Concept of Evaluation of Mineral Additives’ Effect on Cement Pastes’ Durability and Environmental Suitability
Abstract
1. Introduction
- (a)
- technical—representing material functionality,
- (b)
- environmental—linked to saving of natural resources through prolongation of the construction lifetime, and
- (c)
- High porosity and high permeability (up left)—increases water absorption, intensifies liquid migration, and has a negative effect on strength and deformation.
- High porosity and low permeability (up right)—negatively affects water absorption and strength and deformation, but leads to lower liquid migration.
- Low porosity and high permeability (down left)—decreases water absorption and increases liquid migration.
- Low porosity and low permeability (down right)—this is the ideal case, wherein the lowest water absorption and liquid migration are achieved as well as the highest strength and lowest deformations.
2. Materials and Methods
2.1. Input Materials
2.2. Mix Design and Labelling
2.3. Fresh Cement Pastes and Sample Preparation
2.4. Water Absorption
- md—oven dried constant mass of sample rounded to 0.1 (g);
- mssd—mass of saturated sample rounded to 0.1 (g);
- WA—water absorption (wt.%).
2.5. Compressive and Flexural Strength
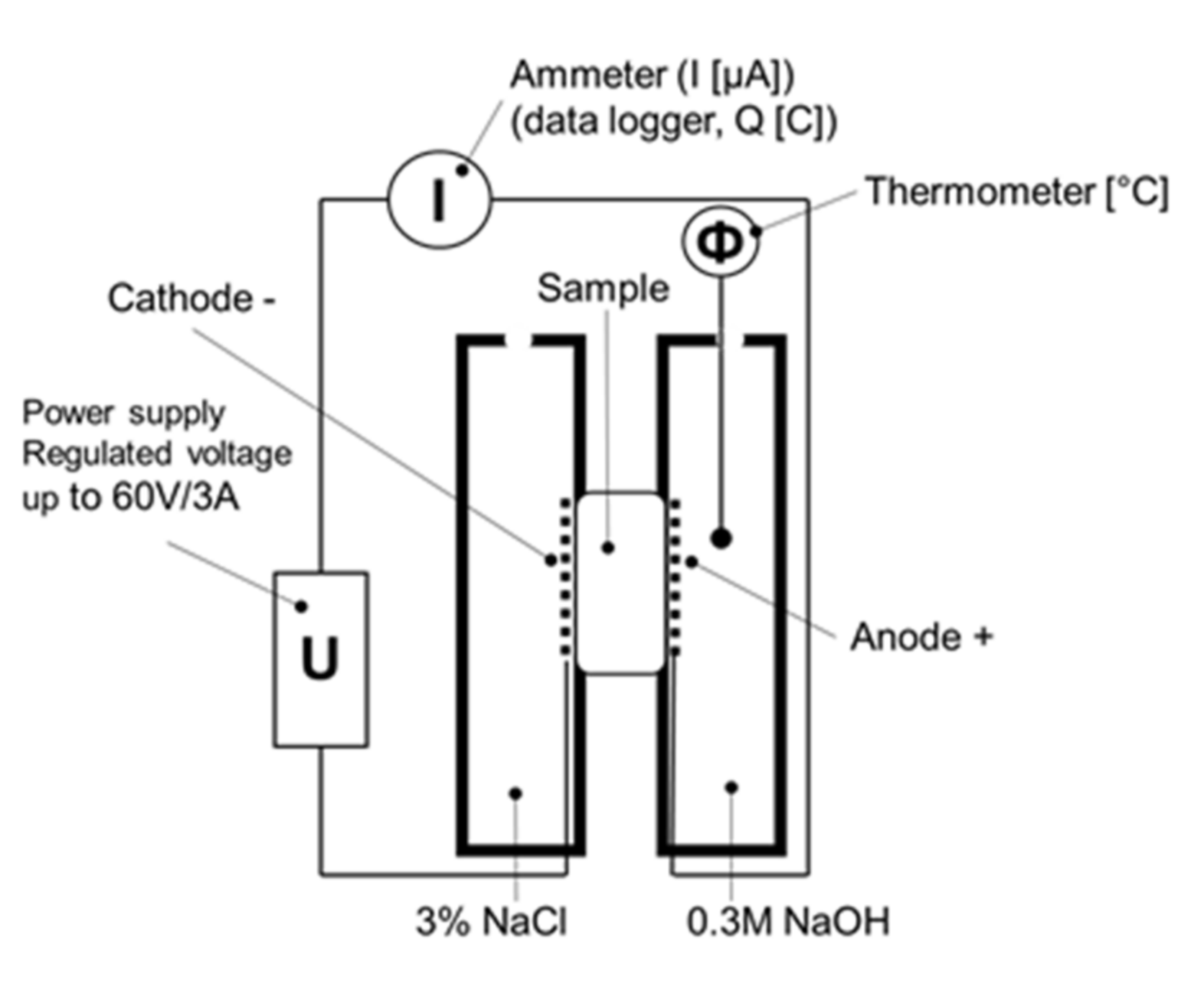
2.6. Rapid Chloride Penetration Test
- Q—overall passing charge (C);
- I—measured electrical current (A);
- t—time (min).
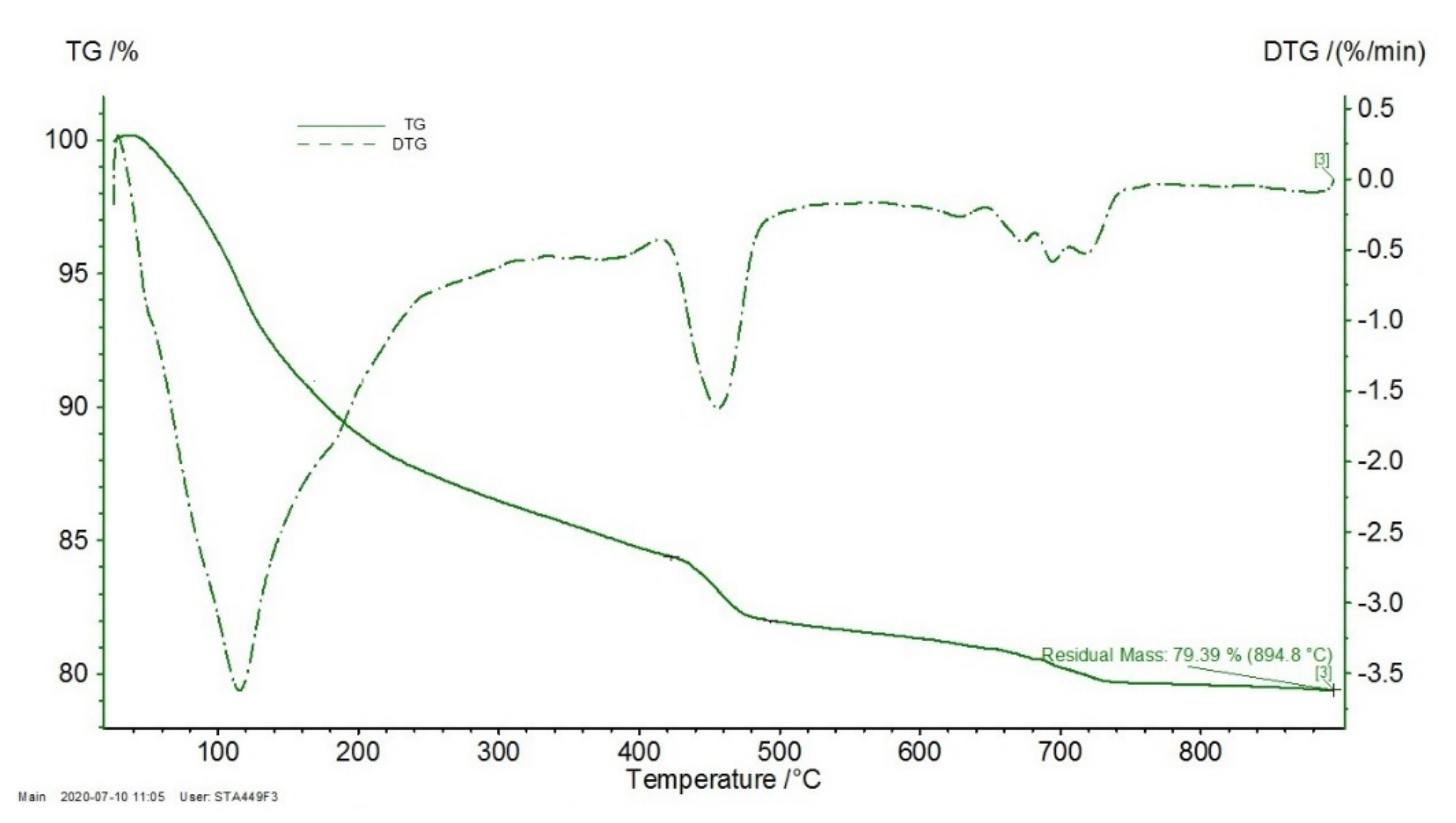
2.7. Thermal Analysis
3. Results and Discussion
3.1. Workability
- (a)
- (b)
- the structure of zeolite (clinoptilolite) crystalline grid-cavities, interconnected by channels, enables water to be stored, leading to a decrease in consistency [81];
- (c)
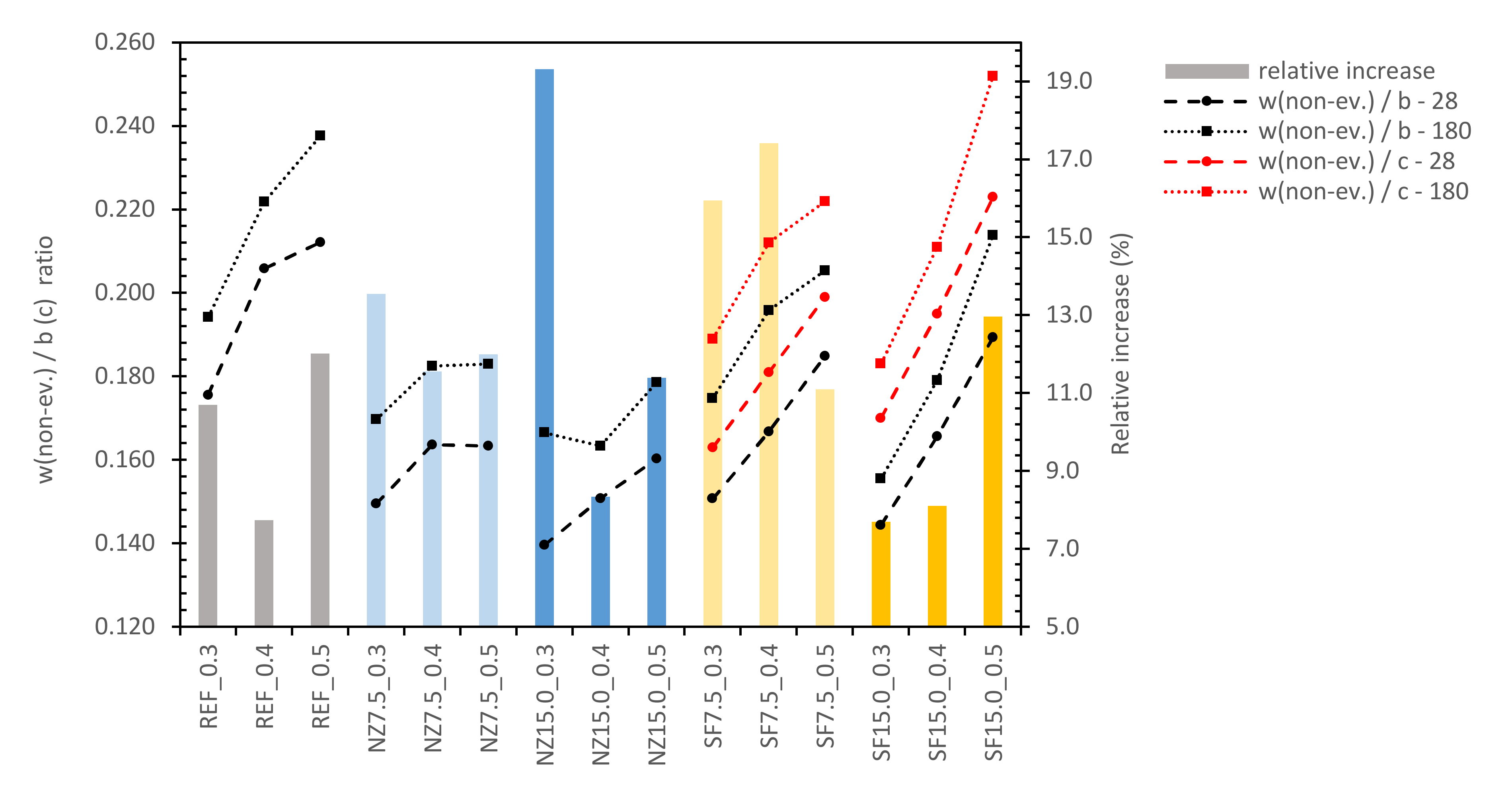
- a less amount of water (w/b = 0.3) [82]. The same is also seen when the results for zeolite and silica fume are compared. SF grains were not fully de-agglomerated and acted as the ball bearings and filler. This also impacted other properties, as will be discussed further. Therefore, the w/b ratio for SF had to be replaced by the w/c ratio, which is higher.
