Thermal Stability of TiN Coated Cubic Boron Nitride Powder
Abstract
1. Introduction
2. Materials and Methods
3. Results
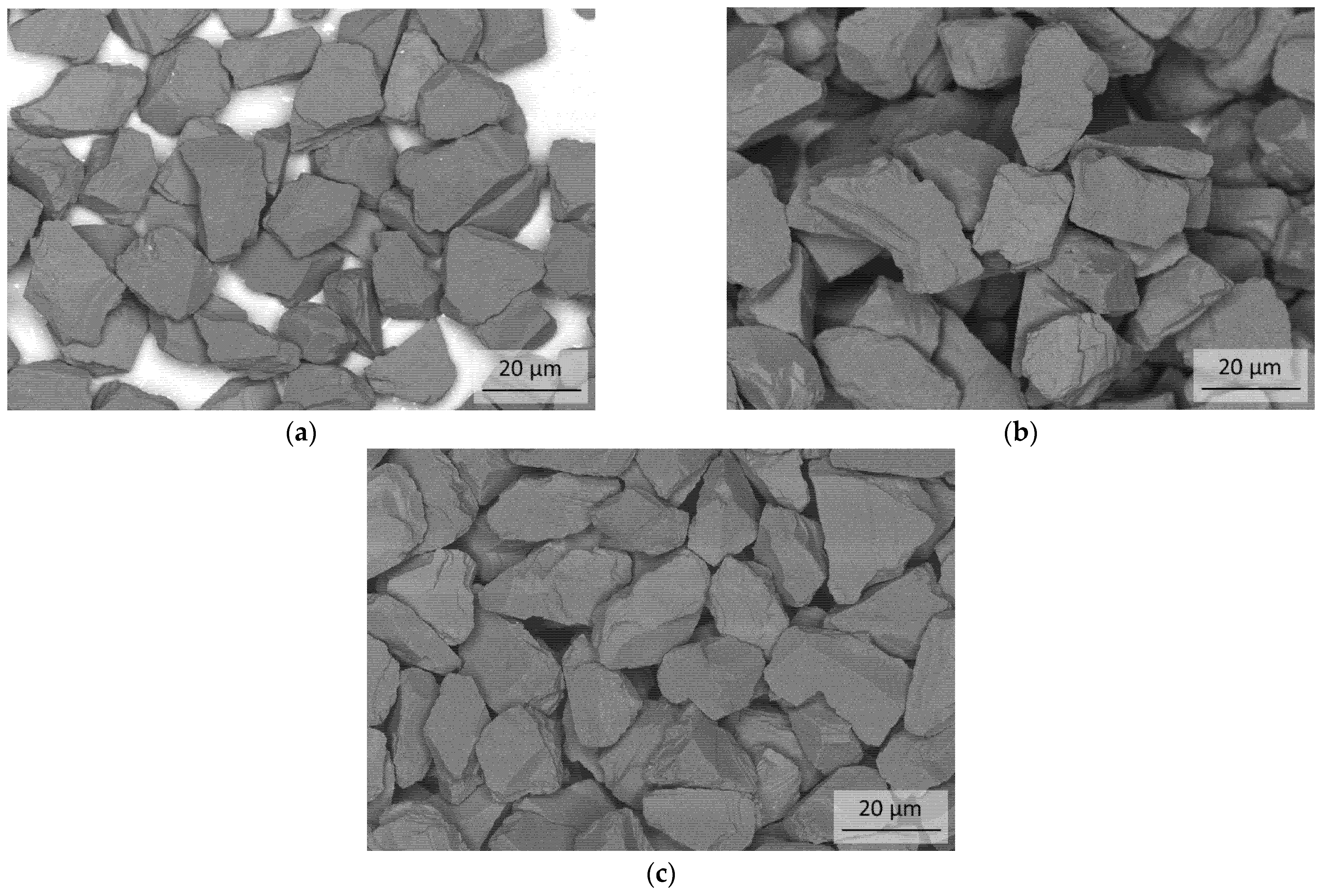
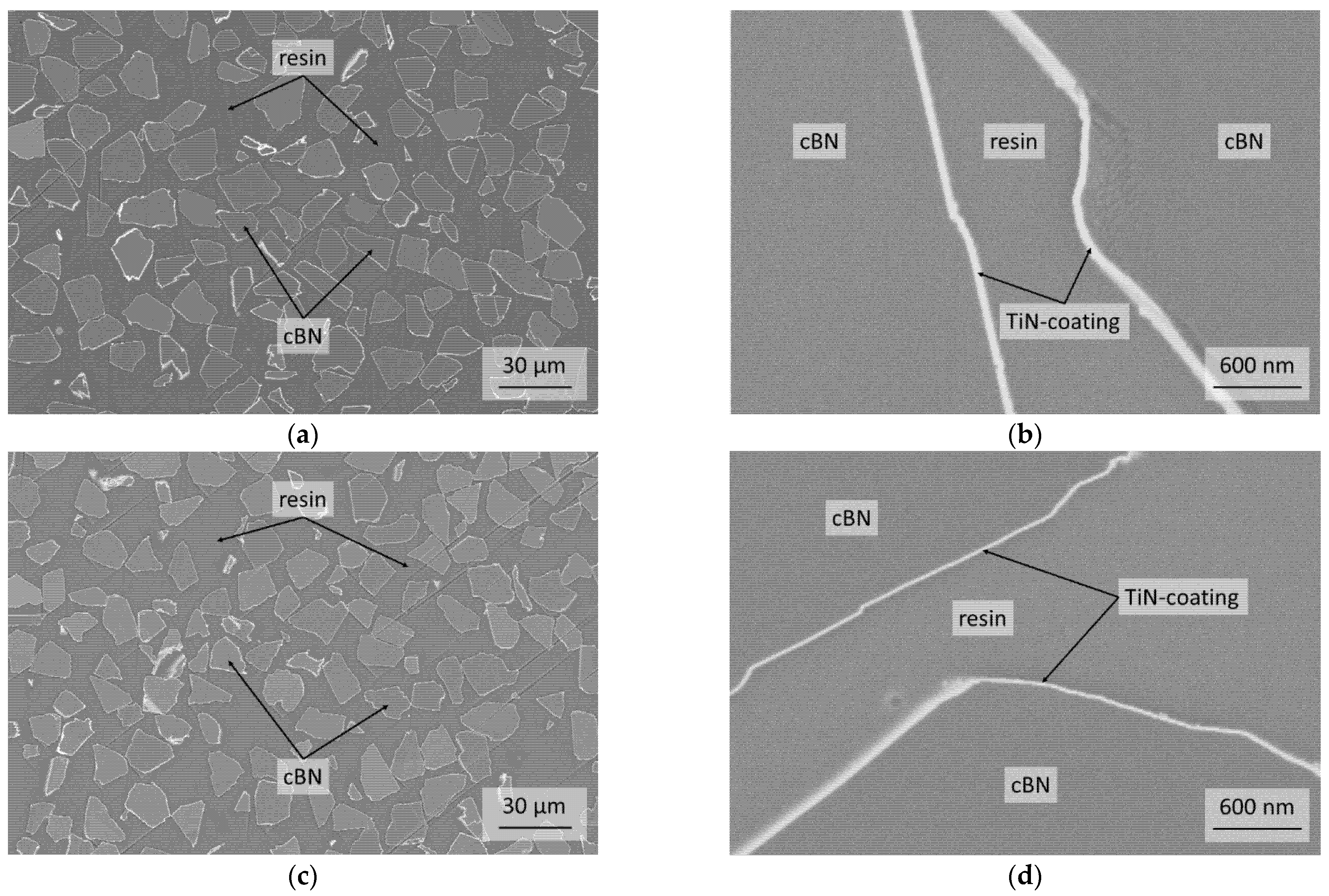
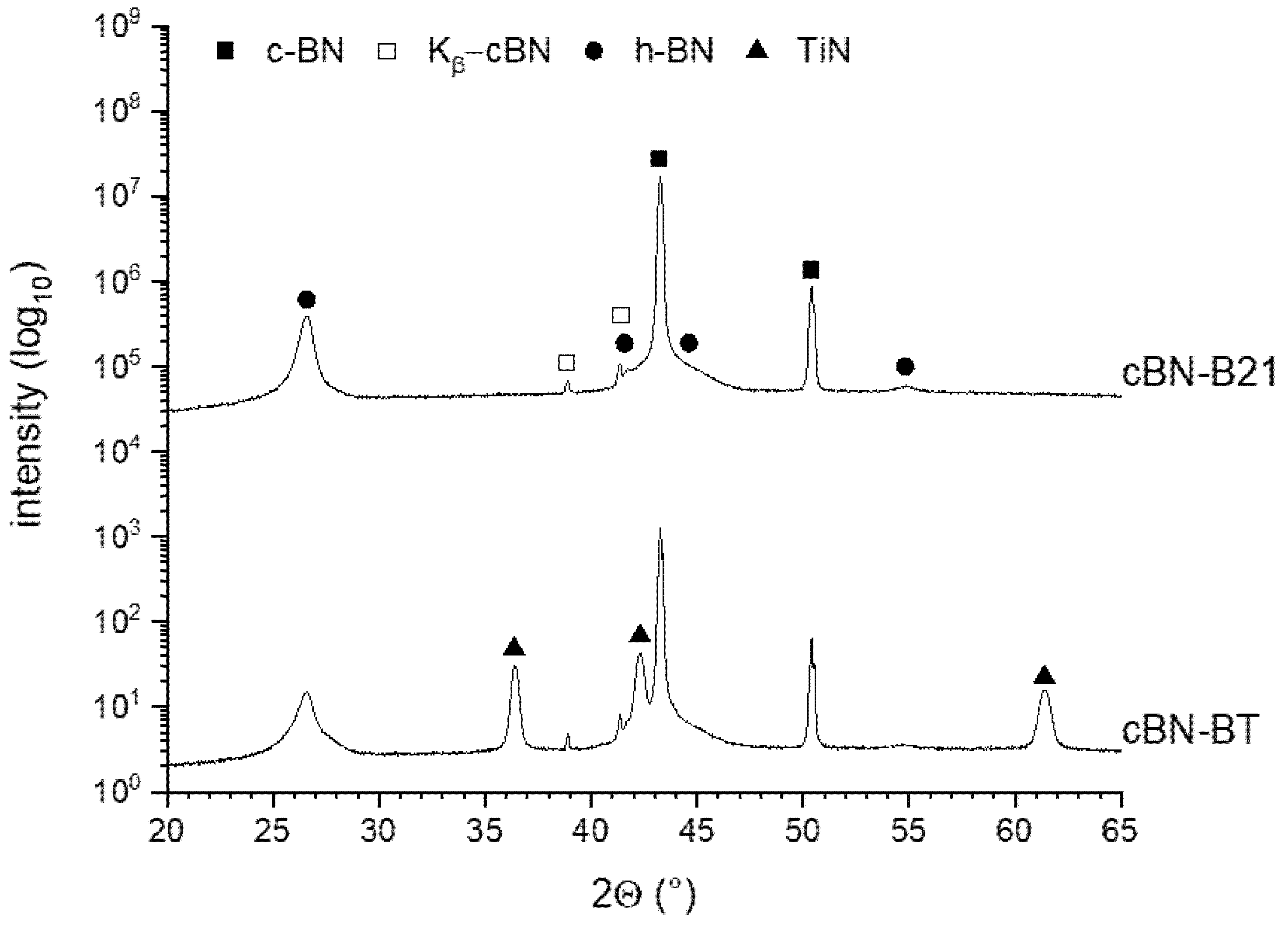
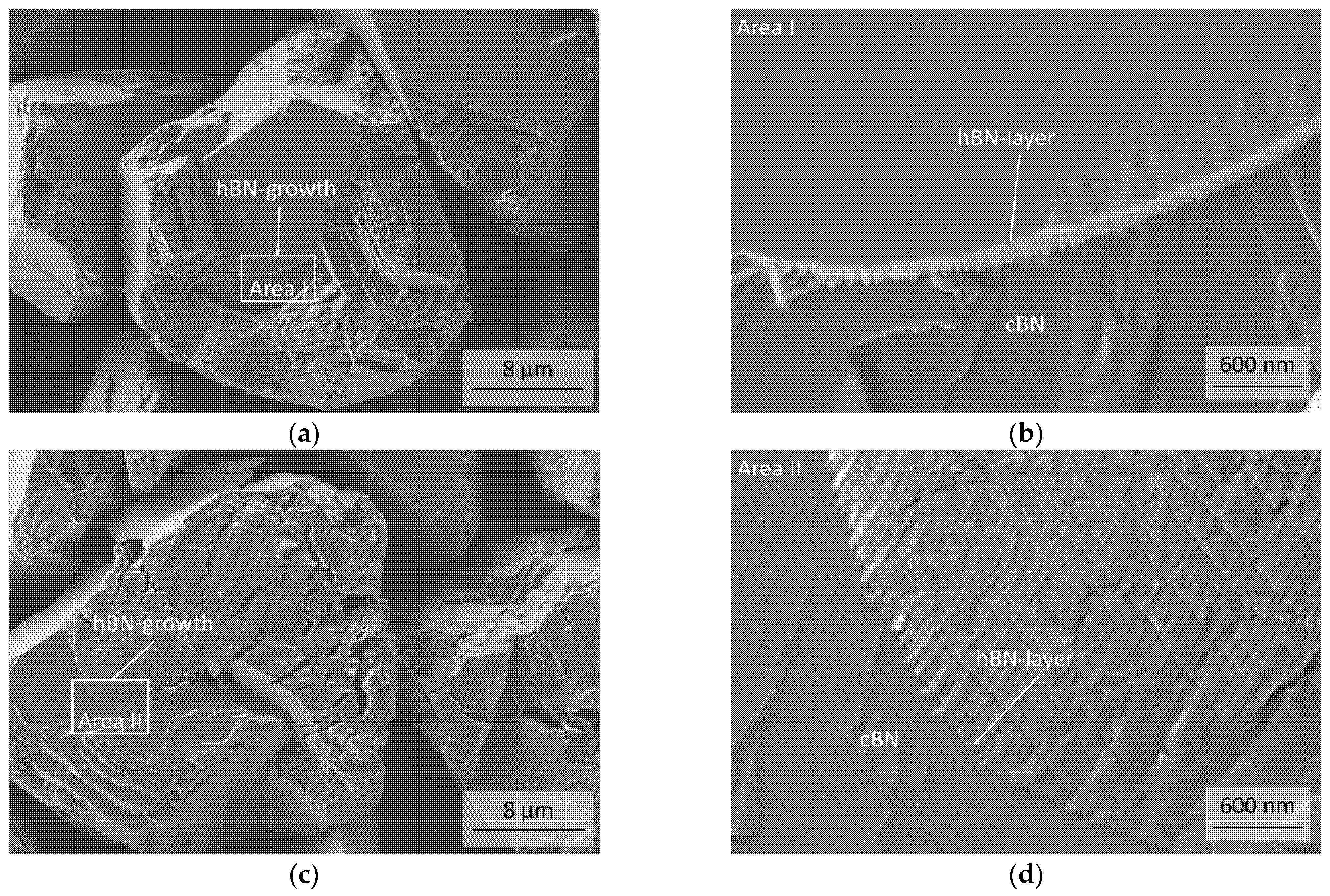
3.1. Characterization of the Initial cBN Powders
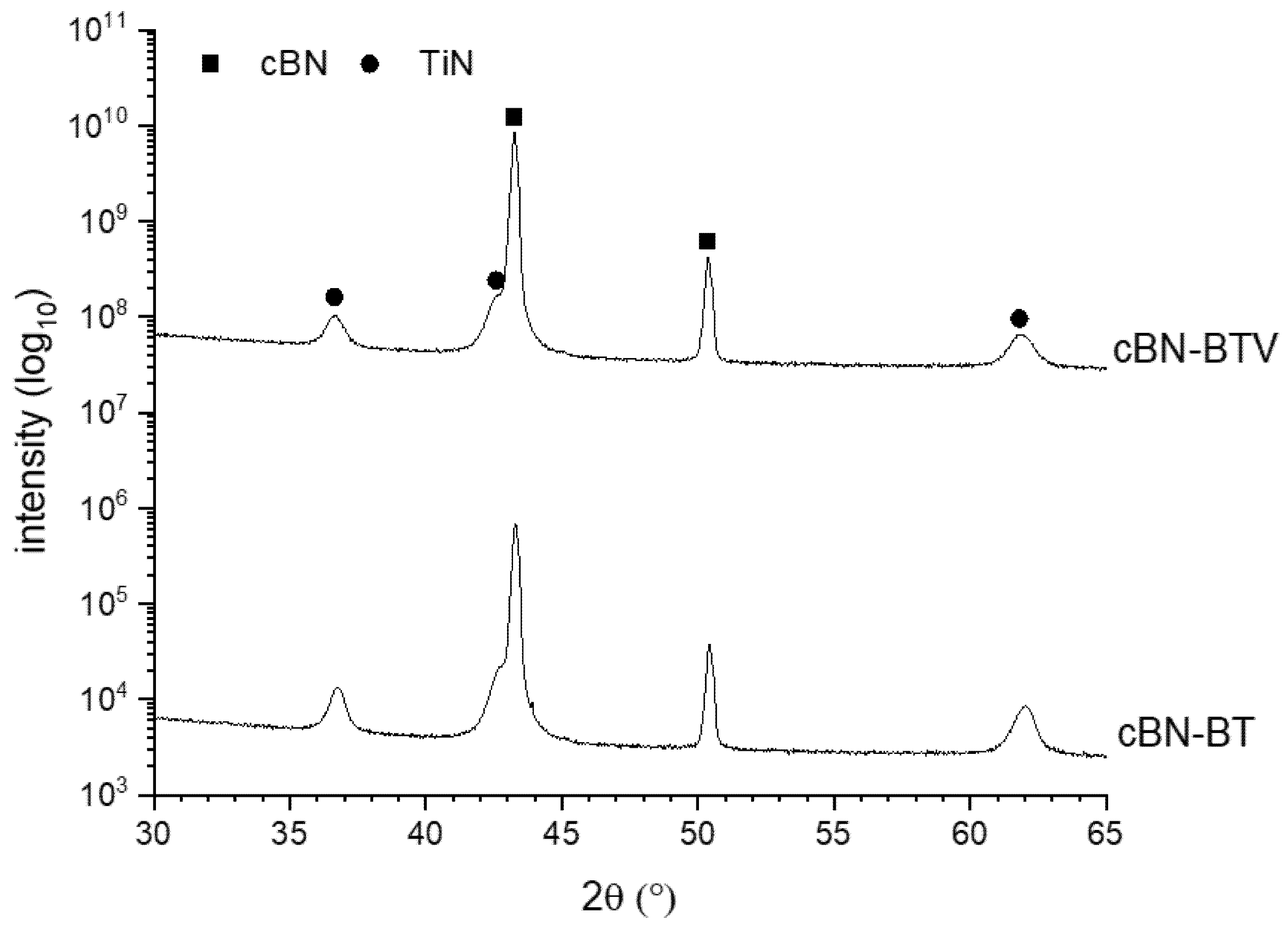
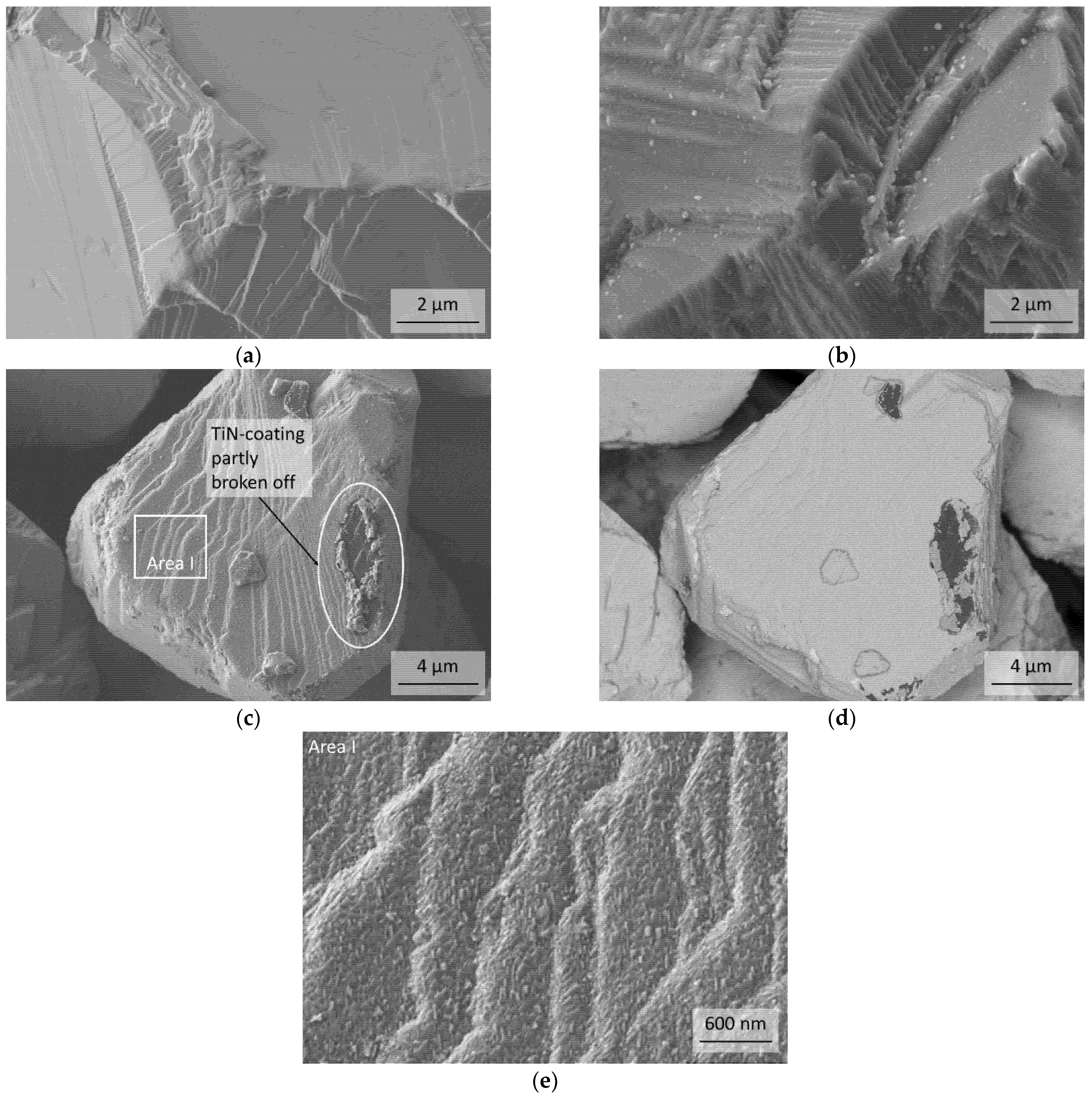
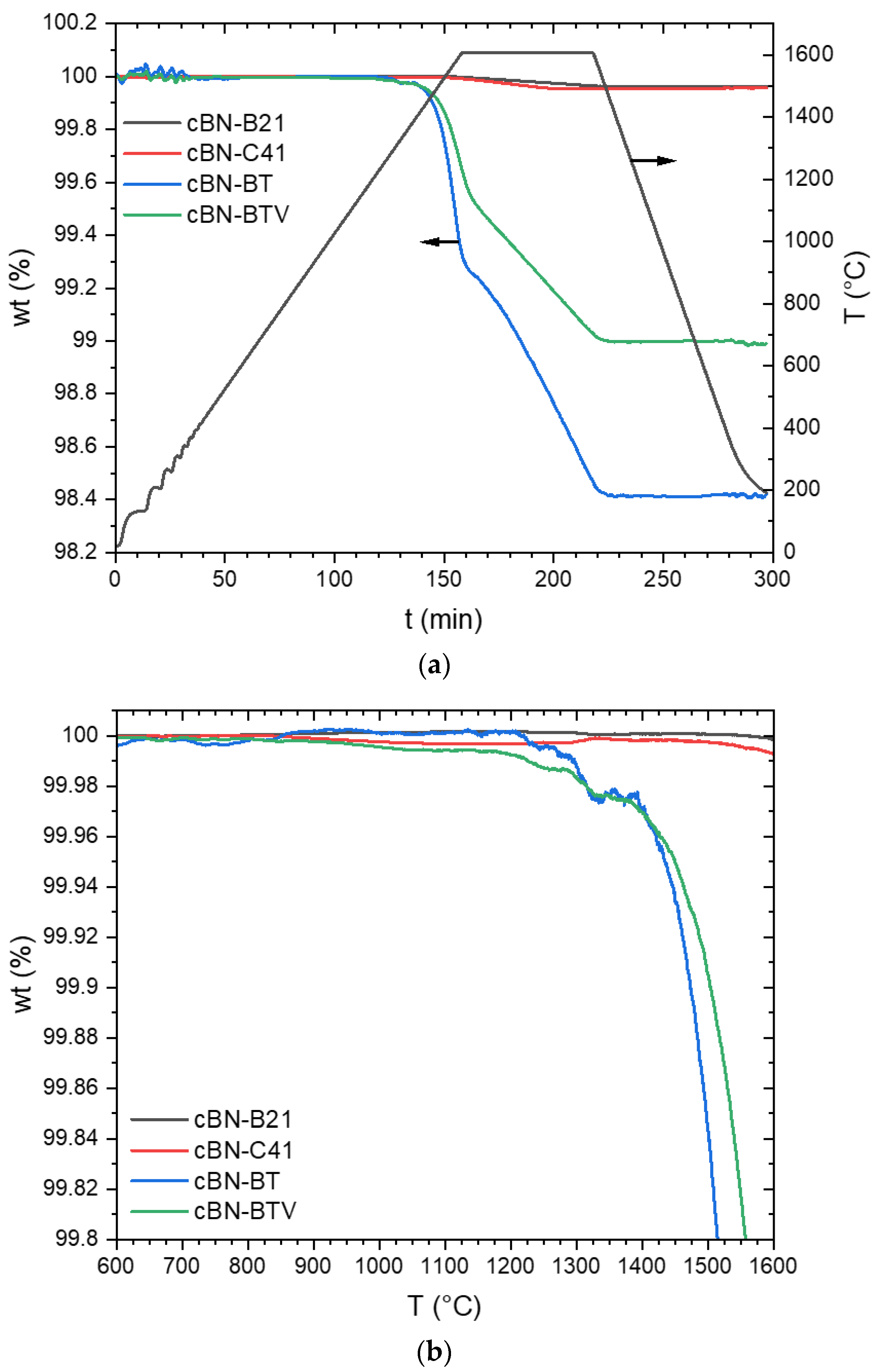
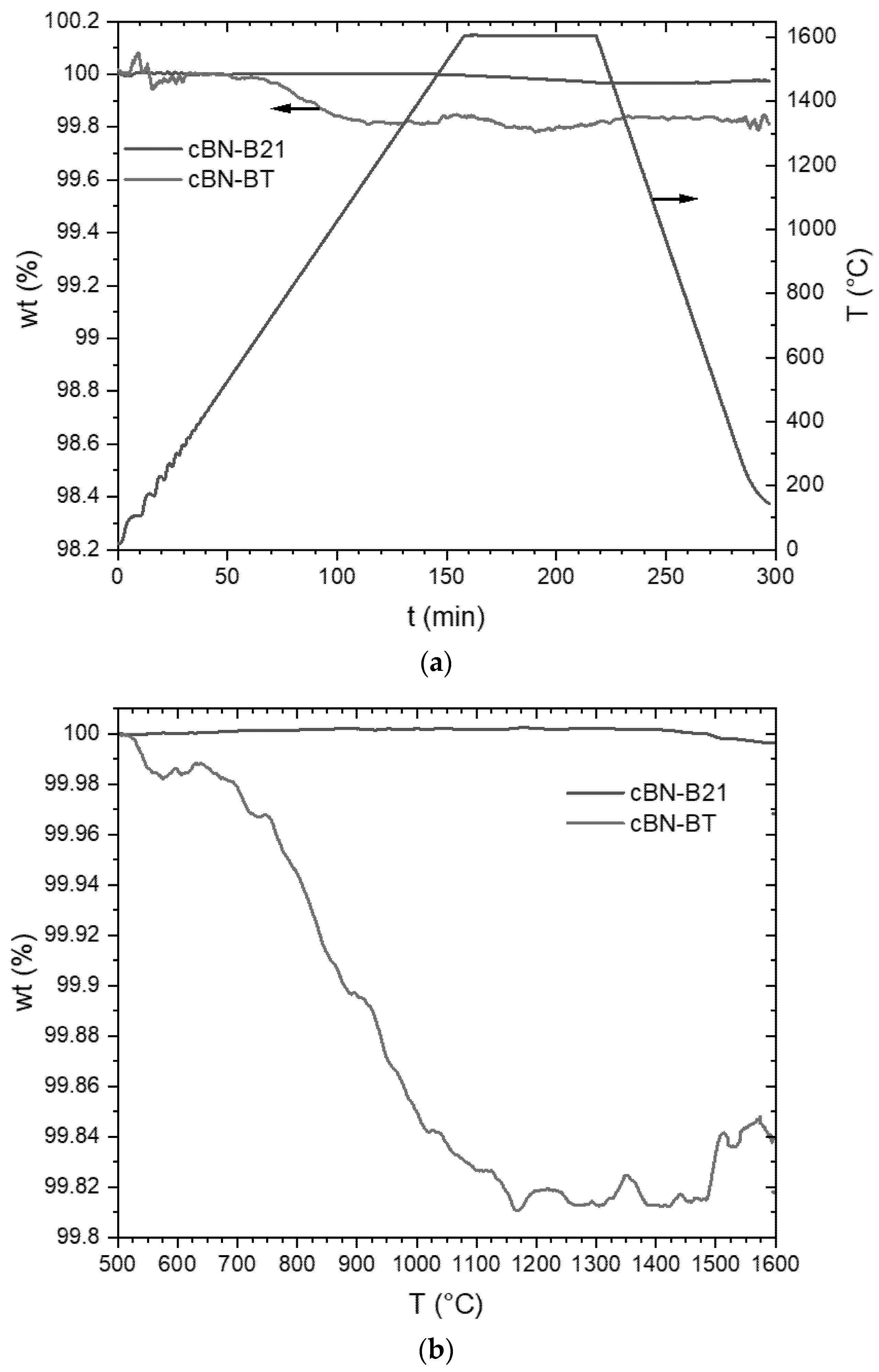
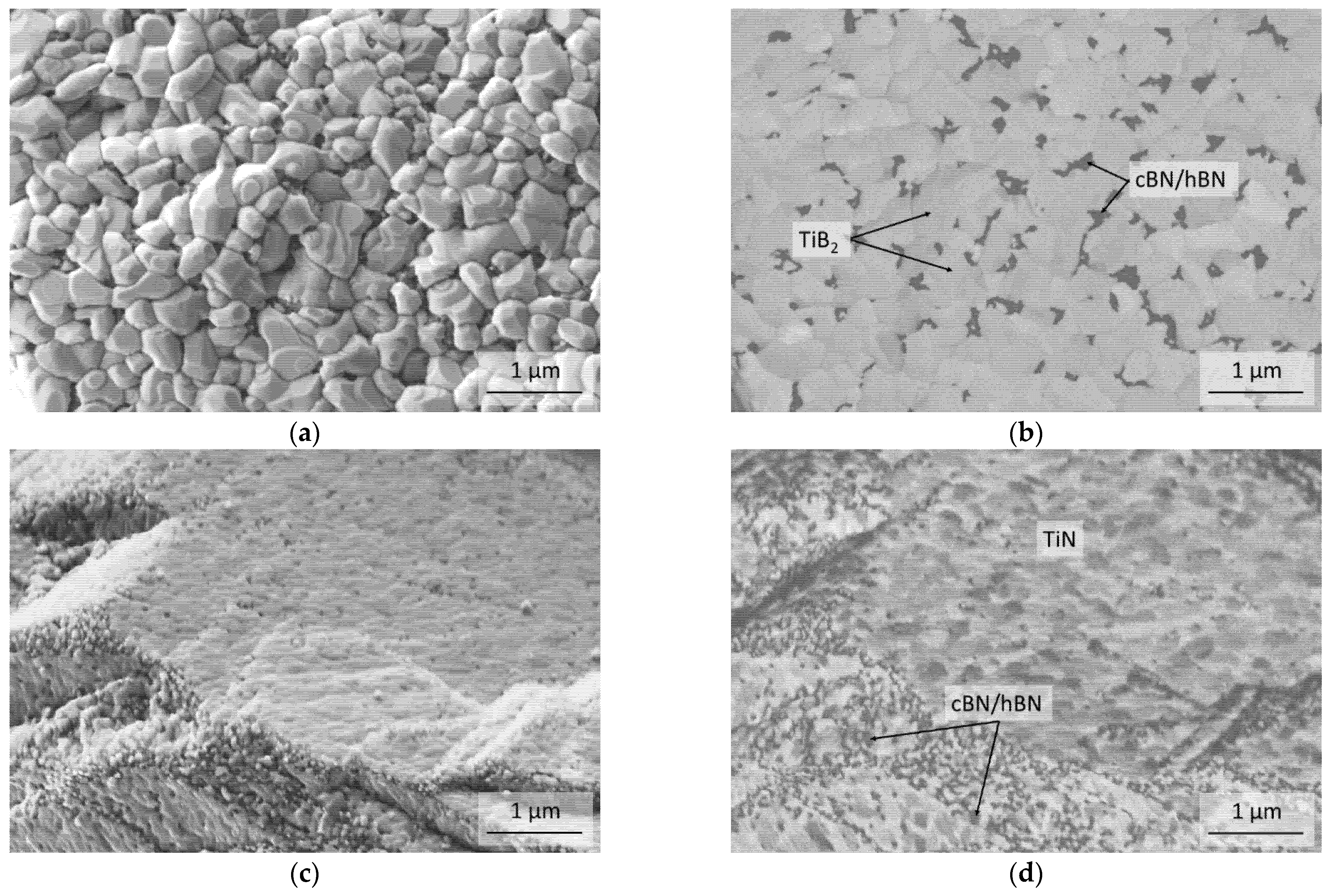
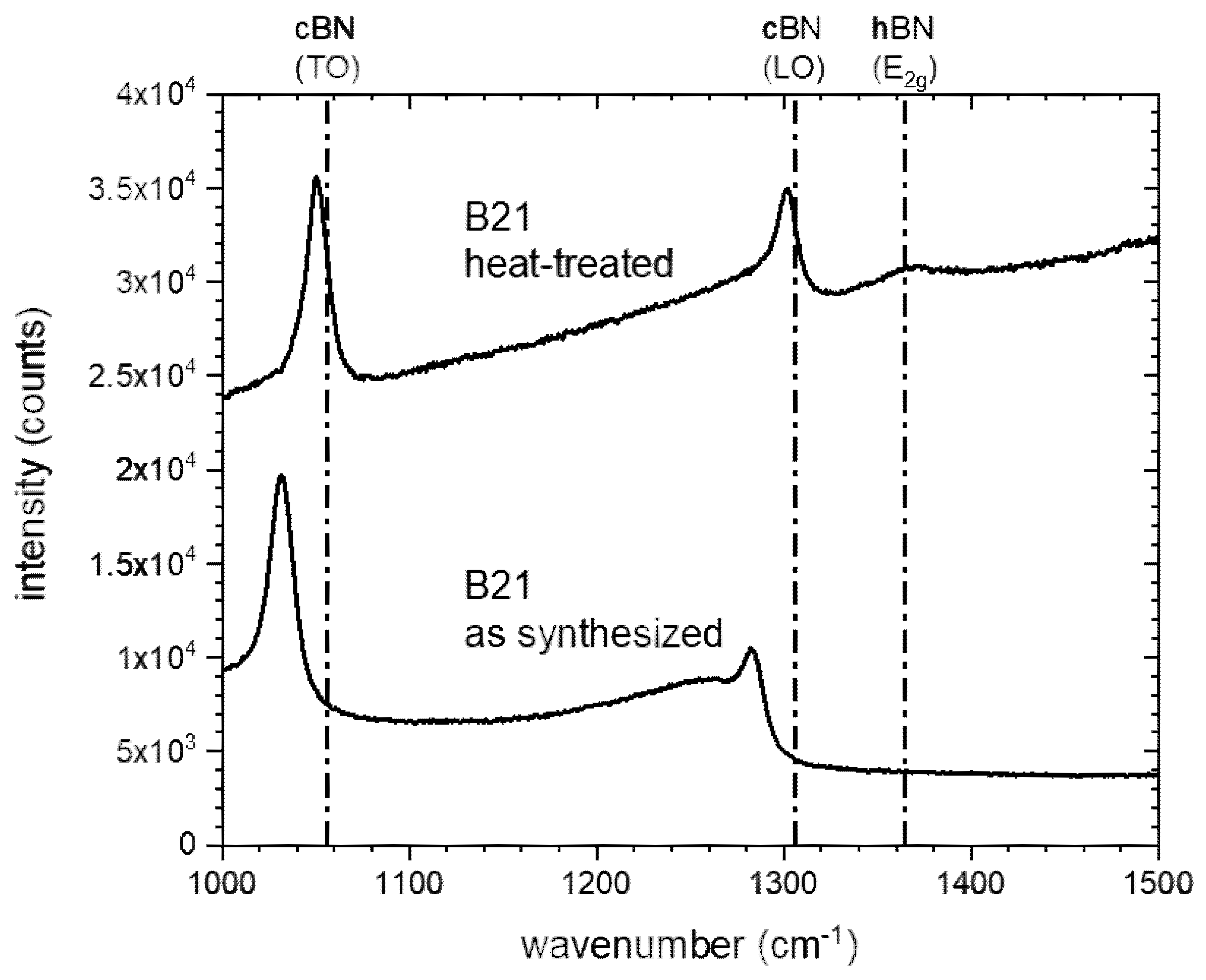
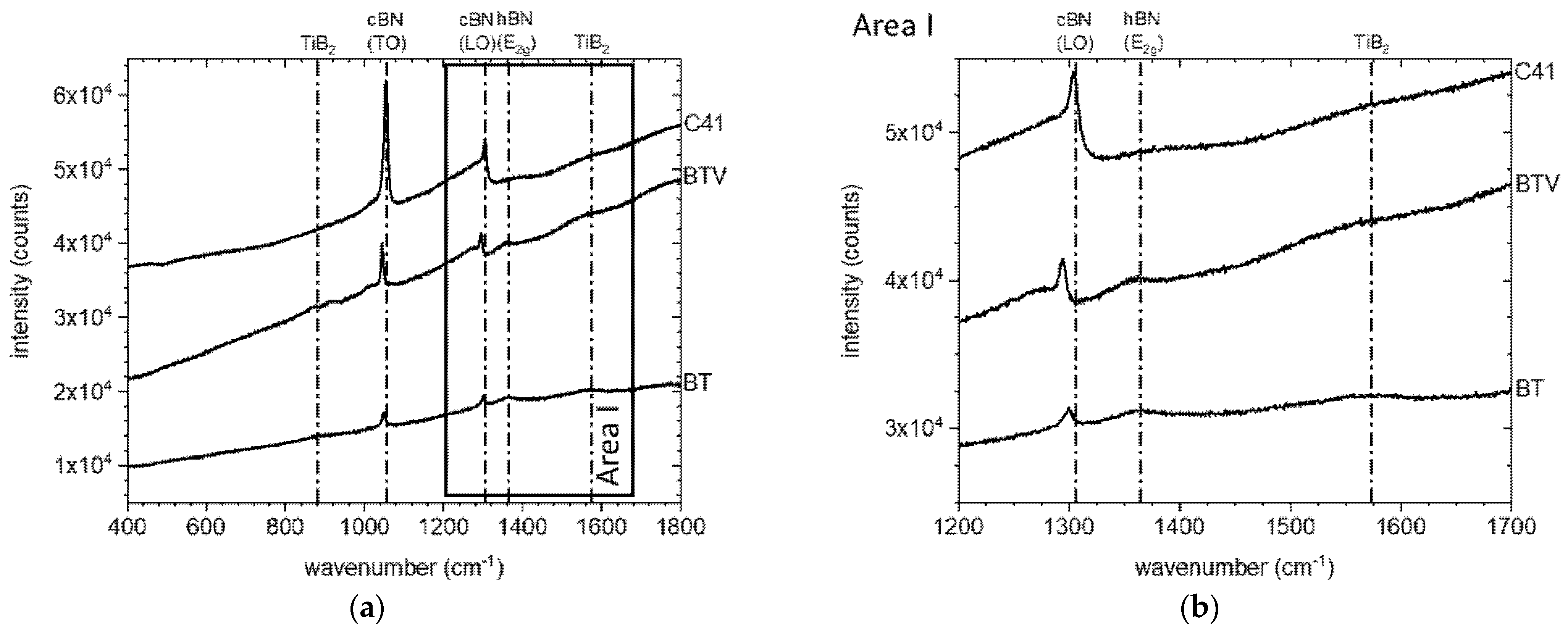
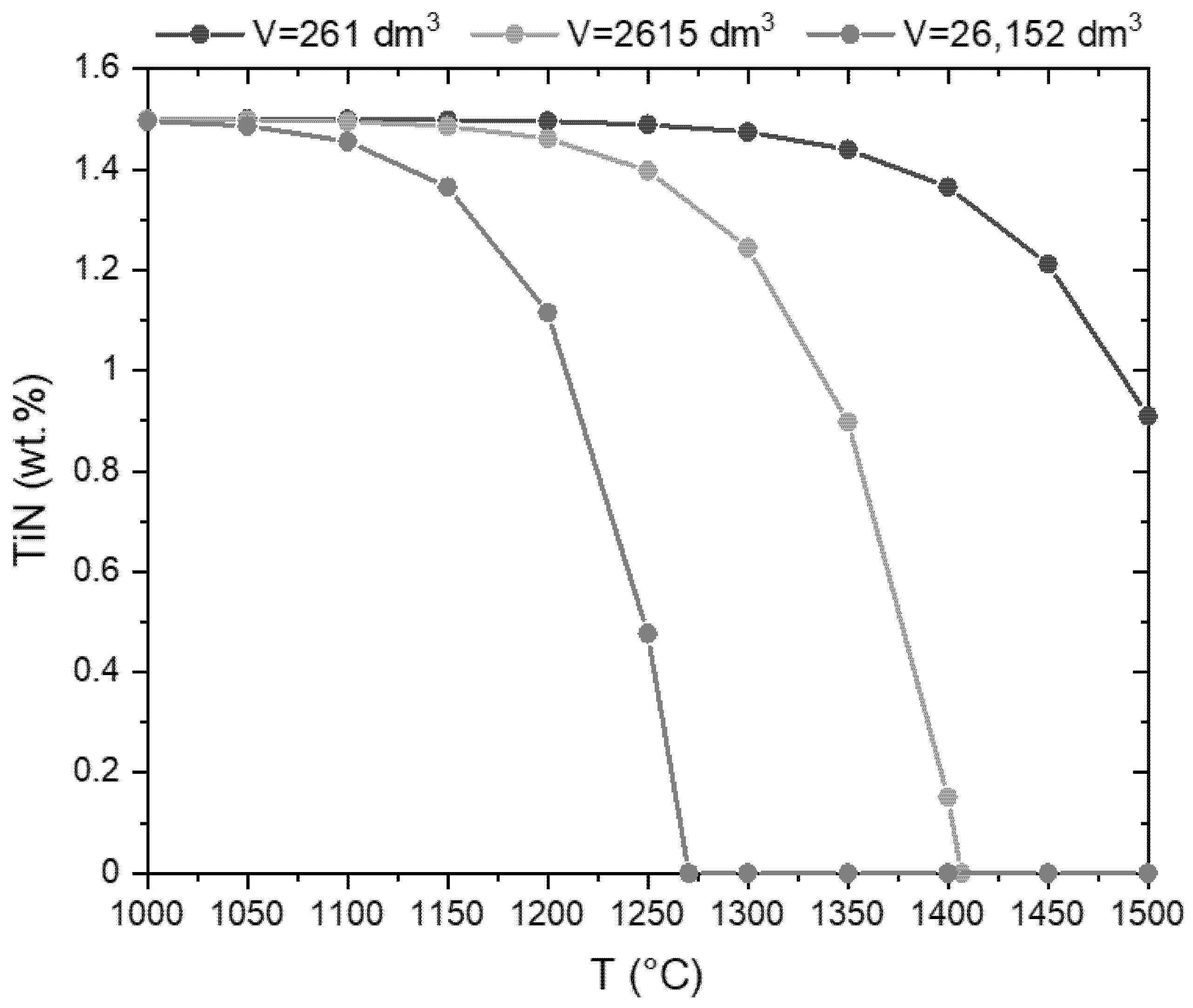
3.2. Thermal Stability of the cBN Powders
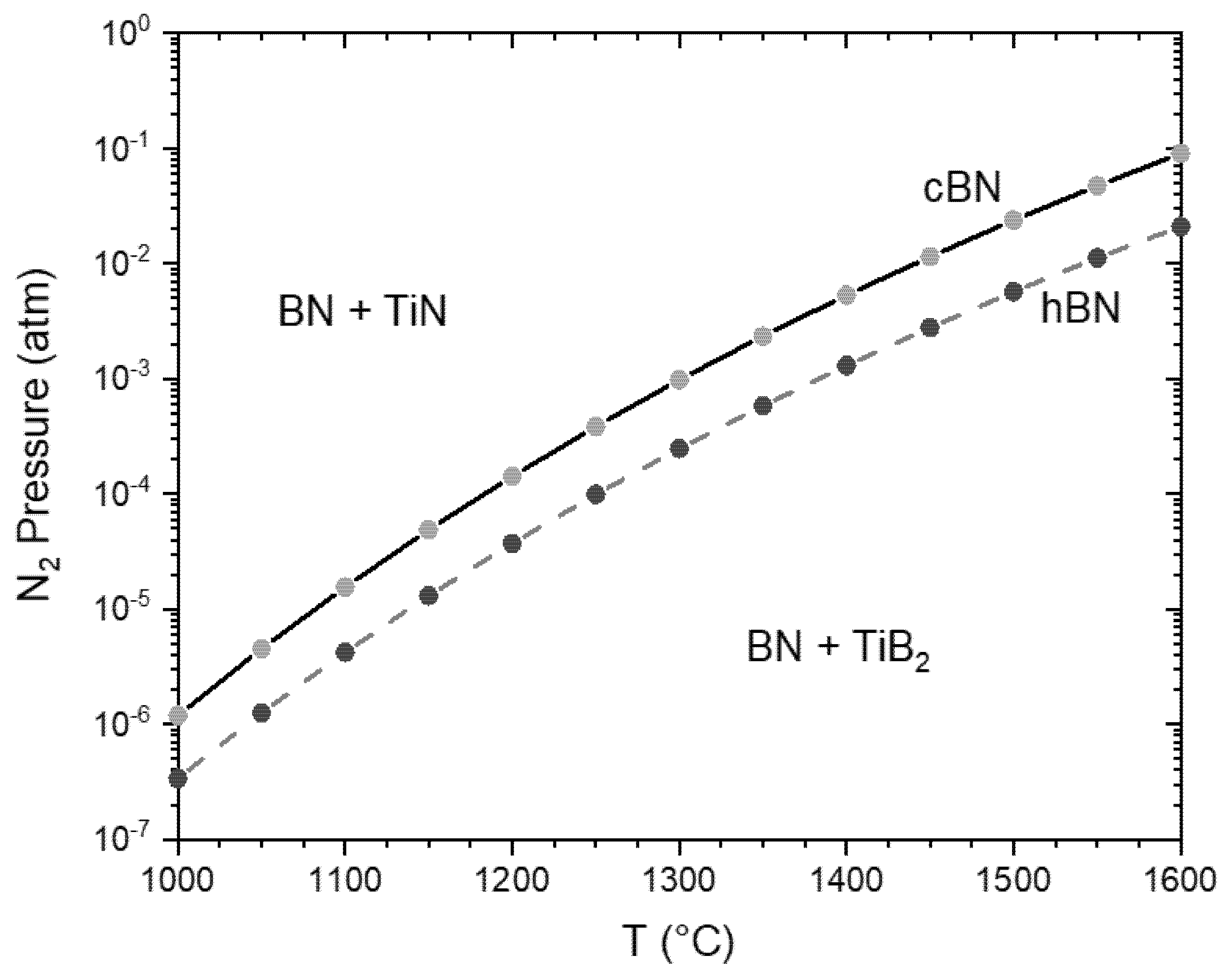
4. Discussion
5. Conclusions
- The stability of TiN coatings on cBN particles strongly depend on the nitrogen pressure. The decomposition into TiB2 was observed in Ar at temperatures above approximately 1200 °C. This reaction results in the formation of pores in the originally dense coating. Therefore, the layer could not more work as a protection layer.
- In a nitrogen atmosphere (1 atm pressure) no interaction of TiN with cBN was observed up to 1600 °C. However, the investigated very thin fine-grained TiN coatings, produced by ALD, showed after the heat treatment at 1600 °C a recrystallization, resulting also in very fine pores. Further investigations are necessary to clarify whether these processes also take place at 1300–1400 °C, at which composites are typically sintered with cBN. Additionally, the influence of the thickness and crystallite size of the coating needs further investigations.
