Full Optimization of an Electroless Nickel Solution: Boosting the Performance of Low-Phosphorous Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Electroless Nickel Bath and Operating Conditions
2.2. Substrate Conditioning
2.3. Selected Orthogonal Array
2.4. Characterization of the Coatings
3. Results and Discussion
3.1. Univariate Optimization
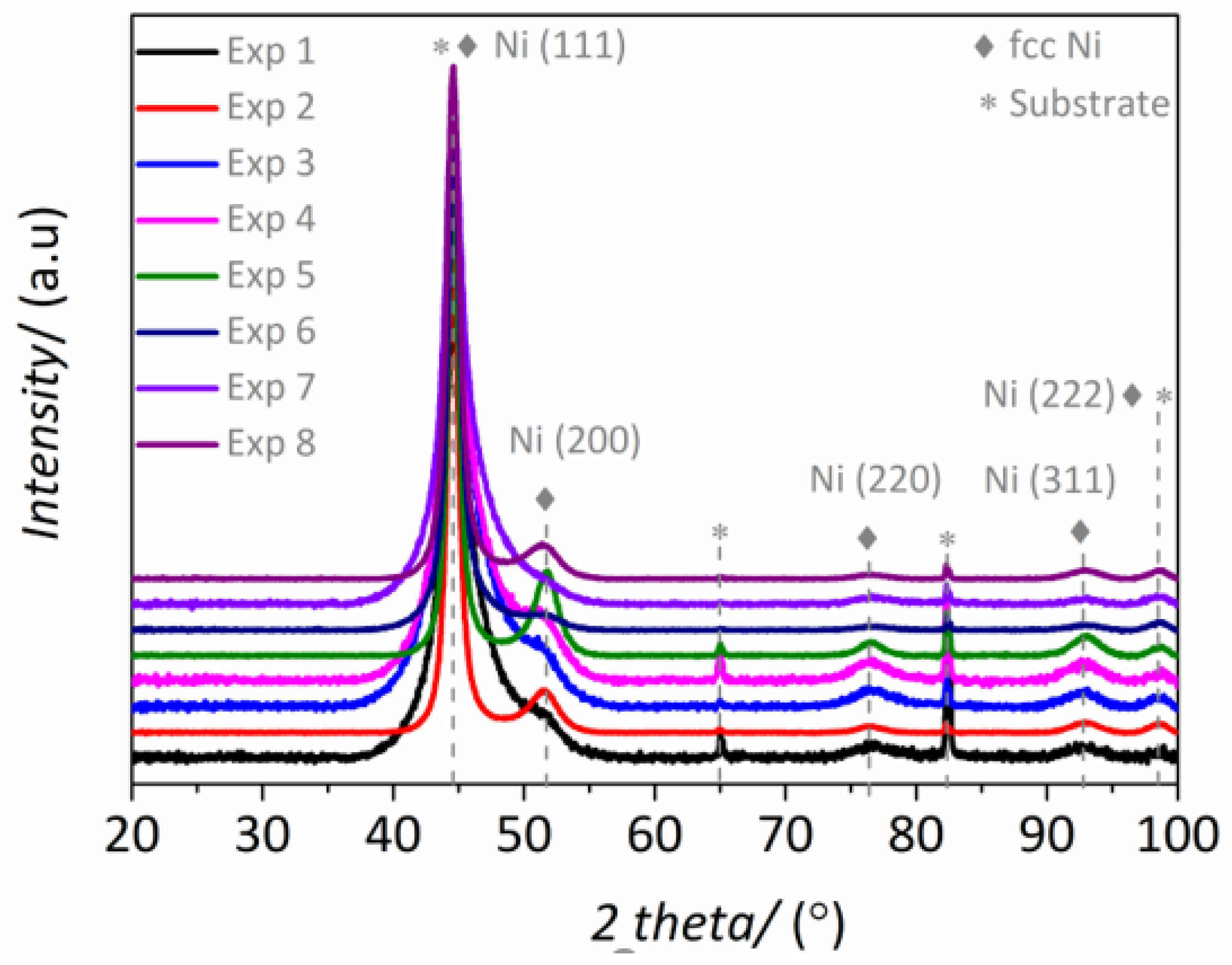
3.1.1. Composition and Crystallographic Structure
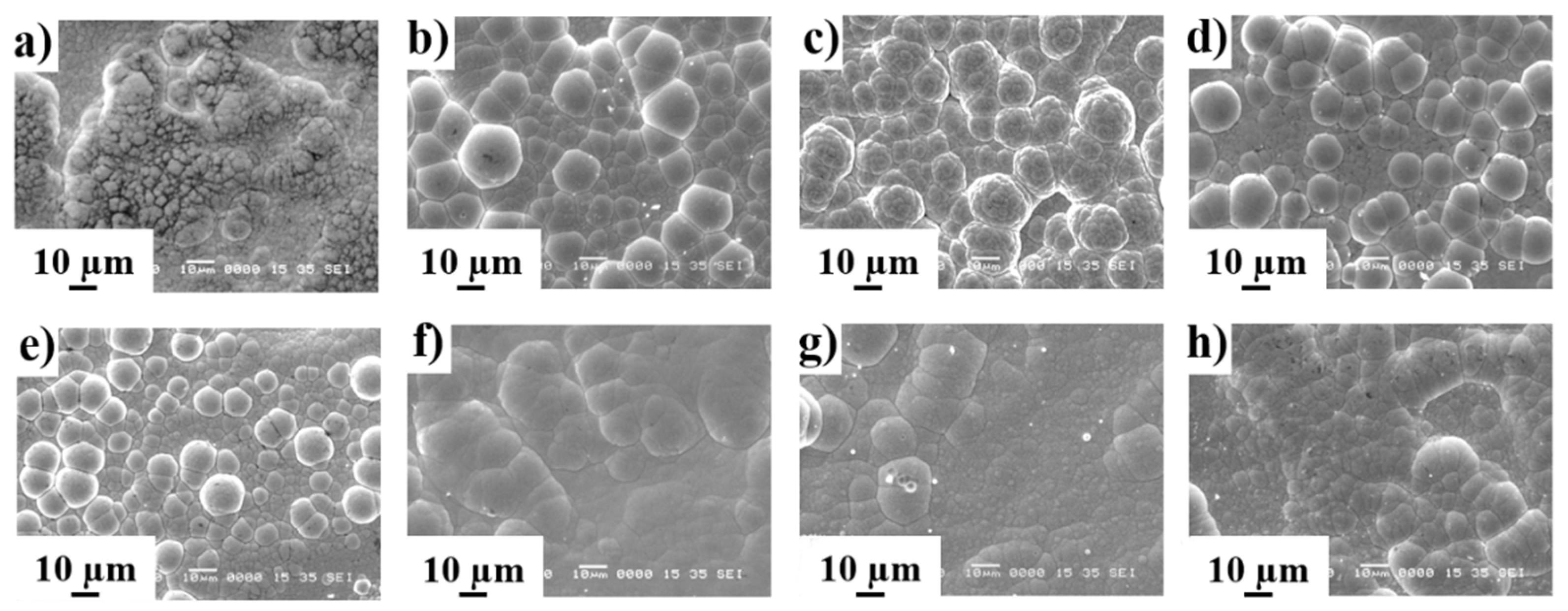
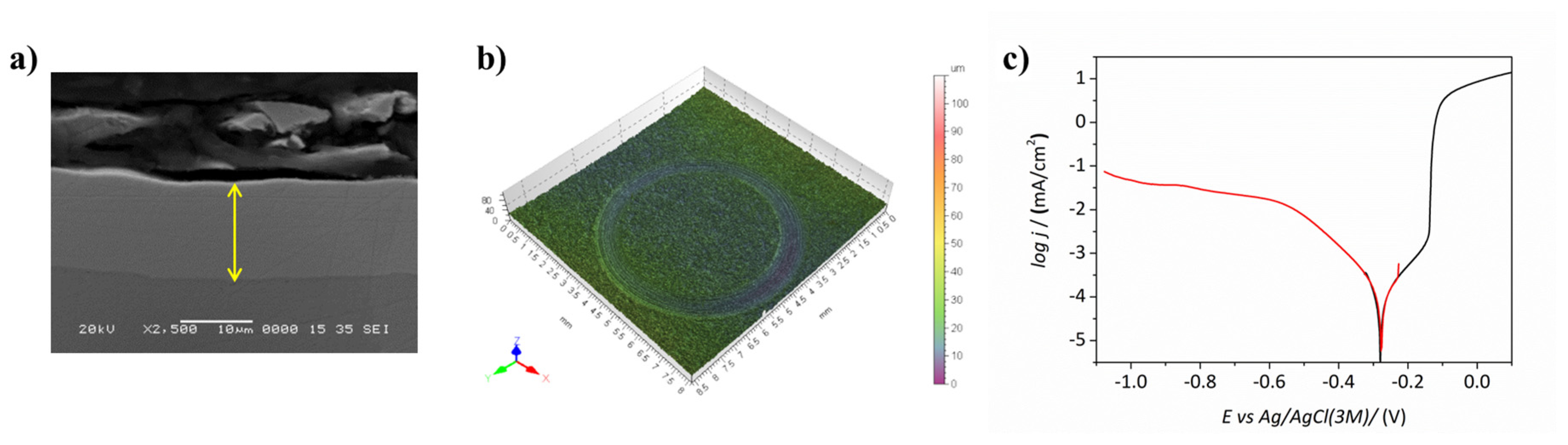
3.1.2. Thickness and Surface Morphology
3.1.3. Hardness
3.1.4. Wear Resistance
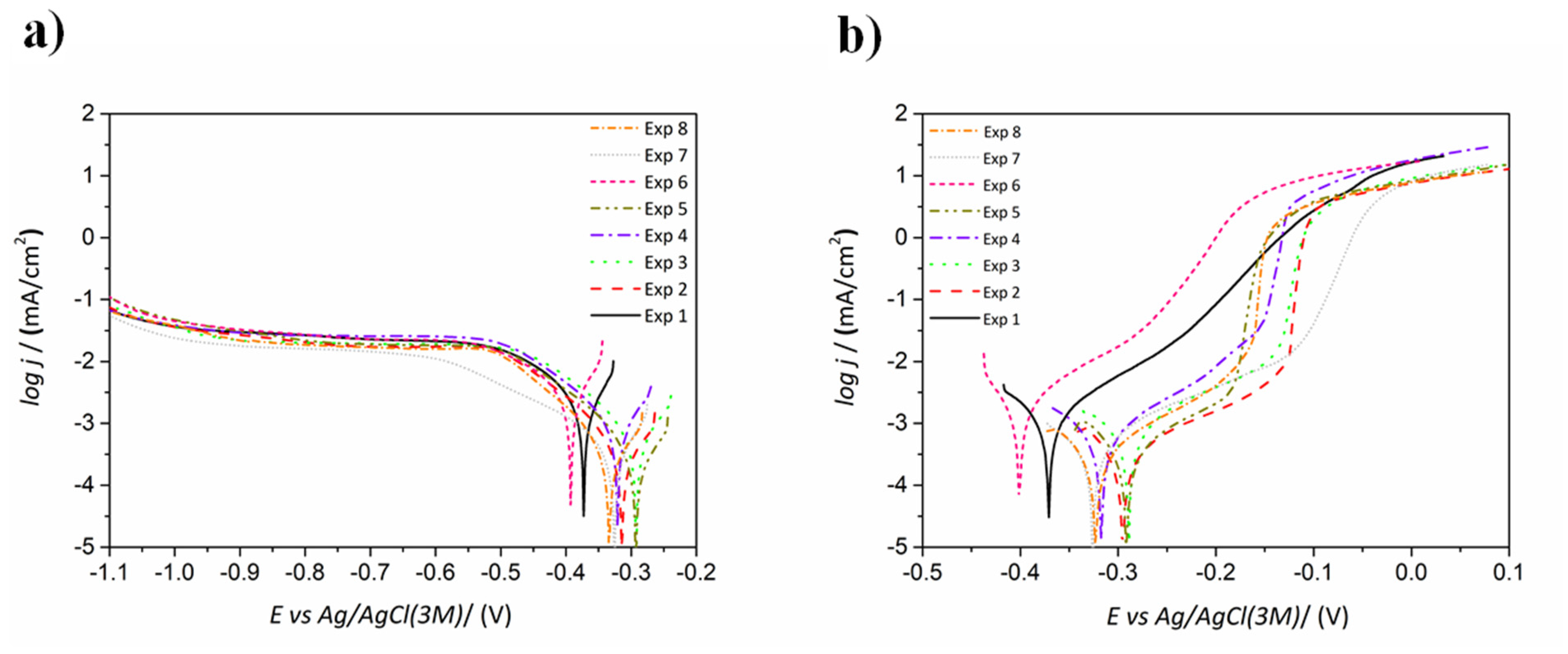
3.1.5. Corrosion Resistance
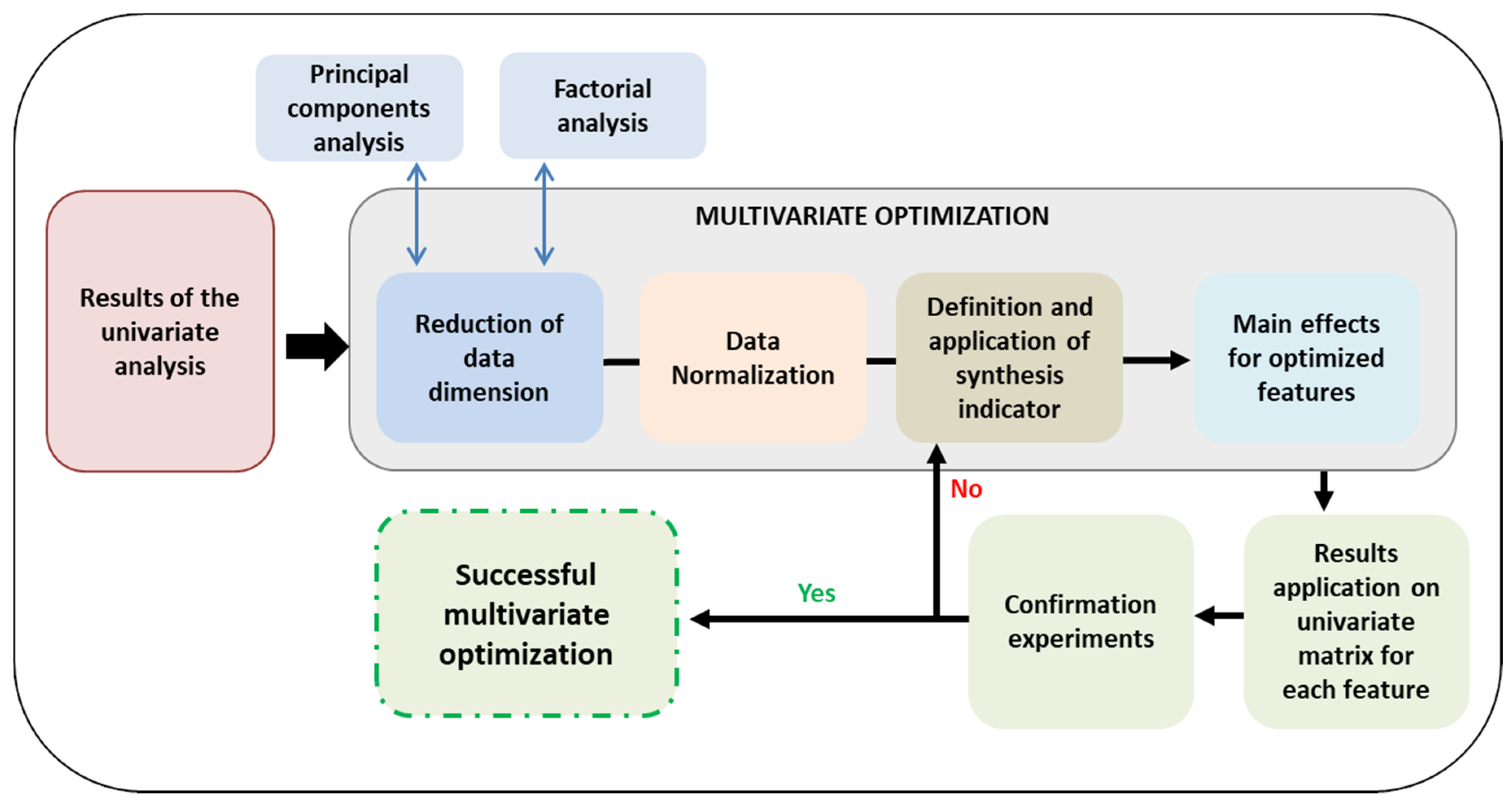
3.2. Multivariate Optimization
Results of the Confirmation Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, A.; Riddell, G. Deposition of nickel and cobalt by chemical reduction. J. Res. Natl. Inst. Stand. Technol. 1947, 39, 385. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings-A critical review. J. Alloy. Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Diegle, R.B.; Sorensen, N.R.; Clayton, C.R.; Helfand, M.A.; Yu, Y.C. An XPS Investigation into the Passivity of an Amorphous Ni-20P Alloy. J. Electrochem. Soc. 1988, 135, 1085–1092. [Google Scholar] [CrossRef]
- Elsener, B.; Crobu, M.; Scorciapino, M.A.; Rossi, A. Electroless deposited Ni–P alloys: Corrosion resistance mechanism. J. Appl. Electrochem. 2008, 38, 1053–1060. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, J.; Lin, N.; Tang, B. Review on electroless plating Ni–P coatings for improving surface performance of steel. Surf. Rev. Lett. 2014, 21, 1–13. [Google Scholar] [CrossRef]
- Zhang, B. Amorphous and Nano Alloys Electroless Depositions; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Riedel, W. Electroless Nickel Plating; ASM International: Cleveland, OH, USA, 1991. [Google Scholar]