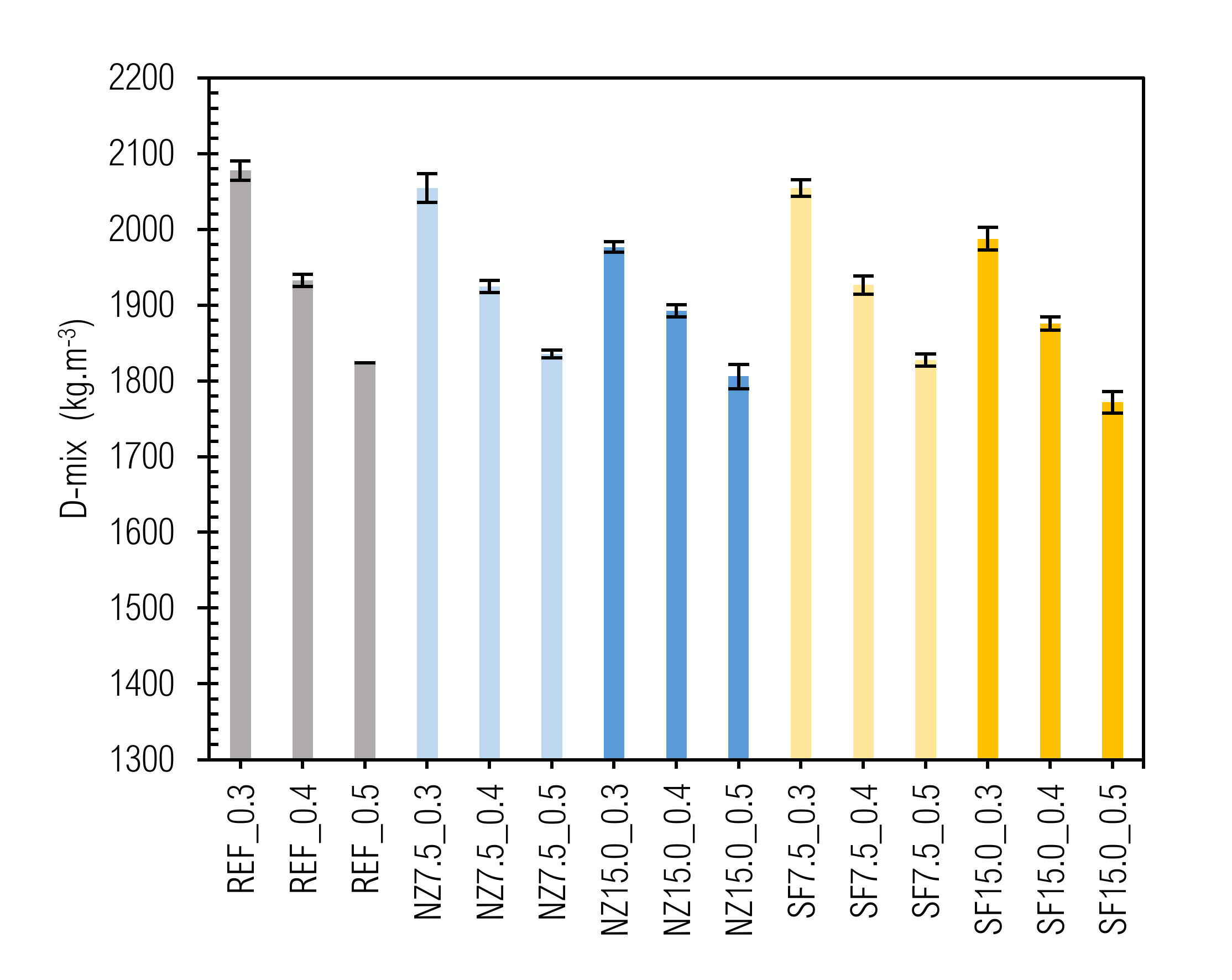
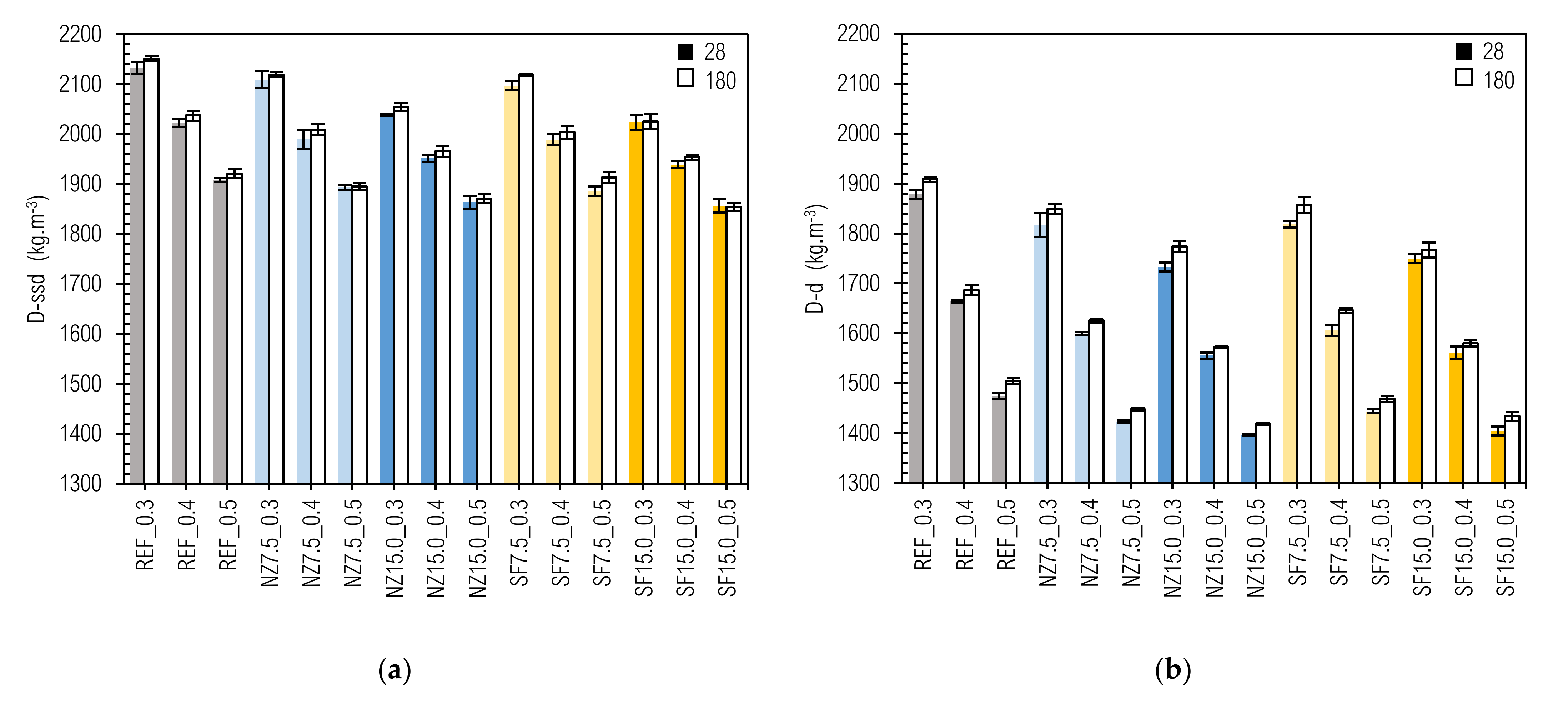
3.2. Density
- m(cp), m(agg), m(c), and m(w)—mass of cement paste, aggregate, cement, and water, respectively;
- x—water to cement ratio;
- y—cement paste to aggregate ratio.
- m(w)—content of water in fresh CP (kg·m−3);
- D(ssd)—density of CP in saturated-surface-dry state (kg·m−3);
- D(d)—density of CP in oven-dried state (kg·m−3).