- The observed results could be predicted by the thermodynamic calculations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfrum, A.-K. Verdichtung und Eigenschaften von Hartstoffverstärkten Siliciumnitridwerkstoffen. Ph.D. Dissertation, Technische Universität Dresden, Dresden, Germany, 2019. [Google Scholar]
- Hotta, M.; Goto, T. Preparation of β SiAlON-cBN composites by spark plasma sintering. Key Eng. Mater. 2008, 403, 241–242. [Google Scholar] [CrossRef]
- Michalski, A.; Rosiński, M.; Płocińska, M.; Szawłowski, J. Synthesis and characterization of cBN/WCCo composites obtained by the pulse plasma sintering (PPS) method. IOP Conf. Ser. 2011, 18, 202016. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Densification, microstructure and mechanical properties of SiO2–cBN composites by spark plasma sintering. Ceram. Int. 2012, 38, 351–356. [Google Scholar] [CrossRef]
- Zhang, J.F.; Tu, R.; Goto, T. Spark plasma sintering and characterization of WC-Co-cBN composites. Key Eng. Mater. 2014, 616, 194–198. [Google Scholar] [CrossRef]
- Klimczyk, P.; Wyżga, P.; Cyboroń, J.; Laszkiewicz-Łukasik, J.; Podsiadło, M.; Cygan, S.; Jaworska, L. Phase stability and mechanical properties of Al2O3-cBN composites prepared via spark plasma sintering. Diam. Relat. Mater. 2020, 104, 107762. [Google Scholar] [CrossRef]