- Gawrilov, G.G. Chemical (Electroless) Nickel Plating; Portcullis Press Ltd.: London, UK, 1979. [Google Scholar]
- Yanhai, C.; Hengyang, C.; Zhencai, Z.; Yong, Z.; Yuxing, P. Tribological Behavior of Ni-P Deposits on Dry Condition. Rare Met. Mater. Eng. 2014, 43, 11–16. [Google Scholar] [CrossRef]
- Taheri, R.; Oguocha, I.; Yannacopoulos, S. The tribological characteristics of electroless NiP coatings. Wear 2001, 249, 389–396. [Google Scholar] [CrossRef]
- Mimani, T.; Mayanna, S. The effect of microstructure on the corrosion behaviour of electroless Ni P alloys in acidic media. Surf. Coat. Technol. 1996, 79, 246–251. [Google Scholar] [CrossRef]
- Shen, Y.; Xue, W.; Liu, Z.; Zuo, L. Nanoscratching deformation and fracture toughness of electroless Ni–P coatings. Surf. Coat. Technol. 2010, 205, 632–640. [Google Scholar] [CrossRef]
- Hamada, A.; Sahu, P.; Porter, D. Indentation property and corrosion resistance of electroless nickel–phosphorus coatings deposited on austenitic high-Mn TWIP steel. Appl. Surf. Sci. 2015, 356, 1–8. [Google Scholar] [CrossRef]
- SriBalaji, M.; Arunkumar, P.; Babu, K.S.; Keshri, A.K. Crystallization mechanism and corrosion property of electroless nickel phosphorus coating during intermediate temperature oxidation. Appl. Surf. Sci. 2015, 355, 112–120. [Google Scholar] [CrossRef]
- Farzaneh, A.; Ehteshamzadeh, M.; Mohammadi, M. Corrosion performance of the electroless Ni–P coatings prepared in different conditions and optimized by the Taguchi method. J. Appl. Electrochem. 2010, 41, 19–27. [Google Scholar] [CrossRef]
- Khosrow, D. Quality Control, Robust Design, and the Taguchi Method; Springer: Berlin, Germany, 1988. [Google Scholar]
