Impact of TiO2 Nanostructures on Dye-Sensitized Solar Cells Performance
Abstract
1. Introduction
2. Experimental
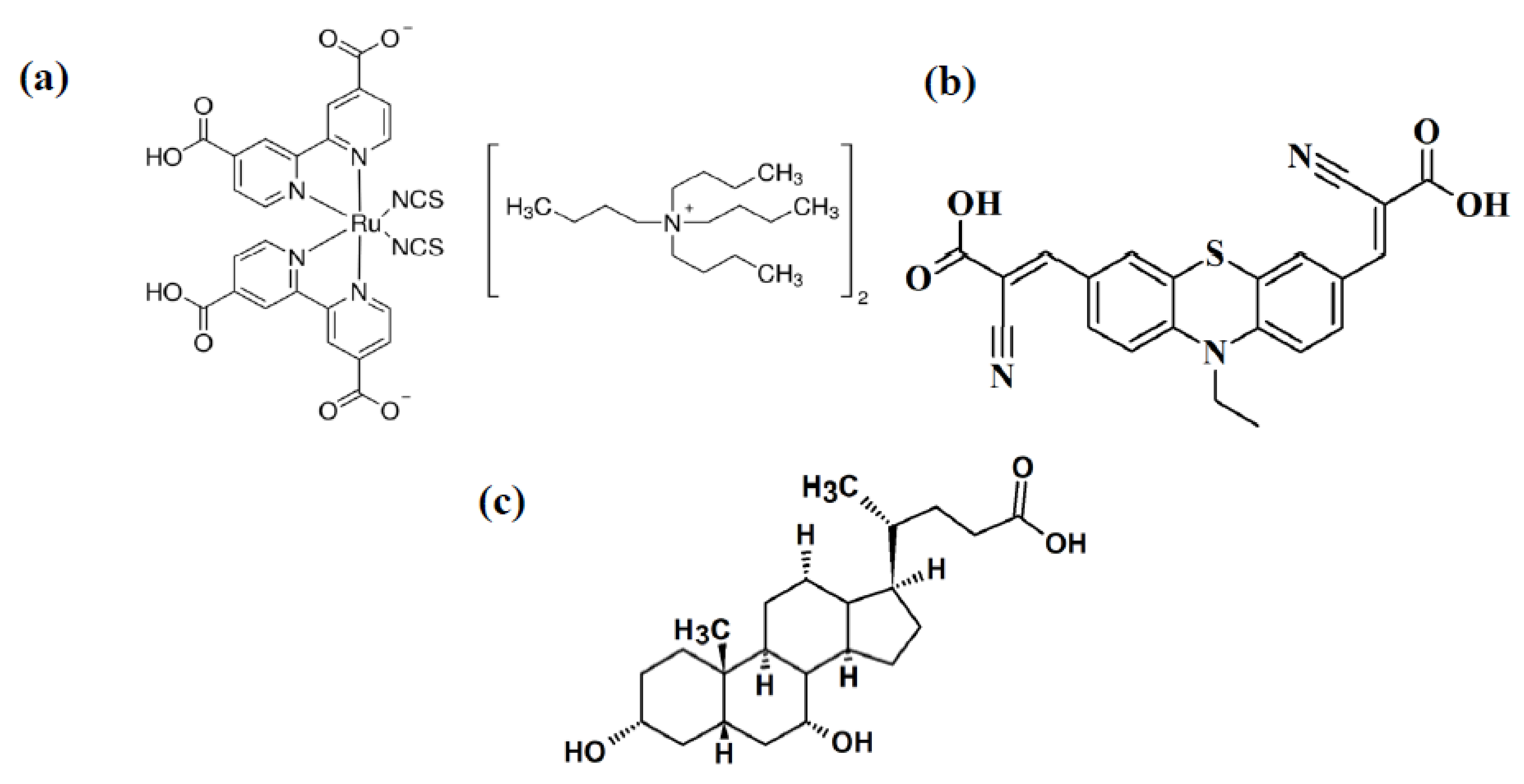
2.1. Materials and Methods
- titania paste consisting of NPs (18 NR-T, Greatcell Solar, Queanbeyan, Australia),
- titania paste consisting of NPs with addition of TiO2 NW (3D Nano) (in the ratio of 2.350g NPs paste to 0.005g NWs).
- titania paste consisting of NPs with addition of TiO2 NTs (3D Nano) (in the ratio of 3.720 g NPs paste to 0.008g NTs).
