Influence of Aging on Corrosion Behaviour of the 6061 Cast Aluminium Alloy
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Heat-Treatment Procedures and Hardness Testing
2.3. Corrosion Testing
2.3.1. Electrochemical Corrosion Testing
2.3.2. In Situ Observation by Confocal Laser Scanning Microscopy
2.4. Transmission Electron Microscopy (TEM) Measurements
2.5. Scanning Kelvin Probe Force Microscopy (SKPFM) Measurements
2.5.1. Sample Preparation
2.5.2. Testing Parameters
2.6. Electron Probe Microanalysis (EPMA)
3. Results
3.1. Electrochemical Behaviour in 3.5% NaCl Solution
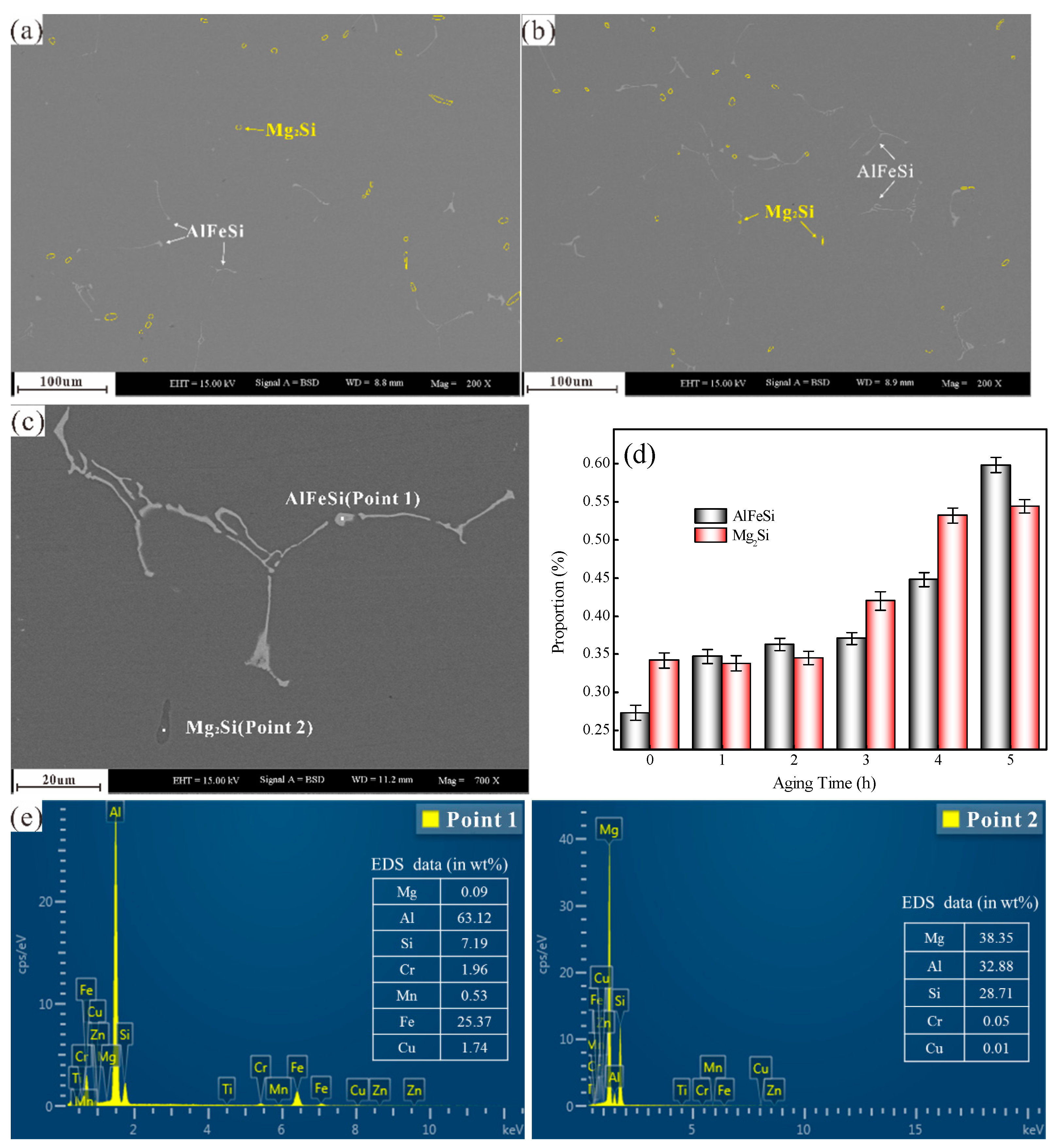
3.2. Mg2Si Phase Characterization under Different Aged Times
3.2.1. Age-Hardening Behaviour
3.2.2. TEM Investigation
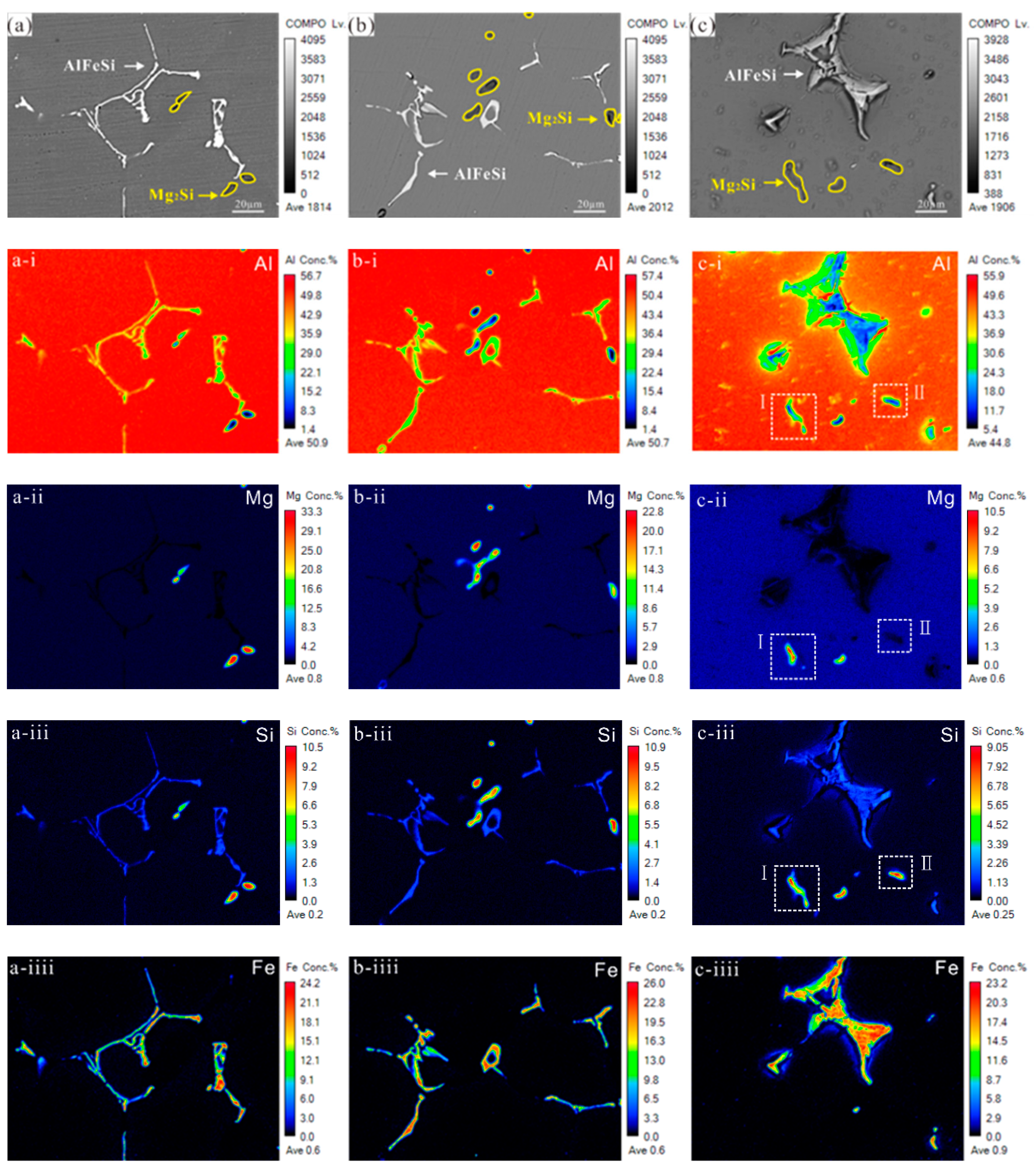
3.3. AlFeSi Phase Characterization
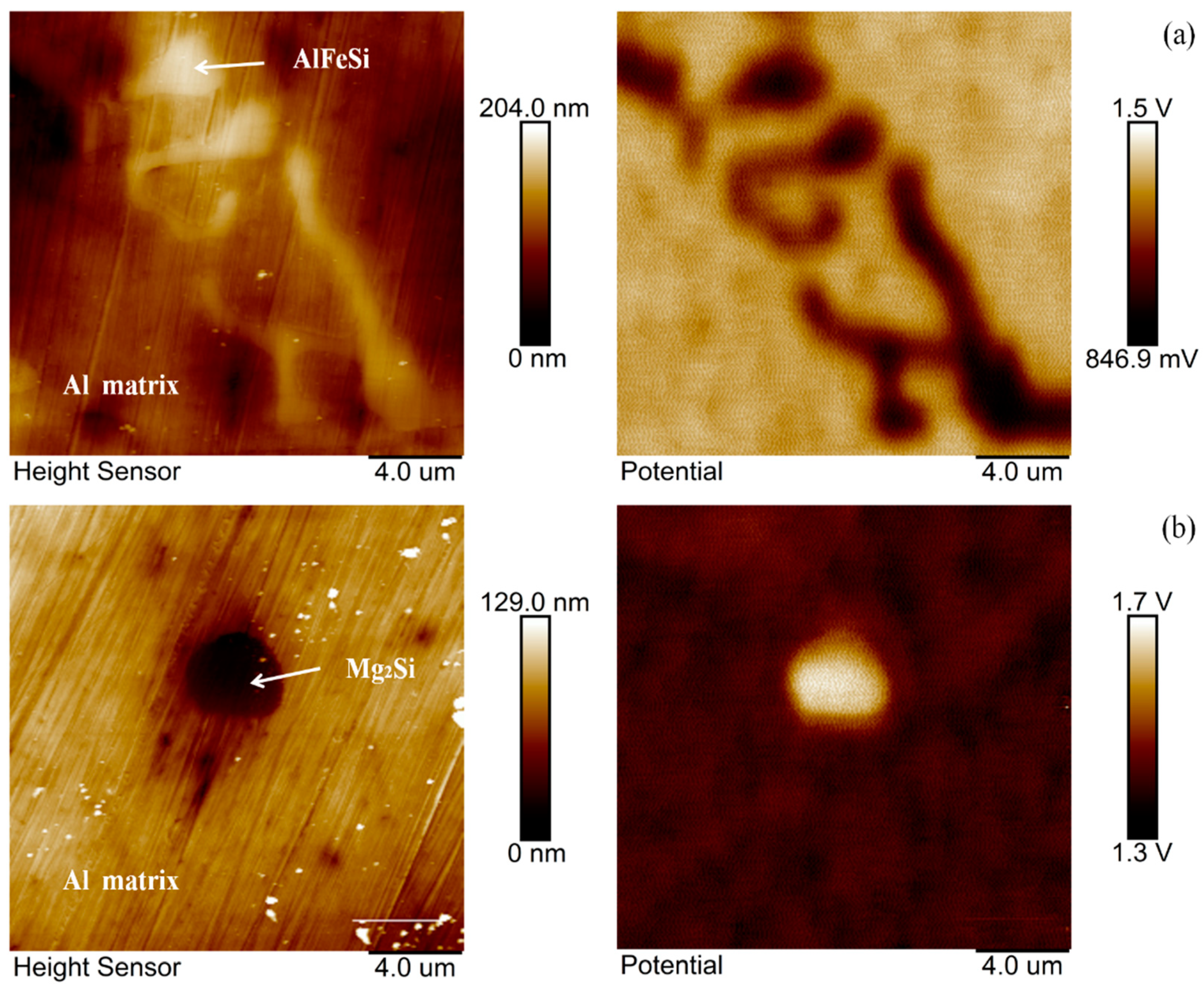
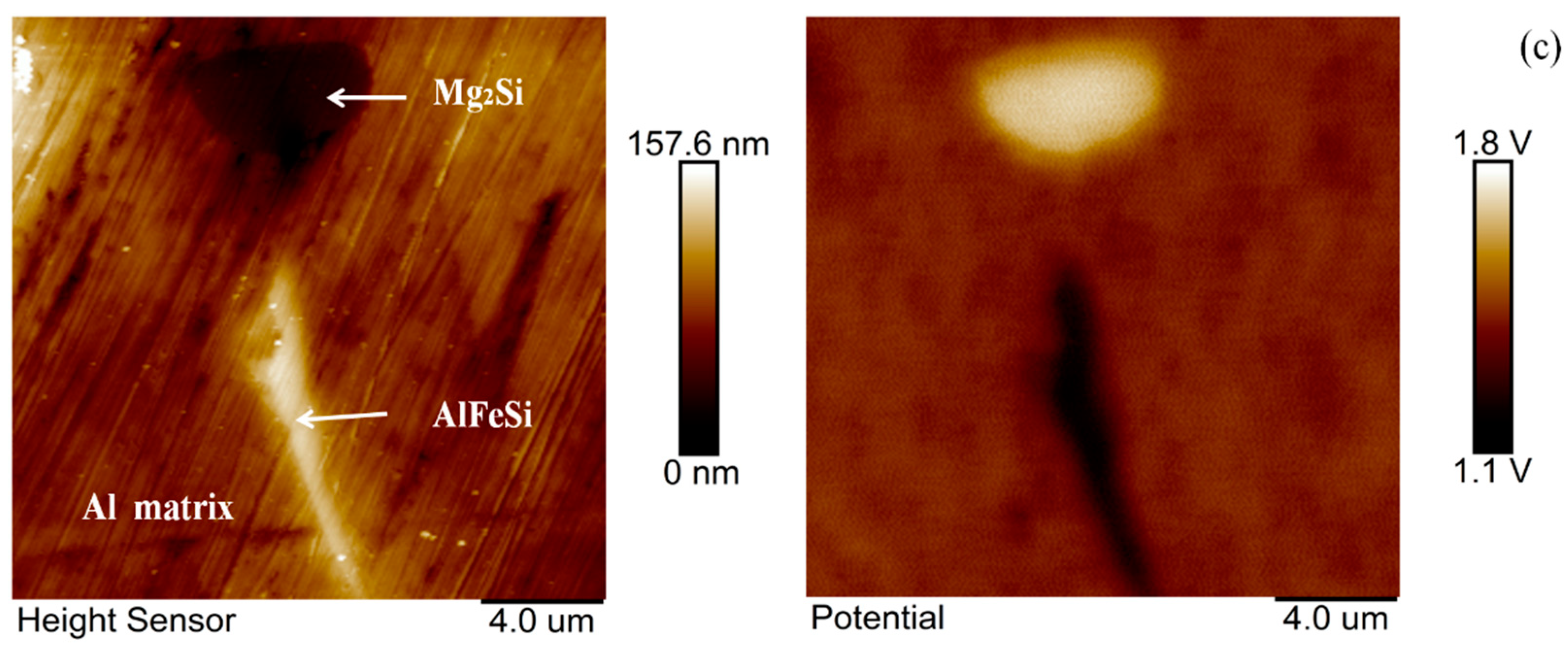
3.4. SKPFM-Measured Volta Potential Map
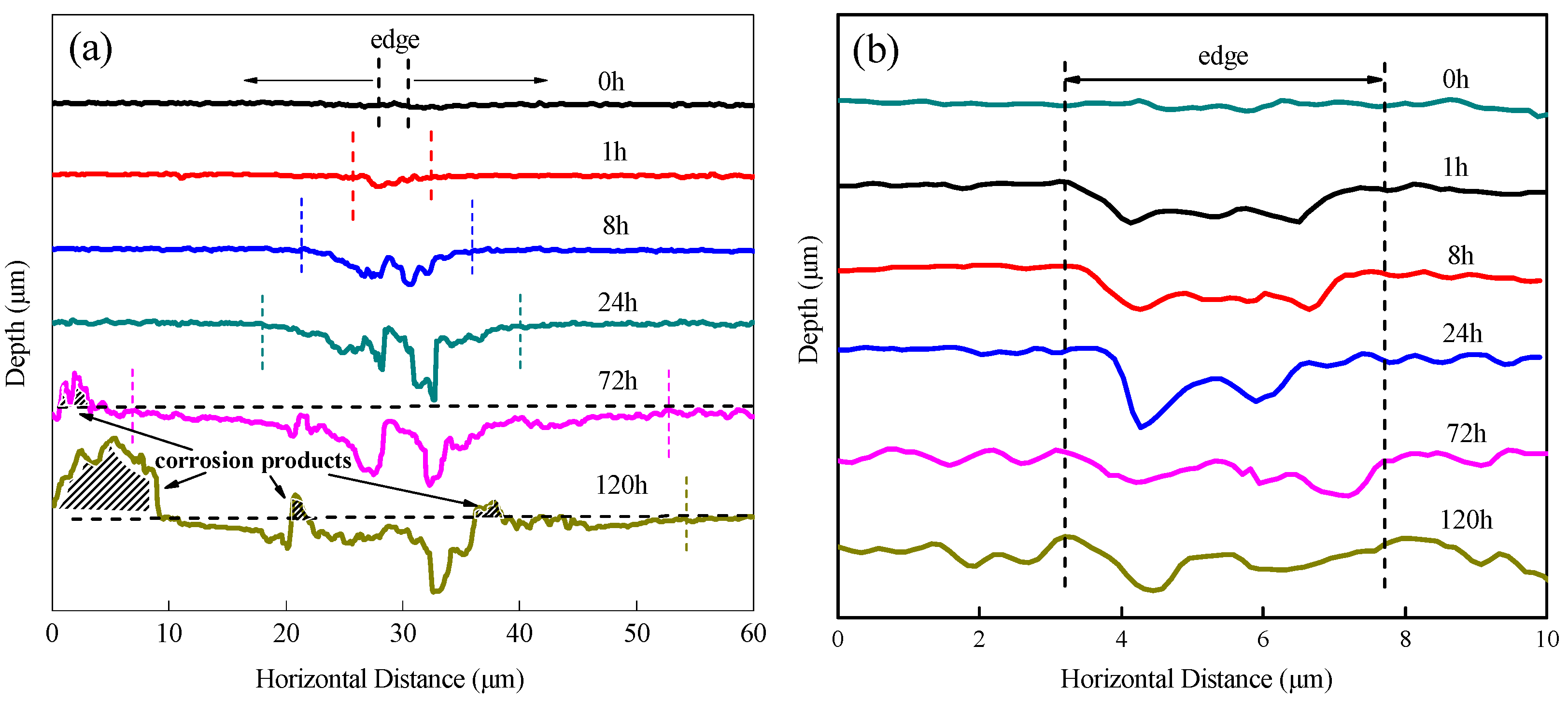
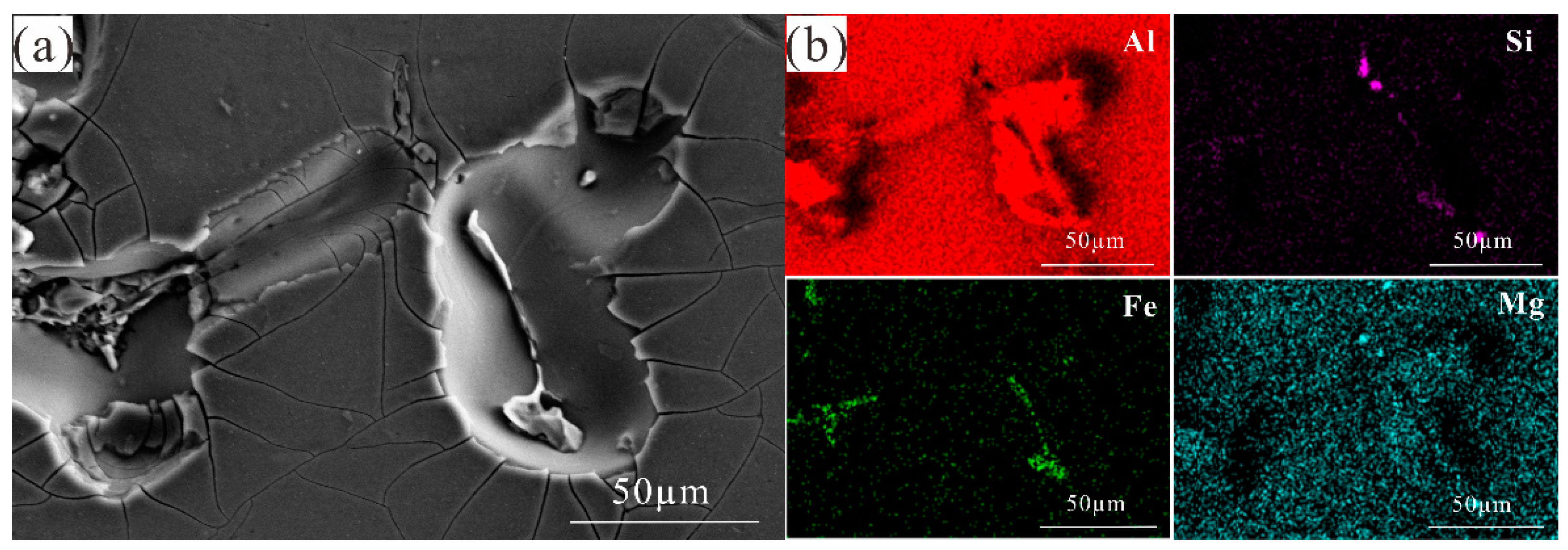
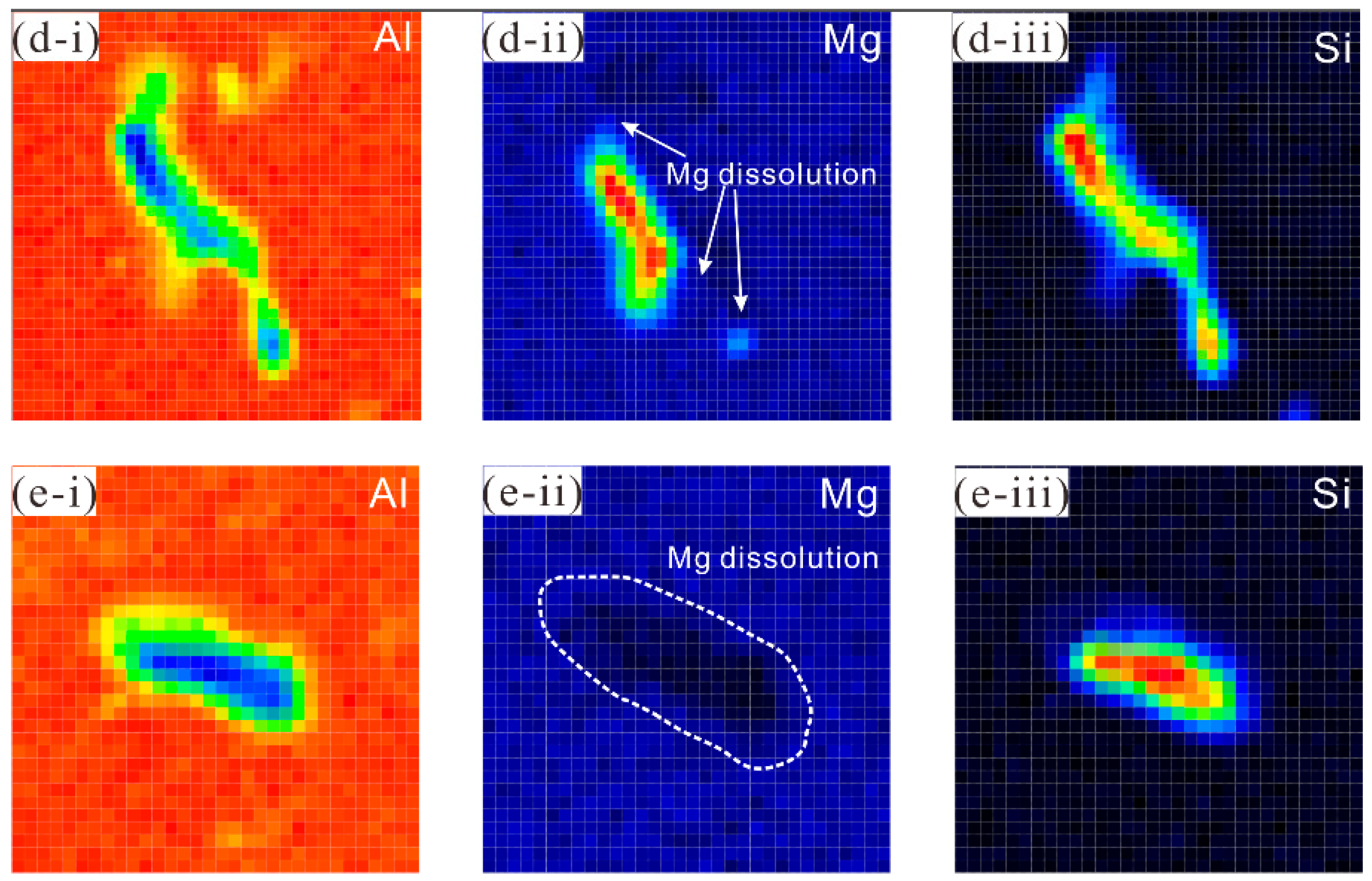
3.5. In Situ Observation by Confocal Laser Scanning Microscopy
3.6. EPMA Element Distribution Characterisation
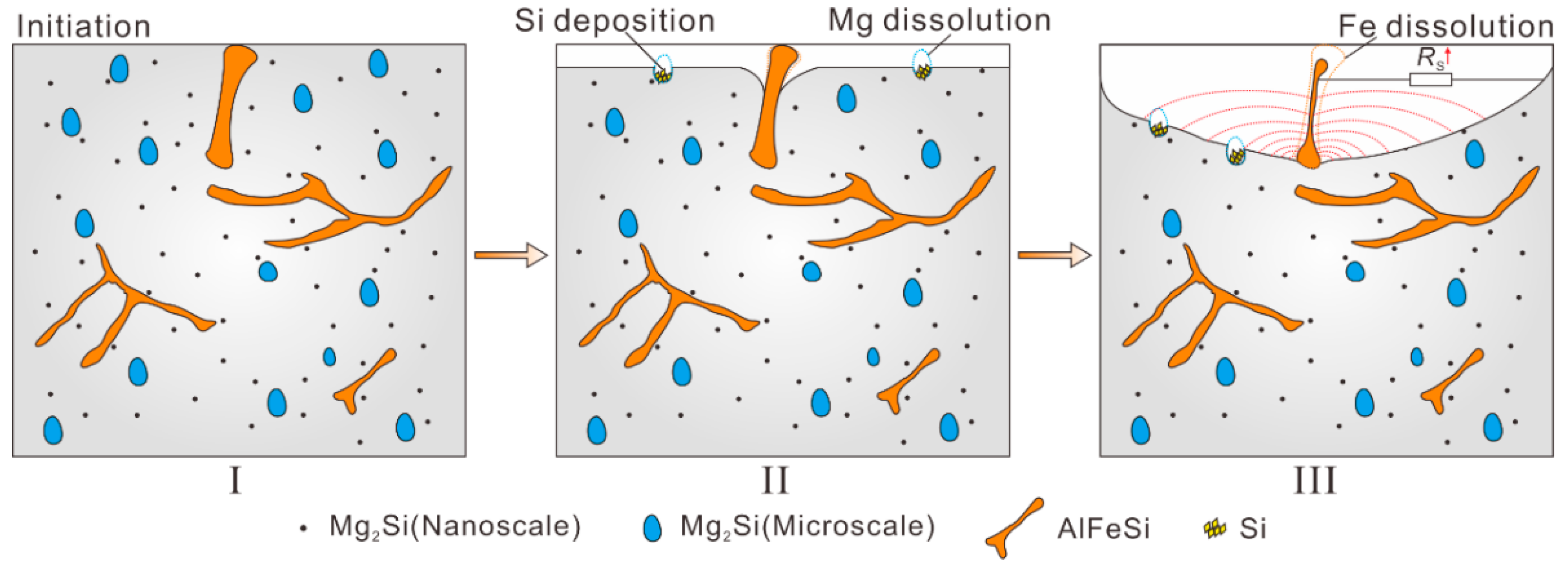
