Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formation of PLA-Based Films
2.3. Fourier Transform Infrared Analysis Analysis
2.4. Scanning Electron Microscopy (SEM)
2.5. Atomic Force Microscopy (AFM)
2.6. Thermogravimetric Analysis
2.7. Differential Scanning Calorimetry Method
2.8. Mechanical Properties
2.9. Transparency
2.10. Colour Measurement
2.11. Analysis of Antibacterial Properties
3. Results and Discussion
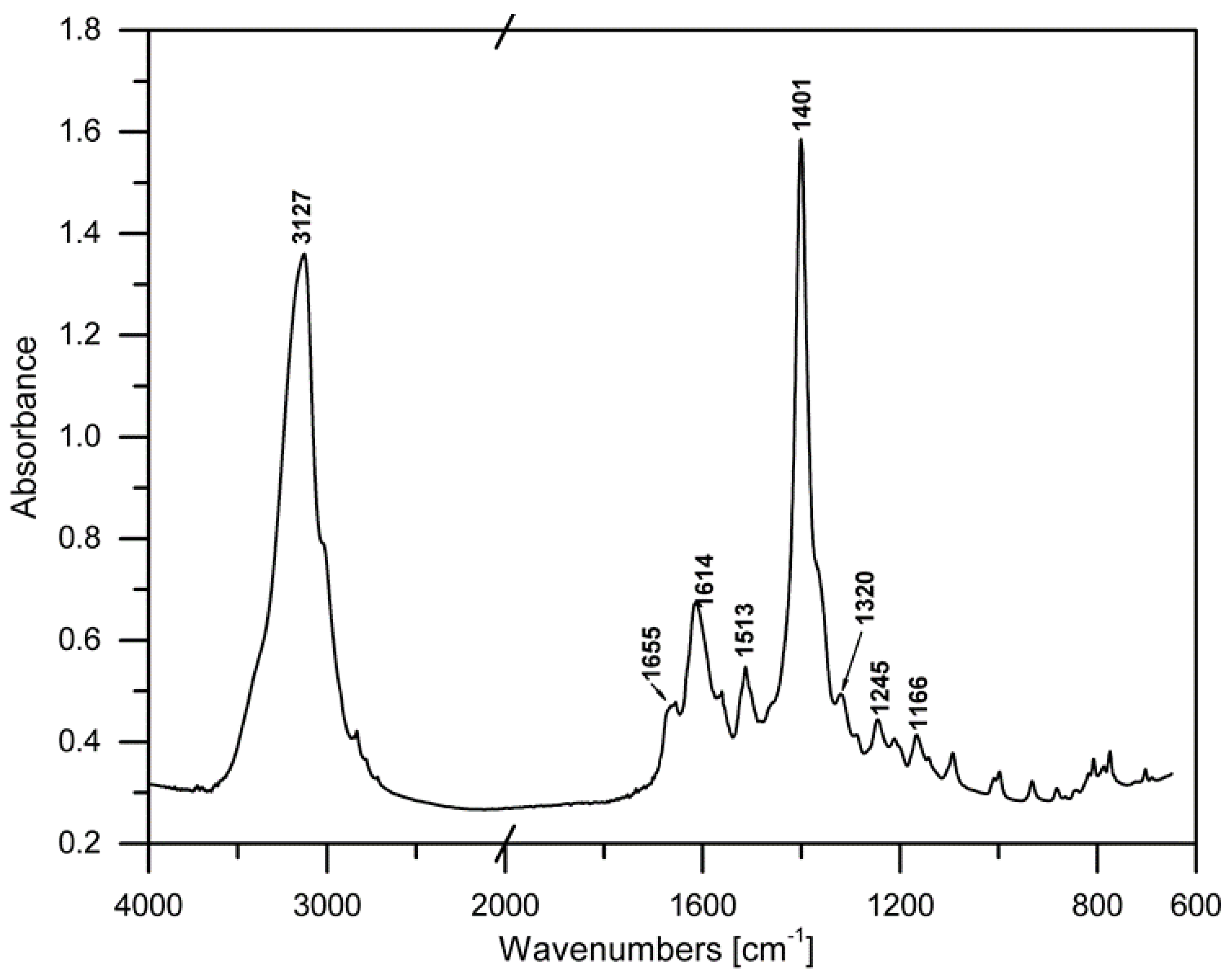
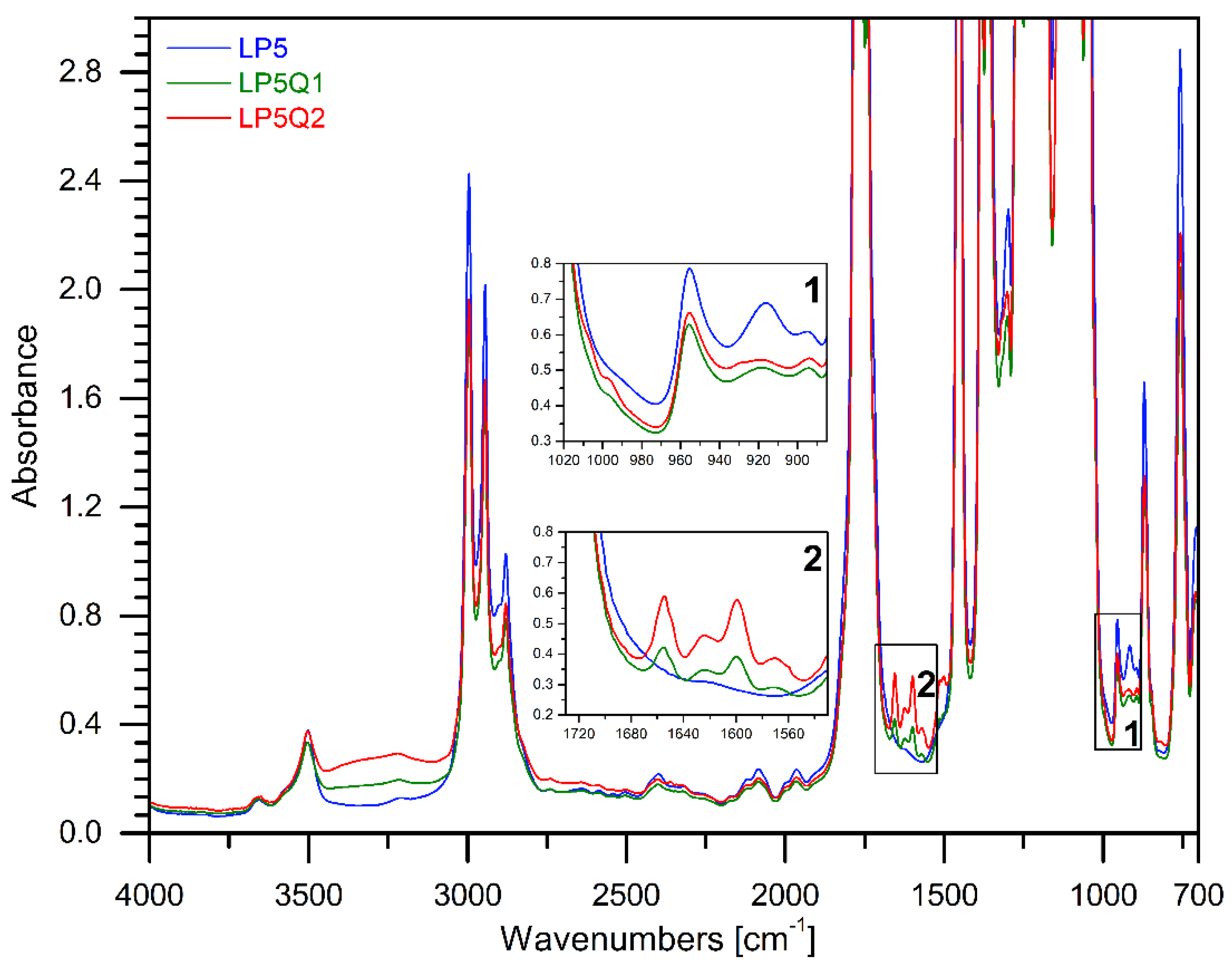
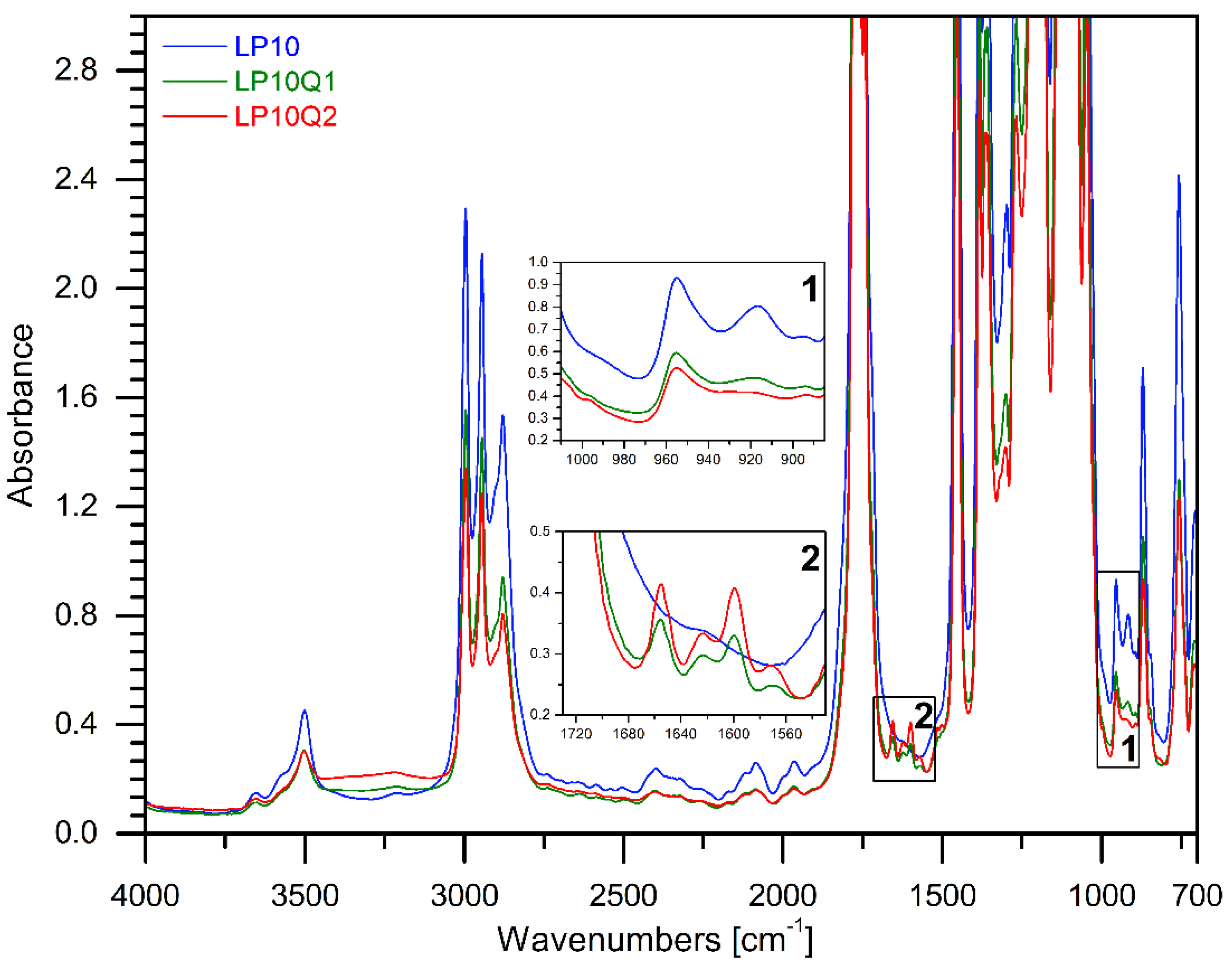
3.1. FTIR Analysis
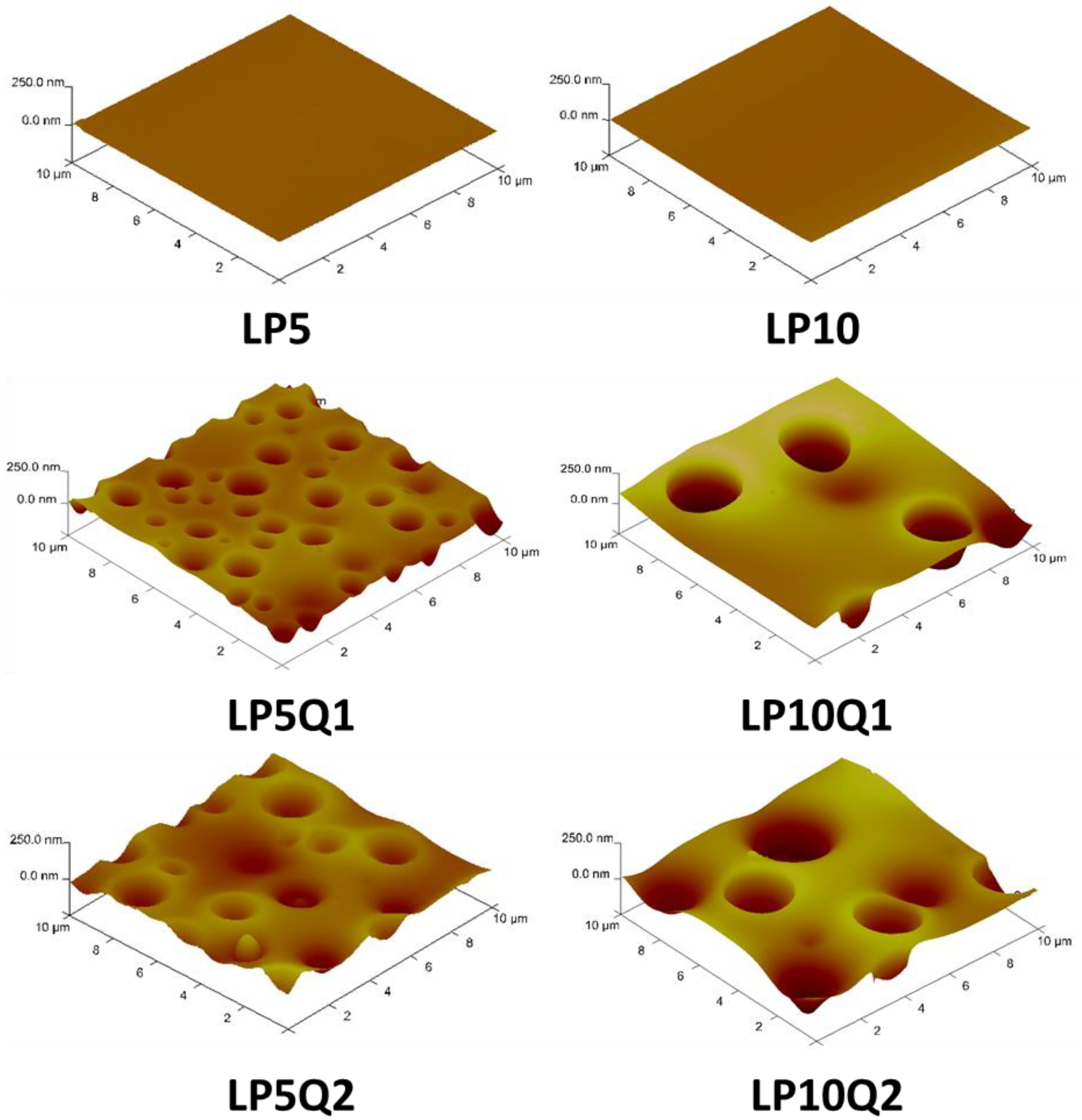
3.2. SEM and AFM Analyses
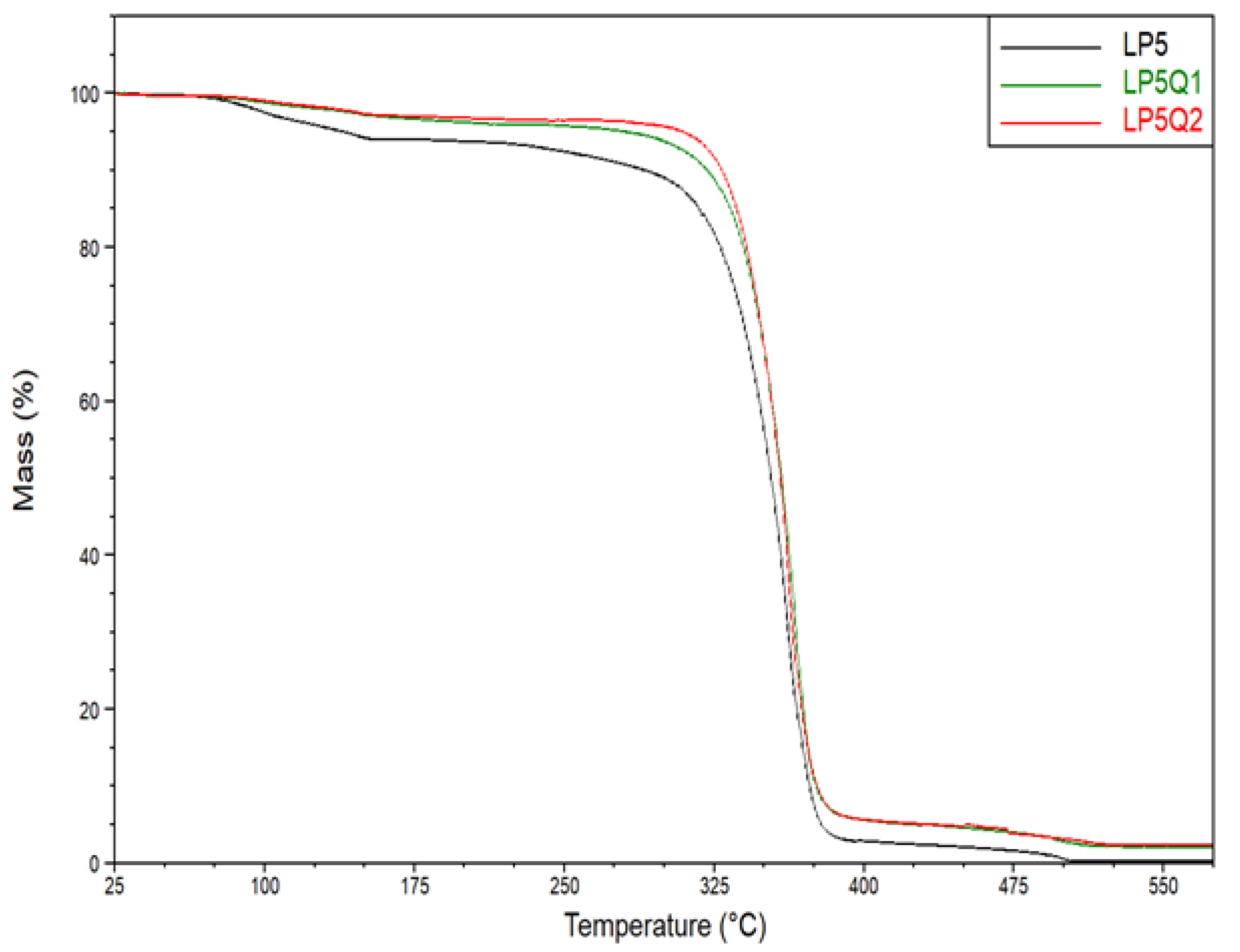
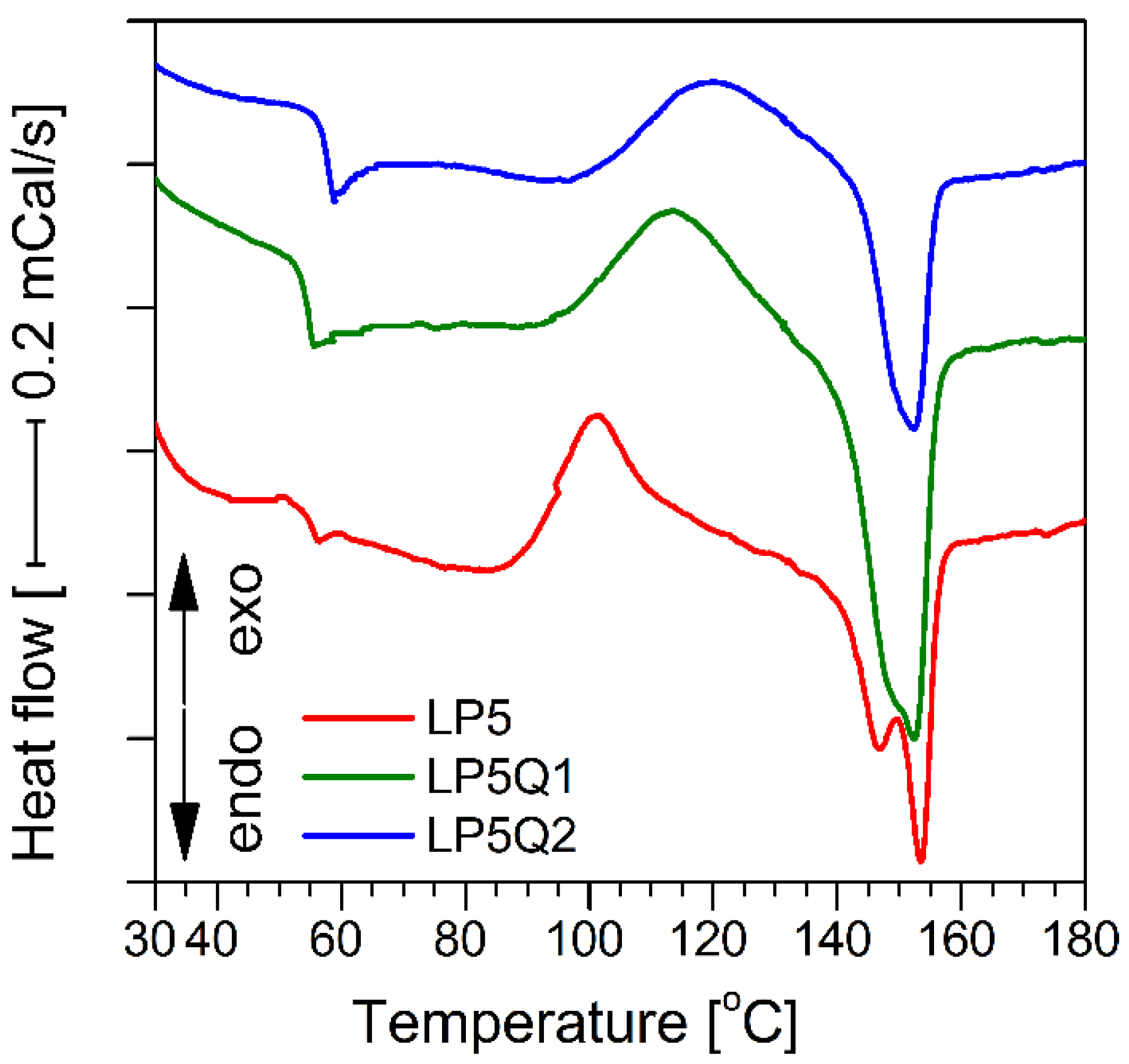
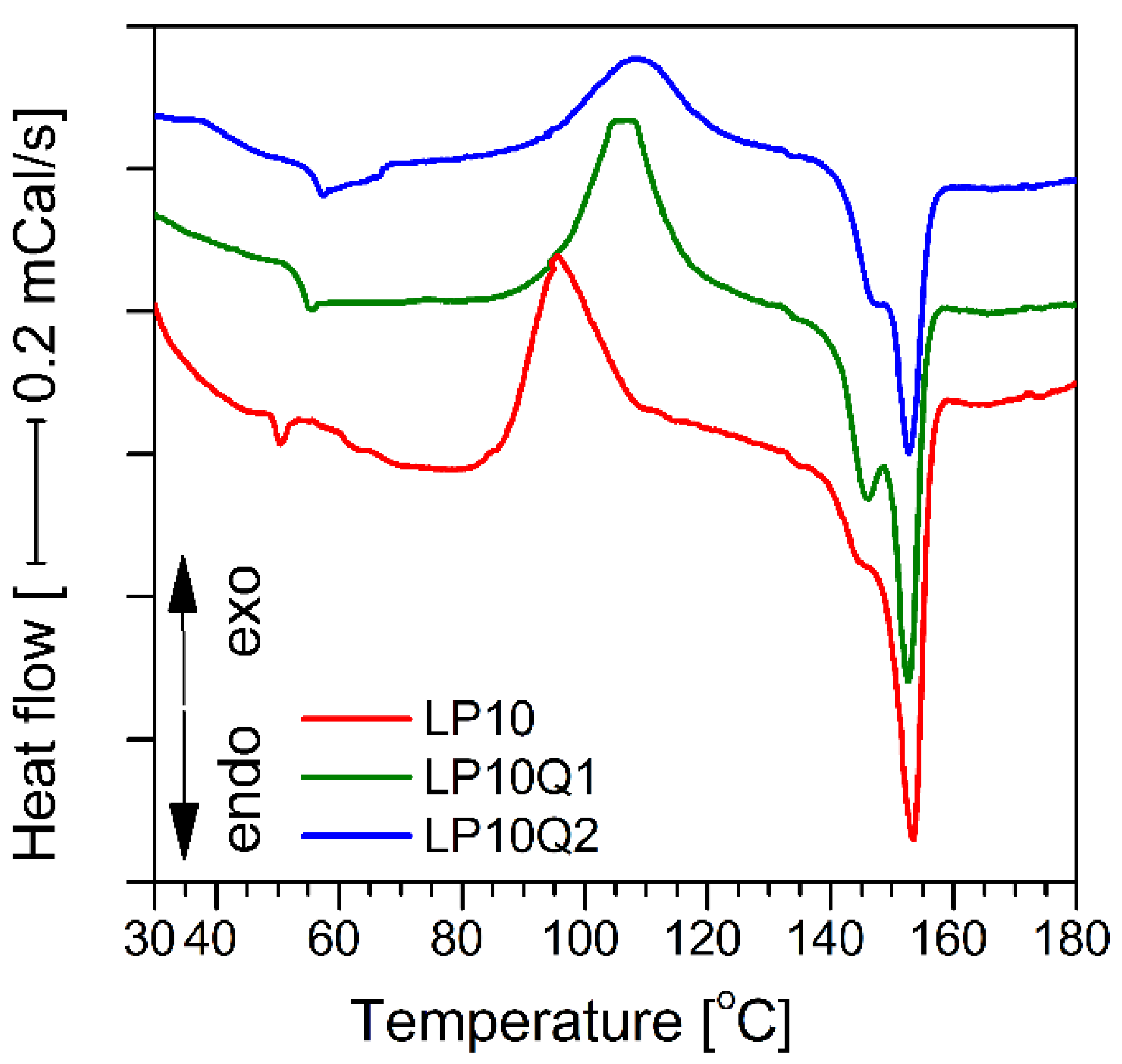
3.3. Determination of Thermal Properties of Polylactide-Based Materials
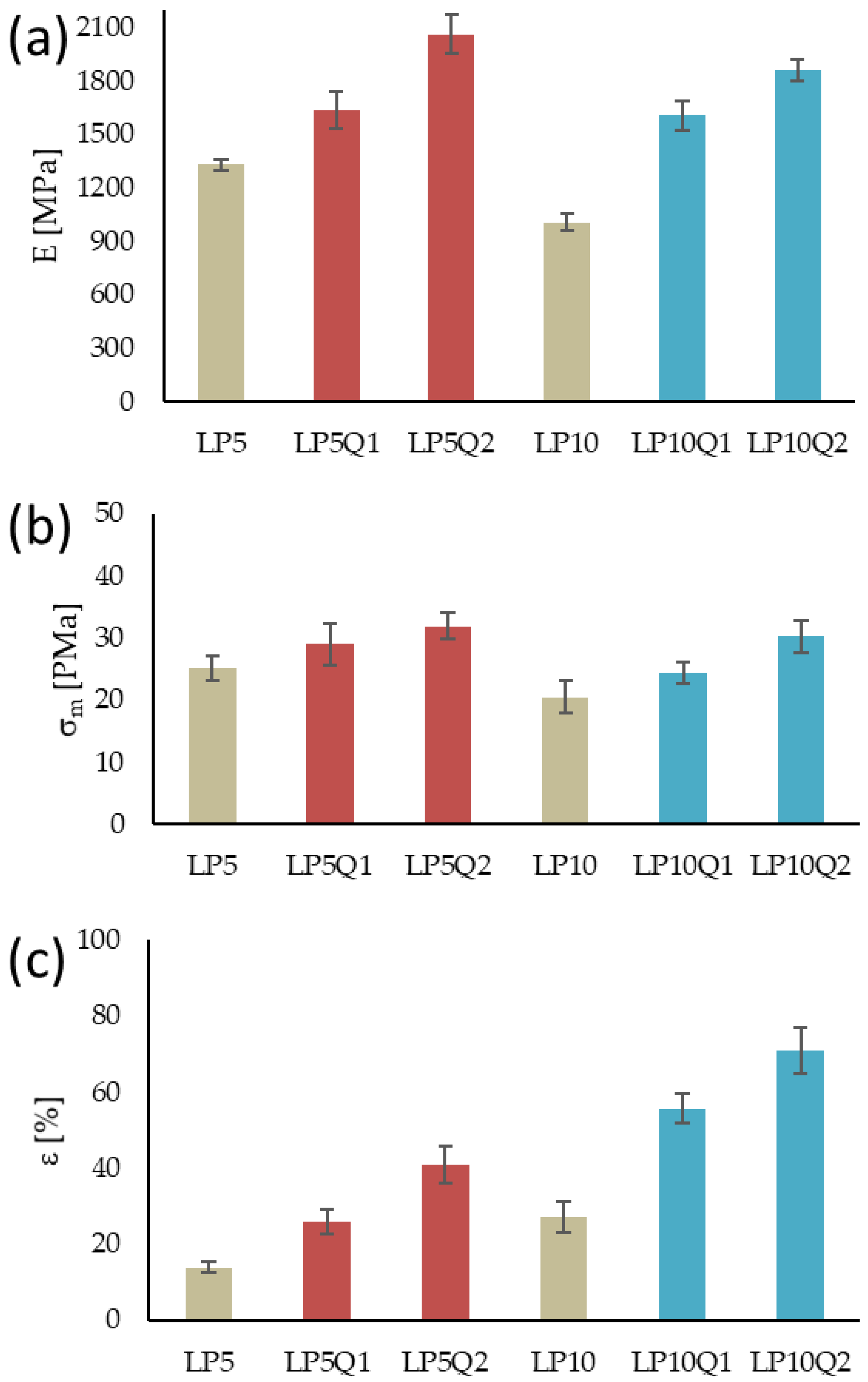
3.4. Evaluation of Mechanical Properties
3.5. Thickness and Transparency of Studied Materials
3.6. Differences in Colour of Studied Materials
3.7. Examination of Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Rojas, A.; Arango Ospina, A.; Rodríguez-Vélez, P.; Arana-Florez, R. What is the new about food packaging material? A bibliometric review during 1996–2016. Trends Food Sci. Technol. 2019, 85, 252–261. [Google Scholar] [CrossRef]
- Barlow, C.Y.; Morgan, D.C. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial food packaging based on sustainable bio-based materials for reducing foodborne pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of active films poly (butylene adipate co-terephthalate)—PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind. Crop. Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Przybytek, A.; Sienkiewicz, M.; Kucińska-Lipka, J.; Janik, H. Preparation and characterization of biodegradable and compostable PLA/TPS/ESO compositions. Ind. Crop. Prod. 2018, 122, 375–383. [Google Scholar] [CrossRef]