- Garrett, J.C.; Sigalas, I.; Herrmann, M.; Olivier, E.J.; O’Connell, J.H. cBN reinforced Y-α-SiAlON composites. J. Eur. Ceram. Soc. 2013, 33, 2191–2198. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, X.; Chang, R.; Li, T.; Zang, J.; Wang, Y.; Yu, Y.; Lu, J.; Xu, X. Reactive sintering cBN-Ti-Al composites by spark plasma sintering. Diam. Relat. Mater. 2016, 69, 138–143. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Cubic boron nitride-containing ceramic matrix composites for cutting tools. Adv. Ceram. Matrix Compos. 2014, 570–586. [Google Scholar] [CrossRef]
- Scheffler, M. Zukunftspotenziale von Hochleistungskeramiken: Expertenstudie; DKG: Köln, Germany, 2014; ISBN 978-3-00-045777-7. [Google Scholar]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Bundy, F.P.; Wentorf, R.H. Direct transformation of hexagonal boron nitride to denser forms. J. Chem. Phys. 1963, 38, 1144–1149. [Google Scholar] [CrossRef]
- Corrigan, F.R.; Bundy, F.P. Direct transitions among the allotropic forms of boron nitride at high pressures and temperatures. J. Chem. Phys. 1975, 63, 3812. [Google Scholar] [CrossRef]
- Vereshchagin, L.F.; Gladkaya, I.S.; Dubitskii, G.A.; Slesarev, V.N. Synthesis of cubic boron nitride single crystals in systems containing hydrogen. Izv. Akad. Nauk. SSSR Neorg. Mater. 1979, 15, 256–259. [Google Scholar]
- Maki, J.; Ikawa, H.; Fukunaga, O. Phase equilibrium between cubic and hexagonal boron nitride. In Proceedings of the 2nd New Diamond Science and Technology International Conference, Washington, DC, USA, 23–27 September 1990; pp. 1051–1055. [Google Scholar]