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolobnev, L.B.B.N.I.; Khokhlatova, L.B.; Ryabov, D.K. Structure, Properties and Application of Alloys of the Al–Mg–Si–(Cu) System. Met. Sci. Heat Treat. 2012, 53, 440–444. [Google Scholar] [CrossRef]
- Cui, L.; Guo, M.; Peng, X.; Zhang, Y.; Zhang, J.; Zhuang, L. Influence of Pre-deformation on the Precipitation Behaviors of Al-Mg-Si-Cu Alloy for Automotive Application. Acta Metall. Sin. 2015, 51, 289–297. [Google Scholar]
- Lai, Y.; Fan, W.; Yin, M.; Wu, C.; Chen, J. Structures and formation mechanisms of dislocation-induced precipitates in relation to the age-hardening responses of Al-Mg-Si alloys. J. Mater. Sci. Technol. 2020, 41, 127–138. [Google Scholar] [CrossRef]
- Zhong, H.; Rometsch, P.; Estrin, Y. Effect of alloy composition and heat treatment on mechanical performance of 6xxx aluminum alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 2174–2178. [Google Scholar] [CrossRef]
- Zander, B.D.; Schnatterer, C.; Altenbach, C.; Chaineux, V. Microstructural impact on intergranular corrosion and the mechanical properties of industrial drawn 6056 aluminum wires. Mater. Des. 2015, 83, 49–59. [Google Scholar] [CrossRef]
- Blanc, C.; Mankowski, G. Susceptibility to pitting corrosion of 6056 aluminium alloy. Corros. Sci. 1997, 39, 949–959. [Google Scholar] [CrossRef]