2.2. Measurements
2.3. Dye Loading Analysis
3. Results and Discussion
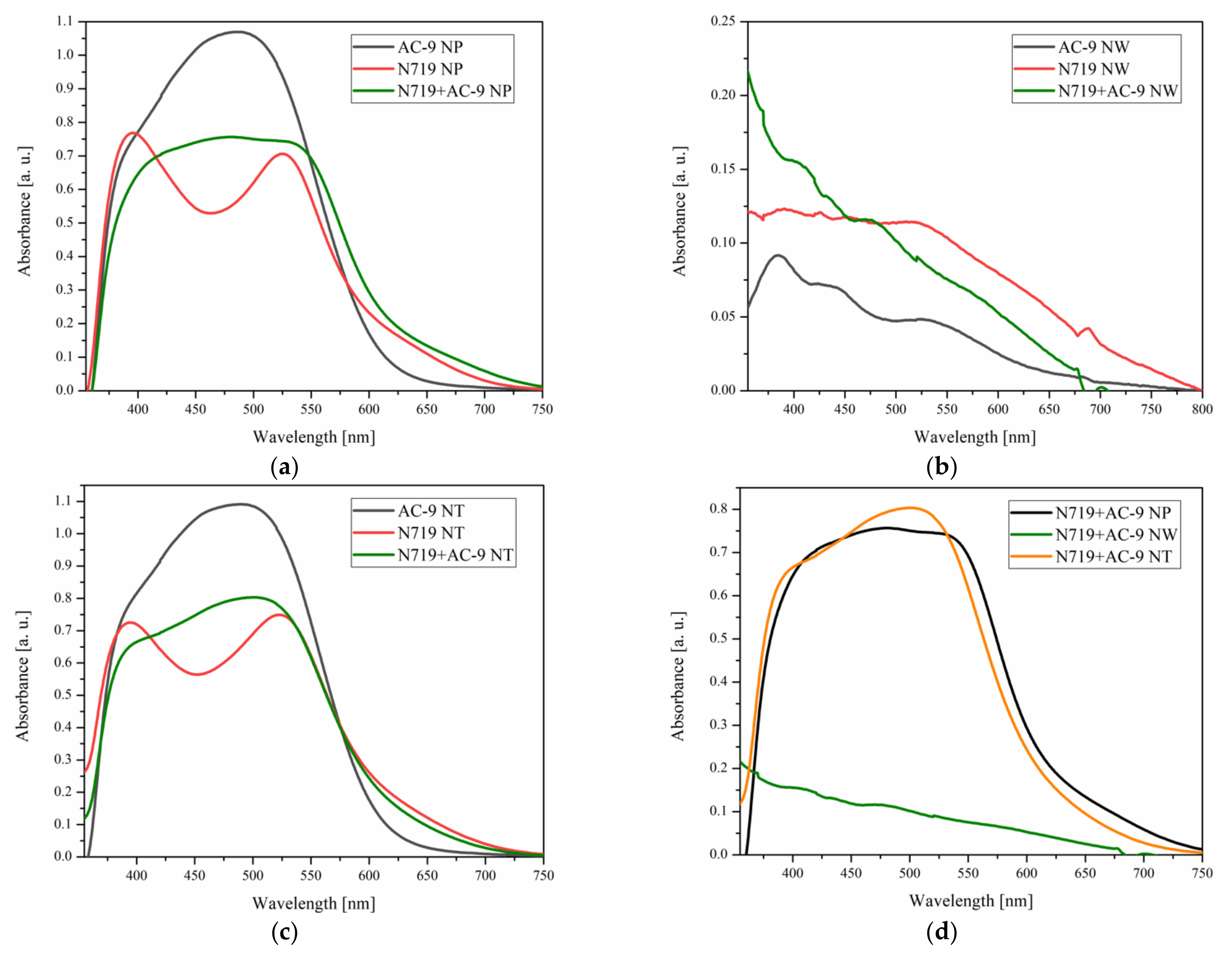
3.1. UV-Vis Absorption of Photoanodes
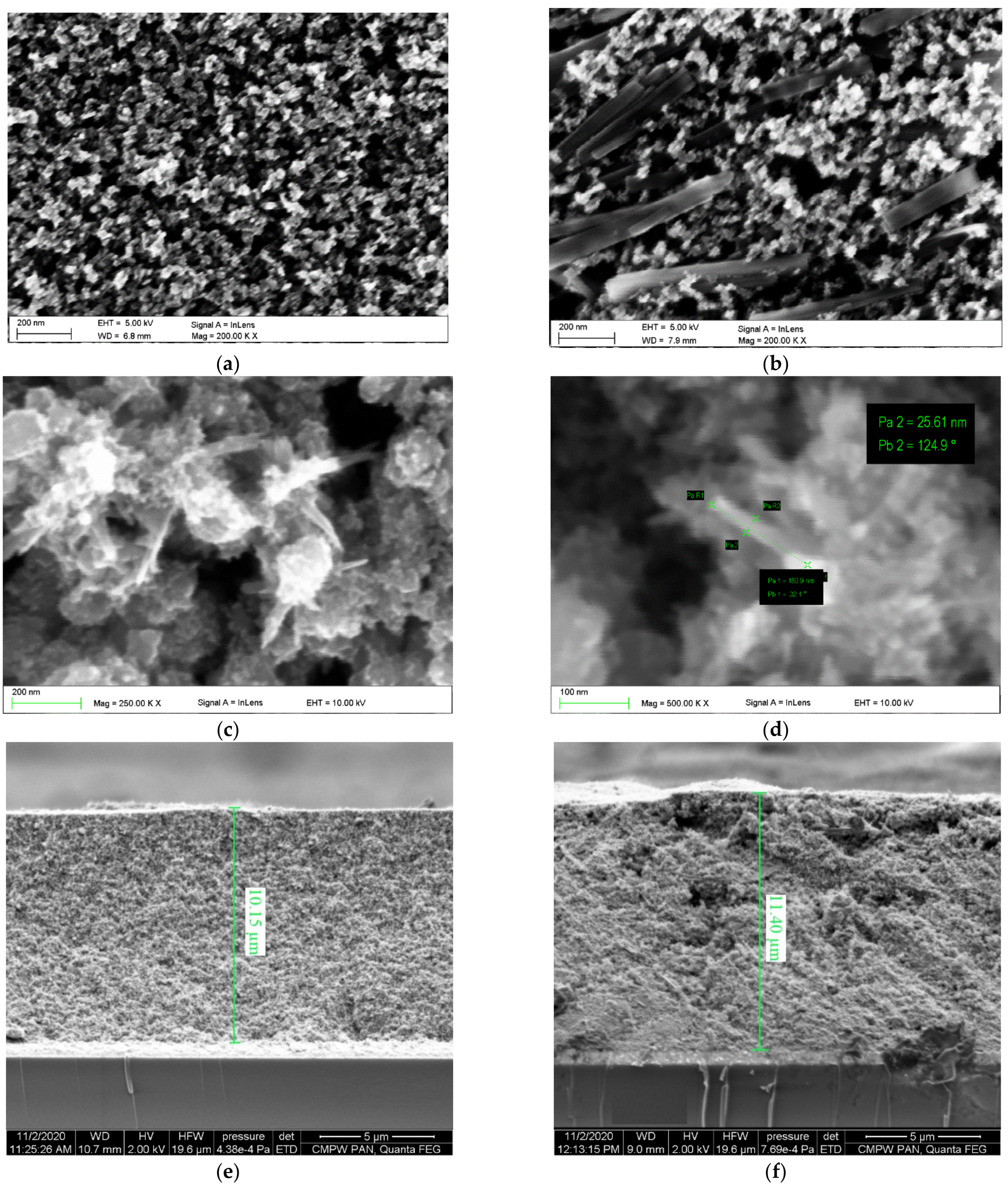
3.2. Morphology and Thickness of Photoanodes
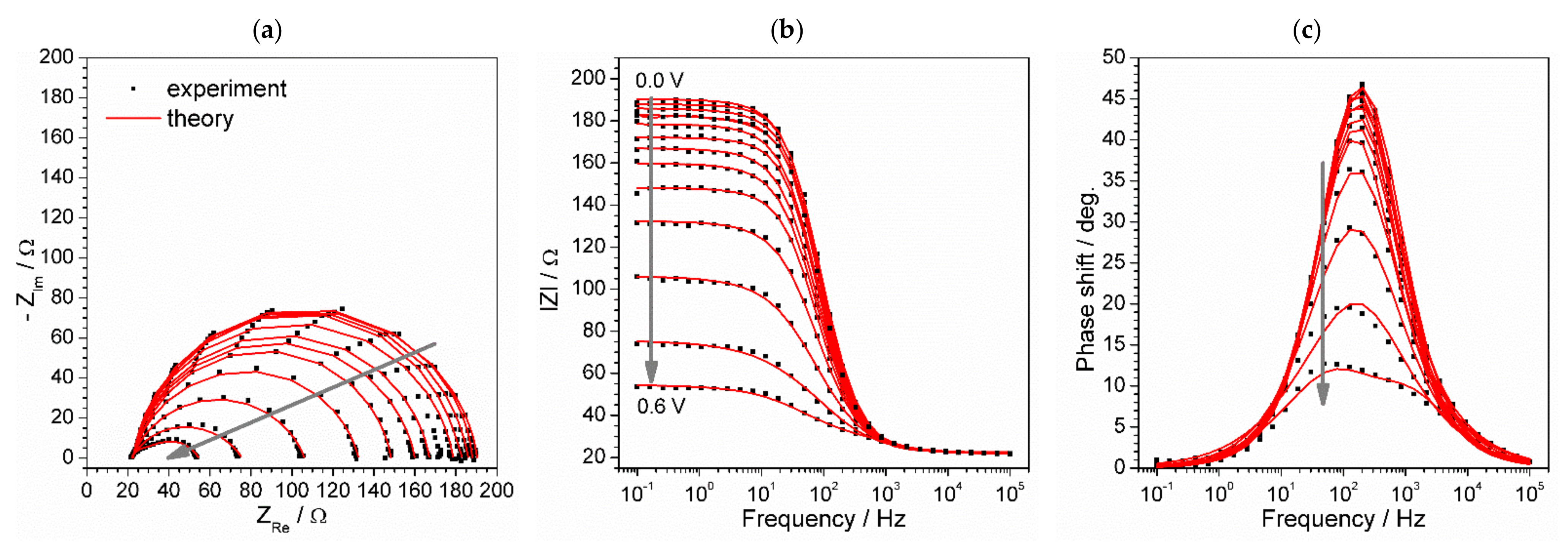
3.3. Photovoltaic Response of DSSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oregan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.Z. Optical properties and applications of hybrid semiconductor nanomaterials. Coord. Chem. Rev. 2009, 253, 3015–3041. [Google Scholar] [CrossRef]
- Meng, Y.M.; Lin, Y.; Yang, J.Y. Synthesis of rod-cluster ZnO nanostructures and their application to dye-sensitized solar cells. Appl. Surf. Sci. 2013, 268, 561–565. [Google Scholar] [CrossRef]
- Fan, J.; Li, Z.; Zhou, W.; Miao, Y.; Zhang, Y.; Hu, J.; Shao, G. Dye-sensitized solar cells based on TiO2 nanoparticles/nanobelts double-layered film with improved photovoltaic performance. Appl. Surf. Sci. 2014, 319, 75–82. [Google Scholar] [CrossRef]
- Babar, F.; Umer, M.; Hafza, A.; Mehdi, M.H.; Ul Haq Khan, A.; Khalid, H.; ul Huda, N.; Fatima, Z. Nanostructured photoanode materials and their deposition methods for efficient and economical third generation dye-sensitized solar cells: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 129, 1–13. [Google Scholar] [CrossRef]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar]
- Mehmood, U.; Malaibari, Z.; Rabani, F.A.; Rehman, A.U.; Ahmad, S.H.A.; Atieh, M.A.; Kamal, M.S. Photovoltaic improvement and charge recombination reduction by aluminum oxide impregnated MWCNTs/TiO2 based photoanode for dyesensitized solar cells. Electrochim. Acta 2016, 203, 162–170. [Google Scholar] [CrossRef]
- Gratzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- Iwata, S.; Shibakawa, S.; Imawaka, N.; Yoshino, K. Stability of the current characteristics of dye-sensitized solar cells in the second quadrant of the current–voltage characteristics. Energy Rep. 2018, 4, 8–12. [Google Scholar] [CrossRef]
- Song, L.; Du, P.; Xiong, J.; Ko, F.; Cui, C. Efficiency enhancement of dye-sensitized solar cells by optimization of electrospun ZnO nanowire/nanoparticle hybrid photoanode and combined modification. Electrochim. Acta 2015, 163, 330–337. [Google Scholar] [CrossRef]
- Wu, C.-T.; Wu, J.-J. Three-dimensional zno nanodendrite/nanoparticle composite solar cells. In Proceedings of the 2010 35th IEEE Photovoltaic Specialists Conference, Honolulu, HI, USA, 20–25 June 2010; pp. 3267–3269. [Google Scholar]
- Ahmad, W.; Mehmood, U.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Aslam, M.Z.; Kamal, M.S.; Shawabkeh, R.A. Synthesis of zinc oxide/titanium dioxide (ZnO/TiO2) nanocomposites by wet incipient wetness impregnation method and preparation of ZnO/TiO2 paste using poly(vinylpyrrolidone) for efficient dye sensitized solar cells. Electrochim. Acta 2016, 222, 473–480. [Google Scholar] [CrossRef]