- Solozhenko, V.L. Boron nitride phase diagram. State of the art. High Press. Res. 1995, 13, 199–214. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Turkevich, V.Z. Thermoanalytical study of the polymorphic transformation of cubic into graphite-like boron nitride. J. Therm. Anal. 1992, 38, 1181–1188. [Google Scholar] [CrossRef]
- Solozhenko, V.L. Inverse drop-calorimetry. A study of metastable and nonequilibrium phases. Thermochim. Acta 1993, 218, 395–400. [Google Scholar] [CrossRef]
- Solozhenko, V.L. Thermodynamics of dense boron nitride modifications and a new phase P.,T diagram for BN. Thermochim. Acta 1993, 218, 221–227. [Google Scholar] [CrossRef]
- Solozhenko, L.; Turkevich, V.Z.; Holzapfel, W.B. Refined phase diagram of boron nitride. J. Phys. Chem. B 1999, 103, 2903–2905. [Google Scholar] [CrossRef]
- Will, G.; Nover, G.; von der Gönna, J. New experimental results on the phase diagram of boron nitride. J. Solid State Chem. 2000, 154, 280–285. [Google Scholar] [CrossRef]
- Fukunaga, O. The equilibrium phase boundary between hexagonal and cubic boron nitride. Diam. Relat. Mater. 2000, 9, 7–12. [Google Scholar] [CrossRef]
- Wolfrum, A.-K.; Matthey, B.; Michaelis, A.; Herrmann, M. On the stability of c-BN-reinforcing particles in ceramic matrix materials. Materials 2018, 11, 255. [Google Scholar] [CrossRef]
- Cahill, J.T.; Du Frane, W.L.; Sio, C.K.; King, G.C.S.; Soderlind, J.C.; Lu, R.; Worsley, A.; Kuntza, J.D. Transformation of boron nitride from cubic to hexagonal under 1-atm helium. Diam. Relat. Mater. 2020, 109, 108078. [Google Scholar] [CrossRef]