- Bonin, L.; Castro, C.; Vitry, V.; Hantson, A.-L.; Delaunois, F. Optimization of electroless NiB deposition without stabilizer, based on surface roughness and plating rate. J. Alloy. Compd. 2018, 767, 276–284. [Google Scholar] [CrossRef]
- American Iron and Steel Institute (AISI). AISI 1010 Carbon Steel. AISI 1010; American Iron and Steel Institute: Washington, DC, USA, 1908. [Google Scholar]
- Ishrat, S.I.; Khan, Z.A.; Siddiquee, A.N.; Badruddin, I.A.; AlGahtani, A.; Javaid, S.; Gupta, R. Optimising Parameters for Expanded Polystyrene Based Pod Production Using Taguchi Method. Mathematics 2019, 7, 847. [Google Scholar] [CrossRef]
- Yang, W.; Tarng, Y. Design optimization of cutting parameters for turning operations based on the Taguchi method. J. Mater. Process. Technol. 1998, 84, 122–129. [Google Scholar] [CrossRef]
- Bruker EVA, Version 2020. Software for the Analysis of 1D and 2D X-ray Datasets Including Visualization, Data Reduction, Phase Identification and Quantification, Statistical Evaluation; Bruker: Billerica, MA, USA, 2020. [Google Scholar]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Clove oil nanoemulsion as an effective antibacterial agent: Taguchi optimization method. Desalination Water Treat. 2015, 57, 18379–18390. [Google Scholar] [CrossRef]
- Chen, K.; Liu, C.; Whalley, D.C.; Hutt, D.A.; Li, J.F.; Mannan, S.H. Electroless Ni-W-P alloy coatings as barrier metallisations for liquid solder interconnects. In Proceedings of the 2006 1st Electronic Systemintegration Technology Conference, Dresden, Germany, 5–7 September 2006. [Google Scholar]
- Keong, K.G.; Sha, W. Crystallisation and Phase Transformation Behaviour of Electroless Nickel-Phosphorus Deposits and Their Engineering Properties. Surf. Eng. 2002, 18, 329–343. [Google Scholar] [CrossRef]
- Palaniappa, M.; Seshadri, S. Friction and wear behavior of electroless Ni-P and Ni-W-P alloy coatings. Wear 2008, 265, 735–740. [Google Scholar] [CrossRef]