- Behera, K.; Chang, Y.-H.; Yadav, M.; Chiu, F.-C. Enhanced thermal stability, toughness, and electrical conductivity of carbon nanotube-reinforced biodegradable poly(lactic acid)/poly(ethylene oxide) blend-based nanocomposites. Polymer 2020, 186, 122002. [Google Scholar] [CrossRef]
- Komesu, A.; de Oliveira, J.A.R.; da Silva Martins, L.H.; Maciel, M.R.W.; Filho, R.M. Lactic acid production to purification: A review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive surfaces of polylactide and silver nanoparticles for the prevention of microbial contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef]
- Holcapkova, P.; Hurajova, A.; Bazant, P.; Pummerova, M.; Sedlarik, V. Thermal stability of bacteriocin nisin in polylactide-based films. Polym. Degrad. Stab. 2018, 158, 31–39. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Jakubowska, E.; Tarach, I.; Sedlarik, V.; Pummerova, M. Antibacterial films based on PVA and PVA–chitosan modified with poly(hexamethylene guanidine). Polymers 2019, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mulla, M.; Arfat, Y.A. Application of high-pressure processing and polylactide/cinnamon oil packaging on chicken sample for inactivation and inhibition of Listeria monocytogenes and Salmonella Typhimurium, and post-processing film properties. Food Control 2017, 78, 160–168. [Google Scholar] [CrossRef]
- Tarach, I.; Olewnik-Kruszkowska, E.; Richert, A.; Gierszewska, M.; Rudawska, A. Influence of tea tree essential oil and poly(ethylene glycol) on antibacterial and physicochemical properties of polylactide-based films. Materials 2020, 13, 4953. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Maróstica Júnior, M.R.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J. Mol. Model. 2020, 26, 133. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E. Effect of UV-A irradiation and temperature on the antioxidant activity of quercetin studied using ABTS, DPPH and electrochemistry methods. Int. J. Electrochem. Sci. 2015, 10, 5276–5290. [Google Scholar]