- Irshad, H.M.; Ahmed, B.A.; Ehsan, M.A.; Khan, T.I.; Laoui, T.; Yousaf, M.R.; Ibrahim, A.; Hakeem, A.S. Investigation of the structural and mechanical properties of micro-/nano-sized Al2O3 and cBN composites prepared by spark plasma sintering. Ceram. Int. 2017, 43, 10645–10653. [Google Scholar] [CrossRef]
- Irshad, H.M.; Ahmed, B.A.; Ehsan, M.A.; Khan, T.I.; Laoui, T.; Yousaf, M.R.; Ibrahim, A.; Hakeem, A.S. Tribological behaviour of alumina-based nanocomposites reinforced with uncoated and Ni-coated cubic boron nitride. J. Mater. Res. Technol. 2019, 8, 5066–5079. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; GOTO, T. Evaluation of CVD-Deposited SiO2 as a sintering aid for cubic boron nitride consolidated with alumina by spark plasma sintering. J. Am. Ceram. Soc. 2012, 95, 2827–2832. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Spark plasma sintering of TiN-cubic BN composites. J. Ceram. Soc. Jpn. 2010, 118, 137–140. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Densification and microstructure of Al2O3-cBN composites prepared by spark plasma sintering. J. Ceram. Soc. Jpn. 2008, 116, 744–748. [Google Scholar] [CrossRef]
- Hotta, M.; Goto, T. Spark plasma sintering of βSiAlON-cBN composite. Mater. Sci. Forum 2007, 561–565, 599–602. [Google Scholar] [CrossRef]
- Kitiwan, M.; Ito, A.; Goto, T. Phase transformation and densification of hBN-TiN composites fabrication by spark plasma sintering. Key Eng. Mater. 2012, 508, 52–55. [Google Scholar] [CrossRef]
- Xie, H.; Deng, F.; Wang, H.; Liu, J.; Han, S.; Feng, F. Study of the proportioning design method and mechanical properties of a cBN–TiN composite. Int. J. Refract. Met. Hard Mater. 2020, 89, 105209. [Google Scholar] [CrossRef]