- El-Menshawy, K.; El-Sayed, A.-W.A.; El-Bedawy, M.E.; Ahmed, H.A.; El-Raghy, S.M. Effect of aging time at low aging temperatures on the corrosion of aluminum alloy 6061. Corros. Sci. 2012, 54, 167–173. [Google Scholar] [CrossRef]
- van Huis, M.; Chen, J.; Zandbergen, H.; Sluiter, M. Phase stability and structural relations of nanometer-sized, matrix-embedded precipitate phases in Al–Mg–Si alloys in the late stages of evolution. Acta Mater. 2006, 54, 2945–2955. [Google Scholar] [CrossRef]
- Chen, H.; Lu, J.; Kong, Y.; Li, K.; Yang, T.; Meingast, A.; Yang, M.; Lu, Q.; Du, Y. Atomic scale investigation of the crystal structure and interfaces of the B′ precipitate in Al-Mg-Si alloys. Acta Mater. 2020, 185, 193–203. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al–Mg–Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Zeng, F.-L.; Wei, Z.-L.; Li, J.-F.; Li, C.-X.; Tan, X.; Zhang, Z.; Zheng, Z.-Q. Corrosion mechanism associated with Mg2Si and Si particles in Al–Mg–Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Kairy, S.; Rometsch, P.; Davies, C.; Birbilis, N. On the Intergranular Corrosion and Hardness Evolution of 6xxx Series Al Alloys as a Function of Si:Mg Ratio, Cu Content, and Aging Condition. Corrosion 2017, 73, 1280–1295. [Google Scholar] [CrossRef]