- Qiu, L.; Ono, L.K.; Qi, Y. Advances and challenges to the commercialization of organic–inorganic halide perovskite solar cell technology. Mater. Today Energy 2018, 7, 169–189. [Google Scholar] [CrossRef]
- Etxebarria, I.; Ajuria, J.; Pacios, R. Polymer:fullerene solar cells: Materials, processing issues, and cell layouts to reach power conversion efficiency over 10%, a review. J. Photonics Energy 2015, 5, 057214. [Google Scholar] [CrossRef]
- Zhu, P.; Reddy, M.V.; Wu, Y.; Peng, S.; Yang, S.; Nair, A.S.; Loh, K.P.; Chowdari, B.V.R.; Ramakrishana, S. Mesoporous SnO2 agglomerates with hierarchical structures as an efficient dual-functional material for dye-sensitized solar cells. Chem. Commun. 2012, 48, 10865–10867. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Pechy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2 -based solar cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef]
- Dembele, K.T.; Selopal, G.G.; Soldano, C.; Nehcache, R.; Rimada, J.C.; Concina, I.; Sberveglieri, G.; Rosei, F.; Vomiero, A. Hybrid Carbon Nanotubes−TiO2 Photoanodes for High Efficiency Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 14510–14517. [Google Scholar] [CrossRef]
- De Marco, L.; Manca, M.; Giannuzzi, R.; Malara, F.; Melcarne, G.; Ciccarella, G.; Zama, I.; Cingolani, R.; Gigli, G. Novel Preparation Method of TiO2 -Nanorod-Based Photoelectrodes for Dye-Sensitized Solar Cells with Improved LightHarvesting Efficiency. J. Phys. Chem. C 2010, 114, 4228. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Liao, J.-Y.; Chen, H.-Y.; Yu, X.-Y.; Su, C.-Y.; Kuang, D.-B. Dye-sensitized solar cells based on a double layered TiO2 photoanode consisting of hierarchical nanowire arrays and nanoparticles with greatly improved photovoltaic performance. J. Mater. Chem. 2012, 22, 18057. [Google Scholar] [CrossRef]
- Tan, B.; Wu, Y. Dye-Sensitized Solar Cells Based on Anatase TiO2 Nanoparticle / Nanowire Composites. J. Phys. Chem. B 2006, 110, 15932. [Google Scholar] [CrossRef]
- Luo, J.; Gao, L.; Sun, J.; Liu, Y. A bilayer structure of a titania nanoparticle/highlyordered nanotube array for low-temperature dye-sensitized solar cells. RSC Adv. 2012, 2, 1884. [Google Scholar] [CrossRef]
- Sun, K.C.; Qadir, M.B.; Jeong, S.H. Hydrothermal synthesis of TiO2 nanotubes and their application as an over-layer for dye-sensitized solar cells. RSC Adv. 2014, 4, 23223. [Google Scholar] [CrossRef]
- Lin, Y.P.; Lin, S.Y.; Lee, Y.C.; Chen-Yang, Y.W. High surface area electrospun prickle-like hierarchical anatase TiO2 nanofibers for dye-sensitized solar cell photoanodes. J. Mater. Chem. A 2013, 1, 9875. [Google Scholar] [CrossRef]