- Umer, M.A.; Sub, P.H.; Lee, D.J.; Ryu, H.J.; Hong, S.H. Polycrystalline cubic boron nitride sintered compacts prepared from nanocrystalline TiN coated cBN powder. Mater. Sci. Eng. 2012, 552, 151–156. [Google Scholar] [CrossRef]
- Umer, M.A.; Park, H.S.; Lee, D.J.; Ryu, H.J.; Hong, S.H. A sol–gel route to nanocrystalline TiN coated cubic boron nitride particles. J. Alloys Compd. 2011, 509, 9764–9769. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Longrie, D.; Deduytsche, D.; Detavernier, C. Reactor concepts for atomic layer deposition on agitated particles: A review. J. Vac. Sci. Technol. A 2014, 32, 10802. [Google Scholar] [CrossRef]
- Longrie, D.; Deduytsche, D.; Haemers, J.; Smet, P.F.; Driesen, K.; Detavernier, C. Thermal and plasma-enhanced atomic layer deposition of TiN using TDMAT and NH3 on particles agitated in a rotary reactor. ACS Appl. Mater. Interfaces 2014, 6, 7316–7324. [Google Scholar] [CrossRef]
- Didden, A.; Hillebrand, P.; Wollgarten, M.; Dam, B.; van de Krol, R. Deposition of conductive TiN shells on SiO2 nanoparticles with a fluidized bed ALD reactor. J. Nanopart. Res. 2016, 18, 35. [Google Scholar] [CrossRef]
- Hoehn, S.; Sempf, K.; Herrmann, M. Artefact-free preparation and characterisation of ceramic materials and interfaces. Ceram. Forum Int. 2011, 88, E16–E20. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Tuschel, D. Effect of dopants or impurities on the raman spectrum of the host crystal. Spectroscopy (St. Monica) 2017, 32, 13–18. [Google Scholar]