- Zeid, E.F.A. Mechanical and electrochemical characteristics of solutionized AA 6061, AA6013 and AA 5086 aluminum alloys. J. Mater. Res. Technol. 2019, 8, 1870–1877. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Nilsson, J.-O. Corrosion behaviour of AA6082 Al-Mg-Si alloy extrusion: The influence of quench cooling rate. Corros. Sci. 2019, 150, 100–109. [Google Scholar] [CrossRef]
- Yoshida, T.M. Effect of grain boundary characteristics on intergranular corrosion resistance of 6061 aluminum alloy extrusion. Metall. Mater. Trans. A 2002, 33, 2891–2898. [Google Scholar]
- Xuehong, X.; Deng, Y.; Shuiqing, C.; Xiaobin, G. Effect of interrupted ageing treatment on the mechanical properties and intergranular corrosion behavior of Al-Mg-Si alloys. J. Mater. Res. Technol. 2020, 9, 230–241. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Miao, F.; Sun, W.; Fang, B.; Song, R.; Zheng, Z. Improving the intergranular corrosion resistance of Al–Mg–Si–Cu alloys without strength loss by a two-step aging treatment. Mater. Sci. Eng. A 2014, 590, 267–273. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, B.Z.; Yuan, Y.F.; Guo, S.Y.; Pan, J. Effect of Solution Temperature on the Corrosion Behavior of 6061-T6 Aluminum Alloy in NaCl Solution. J. Mater. Eng. Perform. 2020, 29, 4725–4732. [Google Scholar] [CrossRef]