- Liao, J.-Y.; He, J.-W.; Xu, H.; Kuang, D.B.; Su, C.-Y. Effect of TiO2 morphology on photovoltaic performance of dye-sensitized solar cells: Nanoparticles, nanofibers, hierarchical spheres and ellipsoid spheres. J. Mater. Chem. 2012, 22, 7910–7918. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, F.; Li, J.; Dong, H.; Li, Q.; Jiang, K.; Xu, D. Monodisperse TiO2 hierarchical hollow spheres assembled by nanospindles for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 11665. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, F.; Ren, X.; Wang, Y.; Shao, W.; Li, C.; Huang, G. Efficient Light Scattering from One-Pot Solvothermally Derived TiO2 Nanospindles. Ind. Eng. Chem. Res. 2011, 50, 9003–9008. [Google Scholar] [CrossRef]
- Tański, T.; Jarka, P.; Szindler, M.; Drygała, A.; Matysiak, W.; Libera, M. Study of dye sensitized solar cells photoelectrodes consisting of nanostructures. Appl. Surf. Sci. 2019, 491, 807–813. [Google Scholar] [CrossRef]
- Jarka, P.; Drygała, A.; Szindler, M.; Tański, T.; Matysiak, W. Influence of Screen Printed Nanowires/Nanoparticles TiO2 Nanocomposite Layer on Properties of Dye-Sensitized Solar Cells. Acta Phys. Pol. A 2020, 138, 312–316. [Google Scholar] [CrossRef]
- Lin, J.; Chen, J.; Chen, X. High-efficiency dye-sensitized solar cells based on robust and both-end-open TiO2 nanotube membranes. Nanoscale Res. Lett. 2011, 6, 475. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ghicov, A.; Albu, S.P.; Schmuki, P. Bamboo-type TiO2 nanotubes: Improved conversion efficiency in dye-sensitized solar cells. J. Am. Chem. Soc. 2008, 130, 16454–16455. [Google Scholar] [CrossRef]
- Jalali, M.; Moakhar, R.S.; Kushwaha, A.; Goh, G.K.L.; Riahi-Noori, N.; Sadrnezhaad, S.K. Enhanced dye loading-light harvesting TiO2 photoanode with screen printed nanorod-nanoparticles assembly for highly efficient solar cell. Electrochim. Acta 2015, 169, 395–401. [Google Scholar] [CrossRef]
- Selopal, G.S.; Memarian, N.; Milan, R.; Concina, I.; Sberveglieri, G.; Vomiero, A. Effect of Blocking Layer to Boost Photoconversion Efficiency in ZnO Dye-Sensitized Solar Cells. Appl. Mater. Interfaces 2014, 6, 11236–11244. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian-Sarcheshmeh, H.; Arazi, R.; Mazloum-Ardakani, M. Application of bifunctional photoanode materials in DSSCs. Renew. Sustain. Energy Rev. 2020, 134, 110249. [Google Scholar] [CrossRef]
- Ardakani, M.M.; Firouzabadi, A.D.; Benvidi, A.; Fatemeh Mirjalili, B.B.; Zare, R. Effect of Hydroquinone Dderivatives in Electrolytes on Dye-Sensitized Solar Cell Performance. J. Nanostruct. 2014, 4, 37–44. [Google Scholar]