- Shu, X.; He, Z.; Wang, Y.; Yin, L. Mechanical properties of Ni-based coatings fabricated by electroless plating method. Surf. Eng. 2020, 36, 944–951. [Google Scholar] [CrossRef]
- Popov, K.I.; Djokić, S.S.; Nikolić, N.D.; Jović, V.D. Morphology of Electrochemically and Chemically Deposited Metals; Springer International Publishing: Berlin, Germany, 2016. [Google Scholar]
- Huang, Y.; Cui, F. Effect of complexing agent on the morphology and microstructure of electroless deposited Ni-P alloy. Surf. Coat. Technol. 2007, 201, 5416–5418. [Google Scholar] [CrossRef]
- Rahman, A.; Jayaganthan, R. Effect of pH values on nanostructured Ni-P films. Appl. Nanosci. 2015, 5, 493–498. [Google Scholar] [CrossRef]
- Małecki, A.; Micek-Ilnicka, A. Electroless nickel plating from acid bath. Surf. Coat. Technol. 2000, 123, 72–77. [Google Scholar] [CrossRef]
- Imaz, N.; Ostra, M.; Vidal, M.; Díez, J.; Sarret, M.; García-Lecina, E. Corrosion behaviour of chromium coatings obtained by direct and reverse pulse plating electrodeposition in NaCl aqueous solution. Corros. Sci. 2014, 78, 251–259. [Google Scholar] [CrossRef]
- Imaz, N.; Diez, J.A.; Ostra, M.; Sarret, M.; García-Lecina, E. Advanced Ni–W coatings obtained by the combination of pulse plating and chemometric techniques. Trans. IMF 2014, 92, 305–315. [Google Scholar] [CrossRef]
- Ashtiani, A.A.; Faraji, S.; Iranagh, S.A.; Faraji, A.H. The study of electroless Ni–P alloys with different complexing agents on Ck45 steel substrate. Arab. J. Chem. 2017, 10, S1541–S1545. [Google Scholar] [CrossRef]