- Parhi, B.; Bharatiya, D.; Swain, S.K. Application of quercetin flavonoid based hybrid nanocomposites: A review. Saudi Pharm. J. 2020, 28, 1719–1732. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Physical and antioxidant properties of cassava starch–carboxymethyl cellulose incorporated with quercetin and TBHQ as active food packaging. Polymers 2020, 12, 366. [Google Scholar] [CrossRef]
- López-de-Dicastillo, C.; Alonso, J.M.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Improving the antioxidant protection of packaged food by incorporating natural flavonoids into ethylene−vinyl alcohol copolymer (EVOH) films. J. Agric. Food Chem. 2010, 58, 10958–10964. [Google Scholar] [CrossRef]
- Masek, A.; Latos, M.; Piotrowska, M.; Zaborski, M. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packag. Shelf Life 2018, 16, 51–58. [Google Scholar] [CrossRef]
- Kost, B.; Svyntkivska, M.; Brzeziński, M.; Makowski, T.; Piorkowska, E.; Rajkowska, K.; Kunicka-Styczyńska, A.; Biela, T. PLA/β-CD-based fibres loaded with quercetin as potential antibacterial dressing materials. Colloids Surf. B Biointerfaces 2020, 190, 110949. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-Coated nano-liposomes. LWT-Food Sci. Technol. 2017, 85, 37–44. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Nowaczyk, J.; Kadac, K. Effect of compatibilizig agent on the properties of polylactide and polylactide based composite during ozone exposure. Polym. Test. 2017, 60, 283–292. [Google Scholar] [CrossRef]
- Chow, W.S.; Lok, S.K. Thermal properties of poly(lactic acid)/organo-montmorillonite nanocomposites. J. Therm. Anal. Calorim. 2009, 95, 627–632. [Google Scholar] [CrossRef]