- Ardakani, M.M.; Arazi, R.; Fatemeh Mirjalili, B.B.; Azad, S. Synthesis and application of Fe3O4@nanocellulose/TiCl as a nanofiller for high performance of quasisolid-based dye-sensitized solar cells. Int. J. Energy Res. 2019, 43, 4483–4494. [Google Scholar] [CrossRef]
- Shao, W.; Gu, F.; Gai, L.L.; Li, C.Z. Planar scattering from hierarchical anatase TiO2 nanoplates with variable shells to improve light harvesting in dye-sensitized solar cells. Chem. Commun. 2011, 47, 5046–5048. [Google Scholar] [CrossRef] [PubMed]
- Ghannadi, S.; Abdizadeha, H.; Rakhsha, A.; Golobostanfard, M.R. Sol-electrophoretic deposition of TiO2 nanoparticle/nanorod array for photoanode of dye-sensitized solar cell. Mater. Chem. Phys. 2021, 258, 123893. [Google Scholar] [CrossRef]
- López-Covarrubias, J.G.; Soto-Muñoz, L.; Iglesias, A.L.; Villarreal-Gómez, L.J. Electrospun Nanofibers Applied to Dye Solar Sensitive Cells: A Review. Materials 2019, 19, 3190. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, Y.; Wang, X.; Zhang, L.; Wang, D.; Fu, Z.; Lina, Y. Branched hierarchical photoanode of anatase TiO2 nanotubes on rutile TiO2 nanorod arrays for efficient quantum dot-sensitized solar cells. J. Mater. Chem. A 2015, 3, 4445–4452. [Google Scholar] [CrossRef]
- Hegazy, A.; Kinadjian, N.; Sadeghimakki, B.; Sivoththaman, S.; Allam, N.K.; Prouzet, E. TiO2 nanoparticles optimized for photoanodes tested in large area Dye-sensitized solar cells (DSSC). Sol. Energy Mater. Sol. Cells 2016, 153, 108–116. [Google Scholar] [CrossRef]
- Hegazy, A.; Prouzet, E.; Chim, C.R. Effect of physical chemistry parameters in photocatalytic properties of TiO2 nanocrystals. Comptes Rendus Chim. 2013, 16, 651–659. [Google Scholar] [CrossRef]
- Zhao, P.; Yao, S.; Wang, M.; Wang, B.; Sun, P.; Liu, F.; Liang, X.; Sun, Y.; Lu, G. High-efficiency dye-sensitized solar cells with hierarchical structures titanium dioxide to transfer photogenerated charge. Electrochim. Acta 2015, 170, 276–283. [Google Scholar] [CrossRef]
- Hardin, B.E.; Hoke, E.T.; Armstrong, P.B.; Yum, J.-H.; Comte, P.; Torres, T.; Fréchet, J.M.J.; Nazeeruddin, M.K.; Grätzel, M.; McGehee, M.D. Increased light harvesting in dye-sensitized solar cells with energy relay dyes. Nat. Photonics 2009, 3, 406. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, D.K.; Ihn, K.J.; Jo, S.M.; Kim, D.Y. Electrospun TiO2 Electrodes for Dye-Sensitized Solar Cells. Nanotechnology 2004, 15, 1861–1865. [Google Scholar] [CrossRef]
- Wang, X.; He, G.; Fong, H.; Zhu, Z. Electron Transport and Recombination in Photoanode of Electrospun TiO2 Nanotubes for Dye-Sensitized Solar Cells. Phys. Chem. C 2013, 117, 1641–1646. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.M.; Smolin, Y.Y.; Hagaman, D.; Soroush, M.; Lau, K.K.S.; Ji, H.F. Suitability of N-propanoic acid spiropyrans and spirooxazines for use as sensitizing dyes in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2017, 19, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Yildirim, E.; Akhtar, J.; Siddiqi, H.M.; El-Shafei, A. A comparative study of the influence of N,N′-dialkyl vs. N,N′-diaryl-based electron donor ancillary ligands on photocurrent and photovoltage in dye-sensitized solar cells (DSSCs). Phys. Chem. Chem. Phys. 2017, 19, 20847–20860. [Google Scholar] [CrossRef] [PubMed]