3.3. Flexural Strength
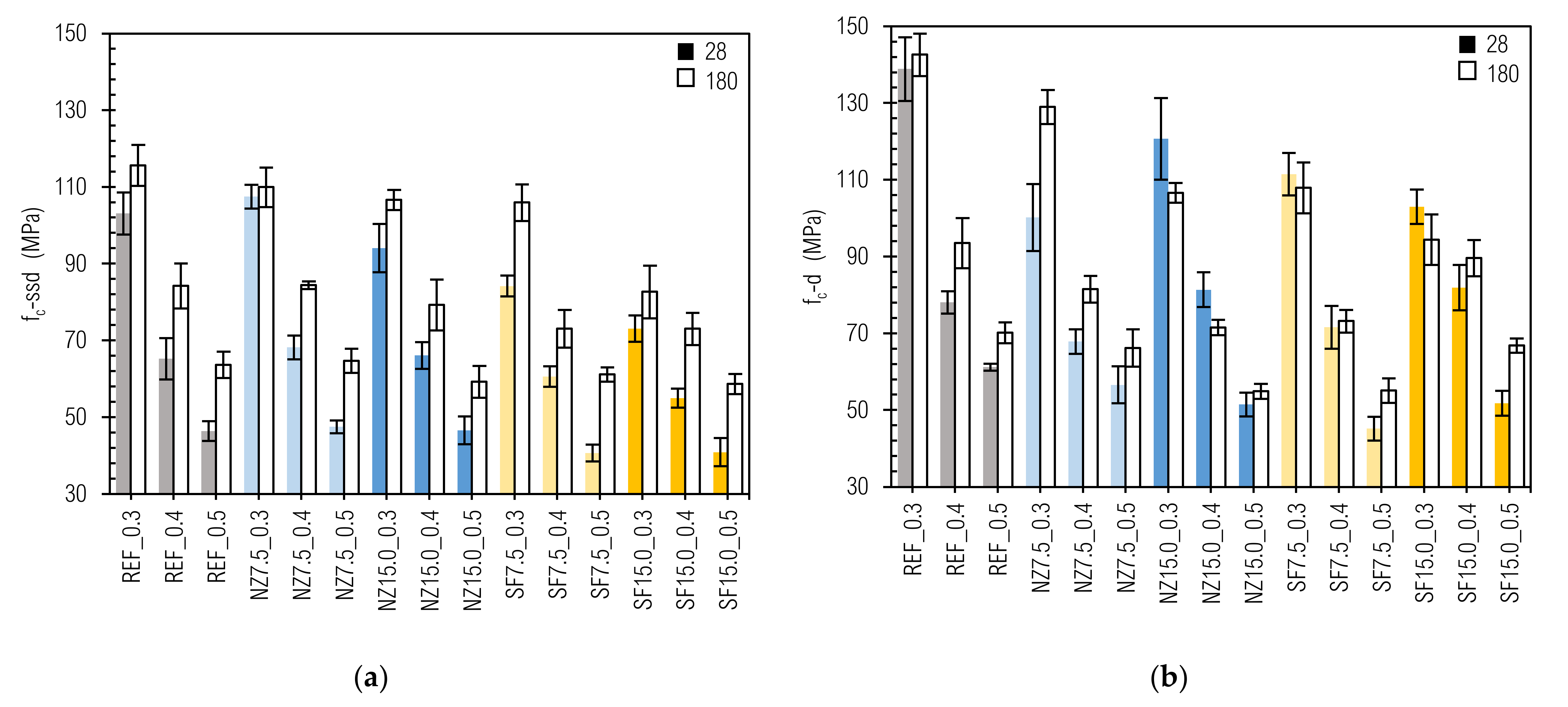
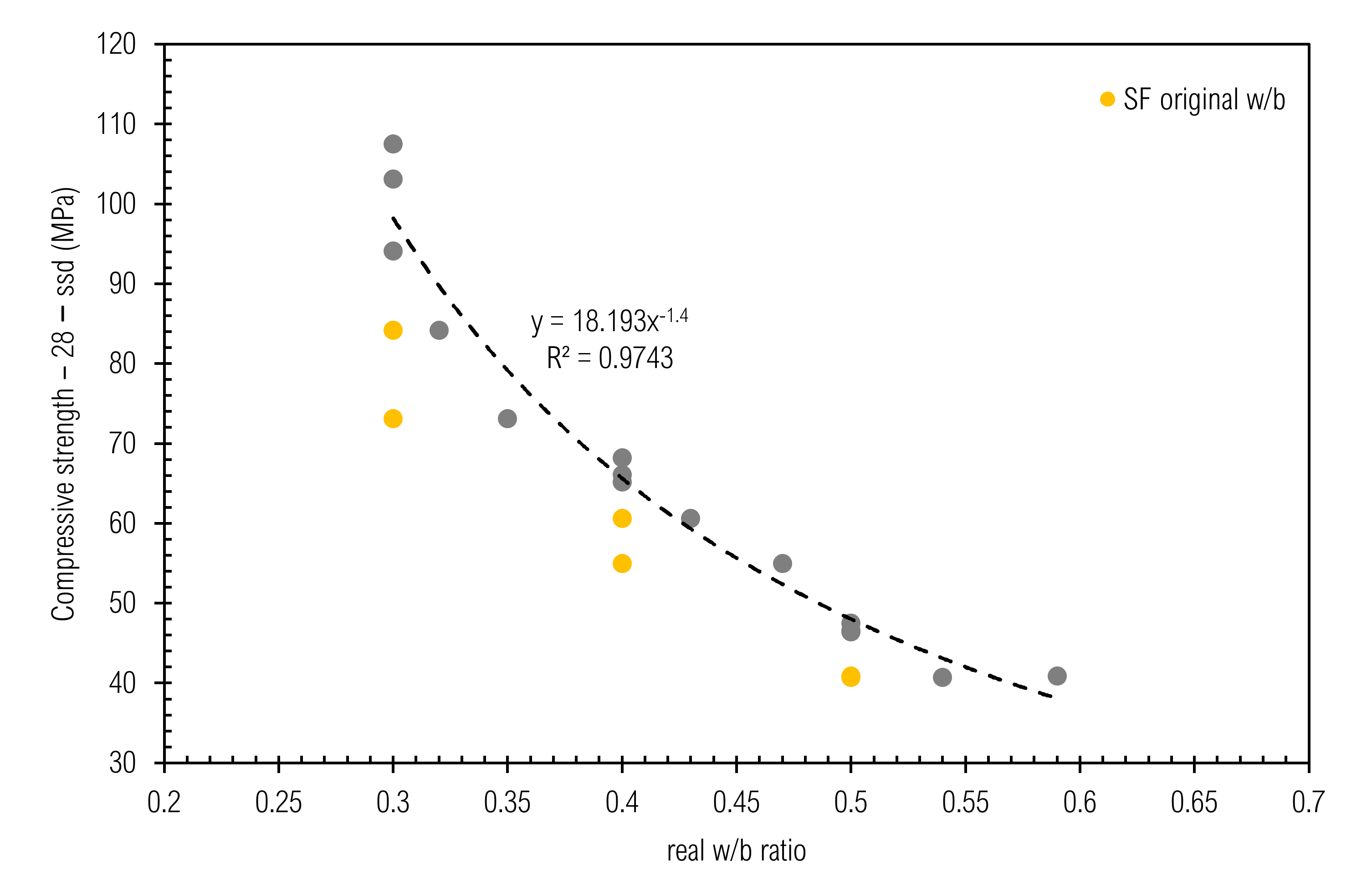
3.4. Compressive Strength
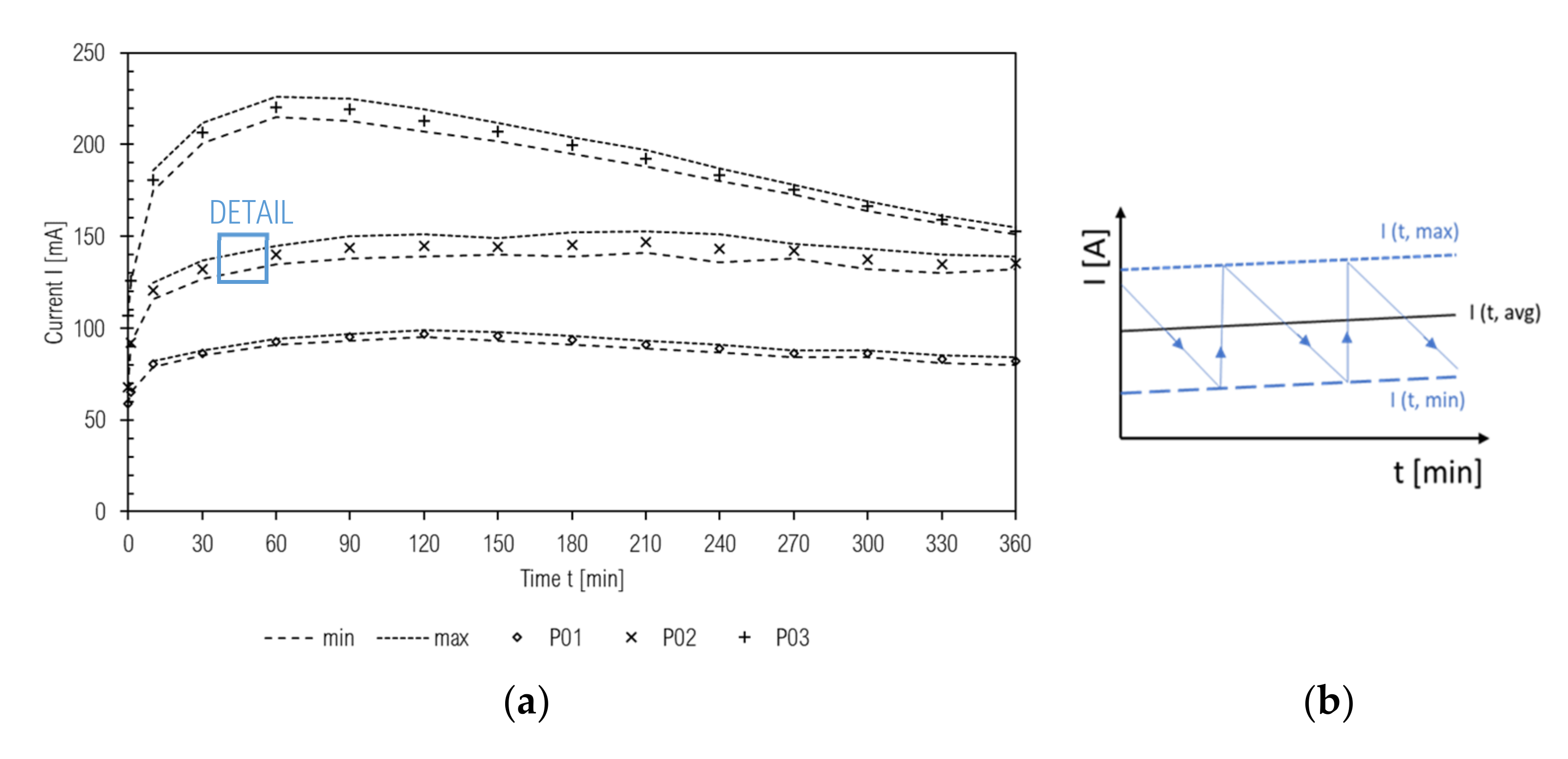
3.5. Rapid Chloride Penetration Test
- (1)
- rapid increase of current and temperature,
- (2)
- stabilization of maximum increase of temperature,
- (3)
- slow current and temperature decrease. These findings can be confirmed by values of initial, maximum, and final current values, while these variances are more disarranged in the case of high permeable samples than those with low permeability.