- Jana, M.; Singh, R.N. A study of evolution of residual stress in single crystal silicon electrode using Raman spectroscopy. Appl. Phys. Lett. 2017, 111, 63901. [Google Scholar] [CrossRef]
- Wdowik, U.D.; Twardowska, A.; Rajchel, B. Vibrational spectroscopy of binary titanium borides: First-principles and experimental studies. Adv. Condens. Matter. Phys. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Gu, L.; Wang, T.; Zhang, W.; Liang, G.; Gu, A.; Yuan, L. Low-cost and facile fabrication of titanium dioxide coated oxidized titanium diboride-epoxy resin composites with high dielectric constant and extremely low dielectric loss. RSC Adv. 2013, 3, 7071. [Google Scholar] [CrossRef]
- Gall, D.; Stoehr, M.; Greene, J.E. Vibrational modes in epitaxial Ti1−xScxN(001) layers: An ab initio calculation and Raman spectroscopy study. Phys. Rev. B 2001, 64, 174302. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Tay, B.K.; Lau, S.P.; Kupfer, H.; Richter, F. Substrate bias dependence of Raman spectra for TiN films deposited by filtered cathodic vacuum arc. J. Appl. Phys. 2002, 92, 1845–1849. [Google Scholar] [CrossRef]
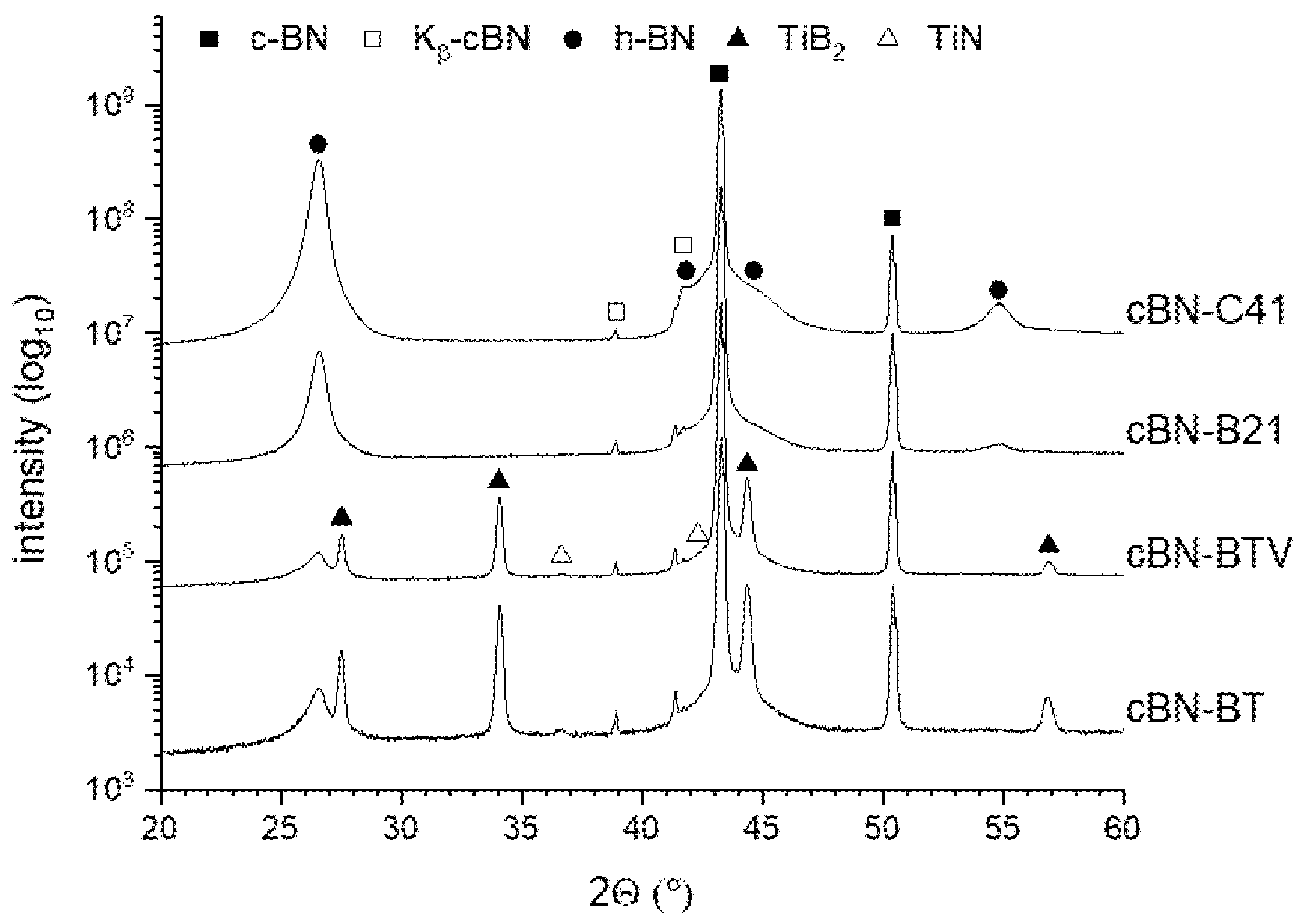
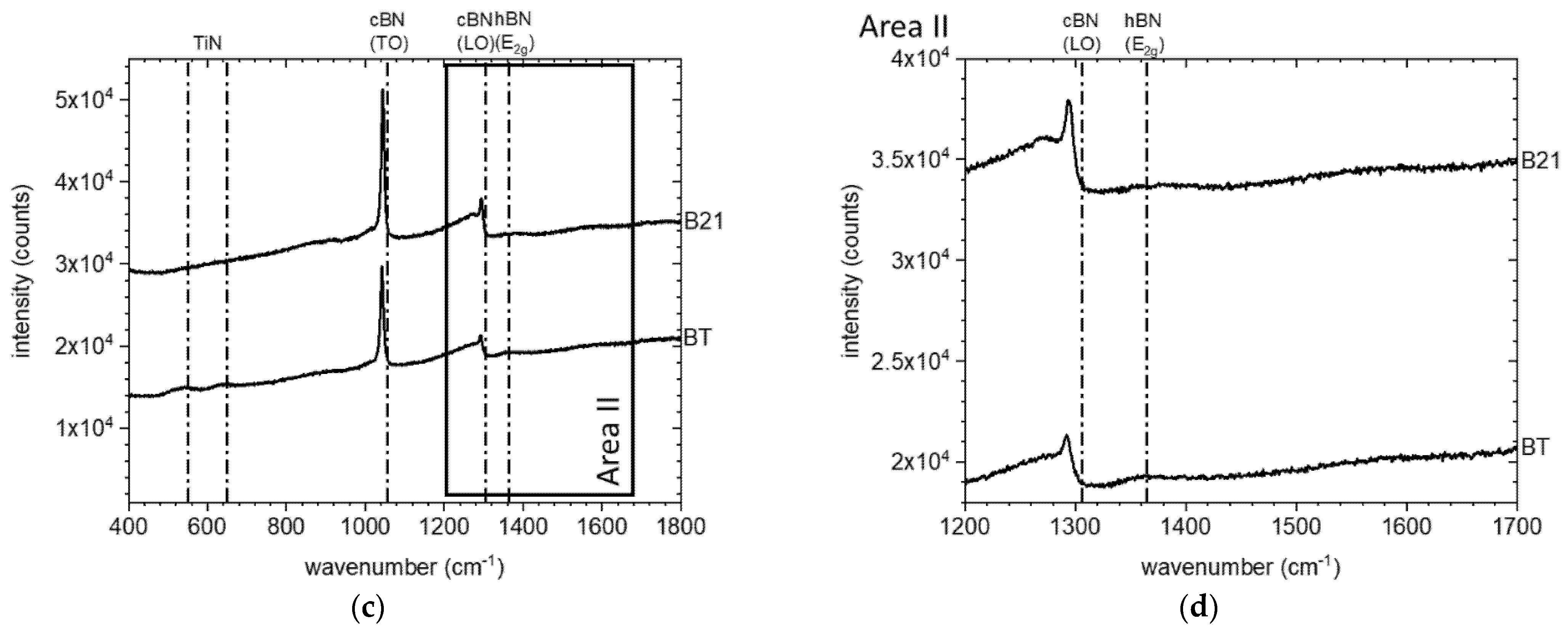
| cBN-Powder | Oxygen Content (wt. %) | Metallic Impurities (wt. %) | TiN-Coating Content (XRF/XRD) (wt. %) | TiN-Coating Thickness (nm) | |||
|---|---|---|---|---|---|---|---|
| Fe | Si | Cl | Cr | ||||
| B20 | 0.122 ± 0.042 | 0.0028 | 0.03 | - | - | - | - |
| B21 | 0.101 ± 0.015 | 0.0027 | 0.02 | - | - | - | - |