- Kula, S.; Szlapa-Kula, A.; Fabiańczyk, A.; Gnida, P.; Libera, M.; Bujak, K.; Siwy, M.; Schab-Balcerzak, E. Effect of thienyl units in cyanoacrylic acid derivatives toward dye-sensitized solar cells. J. Photochem. Photobiol. B Biol. 2019, 197, 111555. [Google Scholar] [CrossRef]
- Wang, X.; Bolag, A.; Yun, W.; Du, Y.; Eerdun, C.; Zhang, X.; Bao, T.; Ning, J.; Alata, H.; Ojiyed, T. Enhanced performance of dye-sensitized solar cells based on a dual anchored diphenylpyranylidene dye and N719 co-sensitization. J. Mol. Struct. 2020, 1206, 127694. [Google Scholar] [CrossRef]
- Fabiańczyk, A.; Gnida, P.; Chulkin, P.; Kula, S.; Filapek, M.; Szlapa-Kula, A.; Szlapa-Kula, A.; Janeczek, H.; Schab-Balcerzak, E. Effect of heterocycle donor in 2-cyanoacrylic acid conjugated derivatives for DSSC applications. Sol. Energy 2020, in press. [Google Scholar] [CrossRef]
- Kotowicz, S.; Sęk, D.; Kula, S.; Fabiańczyk, A.; Małecki, J.G.; Gnida, P.; Maćkowski, S.; Siwy, M.; Schab-Balcerzak, E. Photoelectrochemical and thermal characterization of aromatic hydrocarbons substituted with a dicyanovinyl unit. Dye Pigment 2020, 180. [Google Scholar] [CrossRef]
- Su, R.; Lyu, L.; Elmorsy, M.R.; El-Shafei, A. Structural studies and photovoltaic investigation of indolo [2,3-: B] quinoxaline-based sensitizers/co-sensitizers achieving highly efficient DSSCs. New J. Chem. 2020, 44, 2797–2812. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Z.; Jia, C.; Wang, Y.; Wu, X.; Yao, X. Co-sensitization of Dithiafulvenyl-Phenothiazine Based Organic Dyes with N719 for Efficient Dye-Sensitized Solar Cells. Electrochim. Acta 2016, 211, 364–374. [Google Scholar] [CrossRef]
- Elmorsy, M.R.; Su, R.; Abdel-Latif, E.; Badawy, S.A.; El-Shafei, A.; Fadda, A.A. New cyanoacetanilides based dyes as effective co-sensitizers for DSSCs sensitized with ruthenium (II) complex (HD-2). J. Mater. Sci. Mater. Electron. 2020, 31, 7981–7990. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Y.; Ding, W.; Yu, G.; Hu, Z.; Wang, H.; Liu, S.; Zou, Y.; Pan, C. Photovoltaic performance of long-chain poly(triphenylamine-phenothiazine) dyes with a tunable π-bridge for dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 14217–14227. [Google Scholar] [CrossRef]
- Elmorsy, M.R.; Abdel-Latif, E.; Badawy, S.A.; Fadda, A.A. Molecular geometry, synthesis and photovoltaic performance studies over 2-cyanoacetanilides as sensitizers and effective co-sensitizers for DSSCs loaded with HD-2. J. Photochem. Photobiol. A Chem. 2020, 389, 112239. [Google Scholar] [CrossRef]