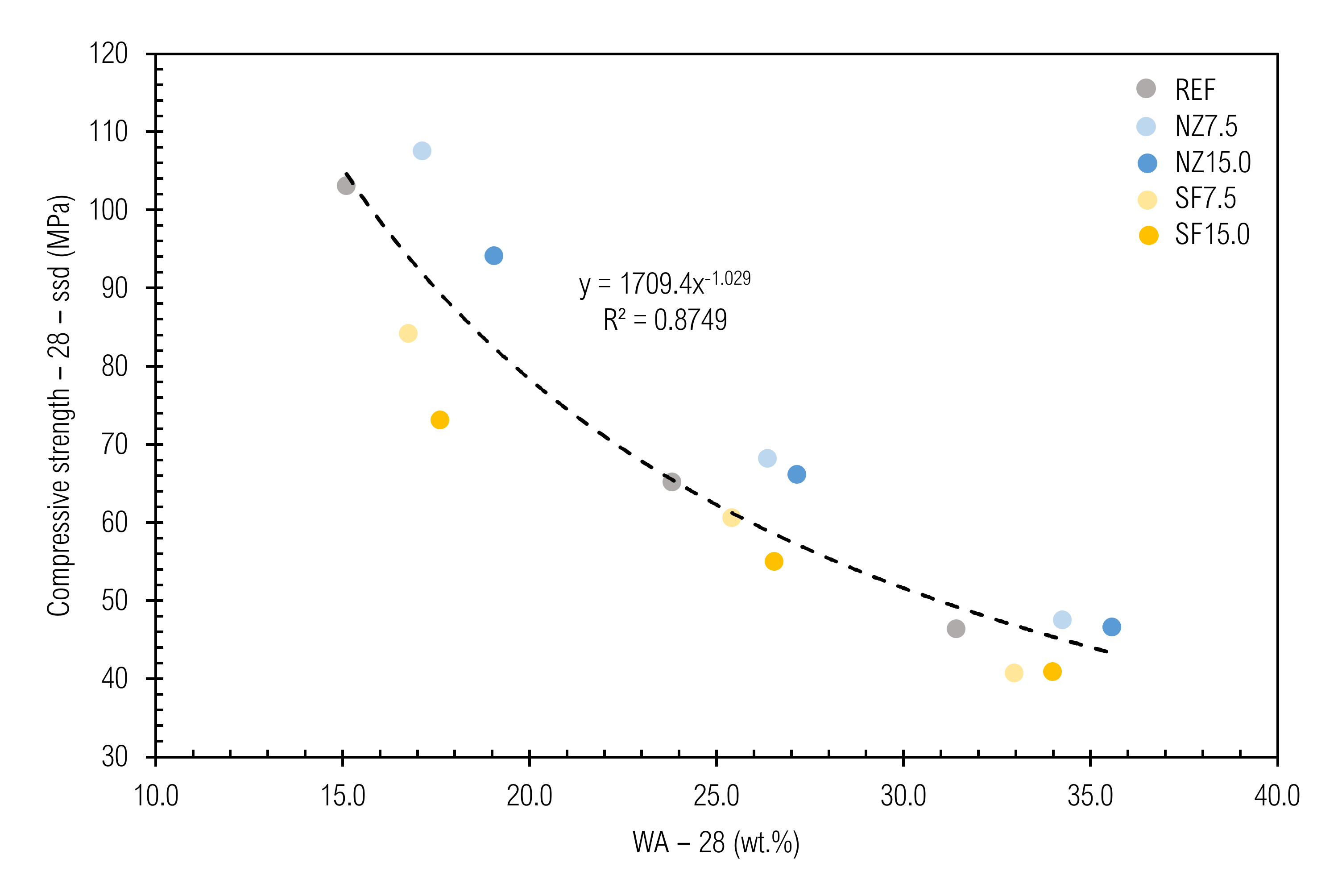
3.6. Water Absorption
- WA(cc)—overall cement composite water absorption (wt.%);
- m(f)—filler (aggregate) content in fresh cement composite (kg·m−3);
- D(cc)—density of cement composite in fresh state (kg·m−3);
- WA(f)—water absorption of filler (wt.%);
- m(cp)—cement paste content in fresh cement composite (kg·m−3);
- WA(cp)—water absorption of cement paste (wt.%).
3.7. Thermal Analysis
3.8. Evaluation of Mineral Additives and Their Effect on the CC with Regards to Durability and Environmental Suitability
3.8.1. Prediction of Consistency
3.8.2. Prediction of Compressive Strength of CC
3.8.3. Prediction of Water Absorption of CC
3.8.4. Prediction of Permeability of CC
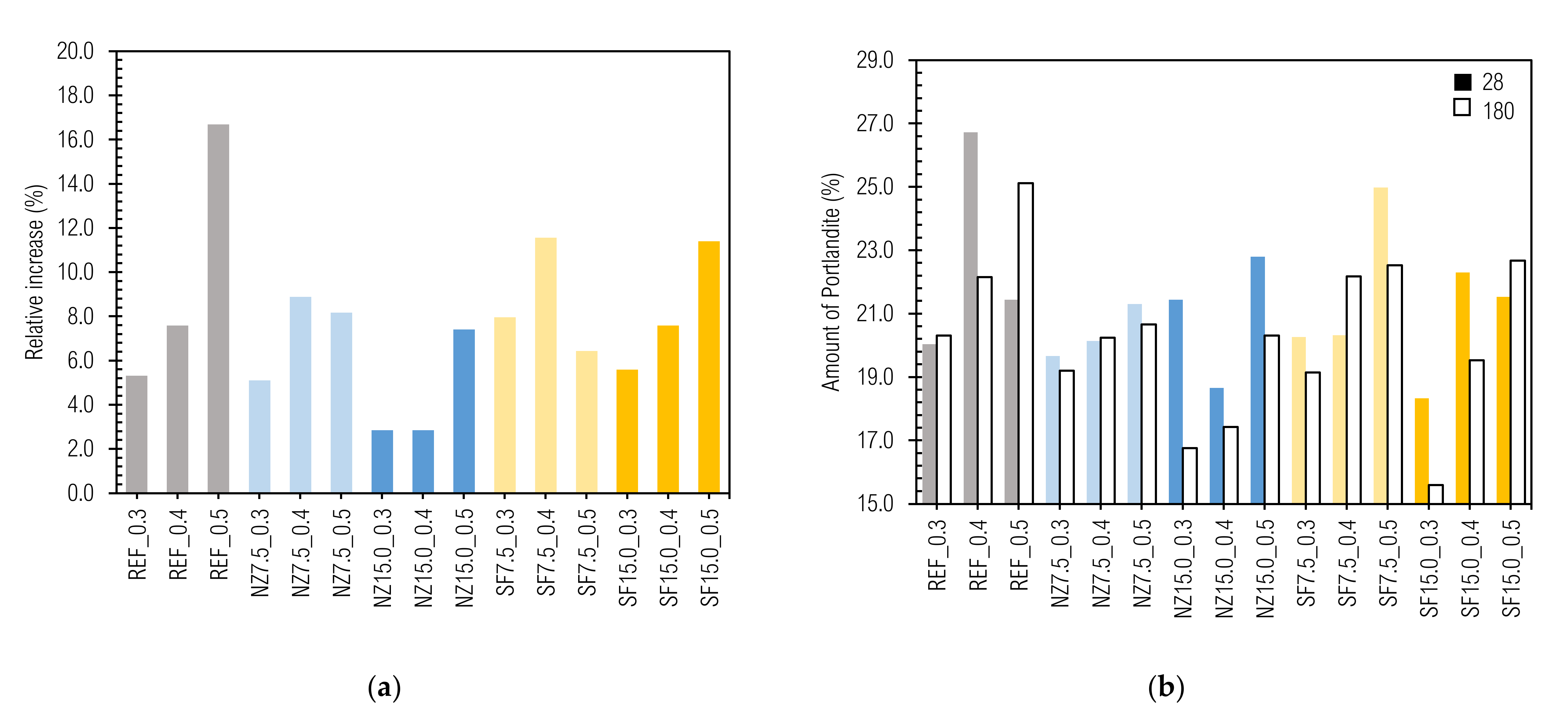
3.8.5. Prediction of Hydration Phases Composition in CC
4. Conclusions
- Monitoring of CC density (in ssd and oven-dried state) over time could provide decent information not only about water absorption but also about the hydration process. The chemically bound water, calculated from densities and thermal analysis, evinces good correlation.
- Flexural strength should not be regarded as a cement paste durability parameter due to its high brittleness, which leads to sudden collapse during loading and thus to low precision of measurements.
- Despite the fact that compressive strength is the most frequently measured property of CC, for complex evaluation of CC durability, it is necessary to perform additional non-mechanical tests, such as permeability and chemical analysis.
- The evaluation of results should undergo statistic comparative analysis and Student’s t-test seems to be suitable enough.
- The moisture content of the specimen significantly affects compressive strength, which could lead to overestimation or underestimation of durability, and thus sustainability of CC.
- The results of the rapid chloride penetration test could be considered an efficient qualificator of cement paste durability evaluation when mineral additives are assessed.
