695–702. [Google Scholar] [CrossRef]
- Kim, D.; Han, J. Remediation of copper contaminated soils using water containing hydrogen nanobubbles. Appl. Sci. 2020, 10, 2185. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. Nanobubble column flotation of fine coal particles and associated fundamentals. Int. J. Miner. Process. 2013, 124, 109–116. [Google Scholar] [CrossRef]
- Kim, M.; Song, S.; Kim, W.; Han, J.G. An experimental study on bubble collapsing effect of nanobubble using ultrasonic wave. J. Nanosci. Nanotechnol. 2020, 20, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Solano-Altamirano, J.M.; Ledez, K.M. 2-Driving Force of Gas-Bubble Growth and Dissolution, Gas Bubble Dynamics in the Hyman Body; Academic Press: Cambridge, MA, USA, 2018; pp. 49–62. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Kukizaki, M.; Goto, M. Size control of nanobubbles generated from Shirasu-porous-glass (SPG) membranes. J. Membr. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Chung Ang University. Apparatus for high Concentratining nanobubbles and highly Concentratining method of nanobubbles. Korea Patent No. 10-173241(4), 15 June 2017.
- ASTM C109/C109M-20b. Standard Test. Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM C1437-20 Standard Test. Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous dispersions of nanobubbles: Generation, properties and features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]
- Ohgaki, K.; Khanh, N.Q.; Joden, Y.; Tsuji, A.; Nakagawa, T. Physicochemical approach to nanobubble solutions. Chem. Eng. Sci. 2010, 65, 1296–1300. [Google Scholar] [CrossRef]
- Kosmatka, S.H.; Kerkhoff, B.; Panarese, W.C. Design and Control of Concrete Mixtures, 14th ed.; Portland Cement Association: Skokie, IL, USA, 2003; p. 8. [Google Scholar]
- Gong, K.; Pan, Z.; Korayem, A.H.; Qiu, L.; Li, D.; Collins, F.; Wang, C.M.; Duan, W. Reinforcing effects of graphene oxide on Portland cement paste. J. Mater. Civ. Eng. 2018, 27, A4014010. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete; Prentice Hall: Englewood Cliffs, NJ, USA, 1981. [Google Scholar]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2002. [Google Scholar]
- Kim, W.K. Enhancement of Durability and Watertightness of Cement Mixture Using Highly Concentrated Hydrogen Nano-Bubble Water. Master Thesis, Chung-Ang University, Seoul, Korea, 2021. [Google Scholar]


| Mixtures | Mixing Ratio (%) | EXP-GW | EXP-H(40) | EXP-H(80) | Curing Time (days) | Test Method * |
|---|---|---|---|---|---|---|
| Cement mortar | 25.4 | Ordinary Portland cement (OPC) | 3, 7, 14, 28 | ①, ② | ||
| 62.2 | Sand | |||||
| 12.4 | General water | HNBW(40) a | HNBW(80) b | |||
| Cement paste | 67.2 | Ordinary Portland cement (OPC) | 7 | ③, ④ | ||
| 32.8 | General water | HNBW(40) | HNBW(80) | |||
| Properties | HNBW(0) | HNBW(40) | HNBW(80) |
|---|---|---|---|
| Osmosis duration (min) | - | 40 | 80 |
| Total concentration (particles/mL) | 131.12 × 106 | 150.29 × 106 | 275.94 × 106 |
| Mean diameter (nm) | 276 | 181 | 182 |
| Mode diameter (nm) | 185 | 158 | 156 |
| Case | Porosity (%) | Average Pore Diameter (nm) | Bulk Density (g/cm3) |
|---|---|---|---|
| EXP-GW | 41.32 | 43.19 | 1.32 |
| EXP-H(40) | 36.94 | 38.50 | 1.42 |
| EXP-H(80) | 31.06 | 29.93 | 1.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-K.; Kim, Y.-H.; Hong, G.; Kim, J.-M.; Han, J.-G.; Lee, J.-Y. Effect of Hydrogen Nanobubbles on the Mechanical Strength and Watertightness of Cement Mixtures. Materials 2021, 14, 1823. https://doi.org/10.3390/ma14081823
Kim W-K, Kim Y-H, Hong G, Kim J-M, Han J-G, Lee J-Y. Effect of Hydrogen Nanobubbles on the Mechanical Strength and Watertightness of Cement Mixtures. Materials. 2021; 14(8):1823. https://doi.org/10.3390/ma14081823
Chicago/Turabian StyleKim, Won-Kyung, Young-Ho Kim, Gigwon Hong, Jong-Min Kim, Jung-Geun Han, and Jong-Young Lee. 2021. "Effect of Hydrogen Nanobubbles on the Mechanical Strength and Watertightness of Cement Mixtures" Materials 14, no. 8: 1823. https://doi.org/10.3390/ma14081823
APA StyleKim, W.-K., Kim, Y.-H., Hong, G., Kim, J.-M., Han, J.-G., & Lee, J.-Y. (2021). Effect of Hydrogen Nanobubbles on the Mechanical Strength and Watertightness of Cement Mixtures. Materials, 14(8), 1823. https://doi.org/10.3390/ma14081823