- Unny, D.; Kandregula, G.R.; Sivanadanam, J.; Ramanujam, K. Molecular engineering of pyrene carbazole dyes with a single bond and double bond as the mode of linkage. New J. Chem. 2020, 44, 16511–16525. [Google Scholar] [CrossRef]
- Su, R.; Elmorsy, M.R.; Abed, M.; Islam, A.; Lord, M.; Fadda, A.A.; El-Shafei, A. A Comparative Study on Two RuII Complexes with Thiophene-Based Ancillary Ligands for High-Efficiency Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2017, 2017, 3690–3697. [Google Scholar] [CrossRef]
- Tanaka, E.; Michaels, H.; Freitag, M.; Robertson, N. Synergy of co-sensitizers in a copper bipyridyl redox system for efficient and cost-effective dye-sensitized solar cells in solar and ambient light. J. Mater. Chem. A 2020, 8, 1279–1287. [Google Scholar] [CrossRef]
- Cole, J.M.; Pepe, G.; Al Bahri, O.K.; Cooper, C.B. Cosensitization in Dye-Sensitized Solar Cells. Chem. Rev. 2019, 119, 7279–7327. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, M.R.; Lyu, L.; Su, R.; Abdel-Latif, E.; Badawy, S.A.; El-Shafei, A.; Fadda, A.A. Co-sensitization of the HD-2 complex with low-cost cyanoacetanilides for highly efficient DSSCs. Photochem. Photobiol. Sci. 2020, 19, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Carella, A.; Borbone, F.; Centore, R. Research progress on photosensitizers for DSSC. Front. Chem. 2018, 6, 1–24. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Smestad, G.P. Education and solar conversion: Demonstrating electron transfer. Sol. Energy Mater. Sol. Cells 1998, 55, 157–178. [Google Scholar] [CrossRef]
- Błaszczyk, A. Strategies to improve the performance of metal-free dye-sensitized solar cells. Dye Pigment 2018, 149, 707–718. [Google Scholar] [CrossRef]
- Słodek, A.; Zych, D.; Szafraniec-Gorol, G.; Gnida, P.; Vasylieva, M.; Schab-Balcerzak, E. Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight. Materials 2020, 13, 2292. [Google Scholar] [CrossRef] [PubMed]
- Bandarenka, A.S.; Ragoisha, G.A. Inverse Problem in Potentiodynamic Electrochemical Impedance Spectroscopy. In Progress in Chemometrics Research; Nova Science Publishers: New York, NY, USA, 2005; pp. 89–102. [Google Scholar]
- Chang, S.-M.; Lin, C.-L.; Chen, Y.-J.; Wang, H.-C.; Chang, W.-C.; Lin, L.-Y. Investigation of benzo (1,2-b:4,5-b′) dithiophene as a spacer in organic dyes for high efficient dye-sensitized solar cell. Org. Electron. 2015, 25, 254–260. [Google Scholar] [CrossRef]