- ISO. Plastics—Determination of tensile properties—Part 1: General principles; 527-1:2020; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- ISO. Plastics—Determination of tensile properties—Part 3: Test conditions for films and sheets; 527-3:2019; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. The role of a deep eutectic solvent in changes of physicochemical and antioxidative properties of chitosan-based films. Carbohyd. Polym. 2021, 255, 117527. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- ISO. Textile fabrics—Determination of antibacterial activity: Agar diffusion plate test; 20645:2006; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Basu, A.; Kundu, S.; Sana, S.; Halder, A.; Abdullah, M.F.; Datta, S.; Mukherjee, A. Edible nano-bio-composite film cargo device for food packaging applications. Food Packag. Shelf Life 2017, 11, 98–105. [Google Scholar] [CrossRef]
- Yan, L.; Wang, R.; Wang, H.; Sheng, K.; Liu, C.; Qu, H.; Ma, A.; Zheng, L. Formulation and characterization of chitosan hydrochloride and carboxymethyl chitosan encapsulated quercetin nanoparticles for controlled applications in foods system and simulated gastrointestinal condition. Food Hydrocoll. 2018, 84, 450–457. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Koter, I.; Skopińska-Wiśniewska, J.; Richert, J. Degradation of polylactide composites under UV irradiation at 254nm. J. Photochem. Photobiol. A 2015, 311, 144–153. [Google Scholar] [CrossRef]
- Moraczewski, K.; Pawłowska, A.; Stepczyńska, M.; Malinowski, R.; Kaczor, D.; Budner, B.; Gocman, K.; Rytlewski, P. Plant extracts as natural additives for environmentally friendly polylactide films. Food Packag. Shelf Life 2020, 26, 100593. [Google Scholar] [CrossRef]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.R. Infrared spectrum of poly(l-lactide): Application to crystallinity studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, rheological, and thermal properties of plasticized polylactide films incorporated with essential oils to inhibit Staphylococcus aureus and Campylobacter jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the processing methods on the performance of polylactide films: Thermocompression versus solvent casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Phaechamud, T.; Chitrattha, S. Pore formation mechanism of porous poly(Dl-lactic acid) matrix membrane. Mater. Sci. Eng. C 2016, 61, 744–752. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The potential of flavonoids as natural antioxidants and UV light stabilizers for polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Younes, H.; Cohn, D. Phase separation in poly(ethylene glycol)/poly(lactic acid) blends. Eur. Polym. J. 1988, 24, 765–773. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Piórkowska, E.; Kuliński, Z.; Gadzinowska, K. Plasticization of polylactide. Polimery 2009, 54, 83–90. [Google Scholar] [CrossRef]
- Tabi, T.; Sajo, I.E.; Szabo, F.; Luyt, A.S.; Kovacs, J.G. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S.K. Properties and characterization of biodegradable poly(lactic acid) (PLA)/poly(ethylene glycol) (PEG) and PLA/PEG/organoclay: A study of crystallization kinetics, rheology, and compostability. J. Thermoplast. Compos. 2014, 29, 443–463. [Google Scholar] [CrossRef]
- Sungsanit, K.; Kao, N.; Bhattacharya, S.N. Properties of linear poly(lactic acid)/polyethylene glycol blends. Polym. Eng. Sci. 2012, 52, 108–116. [Google Scholar] [CrossRef]
- Holcapkova, P.; Hurajova, A.; Kucharczyk, P.; Bazant, P.; Plachy, T.; Miskolczi, N.; Sedlarik, V. Effect of polyethylene glycol plasticizer on long-term antibacterial activity and the release profile of bacteriocin nisin from polylactide blends. Polym. Adv. Technol. 2018, 29, 2253–2263. [Google Scholar] [CrossRef]
- Rubini, K.; Boanini, E.; Menichetti, A.; Bonvicini, F.; Gentilomi, G.A.; Montalti, M.; Bigi, A. Quercetin loaded gelatin films with modulated release and tailored anti-oxidant, mechanical and swelling properties. Food Hydrocolloid 2020, 109, 106089. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Impregnation of poly(lactic acid) with polyphenols of plant origin. Fibres Text. East. Eur. 2020, 28, 15–20. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic acid and quercetin as intelligent and active ingredients in poly(vinyl alcohol) films for food packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Anuar, H.; Azlina, H.N.; Suzana AB, K.; Kaiser, M.R.; Bonnia, N.N.; Surip, S.N.; Abd Razak, S.B. Effect of PEG on impact strength of PLA hybrid biocomposite. In Proceedings of the 2012 IEEE Symposium on Business, Engineering and Industrial Applications, Bandung, Indonesia, 23–26 September 2012; pp. 473–476. [Google Scholar]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- Cruz-Zúñiga, J.M.; Soto-Valdez, H.; Peralta, E.; Mendoza-Wilson, A.M.; Robles-Burgueño, M.R.; Auras, R.; Gámez-Meza, N. Development of an antioxidant biomaterial by promoting the deglycosylation of rutin to isoquercetin and quercetin. Food Chem. 2016, 204, 420–426. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active chicken meat packaging based on polylactide films and bimetallic Ag–Cu nanoparticles and essential oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Ohemeng, K.A.; Podlogar, B.L.; Nguyen, V.N.; Bernstein, J.I.; Krause, H.M.; Hilliard, J.J.; Barrett, J.F. DNA gyrase inhibitory and antimicrobial activities of some diphenic acid monohydroxamides. J. Med. Chem. 1997, 40, 3292–3296. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]




| Sample | Quercetin Content 1 [wt.%] | PEG Content 1 [wt.%] |
|---|---|---|
| LP5 | - | 5 |
| LP10 | - | 10 |
| LP5Q1 | 1 | 5 |
| LP10Q1 | 1 | 10 |
| LP5Q2 | 2 | 5 |
| LP10Q2 | 2 | 10 |
| Braking Zone [mm] the Average Value of Rise | Growth (a) | Description | Rating |
|---|---|---|---|
| >1 | Lack | Inhibition zone above 1, no increase (b) | Good effect |
| 0–1 | Lack | Growth inhibition zone up to 1, no growth (b) | |
| 0 | Lack | No braking zone, no increase (c) | |
| 0 | Weak | Lack of braking zones, only some colonies limited growth almost completely stopped (d) | Limited effect |