| C41 | 0.113 ± 0.023 | 0.0028 | 0.02 | - | - | - | - |
| BT | 0.170 ± 0.023 | 0.02 | 0.03 | 0.05 | 0.0085 | 2.3/2.3 ± 0.1 | 50 |
| BTV | 0.135 ± 0.032 | 0.01 | 0.03 | 0.02 | 0.0080 | 1.4/1.3 ± 0.1 | 20 |
| cBN-Powder | Coating | Atmosphere | Mass Loss (wt. %) |
|---|---|---|---|
| B21 | - | argon | 0.04 |
| C41 | - | 0.04 | |
| BT | TiN | 1.58 | |
| BTV | TiN | 1.01 | |
| B21 | - | nitrogen | 0.02 |
| BT | TiN | 0.17 |
| TiN Coated cBN-Powder | Measured TiN Content before TGA (wt. %) | Measured TiB2 Content after TGA (wt. %) | Calculated TiB2 Content (wt. %) | Calculated Weight Loss (wt. %) | Loss of Mass after TGA (wt. %) |
|---|---|---|---|---|---|
| BT | 2.3 ± 0.1 | 2.8 ± 0.1 | 2.6 | 1.56 | 1.58 |
| BTV | 1.3 ± 0.1 | 1.8 ± 0.1 | 1.5 | 0.88 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hering, B.; Wolfrum, A.-K.; Gestrich, T.; Herrmann, M. Thermal Stability of TiN Coated Cubic Boron Nitride Powder. Materials 2021, 14, 1642. https://doi.org/10.3390/ma14071642
Hering B, Wolfrum A-K, Gestrich T, Herrmann M. Thermal Stability of TiN Coated Cubic Boron Nitride Powder. Materials. 2021; 14(7):1642. https://doi.org/10.3390/ma14071642
Chicago/Turabian StyleHering, Benjamin, Anne-Kathrin Wolfrum, Tim Gestrich, and Mathias Herrmann. 2021. "Thermal Stability of TiN Coated Cubic Boron Nitride Powder" Materials 14, no. 7: 1642. https://doi.org/10.3390/ma14071642
APA StyleHering, B., Wolfrum, A.-K., Gestrich, T., & Herrmann, M. (2021). Thermal Stability of TiN Coated Cubic Boron Nitride Powder. Materials, 14(7), 1642. https://doi.org/10.3390/ma14071642