- Monitoring the parameters’ progress over time is inevitable in the evaluation of mineral additives’ activity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, E.B. Intergenerational Equity in a Kaleidoscopic World. Environ. Policy Law 2019, 49, 3–11. [Google Scholar] [CrossRef]
- Jahren, P.; Sui, T. Concrete and Sustainability; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–53. [Google Scholar]
- Aïtcin, P.-C.; Mindess, S. Sustainability of Concrete; Spon Press: New York, NY, USA, 2011; pp. 1–10. [Google Scholar]
- Mather, B. Concrete Durability. Cem. Concr. Compos. 2004, 26, 3–4. [Google Scholar] [CrossRef]
- Alexander, M.; Bentur, A.; Mindess, S. Durability of Concrete, Design and Construction; CRC Press: Boca Raton, FL, USA, 2017; pp. 160–178. [Google Scholar]
- Hooton, R.D.; Bickley, J.A. Design for durability: The key to improving concrete sustainability. Constr. Build. Mater. 2014, 67, 422–430. [Google Scholar] [CrossRef]
- Hooton, R.D. Future directions for design, specification, testing, and construction of durable concrete structures. Cem. Concr. Res. 2019, 124, 105827. [Google Scholar] [CrossRef]
- Alexander, M.; Beushausen, H. Durability, service life prediction, and modelling for reinforced concrete structures—Review and critique. Cem. Concr. Res. 2019, 122, 17–29. [Google Scholar] [CrossRef]
- Ouyang, X.; Shi, C.; Wu, Z.; Li, K.; Shan, B.; Shi, J. Experimental investigation and prediction of elastic modulus of ultra-high performance concrete (UHPC) based on its composition. Cem. Concr. Res. 2020, 138, 106241. [Google Scholar] [CrossRef]
- Unger, J.F.; Eckardt, S. Multiscale modeling of concrete. Arch. Comput. Methods Eng. 2011, 18, 341–393. [Google Scholar] [CrossRef]
- Patel, R.A. Lattice Boltzmann Method Based Framework for Simulating Physico-Chemical Processes in Heterogeneous Porous Media and Its Application to Cement Paste. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- Mehta, P.K.; Monteiro, P.J.M. Concrete, Microstructure, Properties and Materials, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006; pp. 21–67. [Google Scholar]
- Haach, V.G.; Vasconcelos, G.; Lourenço, P.B. Influence of aggregates grading and water/cement ratio in workability and hardened properties of mortars. Constr. Build. Mater. 2011, 25, 2980–2987. [Google Scholar] [CrossRef]
- Singh, S.B.; Munjal, P.; Thammishetti, N. Role of water/cement ratio on strength development of cement mortar. J. Build. Eng. 2015, 4, 94–100. [Google Scholar] [CrossRef]
- Kovler, K.; Roussel, N. Properties of fresh and hardened concrete. Cem. Concr. Res. 2011, 41, 775–792. [Google Scholar] [CrossRef]
- Chia, K.S.; Zhang, M.-H. Water permeability and chloride penetrability of high-strength lightweight aggregate concrete. Cem. Concr. Res. 2002, 32, 639–645. [Google Scholar] [CrossRef]
- Cui, L.; Cahyadi, J.H. Permeability and pore structure of OPC paste. Cem. Concr. Res. 2001, 31, 277–282. [Google Scholar] [CrossRef]
- Yu, Z.; Ni, C.; Tang, M.; Shen, X. Relationship between water permeability and pore structure of Portland cement paste blended with fly ash. Constr. Build. Mater. 2018, 175, 458–466. [Google Scholar] [CrossRef]
- Angelucci, M.; Beushausen, H.; Alexander, M.G.; Mackechnie, J.R. Specifying cement content for concrete durability: Why less is more. Concr. Beton 2017, 150, 12–17. [Google Scholar]
- Meddah, M.S.; Zitouni, S.; Belâabes, S. Effect of content and particle size distribution of coarse aggregate on the compressive strength of concrete. Constr. Build. Mater. 2010, 24, 505–512. [Google Scholar] [CrossRef]
- Figmig, R.; Kovac, M. The influence of aggregate gradation on the fresh and hardened concrete properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 566, 012007. [Google Scholar] [CrossRef]
- Ulas, M.A.; Alyamac, K.E.; Ulucan, Z.C. Influence of Aggregate Gradation on the Workability, Mechanical Properties and Cost of Steel Fiber-Reinforced Concrete. Adv. Civ. Eng. Mater. 2019, 8, 209–223. [Google Scholar] [CrossRef]
- Antunes, R.; Tia, M. Effects of Aggregate Packing on Concrete Strength and Consistency. Adv. Civ. Eng. Mater. 2018, 7, 479–495. [Google Scholar] [CrossRef]
- Figmig, R. Influence of Aggregate on Properties of Semi-dry Concrete in Hardened State. In Proceedings of the CEE 2019, Proceedings of the International Conference Current Issues of Civil and Environmental Engineering Lviv-Košice-Rzeszów, Liviv, Ukraine, 11–13 September 2019; Blikharskyy, Z., Koszelnik, P., Mesaros, P., Eds.; Springer: Cham, Switzerland, 2020; Volume 47, pp. 87–94. [Google Scholar] [CrossRef]
- Skoczylas, K.; Rucińska, T. Influence of Grain Shape of Waste Glass Aggregate on the Properties of Cement Mortar. J. Ecol. Eng. 2020, 21, 148–159. [Google Scholar] [CrossRef]
- Dilek, U.; Leming, M.L. Relationship between particle shape and void content of fine aggregate. Cem. Concr. Aggreg. 2004, 26, 1–7. [Google Scholar] [CrossRef]
- Jones, M.R.; Zheng, L.; Newlands, M.D. Comparison of particle packing models for proportioning concrete constitutents for minimum voids ratio. Mater. Struct. 2002, 35, 301–309. [Google Scholar] [CrossRef]
- De Grazia, M.T.; Sanchez, L.F.; Romano, R.C.; Pileggi, R.G. Investigation of the use of continuous particle packing models (PPMs) on the fresh and hardened properties of low-cement concrete (LCC) systems. Constr. Build. Mater. 2019, 195, 524–536. [Google Scholar] [CrossRef]
- Campos, H.F.; Klein, N.S.; Marques Filho, J. Proposed mix design method for sustainable high-strength concrete using particle packing optimization. J. Clean. Prod. 2020, 265, 121907. [Google Scholar] [CrossRef]
- Pradhan, S.; Tiwari, B.R.; Kumar, S.; Barai, S.V. Comparative LCA of recycled and natural aggregate concrete using Particle Packing Method and conventional method of design mix. J. Clean. Prod. 2019, 228, 679–691. [Google Scholar] [CrossRef]
- Shuldyakov, K.; Trofimov, B.; Kramar, L. Stable microstructure of hardened cement paste—A guarantee of the durability of concrete. Case Stud. Constr. Mater. 2020, 12, e00351. [Google Scholar] [CrossRef]
- Sharma, A.; Angadi, P.; Sirotiak, T.; Wang, X.; Taylor, P.; Borowicz, P.; Payne, S. Characterization of paste microstructure for durability properties of concrete. Constr. Build. Mater. 2020, 248, 118570. [Google Scholar] [CrossRef]
- Figmig, R.; Eštoková, A.; Sičáková, A. The Temperature Impact on the Various Cement Type Consistency. Key Eng. Mater. 2020, 838, 46–52. [Google Scholar] [CrossRef]
- Ghafoori, N.; Diawara, H. Influence of temperature on fresh performance of self-consolidating concrete. Constr. Build. Mater. 2010, 24, 946–955. [Google Scholar] [CrossRef]
- Beushausen, H.; Torrent, R.; Alexander, M.G. Performance-based approaches for concrete durability: State of the art and future research needs. Cem. Concr. Res. 2019, 119, 11–20. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Girskas, G.; Skripkiūnas, G. Properties of concrete modified with mineral additives. Constr. Build. Mater. 2017, 135, 37–42. [Google Scholar] [CrossRef]
- Aïtcin, P.-C. Entrained air in concrete: Rheology and freezing resistance. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead: Cambridge, UK, 2016; pp. 87–95. [Google Scholar]
- Ruan, S.; Unluer, C. Effect of air entrainment on the performance of reactive MgO and PC mixes. Constr. Build. Mater. 2017, 142, 221–232. [Google Scholar] [CrossRef]
- Wong, H.S.; Pappas, A.M.; Zimmerman, R.W.; Buenfeld, N.R. Effect of entrained air voids on the microstructure and mass transport properties of concrete. Cem. Concr. Res. 2011, 41, 1067–1077. [Google Scholar] [CrossRef]
- Toutanji, M. Technical note the influence of air entrainment on the properties of silica fume concrete. Adv. Cem. Res. 1998, 10, 135–139. [Google Scholar] [CrossRef]
- Zhang, P.; Li, D.; Qiao, Y.; Zhang, S.; Sun, C.; Zhao, T. Effect of air entrainment on the mechanical properties, chloride migration, and microstructure of ordinary concrete and fly ash concrete. J. Mater. Civ. Eng. 2018, 30, 04018265. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Habert, G. Environmental impact of Portland cement production. In Eco-Efficient Concrete; Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V.M., Eds.; Woodhead: Cambridge, UK, 2013; pp. 3–25. [Google Scholar]