- Ahmad, Z. Aluminium Alloys—New Trends in Fabrication and Applications. Alum. Alloys New Trends Fabr. Appl. 2012, 10. [Google Scholar] [CrossRef]
- Cheng, T.; Shi, W.; Xiang, S.; Ballingerc, R.G. Volta potential mapping of the gradient strengthened layer in 20CrMnTi by using SKPFM. J. Mater. Sci. 2020, 55, 12403–12420. [Google Scholar] [CrossRef]
- Cook, A.; Barrett, Z.; Lyon, S.; McMurray, H.; Walton, J.; Williams, G. Calibration of the scanning Kelvin probe force microscope under controlled environmental conditions. Electrochim. Acta 2012, 66, 100–105. [Google Scholar] [CrossRef]
- Liang, W.; Rometsch, P.; Cao, L.; Birbilis, N. General aspects related to the corrosion of 6xxx series aluminium alloys: Exploring the influence of Mg/Si ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Vallabhaneni, R.; Stannard, T.J.; Kaira, C.S.; Chawla, N. 3D X-ray microtomography and mechanical characterization of corrosion-induced damage in 7075 aluminium (Al) alloys. Corros. Sci. 2018, 139, 97–113. [Google Scholar] [CrossRef]
- Weng, Y.; Ding, L.; Zhang, Z.; Jia, Z.; Wen, B.; Liu, Y.; Muraishi, S.; Li, Y.; Liu, Q. Effect of Ag addition on the precipitation evolution and interfacial segregation for Al–Mg–Si alloy. Acta Mater. 2019, 180, 301–316. [Google Scholar] [CrossRef]
- Weng, Y.; Ding, L.; Jia, Z.; Liu, Q. Effect of combined addition of Ag and Cu on the precipitation behavior for an Al-Mg-Si alloy. Mater. Charact. 2021, 171, 110736. [Google Scholar] [CrossRef]
- Lu, Q.; Li, K.; Chen, H.; Yang, M.; Lan, X.; Yang, T.; Liu, S.; Song, M.; Cao, L.; Du, Y. Simultaneously enhanced strength and ductility of 6xxx Al alloys via manipulating meso-scale and nano-scale structures guided with phase equilibrium. J. Mater. Sci. Technol. 2020, 41, 139–148. [Google Scholar] [CrossRef]
- Yang, W.; Huang, L.; Zhang, R.; Wang, M.; Li, Z.; Jia, Y.; Lei, R.; Sheng, X. Electron microscopy studies of the age-hardening behaviors in 6005A alloy and microstructural characterizations of precipitates. J. Alloy. Compd. 2012, 514, 220–233. [Google Scholar] [CrossRef]
- Alvarez-Antolin, F.; Asensio-Lozano, J.; Cofiño-Villar, A.; Gonzalez-Pociño, A. Analysis of Different Solution Treatments in the Transformation of β-AlFeSi Particles into α-(FeMn)Si and Their Influence on Different Ageing Treatments in Al–Mg–Si Alloys. Metals 2020, 10, 620. [Google Scholar] [CrossRef]