- Cui, G.; Li, N.; Li, D.; Chi, M. Study of Optimized Complexing Agent for Low-Phosphorus Electroless Nickel Plating Bath. J. Electrochem. Soc. 2005, 152, C669–C674. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, S.K. Tribology of electroless nickel coatings–A review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Apachitei, I.; Duszczyk, J. Autocatalytic nickel coatings on aluminium with improved abrasive wear resistance. Surf. Coat. Technol. 2000, 132, 89–98. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, H.J.; Hao, L.; Lin, A.; Gan, F.X. Electrochemical corrosion behavior of electroless Ni-P coating in NaCl and H2SO4 solutions. Mater. Corros. 2010, 62, 1003–1007. [Google Scholar] [CrossRef]
- Crobu, M.; Scorciapino, A.; Elsener, B.; Rossi, A.; Scorciapino, M.A. The corrosion resistance of electroless deposited nano-crystalline Ni–P alloys. Electrochim. Acta 2008, 53, 3364–3370. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Yao, M. Studies of Electroless Nickel Deposits with Low Phosphorus Content. Trans. IMF 1999, 77, 78–83. [Google Scholar] [CrossRef]
- Lo, P.-H.; Tsai, W.-T.; Lee, J.-T.; Hung, M.-P. Role of phosphorus in the electrochemical behavior of electroless Ni-P alloys in 3.5 wt.% NaCl solutions. Surf. Coat. Technol. 1994, 67, 27–34. [Google Scholar] [CrossRef]
- Van Gool, A.P.; Boden, P.J.; Harris, S.J. Corrosion Behaviour of some Electroless Nickel—Phosphorus Coatings. Trans. IMF 1987, 65, 108–114. [Google Scholar] [CrossRef]
- Bahramian, A.; Eyraud, M.; Vacandio, F.; Knauth, P. Improving the corrosion properties of amorphous Ni-P thin films using different additives. Surf. Coat. Technol. 2018, 345, 40–52. [Google Scholar] [CrossRef]
- Dearden, J. Electroless Plating—Its Applications in Resistor Technology. Electrocompon. Sci. Technol. 1976, 3, 103–111. [Google Scholar] [CrossRef]
- Zeller, R.L. Electrochemical Corrosion Testing of High Phosphorus Electroless Nickel in 5% NaCl. Corrosion 1991, 47, 692–702. [Google Scholar] [CrossRef]
- Flis, J.; Duquette, D.J. Effect of Phosphorus on Anodic Dissolution and Passivation of Nickel in Near-Neutral Solutions. Corrosion 1985, 41, 700–706. [Google Scholar] [CrossRef]