- Uam, H.-S.; Jung, Y.-S.; Jun, Y.; Kim, K.-J. Relation of Ru(II) dye desorption from TiO2 film during illumination with photocurrent decrease of dye-sensitized solar cells. J. Photochem. Photobiol. A 2010, 212, 122–128. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Orwat, B.; Zalas, M.; Ledwon, P.; Kownacki, I.; Tejchman, W. 2-Thiohydantoin Moiety as a Novel Acceptor/Anchoring Group of Photosensitizers for Dye-Sensitized Solar Cells. Materials 2020, 13, 2065. [Google Scholar] [CrossRef]


| TiO2 Nanoparticles | Sensitizers | AFM | Optical Microscope | SEM |
|---|---|---|---|---|
| RMS (nm) | Thickness (µm) | Thickness (µm) | ||
| Nanoparticles | - | 20 | - | 10.15 |
| N719 | 16 | 10.3 | - | |
| AC-9 | 15 | 14 | - | |
| N719 + AC-9 | 16 | 9.5 | - | |
| N719 + AC-9 + CDCA | 18 | 10.7 | - | |
| Nanoparticles/Nanowires | - | 150 | - | 11.4 |
| N719 | 135 | 12.5 | - | |
| AC-9 | 95 | 10.3 | - | |
| N719 + AC-9 | 130 | 10 | - | |
| N719 + AC9 + CDCA | 138 | 11.1 | - | |
| Nanoparticles/Nanotubes | - | 35 | - | 11.15 |
| N719 | 30 | 11.3 | - | |
| AC-9 | 28 | 9.5 | - | |
| N719 + AC-9 | 30 | 11.4 | - | |
| N719 + AC-9 + CDCA | 33 | 8.2 | - |
| TiO2 Nanostructure | Compounds | Voc (mV) | Jsc | FF | PCE (%) | Dye Loading |
|---|---|---|---|---|---|---|
| (mA cm−2) | (-) | (mol cm−2) | ||||
| Nanoparticles | N719 | 720 | 15.8 | 0.44 | 5.1 | 4.04 × 10−8 |
| AC-9 | 675 | 10.54 | 0.58 | 4.21 | - | |
| N719 + AC-9 | 730 | 15.06 | 0.54 | 6.1 | - | |
| N719 + AC-9 + CDCA | 732 | 15.2 | 0.59 | 6.69 | - | |
| Nanoparticles/Nanowires | N719 | 738 | 8.89 | 0.62 | 4.15 | 3.77 × 10−8 |
| AC-9 | 679 | 5.9 | 0.61 | 2.5 | - | |
| N719 + AC-9 | 740 | 11.48 | 0.57 | 4.9 | - | |
| N719 + AC-9 + CDCA | 740 | 11.34 | 0.63 | 5.44 | - | |
| Nanoparticles/Nanotubes | N719 | 725 | 16.27 | 0.46 | 5.56 | 4.45 × 10−8 |
| AC-9 | 678 | 10.71 | 0.61 | 4.56 | - | |
| N719 + AC-9 | 714 | 16.33 | 0.54 | 6.48 | - | |
| N719 + AC-9 + CDCA | 711 | 16.6 | 0.58 | 6.97 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnida, P.; Jarka, P.; Chulkin, P.; Drygała, A.; Libera, M.; Tański, T.; Schab-Balcerzak, E. Impact of TiO2 Nanostructures on Dye-Sensitized Solar Cells Performance. Materials 2021, 14, 1633. https://doi.org/10.3390/ma14071633
Gnida P, Jarka P, Chulkin P, Drygała A, Libera M, Tański T, Schab-Balcerzak E. Impact of TiO2 Nanostructures on Dye-Sensitized Solar Cells Performance. Materials. 2021; 14(7):1633. https://doi.org/10.3390/ma14071633
Chicago/Turabian StyleGnida, Paweł, Paweł Jarka, Pavel Chulkin, Aleksandra Drygała, Marcin Libera, Tomasz Tański, and Ewa Schab-Balcerzak. 2021. "Impact of TiO2 Nanostructures on Dye-Sensitized Solar Cells Performance" Materials 14, no. 7: 1633. https://doi.org/10.3390/ma14071633
APA StyleGnida, P., Jarka, P., Chulkin, P., Drygała, A., Libera, M., Tański, T., & Schab-Balcerzak, E. (2021). Impact of TiO2 Nanostructures on Dye-Sensitized Solar Cells Performance. Materials, 14(7), 1633. https://doi.org/10.3390/ma14071633