- Örnek, C.; Leygraf, C.; Pan, J. On the Volta potential measured by SKPFM—fundamental and practical aspects with relevance to corrosion science. Corros. Eng. Sci. Technol. 2019, 54, 185–198. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y.; Chen, J.; Dai, Q.; Guo, X. Effects of grain structure related precipitation on corrosion behavior and corrosion fatigue property of Al–Mg–Si alloy. J. Mater. Res. Technol. 2020, 9, 5391–5402. [Google Scholar] [CrossRef]
- Bacaicoa, I.; Luetje, M.; Wicke, M.; Geisert, A.; Zeismann, F.; Fehlbier, M.; Brueckner-Foit, A. 3D Morphology of Al5FeSi inclusions in high Fe-content Al-Si-Cu Alloys. Procedia Struct. Integr. 2016, 2, 2269–2276. [Google Scholar] [CrossRef]
- Brito, C.; Vida, T.; Freitas, E.; Cheung, N.; Spinelli, J.E.; Garcia, A. Cellular/dendritic arrays and intermetallic phases affecting corrosion and mechanical resistances of an Al–Mg–Si alloy. J. Alloy. Compd. 2016, 673, 220–230. [Google Scholar] [CrossRef]
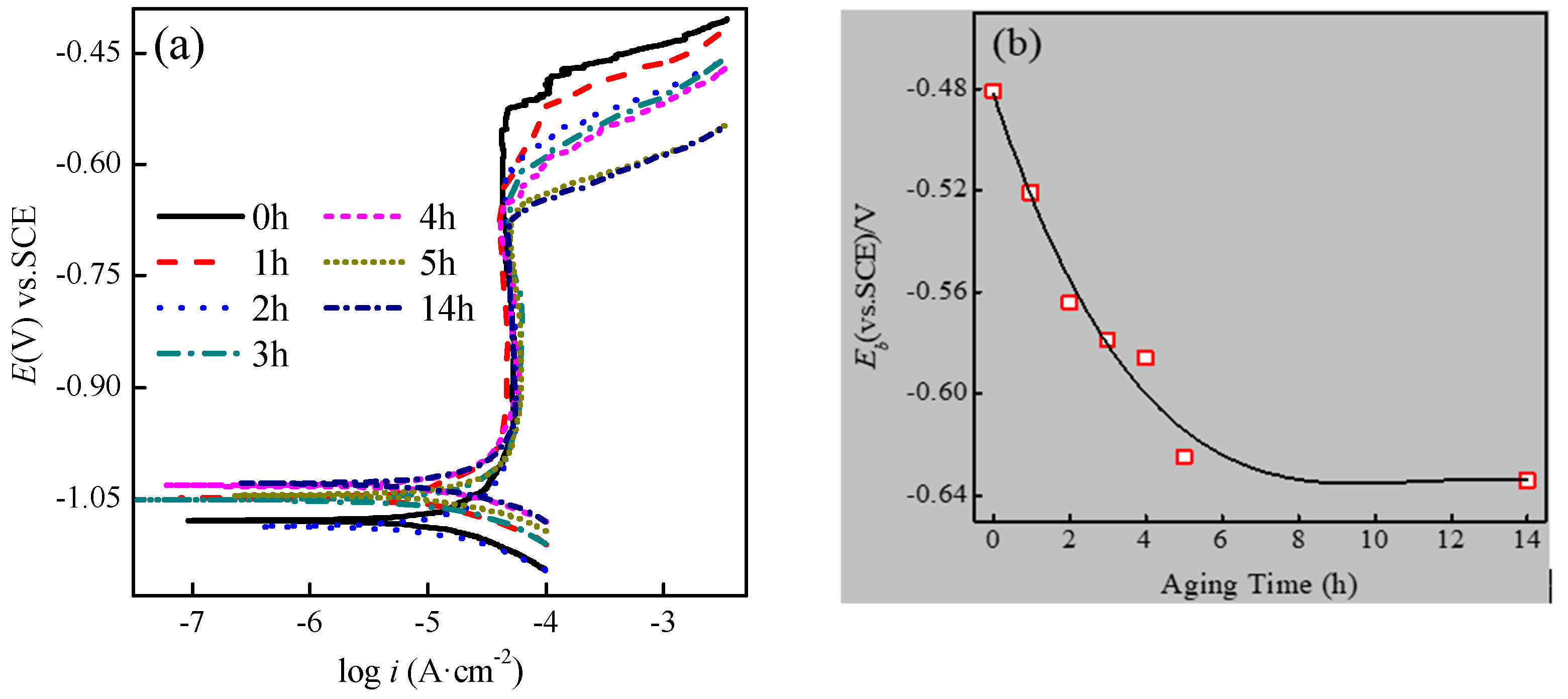
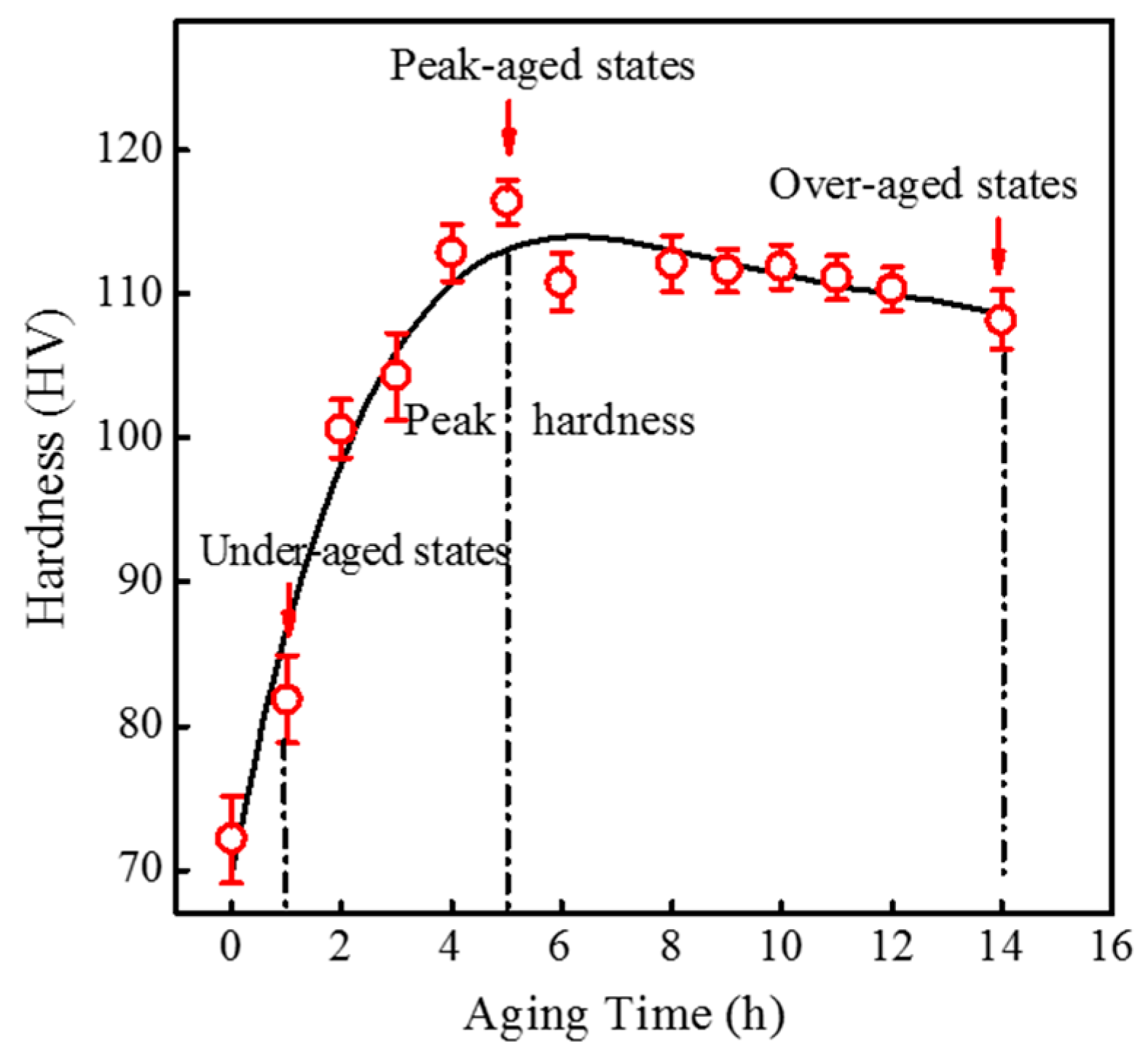
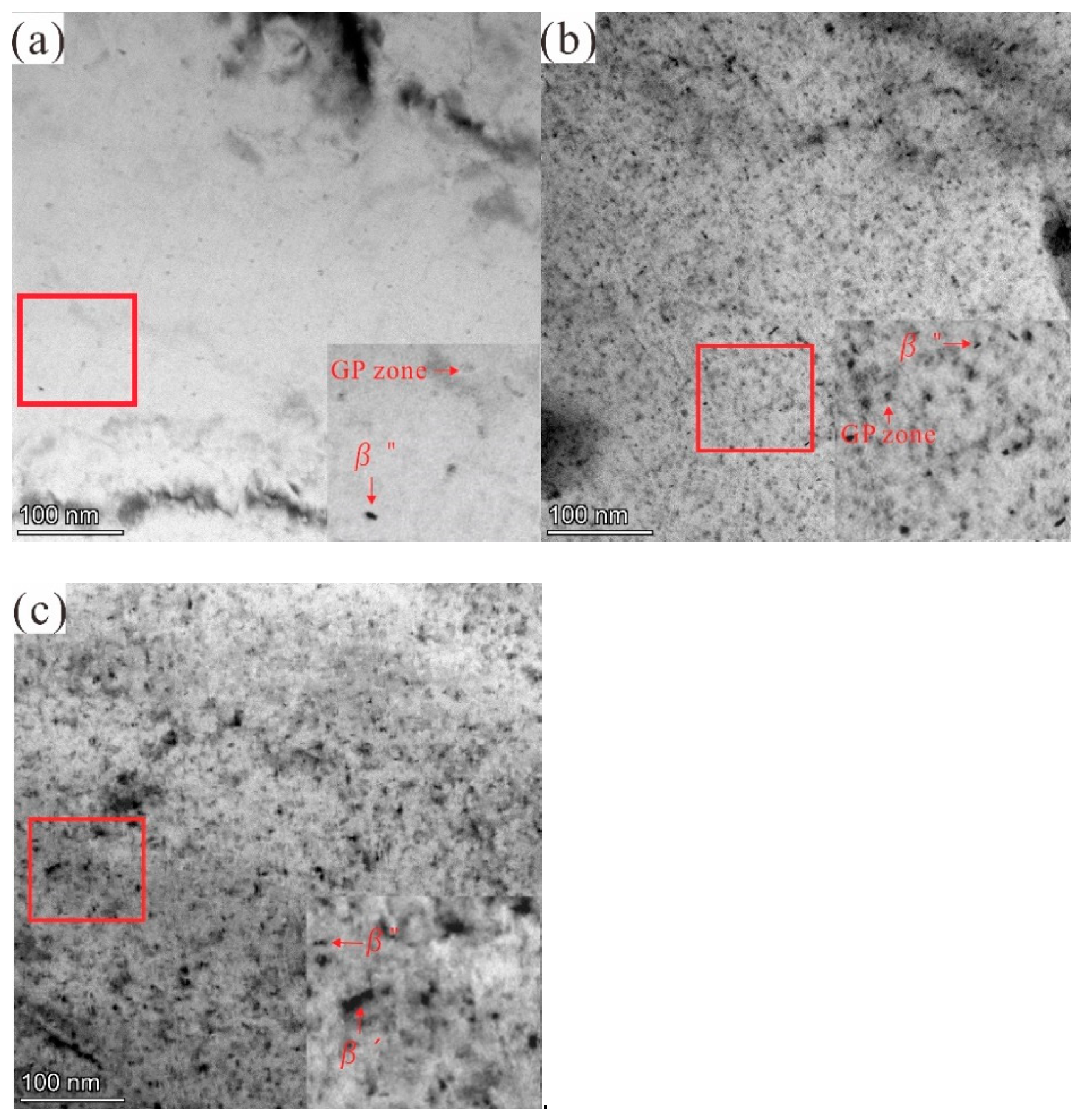
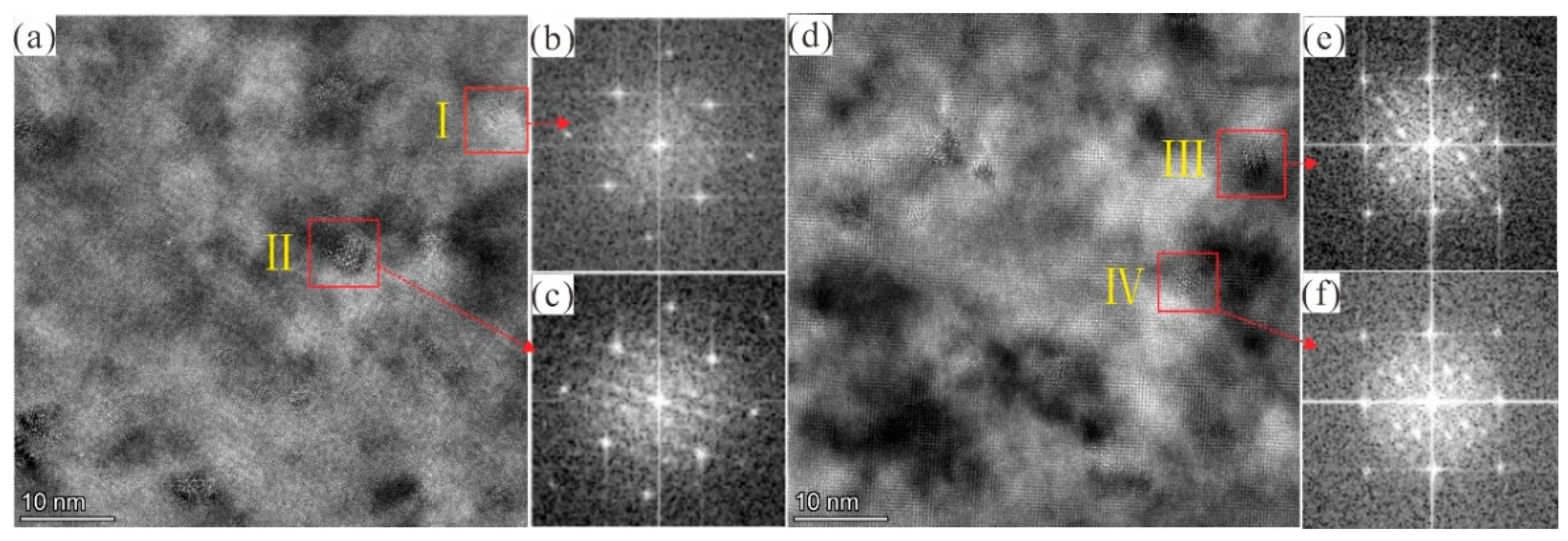

| Aging Time (h) | Ecorr (V) | icorr (μA/cm2) | βa (mV) | βc (mV) | Corrosion Rate (mm/a) |