- Wu, W.-N.; Liu, X.Y.; Hu, Z.; Zhang, R.; Lu, X.Y. Improving the sustainability of cement clinker calcination process by assessing the heat loss through kiln shell and its influencing factors: A case study in China. J. Clean. Prod. 2019, 224, 132–141. [Google Scholar] [CrossRef]
- Mindess, S. Sustainability of concrete. In Developments in the Formulation and Reinforcement of Concrete, 2nd ed.; Mindess, S., Ed.; Woodhead: Duxford, UK, 2019; pp. 3–17. [Google Scholar]
- Daneti, S.B.; Tam, C.T. Sustainability of Concrete Constructions: The Role of Materials and Practices. In Emerging Trends in Civil Engineering; Babu, K., Rao, H., Amarnath, Y., Eds.; Springer: Singapore, 2020; Volume 61, pp. 381–395. [Google Scholar] [CrossRef]
- Rahla, K.M.; Mateus, R.; Bragança, L. Comparative sustainability assessment of binary blended concretes using Supplementary Cementitious Materials (SCMs) and Ordinary Portland Cement (OPC). J. Clean. Prod. 2019, 220, 445–459. [Google Scholar] [CrossRef]
- Juhart, J.; David, G.A.; Saade, M.R.M.; Baldermann, C.; Passer, A.; Mittermayr, F. Functional and environmental performance optimization of Portland cement-based materials by combined mineral fillers. Cem. Concr. Res. 2019, 122, 157–178. [Google Scholar] [CrossRef]
- Tran, Y.T.; Lee, J.; Kumar, P.; Kim, K.H.; Lee, S.S. Natural zeolite and its application in concrete composite production. Compos. Part B Eng. 2019, 165, 354–364. [Google Scholar] [CrossRef]
- Shahmansouri, A.A.; Yazdani, M.; Ghanbari, S.; Bengar, H.A.; Jafari, A.; Ghatte, H.F. Artificial neural network model to predict the compressive strength of eco-friendly geopolymer concrete incorporating silica fume and natural zeolite. J. Clean. Prod. 2020, 279, 123697. [Google Scholar] [CrossRef]
- Azad, A.; Saeedian, A.; Mousavi, S.F.; Karami, H.; Farzin, S.; Singh, V.P. Effect of zeolite and pumice powders on the environmental and physical characteristics of green concrete filters. Constr. Build. Mater. 2020, 240, 117931. [Google Scholar] [CrossRef]
- Nas, M.; Kurbetci, S. Mechanical, durability and microstructure properties of concrete containing natural zeolite. Comput. Concr. 2018, 22, 449–459. [Google Scholar] [CrossRef]
- Ahmad, O.A. Production of high-performance silica fume concrete. Am. J. Appl. Sci. 2017, 14, 1031–1038. [Google Scholar] [CrossRef]
- Sasanipour, H.; Aslani, F.; Taherinezhad, J. Effect of silica fume on durability of self-compacting concrete made with waste recycled concrete aggregates. Constr. Build. Mater. 2019, 227, 116598. [Google Scholar] [CrossRef]
- Khodabakhshian, A.; Ghalehnovi, M.; De Brito, J.; Shamsabadi, E.A. Durability performance of structural concrete containing silica fume and marble industry waste powder. J. Clean. Prod. 2018, 170, 42–60. [Google Scholar] [CrossRef]
- Figmig, R.; Kováč, M. Study on utilization of zeolite in concrete precast industry. Sel. Sci. Pap. J. Civ. Eng. 2019, 14, 93–102. [Google Scholar] [CrossRef]
- Berenguer, R.; Lima, N.; Valdés, A.C.; Medeiros, M.H.F.; Lima, N.B.D.; Delgado, J.M.P.Q.; Rangel, B. Durability of Concrete Structures with Sugar Cane Bagasse Ash. Adv. Mater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Pan, G.; Li, P.; Chen, L.; Liu, G. A study of the effect of rheological properties of fresh concrete on shotcrete-rebound based on different additive components. Constr. Build. Mater. 2019, 224, 1069–1080. [Google Scholar] [CrossRef]
- EN 1008:2002-06. Mixing Water for Concrete—Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, as Mixing Water for Concrete; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- EN 1015-3:1999-02. Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table); European Committee for Standardization: Brussels, Belgium, 1999. [Google Scholar]
- EN 196-1:2016-04. Methods of Testing Cement—Part 1: Determination of Strength; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- STN 731316:1989-05. Determination of Moisture Content, Absorptivity and Capillarity of Concrete (in Czech); Federal Bureau for Standardization and Metrology: Prague, Czech Republic, 1989. [Google Scholar]
- British Standard. Testing Hardened Concrete-Part 5: Flexural Strength of Test Specimens; European Committee for Standardization: Brussels, Belgium, 2019. [Google Scholar]
- EN 12390-3:2019-07. Testing Hardened Concrete-Part 3: Compressive Strength of Test Specimens; European Committee for Standardization: Brussels, Belgium, 2019. [Google Scholar]
- ASTM International. Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Bentz, D.P. A virtual rapid chloride permeability test. Cem. Concr. Compos. 2007, 29, 723–731. [Google Scholar] [CrossRef]
- Julio-Betancourt, G.A.; Hooton, R.D. Study of the Joule effect on rapid chloride permeability values and evaluation of related electrical properties of concretes. Cem. Concr. Res. 2004, 34, 1007–1015. [Google Scholar] [CrossRef]
- Andrade, C. Calculation of chloride diffusion coefficients in concrete from ionic migration measurement. Cem. Concr. Res. 1993, 23, 724–742. [Google Scholar] [CrossRef]
- Shi, C. Effect of mixing proportions of concrete on its electrical conductivity and the rapid chloride permeability test (ASTM C 1202 or ASSHTO T 277) results. Cem. Concr. Res. 2004, 34, 537–545. [Google Scholar] [CrossRef]
- Adesina, A. Overview of Workability and Mechanical Performance of Cement-Based Composites Incorporating Nanomaterials. Silicon 2020, 1–10. [Google Scholar] [CrossRef]
- Schlumpf, J.; Bicher, B.; Schwoon, O. Sika Concrete Handbook; Sika: Zurich, Schwitzerland, 2020; p. 236. [Google Scholar]
- Bodnarova, L.; Jarolím, T.; Hela, R. Study of effect of various types of cement on properties of cement pastes. Adv. Mater. Res. 2014, 897, 224–229. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Roussel, N.; Stéfani, C.; Leroy, R. From mini-cone test to Abrams cone test: Measurement of cement-based materials yield stress using slump tests. Cem. Concr. Res. 2005, 35, 817–822. [Google Scholar] [CrossRef]
- Nagrockiene, D.; Pundienė, I.; Kicaite, A. The effect of cement type and plasticizer addition on concrete properties. Constr. Build. Mater. 2013, 45, 324–331. [Google Scholar] [CrossRef]
- Skripkiunas, G.; Karpova, E.; Bendoraitiene, J.; Barauskas, I. Effect of MWCNT and PCE plasticizer on the properties of cement pastes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 660, 012032. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Li, M. Influence of nano-SiO2 on the consistency, setting time, early-age strength, and shrinkage of composite cement pastes. Adv. Mater. Sci. Eng. 2016. [Google Scholar] [CrossRef]
- Markiv, T.; Sobol, K.; Franus, M.; Franus, W. Mechanical and durability properties of concretes incorporating natural zeolite. Arch. Civ. Mech. Eng. 2016, 16, 554–562. [Google Scholar] [CrossRef]
- Chen, J.J.; Ng, P.L.; Li, L.G.; Kwan, A.K.H. Use of superfine zeolite in conjunction with silica fume—Effects on rheology and strength of cementitious paste. Powder Technol. 2018, 328, 75–83. [Google Scholar] [CrossRef]
- Ghasemi, M.; Rasekh, H.; Berenjian, J.; Azari Jafari, H. Dealing with workability loss challenge in SCC mixtures incorporating natural pozzolans: A study of natural zeolite and pumice. Constr. Build. Mater. 2019, 222, 424–436. [Google Scholar] [CrossRef]
- Rahhal, V.F.; Pavlík, Z.; Tironi, A.; Castellano, C.C.; Trezza, M.A.; Černý, R.; Irassar, E.F. Effect of cement composition on the early hydration of blended cements with natural zeolite. J. Therm. Anal. Calorim. 2017, 128, 721–733. [Google Scholar] [CrossRef]
- Živica, V. Effects of the very low water/cement ratio. Constr. Build. Mater. 2009, 23, 3579–3582. [Google Scholar] [CrossRef]
- Ouda, A.S. Development of high-performance heavy density concrete using different aggregates for gamma-ray shielding. Prog. Nucl. Energy 2015, 79, 48–55. [Google Scholar] [CrossRef]