- Meng, M.; Leech, A.; Le, H. Mechanical properties and tribological behavior of electroless Ni–P–Cu coatings on corrosion-resistant alloys under ultrahigh contact stress with sprayed nanoparticles. Tribol. Int. 2019, 139, 59–66. [Google Scholar] [CrossRef]
- Panja, B.; Sahoo, P. Tribo-corrosion Behavior of Electroless Ni-P Coatings in Alkaline Corrosive Environment. Port. Electrochim. Acta 2014, 32, 303–313. [Google Scholar] [CrossRef]
- Lee, J.-H.; H, W.; B, Y.C.; T, C.K.; Y, H.C. Parametric Optimization for Electroless Ni–P on Low Carbon Steel. J. Comput. Theor. Nanosci. 2016, 13, 1640–1648. [Google Scholar] [CrossRef]
- Jeyapaul, R.; Shahabudeen, P.; Krishnaiah, K. Simultaneous optimization of multi-response problems in the Taguchi method using genetic algorithm. Int. J. Adv. Manuf. Technol. 2006, 30, 870–878. [Google Scholar] [CrossRef]



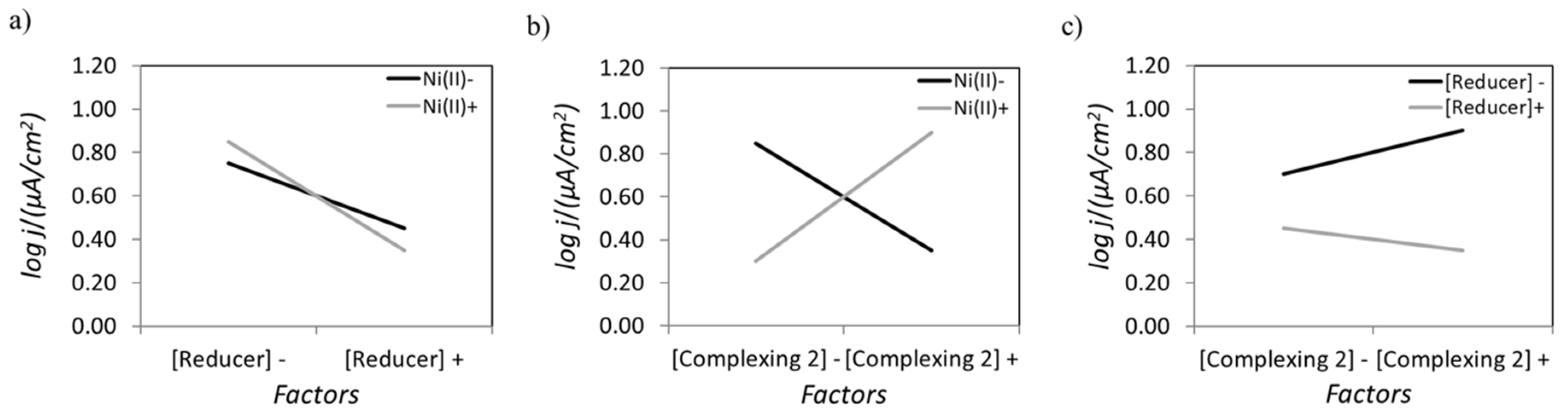
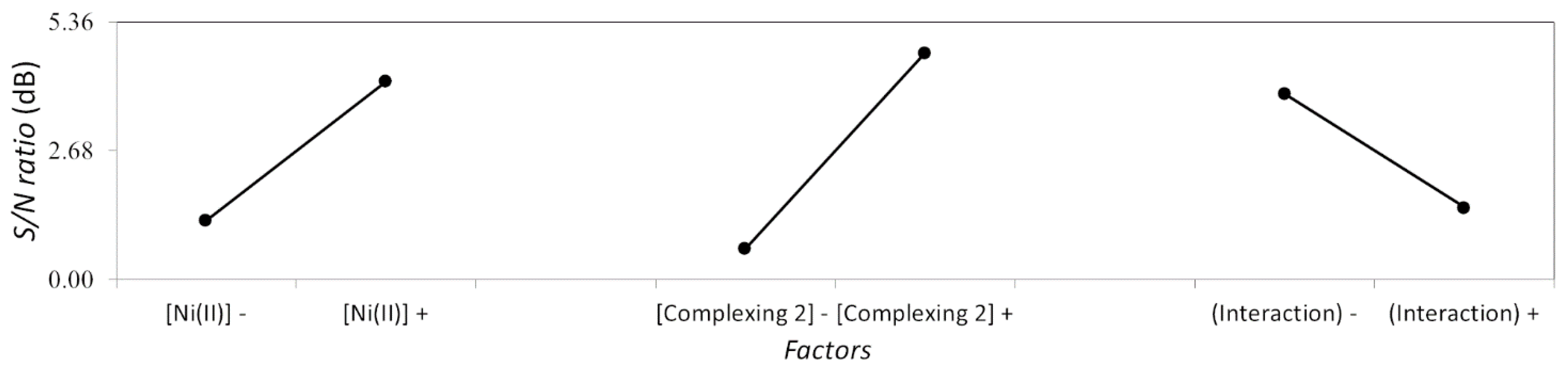

| Factors | A | B | C Interaction A-B | D | E Interaction A-D | F Interaction B-D | G |
|---|---|---|---|---|---|---|---|
| Experiment | Ni (II) g/L | Reducer g/L | Complexing 1 g/L | Complexing 2 g/L | Stirring rpm | T °C | pH |
| 1 | 4.8 | 20.0 | 1.0 | 7.0 | 100 | 75 | 5.2 |
| 2 | 4.8 | 20.0 | 1.0 | 12.0 | 250 | 90 | 6.5 |
| 3 | 4.8 | 30.0 | 3.0 | 7.0 | 100 | 90 | 6.5 |
| 4 | 4.8 | 30.0 | 3.0 | 12.0 | 250 | 75 | 5.2 |
| 5 | 6.5 | 20.0 | 3.0 | 7.0 | 250 | 75 | 6.5 |
| 6 | 6.5 | 20.0 | 3.0 | 12.0 | 100 | 90 | 5.2 |
| 7 | 6.5 | 30.0 | 1.0 | 7.0 | 250 | 90 | 5.2 |
| 8 | 6.5 | 30.0 | 1.0 | 12.0 | 100 | 75 | 6.5 |
| Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| P content (wt.%) | 7.0 | 3.2 | 7.8 | 6.4 | 2.2 | 6.7 | 8.5 | 4.0 |
| S.D. | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 |
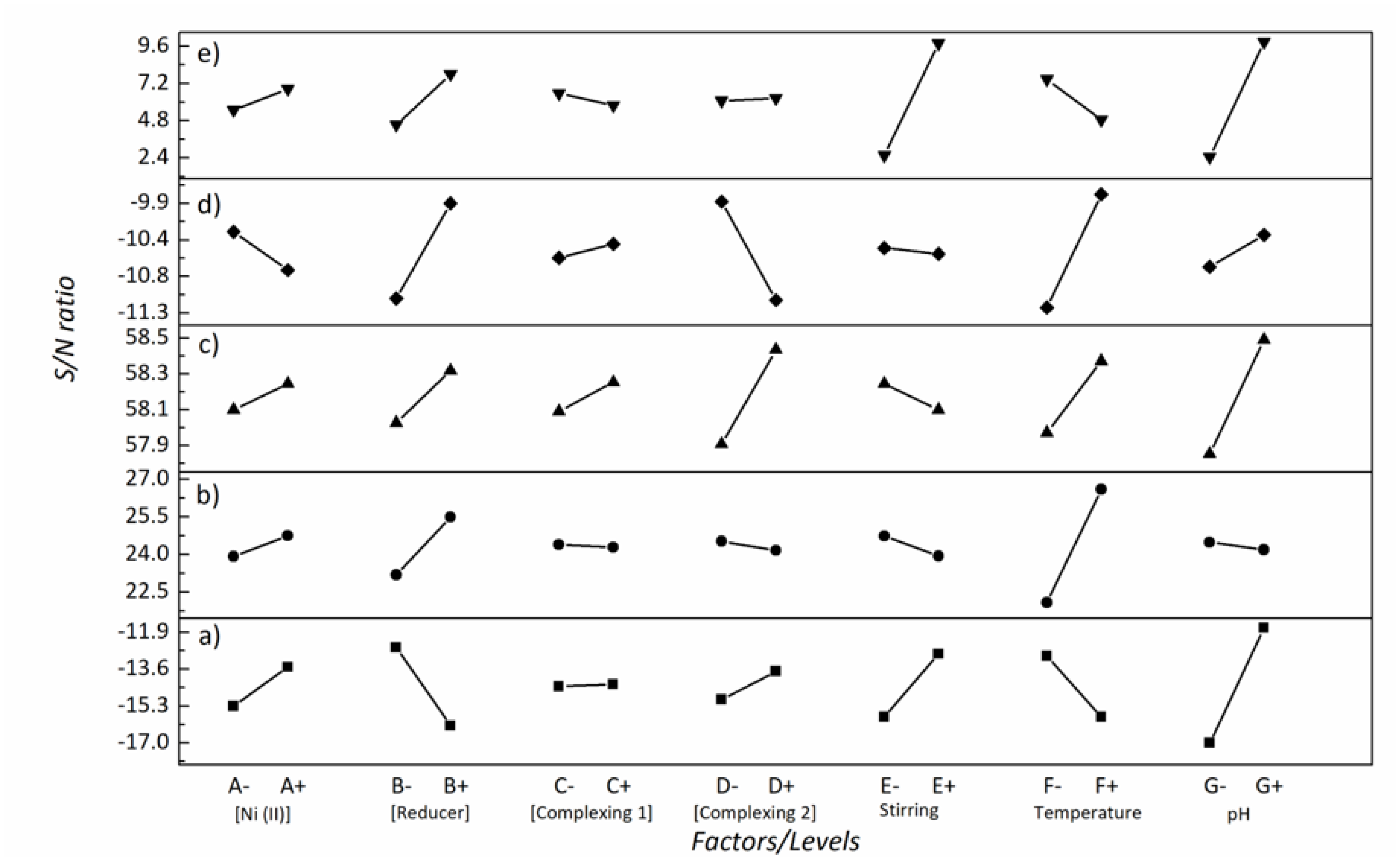