|---|---|---|---|---|---|
| 0 | −1.079 | 24.395 | 174.241 | 78.745 | 0.744 |
| 1 | −1.049 | 24.340 | 326.920 | 84.048 | 0.743 |
| 2 | −1.087 | 28.385 | 235.262 | 92.201 | 0.866 |
| 3 | −1.051 | 29.491 | 172.450 | 90.886 | 0.900 |
| 4 | −1.032 | 31.576 | 244.220 | 76.776 | 0.963 |
| 5 | −1.045 | 34.239 | 198.951 | 83.748 | 1.045 |
| 14 | −1.029 | 34.723 | 232.833 | 84.178 | 1.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Shi, W.; Xiang, S.; Huang, C.; Ballinger, R.G. Influence of Aging on Corrosion Behaviour of the 6061 Cast Aluminium Alloy. Materials 2021, 14, 1821. https://doi.org/10.3390/ma14081821
He T, Shi W, Xiang S, Huang C, Ballinger RG. Influence of Aging on Corrosion Behaviour of the 6061 Cast Aluminium Alloy. Materials. 2021; 14(8):1821. https://doi.org/10.3390/ma14081821
Chicago/Turabian StyleHe, Ting, Wei Shi, Song Xiang, Chaowen Huang, and Ronald G. Ballinger. 2021. "Influence of Aging on Corrosion Behaviour of the 6061 Cast Aluminium Alloy" Materials 14, no. 8: 1821. https://doi.org/10.3390/ma14081821
APA StyleHe, T., Shi, W., Xiang, S., Huang, C., & Ballinger, R. G. (2021). Influence of Aging on Corrosion Behaviour of the 6061 Cast Aluminium Alloy. Materials, 14(8), 1821. https://doi.org/10.3390/ma14081821