- Thomas, C.; Setién, J.; Polanco, J.; Alaejos, P.; De Juan, M.S. Durability of recycled aggregate concrete. Constr. Build. Mater. 2013, 40, 1054–1065. [Google Scholar] [CrossRef]
- Kurda, R.; De Brito, J.; Silvestre, J.D. Concrete with High Volume of Recycled Concrete Aggregates and Fly Ash: Shrinkage Behavior Modeling. ACI Mater. J. 2019, 116, 83–94. [Google Scholar] [CrossRef]
- Iffat, S. Relation between density and compressive strength of hardened concrete. Concr. Res. Lett. 2015, 6, 182–189. [Google Scholar]
- Kosmatka, S.H.; Wilson, M.L. Design and Control of Concrete Mixtures, 15th ed.; PCA: Skokie, IL, USA, 2011; pp. 241–260. [Google Scholar]
- Chaubey, A. Practical Concrete Mix Design; CRC Press: New York, NY, USA, 2020; pp. 27–29. [Google Scholar]
- Gruyaert, E.; Maes, M.; De Belie, N. Performance of BFS concrete: K-Value concept versus equivalent performance concept. Constr. Build. Mater. 2013, 47, 441–455. [Google Scholar] [CrossRef]
- Lura, P.; Winnefeld, F.; Fang, X. A simple method for determining the total amount of physically and chemically bound water of different cements. J. Therm. Anal. Calorim. 2017, 130, 653–660. [Google Scholar] [CrossRef]
- Prochon, P.; Piotrowski, T. Bound water content measurement in cement pastes by stoichiometric and gravimetric analyses. J. Build. Chem. 2016, 1, 18–25. [Google Scholar] [CrossRef]
- Liska, M.; Wilson, A.; Bensted, J. Special Cements. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P.C., Liska, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; p. 618. [Google Scholar]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869–874. [Google Scholar] [CrossRef]
- Farzadnia, N.; Ali, A.A.A.; Demirboga, R. Characterization of high strength mortars with nano alumina at elevated temperatures. Cem. Concr. Res. 2013, 54, 43–54. [Google Scholar] [CrossRef]
- Zhang, B.; Bicanic, N.; Pearce, C.J.; Phillips, D.V. Relationship between brittleness and moisture loss of concrete exposed to high temperatures. Cem. Concr. Res. 2002, 32, 363–371. [Google Scholar] [CrossRef]
- Cao, M.; Ming, X.; Yin, H.; Li, L. Influence of high temperature on strength, ultrasonic velocity and mass loss of calcium carbonate whisker reinforced cement paste. Compos. Part B Eng. 2019, 163, 438–446. [Google Scholar] [CrossRef]
- Pantazopoulou, S.J.; Mills, R.H. Microstructural aspects of the mechanical response of plain concrete. ACI Mater. J. 1995, 92, 605–616. [Google Scholar]
- Yajun, J.; Cahyadi, J.H. Effects of densified silica fume on microstructure and compressive strength of blended cement pastes. Cem. Concr. Res. 2003, 33, 1543–1548. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Yan, P. Comparative study of effect of raw and densified silica fume in the paste, mortar and concrete. Constr. Build. Mater. 2016, 105, 82–93. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shekarchi, M. Use of natural zeolite as a supplementary cementitious material. Cem. Concr. Compos. 2010, 32, 134–141. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Mousavi, R.; Kalhori, M.; Sobhani, J.; Najimi, M. Micro and macro level properties of natural zeolite contained concretes. Constr. Build. Mater. 2015, 101, 347–358. [Google Scholar] [CrossRef]
- Valipour, M.; Yekkalar, M.; Shekarchi, M.; Panahi, S. Environmental assessment of green concrete containing natural zeolite on the global warming index in marine environments. J. Clean. Prod. 2014, 65, 418–423. [Google Scholar] [CrossRef]
- Kondraivendhan, B.; Bhattacharjee, B. Flow behavior and strength for fly ash blended cement paste and mortar. Int. J. Sustain. Built Environ. 2015, 4, 270–277. [Google Scholar] [CrossRef]
- Figmig, R. Efficiency of the crystallizing waterproofing admixture in lower-quality concrete. IOP Conf. Ser. Mater. Sci. Eng. 2020, 867, 012007. [Google Scholar] [CrossRef]
- Shen, J.; Xu, Q. Effect of moisture content and porosity on compressive strength of concrete during drying at 105 C. Constr. Build. Mater. 2019, 195, 19–27. [Google Scholar] [CrossRef]
- Popovics, S. Effect of curing method and final moisture condition on compressive strength of concrete. J. Proc. 1986, 83, 650–657. [Google Scholar]
- Bartlett, F.M.; MacGregor, J.G. Effect of moisture condition on concrete core strengths. ACI Mater. J. 1994, 91, 227–236. [Google Scholar]
- Angelin, A.F.; Lintz, R.C.C.; Gachet-Barbosa, L.A.; Osorio, W.R. The effects of porosity on mechanical behavior and water absorption of an environmentally friendly cement mortar with recycled rubber. Constr. Build. Mater. 2017, 151, 534–545. [Google Scholar] [CrossRef]
- Özen, S.; Göncüoğlu, M.C.; Liguori, B.; De Gennaro, B.; Cappelletti, P.; Gatta, G.D.; Colella, C. A comprehensive evaluation of sedimentary zeolites from Turkey as pozzolanic addition of cement-and lime-based binders. Constr. Build. Mater. 2016, 105, 46–61. [Google Scholar] [CrossRef]
- Wee, T.H.; Suryavanshi, A.K. Influence of aggregate fraction in the mix on the reliability of the rapid chloride permeability test. Cem. Concr. Compos. 1999, 21, 59–72. [Google Scholar] [CrossRef]
- Feng, N.; Feng, X.; Hao, T.; Xing, F. Effect of ultrafine mineral powder on the charge passed of the concrete. Cem. Concr. Res. 2002, 32, 623–627. [Google Scholar] [CrossRef]
- Stanish, K.D.; Hooton, R.D.; Thomas, M.D.A. Testing the Chloride Penetration Resistance of Concrete: A Literature Review; National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 1997. [Google Scholar]
- Feldman, R.; Prudencio, L.R., Jr.; Chan, G. Rapid chloride permeability test on blended cement and other concretes: Correlations between charge, initial current and conductivity. Constr. Build. Mater. 1999, 13, 149–154. [Google Scholar] [CrossRef]
- Moghadam, H.A.; Mirzaei, A.; Dehghi, Z.A. The relation between porosity, hydration degree and compressive strength of Portland cement pastes in the presence of aluminum chloride additive. Constr. Build. Mater. 2020, 250, 118884. [Google Scholar] [CrossRef]
- Kolias, S.; Georgiou, C. The effect of paste volume and of water content on the strength and water absorption of concrete. Cem. Concr. Compos. 2005, 27, 211–216. [Google Scholar] [CrossRef]
- Piasta, W.; Zarzycki, B. The effect of cement paste volume and w/c ratio on shrinkage strain, water absorption and compressive strength of high performance concrete. Constr. Build. Mater. 2017, 140, 395–402. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Lee, K.M.; Bang, J.W.; Kwon, S.J. Effect of W/C ratio on durability and porosity in cement mortar with constant cement amount. Adv. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef]
- STN EN 206+A1:2017-05. Concrete, Specification, Performance, Production and Conformity (in Slovak); Slovak Institute of Technical Standardization: Bratislava, Slovakia, 2017. [Google Scholar]
- EN 1097-6:2013-07. Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption; European Committee for Standardization: Brussels, Belgium, 2013. [Google Scholar]
- Bhatty, J.I.; Reid, K.J. Use of thermal analysis in the hydration studies of a type 1 portland cement produced from mineral tailings. Thermochim. Acta 1985, 91, 95–105. [Google Scholar] [CrossRef]
- Lothenbach, B.; Durdziński, P.; De Weerdt, K. Thermogravimetric analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 177–211. [Google Scholar]
- Tavares, L.R.C.; Junior, J.F.T.; Costa, L.M.; Da Silva Bezerra, A.C.; Cetlin, P.R.; Aguilar, M.T.P. Influence of quartz powder and silica fume on the performance of Portland cement. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tokyay, M. Cement and Concrete Mineral Admixtures; CRC Press: Boca Raton, FL, USA, 2016; p. 46. [Google Scholar]
- Snellings, R. X-ray powder diffraction applied to cement. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 107–176. [Google Scholar]
- Degirmenci, N.; Yilmaz, A. Use of diatomite as partial replacement for Portland cement in cement mortars. Constr. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Gram, A.; Silfwerbrand, J.; Lagerblad, B. Obtaining rheological parameters from flow test—Analytical, computational and lab test approach. Cem. Concr. Res. 2014, 63, 29–34. [Google Scholar] [CrossRef]