| 0 | Average | No braking zone, height reduced to half compared to control (e) | |
| 0 | Strong | Lack of braking zones, the absence of a reduction in growth compared to the control, or only a slight reduction in growth | Insufficient effect |
| Sample | Rq [nm] | Ra [nm] | Rmax [nm] |
|---|---|---|---|
| LP5 | 1.4 ± 0.1 | 1.0 ± 0.1 | 10.5 ± 0.5 |
| LP10 | 3.8 ± 0.2 | 3.1 ± 0.2 | 23.1 ± 1.1 |
| LP5Q1 | 32.2 ± 0.5 | 22.0 ± 0.4 | 232.0 ± 2.0 |
| LP5Q2 | 34.1 ± 0.6 | 26.8 ± 0.5 | 237.0 ± 2.0 |
| LP10Q1 | 69.5 ± 1.2 | 51.4 ± 1.0 | 344.0 ± 4.0 |
| LP10Q2 | 71.9 ± 1.4 | 55.40 ± 1.0 | 364.0 ± 7.0 |
| Samples | Temperature (°C) at Mass Loss | ||
|---|---|---|---|
| 10% | 30% | 50% | |
| LP5 | 289.5 ± 0.4 | 340.1 ± 1.5 | 353.7 ± 1.5 |
| LP10 | 287.2 ± 0.5 | 335.6 ± 1.3 | 350.9 ± 1.4 |
| LP5Q1 | 322.6 ± 0.7 | 347.9 ± 1.0 | 358.5 ± 1.4 |
| LP5Q2 | 329.8 ± 1.2 | 348.3 ± 1.0 | 358.3 ± 1.6 |
| LP10Q1 | 320.0 ± 1.2 | 340.8 ± 1.3 | 352.4 ± 1.7 |
| LP10Q2 | 325.5 ± 1.1 | 345.1 ± 1.3 | 356.1 ± 1.7 |
| Sample | Tg [°C] | Tc [°C] | ΔHc [J/g] | Tm [°C] | ΔHm [J/g] | χm [%] |
|---|---|---|---|---|---|---|
| LP5 | 54.6 ± 0.2 | 101.4 ± 0.5 | 18.4 ± 0.3 | 146.8/153.4 ± 0.2/0.4 | 24.1 ± 0.4 | 23.3 |
| LP5Q1 | 54.5 ± 0.2 | 112.4 ± 0.7 | 16.1 ± 0.1 | 153.8 ± 0.2 | 22.0 ± 0.4 | 21.5 |
| LP5Q2 | 58.6 ± 0.4 | 119.6 ± 0.8 | 15.4 ± 0.1 | 152.3 ± 0.2 | 21.0 ± 0.3 | 19.7 |
| LP10 | 49.5 ± 0.1 | 95.5 ± 0.5 | 17.9 ± 0.2 | 153.5 ± 0.3 | 26.6 ± 0.5 | 27.1 |
| LP10Q1 | 54.0 ± 0.2 | 106.5 ± 0.6 | 15.0 ± 0.2 | 146.2/152.6 ± 0.2/0.3 | 24.8 ± 0.4 | 25.6 |
| LP10Q2 | 54.8 ± 0.2 | 108.9 ± 0.6 | 12.8 ± 0.1 | 146.7/152.8 ± 0.3/0.3 | 16.6 ± 0.2 | 17.3 |
| Sample | Thickness [mm] | T [mm−1] |
|---|---|---|
| LP5 | 0.071 ± 0.002 | 0.76 ± 0.1 |
| LP10 | 0.086 ± 0.002 | 1.54 ± 0.1 |
| LP5Q1 | 0.065 ± 0.003 | 3.34 ± 0.1 |
| LP5Q2 | 0.066 ± 0.001 | 6.04 ± 0.1 |
| LP10Q1 | 0.070 ± 0.004 | 3.92 ± 0.1 |
| LP10Q2 | 0.080 ± 0.004 | 6.58 ± 0.2 |