| Factor | [Ni (II)] | [Reducer] | [Complexing 1] | [Complexing 2] | Stirring | T | pH |
|---|---|---|---|---|---|---|---|
| − | −15.3 | −12.6 | −14.4 | −15.0 | −15.8 | −13.0 | −17.0 |
| + | −13.5 | −16.2 | −14.3 | −13.7 | −12.9 | −15.8 | −11.7 |
| Difference | 1.8 | 3.6 | 0.1 | 1.3 | 2.9 | 2.8 | 5.4 |
| Rank | 5 | 2 | 7 | 6 | 3 | 4 | 1 |
| L8 | P wt.% | Thickness µm | Hardness Hv0.1 | Worn Volume (×10−3) mm3 | Corrosion Rate µA/cm2 |
|---|---|---|---|---|---|
| 1 | 7.1 ± 0.1 | 11.6 ± 0.1 | 714 ± 32 | 0.049 ± 0.005 | 1.20 ± 0.04 |
| 2 | 3.2 ± 0.2 | 16.5 ± 0.3 | 853 ± 23 | 0.068 ± 0.012 | 0.30 ± 0.01 |
| 3 | 7.8 ± 0.1 | 24.4 ± 1.6 | 860 ± 7 | 0.230 ± 0.085 | 0.50 ± 0.02 |
| 4 | 6.4 ± 0.1 | 13.1 ± 0.3 | 792 ± 7 | 0.051 ± 0.003 | 0.40 ± 0.01 |
| 5 | 2.2 ± 0.3 | 11.1 ± 0.2 | 788 ± 10 | 0.050 ± 0.004 | 0.20 ± 0.01 |
| 6 | 6.7 ± 0.1 | 20.3 ± 0.3 | 834 ± 5 | 0.045 ± 0.008 | 1.50 ± 0.06 |
| 7 | 8.5 ± 0.3 | 25.6 ± 1.0 | 777 ± 28 | 0.150 ± 0.021 | 0.40 ± 0.03 |
| 8 | 4.0 ± 0.1 | 15.4 ± 0.4 | 873 ± 31 | 0.045 ± 0.005 | 0.30 ± 0.02 |
| Factors and Experiments | A Ni (II) g/L | B Reducer g/L | Interaction A-B |
|---|---|---|---|
| 1 | 4.8 | 7 | - |
| 2 | 4.8 | 12 | - |
| 3 | 6.5 | 7 | - |
| 4 | 6.5 | 12 | - |
| Feature | Units | Equation | Predicted | Experimental |
|---|---|---|---|---|
| P content | wt.% | A+B−C+D+E+F−G+ | 3.3 | 3.1 ± 0.2 |
| Thickness | µm | A+B+C−D−E−F+G- | 24.1 | 24.3 ± 1.6 |
| Hardness | Hv0.1 | A+B+C+D+E−F+G+ | 870 | 863 ± 27 |
| Worn volume | (×10−3) mm3 | A+B−C−D+E+F−G− | 0.045 | 0.045 ± 0.005 |
| Corrosion rate | µA/cm2 | A+B+C−D+E+F−G+ | 0.30 | 0.25 ± 0.03 |
| SI | P | Thickness | Hardness | Wear | Corrosion |
|---|---|---|---|---|---|
| value | −0.2 | 0.1 | 0.1 | −0.35 | −0.25 |
| Factor | [Ni (II)] | [Reducer] | [Complexing 1] | [Complexing 2] | Stirring | T | pH |
|---|---|---|---|---|---|---|---|
| − | −0.131 | 0.080 | 0.057 | −0.251 | −0.204 | 0.186 | −0.258 |
| + | 0.131 | −0.080 | −0.057 | 0.251 | 0.204 | −0.186 | 0.258 |
| Main effect | 0.263 | 0.160 | 0.115 | 0.502 | 0.408 | 0.372 | 0.516 |
| Rank | 5 | 6 | 7 | 2 | 3 | 4 | 1 |
| Optimal level | + | − | − | + | + | − | + |
| Feature | Units | Predicted |
|---|---|---|
| P content | wt.% | 2.2 |
| Thickness | µm | 10.8 |
| Hardness | Hv0.1 | 842 |
| Worn volume | (×10−3) mm3 | 0.045 |
| Corrosion rate | µA/cm2 | 0.02 |
| Feature | Units | Predicted | Experimental |
|---|---|---|---|
| P content | wt.% | 2.2 | 2.2 ± 0.2 |
| Thickness | µm | 10.8 | 12.1 ± 0.4 |
| Hardness | Hv0.1 | 842 | 851 ± 27 |
| Wear | (×10−3 mm3) | 0.045 | 0.068 ± 0.010 |
| Corrosion | (µA/cm2) | 0.02 | 0.07 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salicio-Paz, A.; Ugarte, I.; Sort, J.; Pellicer, E.; García-Lecina, E. Full Optimization of an Electroless Nickel Solution: Boosting the Performance of Low-Phosphorous Coatings. Materials 2021, 14, 1501. https://doi.org/10.3390/ma14061501
Salicio-Paz A, Ugarte I, Sort J, Pellicer E, García-Lecina E. Full Optimization of an Electroless Nickel Solution: Boosting the Performance of Low-Phosphorous Coatings. Materials. 2021; 14(6):1501. https://doi.org/10.3390/ma14061501
Chicago/Turabian StyleSalicio-Paz, Asier, Ixone Ugarte, Jordi Sort, Eva Pellicer, and Eva García-Lecina. 2021. "Full Optimization of an Electroless Nickel Solution: Boosting the Performance of Low-Phosphorous Coatings" Materials 14, no. 6: 1501. https://doi.org/10.3390/ma14061501
APA StyleSalicio-Paz, A., Ugarte, I., Sort, J., Pellicer, E., & García-Lecina, E. (2021). Full Optimization of an Electroless Nickel Solution: Boosting the Performance of Low-Phosphorous Coatings. Materials, 14(6), 1501. https://doi.org/10.3390/ma14061501