| Sample | L | a | b | ΔE | C | YI |
|---|---|---|---|---|---|---|
| LP5 | 91.7 ± 0.1 | 1.5 ± 0.1 | −11.6 ± 0.2 | − | 11.7 ± 0.2 | −18.1 ± 0.2 |
| LP10 | 91.5 ± 0.1 | 1.5 ± 0.1 | −12.5 ± 0.3 | 0.9 ± 0.2 | 12.6 ± 0.2 | −19.5 ± 0.4 |
| LP5Q1 | 89.5 ± 0.2 | −5.0 ± 0.1 | 13.4 ± 0.2 | 25.9 ± 0.4 | 14.3 ± 0.3 | 21.4 ± 0.5 |
| LP5Q2 | 88.2 ± 0.3 | −8.9± 0.2 | 18.4 ± 0.5 | 31.9 ± 0.4 | 20.4 ± 0.7 | 29.8 ± 0.8 |
| LP10Q1 | 89.2 ± 0.1 | −6.6 ± 0.4 | 14.9 ± 0.3 | 27.8 ± 0.3 | 16.3 ± 0.7 | 23.9 ± 0.3 |
| LP10Q2 | 87.9 ± 0.2 | −8.6 ± 0.3 | 20.2 ± 0.4 | 33.6 ± 0.2 | 22.0 ± 0.5 | 32.8 ± 0.8 |
| Sample | Diameter of Inhibition Zones of Bacteria Growth [mm] | Bacteria Growth in Direct Contact with Sample | Evaluation of Antibacterial Effect 1 | |||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | S. aureus | E. coli | |
| LP5 | 0 | 0 | Medium | Medium | Insufficient | Insufficient |
| LP10 | 0 | 0 | Medium | Medium | Insufficient | Insufficient |
| LP5Q1 | 0 | 0 | Lack | Weak | Good | Good |
| LP5Q2 | 1 | 0 | Lack | Lack | Good | Good |
| LP10Q1 | 0 | 0 | Lack | Weak | Good | Limited |
| LP10Q2 | 4 | 1 | Lack | Lack | Good | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials 2021, 14, 1643. https://doi.org/10.3390/ma14071643
Olewnik-Kruszkowska E, Gierszewska M, Richert A, Grabska-Zielińska S, Rudawska A, Bouaziz M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials. 2021; 14(7):1643. https://doi.org/10.3390/ma14071643
Chicago/Turabian StyleOlewnik-Kruszkowska, Ewa, Magdalena Gierszewska, Agnieszka Richert, Sylwia Grabska-Zielińska, Anna Rudawska, and Mohamed Bouaziz. 2021. "Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol)" Materials 14, no. 7: 1643. https://doi.org/10.3390/ma14071643
APA StyleOlewnik-Kruszkowska, E., Gierszewska, M., Richert, A., Grabska-Zielińska, S., Rudawska, A., & Bouaziz, M. (2021). Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials, 14(7), 1643. https://doi.org/10.3390/ma14071643