- Rasheed, A.; Usman, M.; Farooq, H.; Hanif, A. Effect of Super-plasticizer Dosages on Fresh State Properties and Early-Age Strength of Concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 431, 062010. [Google Scholar] [CrossRef]
- Nkinamubanzi, P.-C.; Aïtcin, P.-C. Cement and superplasticizer combinations: Compatibility and robustness. Cem. Concr. Aggreg. 2004, 26, 1–8. [Google Scholar] [CrossRef]
- Kanamarlapudi, L.; Jonalagadda, K.B.; Jagarapu, D.C.K.; Eluru, A. Different mineral admixtures in concrete: A review. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Shanahan, N.; Tran, V.; Williams, A.; Zayed, A. Effect of SCM combinations on paste rheology and its relationship to particle characteristics of the mixture. Constr. Build. Mater. 2016, 123, 745–753. [Google Scholar] [CrossRef]
- Celik, F.; Akcuru, O. Rheological and workability effects of bottom ash usage as a mineral additive on the cement based permeation grouting method. Constr. Build. Mater. 2020, 263, 120186. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Park, K.-B. Analysis of compressive strength development of concrete containing high volume fly ash. Constr. Build. Mater. 2015, 98, 810–819. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. Influence of curing conditions on properties of high calcium fly ash geopolymer containing Portland cement as additive. Mater. Des. 2014, 53, 269–274. [Google Scholar] [CrossRef]
- Uysal, M.; Yilmaz, K.; Ipek, M. The effect of mineral admixtures on mechanical properties, chloride ion permeability and impermeability of self-compacting concrete. Constr. Build. Mater. 2012, 27, 263–270. [Google Scholar] [CrossRef]
- Li, Y.-X.; Chen, Y.M.; Wei, J.X.; He, X.Y.; Zhang, H.T.; Zhang, W.S. A study on the relationship between porosity of the cement paste with mineral additives and compressive strength of mortar based on this paste. Cem. Concr. Res. 2006, 36, 1740–1743. [Google Scholar] [CrossRef]
- Chen, J.J.; Kwan, A.K.H.; Jiang, Y. Adding limestone fines as cement paste replacement to reduce water permeability and sorptivity of concrete. Constr. Build. Mater. 2014, 56, 87–93. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Res. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Lothenbach, B.; De Belie, N.; Gruyaert, E.; Skibsted, J.; Snellings, R.; Vollpracht, A. TC 238-SCM: Hydration and microstructure of concrete with SCMs. Mater. Struct. 2015, 48, 835–862. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Hydration behaviour, structure and morphology of hydration phases in advanced cement-based systems containing micro and nanoscale pozzolanic additives. Cem. Concr. Res. 2008, 38, 955–962. [Google Scholar] [CrossRef]









| Main Elements Expressed as Oxide (wt.%) | Specific Gravity (kg·m−3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | K2O | Fe2O3 | MgO | SO3 | Other | ||
| Cement | 18.6 | 3.7 | 62.1 | 0.9 | 2.8 | 2.1 | 4.4 | 5.4 | 3050 |
| Zeolite | 58.7 | 9.0 | 2.8 | 2.6 | 1.4 | 0.7 | 0.1 | 24.7 | 2440 |
| Silica-fume | 92.5 | 0.4 | 0.3 | 1.0 | 1.2 | 1.0 | 0.1 | 3.5 | 2200 |
| Component | Replacement (wt.% of Cement) | w/b Ratio | ||
|---|---|---|---|---|
| 0.3 | 0.4 | 0.5 | ||
| Reference | 0.0 | REF_0.3 | REF_0.4 | REF_0.5 |
| Natural zeolite | 7.5 | NZ7.5_0.3 | NZ7.5_0.4 | NZ7.5_0.4 |
| Natural zeolite | 15.0 | NZ15.0_0.3 | NZ15.0_0.4 | NZ15.0_0.4 |
| Silica-fume | 7.5 | SF7.5_0.3 | SF7.5_0.4 | SF7.5_0.4 |
| Silica-fume | 15.0 | SF15.0_0.3 | SF15.0_0.4 | SF15.0_0.4 |
| Total binder (g) | 5600 | 4800 | 4200 | |
| Water (g) | 1680 | 1920 | 2100 | |
| Label | HWR Admixture | 5 min | 30 min | 60 min |
|---|---|---|---|---|
| (wt.% of Cement) | (mm) | |||
| REF_0.3 | 0.34 | 228 | 240 | 247 |
| REF_0.4 | 0.06 | 220 | 240 | 231 |
| REF_0.5 | 0.00 | 262 | 251 | 260 |
| NZ7.5_0.3 | 0.34 | 270 | 232 (17) * | 252 |
| NZ7.5_0.4 | 0.06 | 230 | 225 | 218 |
| NZ7.5_0.5 | 0.00 | 273 | 260 | 258 |
| NZ15.0_0.3 | 0.70 | 228 | 210 | 192 |
| NZ15.0_0.4 | 0.06 | 198 | 195 | 192 |
| NZ15.0_0.5 | 0.00 | 235 | 231 | 237 |
| SF7.5_0.3 | 0.52 | 277 | 243 (14) * | 243 (14) * |
| SF7.5_0.4 | 0.06 | 242 | 213 | 234 |
| SF7.5_0.5 | 0.00 | 281 | 265 | 279 |
| SF15.0_0.3 | 0.52 | 182 | 225 | 223 |
| SF15.0_0.4 | 0.06 | 220 | 218 | 211 |
| SF15.0_0.5 | 0.00 | 255 | 256 | 251 |
| Label | D-mix avg | Water (w) | Cement (c) | SCM Additive (a) | w/c | w/(c + a) = w/b |
|---|---|---|---|---|---|---|
| (kg m−3) | (kg m−3) | (kg m−3] | (kg m−3) | (-) | (-) | |
| REF_0.3 | 2078 | 480 | 1598 | 0 | 0.30 | 0.30 |
| REF_0.4 | 1933 | 552 | 1381 | 0 | 0.40 | 0.40 |
| REF_0.5 | 1824 | 608 | 1216 | 0 | 0.50 | 0.50 |
| NZ7.5_0.3 | 2055 | 474 | 1462 | 119 | 0.32 | 0.30 |
| NZ7.5_0.4 | 1925 | 550 | 1272 | 103 | 0.43 | 0.40 |
| NZ7.5_0.5 | 1836 | 612 | 1132 | 92 | 0.54 | 0.50 |
| NZ15.0_0.3 | 1977 | 456 | 1293 | 228 | 0.35 | 0.30 |
| NZ15.0_0.4 | 1893 | 541 | 1149 | 203 | 0.47 | 0.40 |
| NZ15.0_0.5 | 1806 | 602 | 1023 | 181 | 0.59 | 0.50 |
| SF7.5_0.3 | 2055 | 474 | 1462 | 119 | 0.32 | 0.30 |
| SF7.5_0.4 | 1927 | 551 | 1273 | 103 | 0.43 | 0.40 |
| SF7.5_0.5 | 1828 | 609 | 1128 | 91 | 0.54 | 0.50 |
| SF15.0_0.3 | 1988 | 459 | 1300 | 229 | 0.35 | 0.30 |
| SF15.0_0.4 | 1876 | 536 | 1139 | 201 | 0.47 | 0.40 |
| SF15.0_0.5 | 1772 | 591 | 1004 | 177 | 0.59 | 0.50 |
| fflex-28-ssd | Variation | fflex-28-d | Variation | fflex-180-ssd | Variation | fflex-180-d | Variation | |
|---|---|---|---|---|---|---|---|---|
| (MPa) | (rel. %) | (MPa) | (rel. %) | (MPa) | (rel. %) | (MPa) | (rel. %) | |
| REF_0.3 | 9.5 | 5.3 | 4.7 | 4.3 | 11.1 | 4.5 | 5.2 | 7.7 |
| REF_0.4 | 5.7 | 3.5 | 4.0 | 2.5 | 9.1 | na | 2.8 | 7.1 |
| REF_0.5 | 7.1 | 18.3 | 3.4 | 5.9 | 7.2 | 2.8 | 1.5 | 6.7 |
| NZ7.5_0.3 | 7.1 | 7.0 | 6.1 | 24.6 | 5.4 | 7.4 | 4.9 | 13.4 |
| NZ7.5_0.4 | 3.7 | 8.1 | 2.6 | 3.8 | 3.3 | 3.0 | 4.7 | 8.0 |
| NZ7.5_0.5 | 3.0 | 0.0 | 4.4 | 4.5 | 3.8 | 7.9 | 3.6 | 8.6 |
| NZ15.0_0.3 | 7.7 | 11.7 | 3.0 | 16.7 | 5.1 | 9.8 | 3.0 | 6.0 |
| NZ15.0_0.4 | 5.1 | 2.0 | 4.5 | 4.4 | 3.7 | 5.4 | 3.5 | 11.1 |
| NZ15.0_0.5 | 2.9 | 3.4 | 3.8 | 18.4 | 2.8 | 7.1 | 2.7 | 8.7 |
| SF7.5_0.3 | 10.6 | 5.7 | 1.9 | 15.8 | 6.5 | 7.7 | 4.4 | 7.6 |
| SF7.5_0.4 | 6.7 | 11.9 | 1.4 | 0.0 | 3.3 | 6.1 | 3.3 | 13.5 |
| SF7.5_0.5 | 4.5 | 8.9 | 1.8 | 22.2 | 4.6 | 13.0 | 4.6 | 5.5 |
| SF15.0_0.3 | 8.6 | 5.8 | 4.2 | 9.5 | 8.0 | 6.3 | 2.9 | 41.4 |
| SF15.0_0.4 | 4.9 | 2.0 | 2.2 | 22.7 | 5.3 | 1.9 | 1.6 | 37.5 |
| SF15.0_0.5 | 3.0 | 6.7 | 3.7 | 13.5 | 5.1 | 13.7 | 1.7 | 11.8 |
| Label | Ettringite 105–160 °C (%) | C-S-H 160– 423 °C (%) | Portlandite 423–500 °C (%) | Phases in Range 500–900 °C (%) | wch.b. (%) | Hydration Degree α (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days of curing | 28 | 180 | 28 | 180 | 28 | 180 | 28 | 180 | 28 | 180 | 28 | 180 |
| REF_0.3 | 3.4 | 3.5 | 5.7 | 6.4 | 3.5 | 3.7 | 3.4 | 3.1 | 14.0 | 14.8 | 58.4 | 61.6 |
| REF_0.4 | 3.4 | 3.9 | 5.3 | 7.1 | 3.0 | 4.1 | 8.7 | 3.3 | 15.2 | 16.4 | 63.5 | 68.3 |
| REF_0.5 | 3.3 | 4.5 | 6.8 | 7.2 | 4.1 | 4.2 | 2.8 | 5.1 | 15.3 | 17.9 | 63.9 | 74.5 |
| NZ7.5_0.3 | 3.4 | 3.7 | 6.0 | 6.5 | 3.2 | 3.4 | 4.0 | 3.2 | 14.2 | 14.9 | 59.1 | 62.1 |
| NZ7.5_0.4 | 3.5 | 4.0 | 6.3 | 7.0 | 3.3 | 3.7 | 3.8 | 3.0 | 14.7 | 16.0 | 61.1 | 66.5 |
| NZ7.5_0.5 | 4.1 | 4.8 | 6.7 | 7.4 | 3.4 | 3.7 | 4.5 | 3.4 | 15.9 | 17.2 | 66.3 | 71.7 |
| NZ15.0_0.3 | 3.9 | 4.3 | 5.7 | 6.9 | 2.4 | 2.9 | 6.9 | 2.8 | 14.9 | 15.3 | 61.9 | 63.7 |
| NZ15.0_0.4 | 4.3 | 4.6 | 6.5 | 6.9 | 2.8 | 2.9 | 4.4 | 3.3 | 15.3 | 15.7 | 63.8 | 65.6 |
| NZ15.0_0.5 | 4.6 | 5.4 | 6.2 | 7.3 | 2.6 | 2.8 | 7.2 | 5.2 | 16.4 | 17.7 | 68.5 | 73.6 |
| SF7.5_0.3 | 3.1 | 3.5 | 5.5 | 6.5 | 2.8 | 3.3 | 5.3 | 3.2 | 13.6 | 14.7 | 56.7 | 61.2 |
| SF7.5_0.4 | 3.4 | 4.1 | 6.6 | 7.2 | 3.5 | 3.8 | 3.7 | 3.8 | 15.0 | 16.7 | 62.4 | 69.6 |
| SF7.5_0.5 | 4.0 | 4.4 | 6.3 | 7.6 | 3.3 | 3.9 | 6.8 | 4.0 | 16.4 | 17.5 | 68.5 | 72.9 |
| SF15.0_0.3 | 3.2 | 3.7 | 5.0 | 5.9 | 2.2 | 2.5 | 5.5 | 3.3 | 12.7 | 13.4 | 52.8 | 55.8 |
| SF15.0_0.4 | 3.8 | 4.4 | 5.7 | 6.9 | 2.7 | 3.0 | 6.8 | 4.2 | 14.9 | 16.0 | 62.1 | 66.9 |
| SF15.0_0.5 | 3.8 | 4.5 | 6.2 | 6.9 | 2.8 | 3.0 | 6.1 | 6.1 | 15.2 | 16.9 | 63.2 | 70.4 |
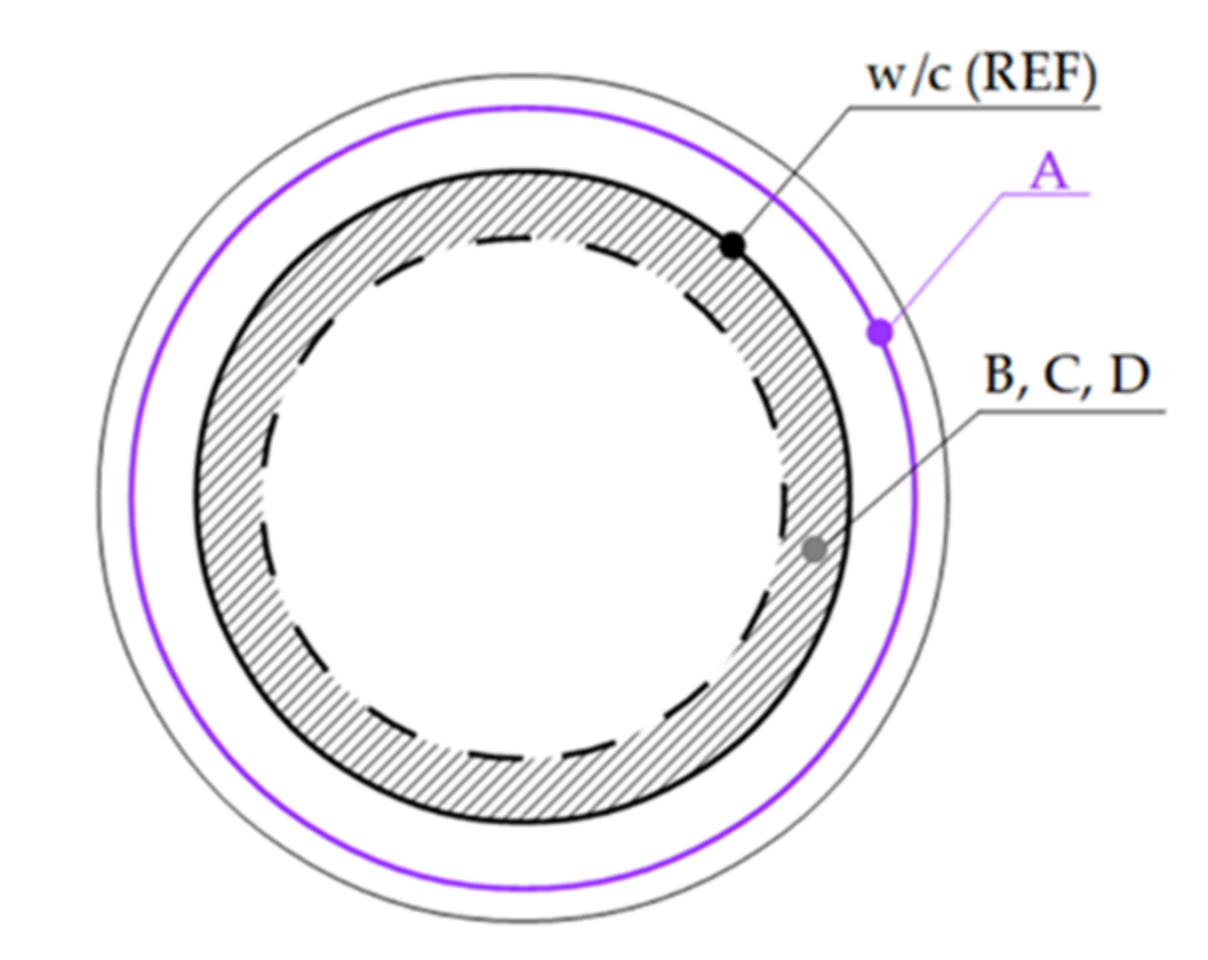
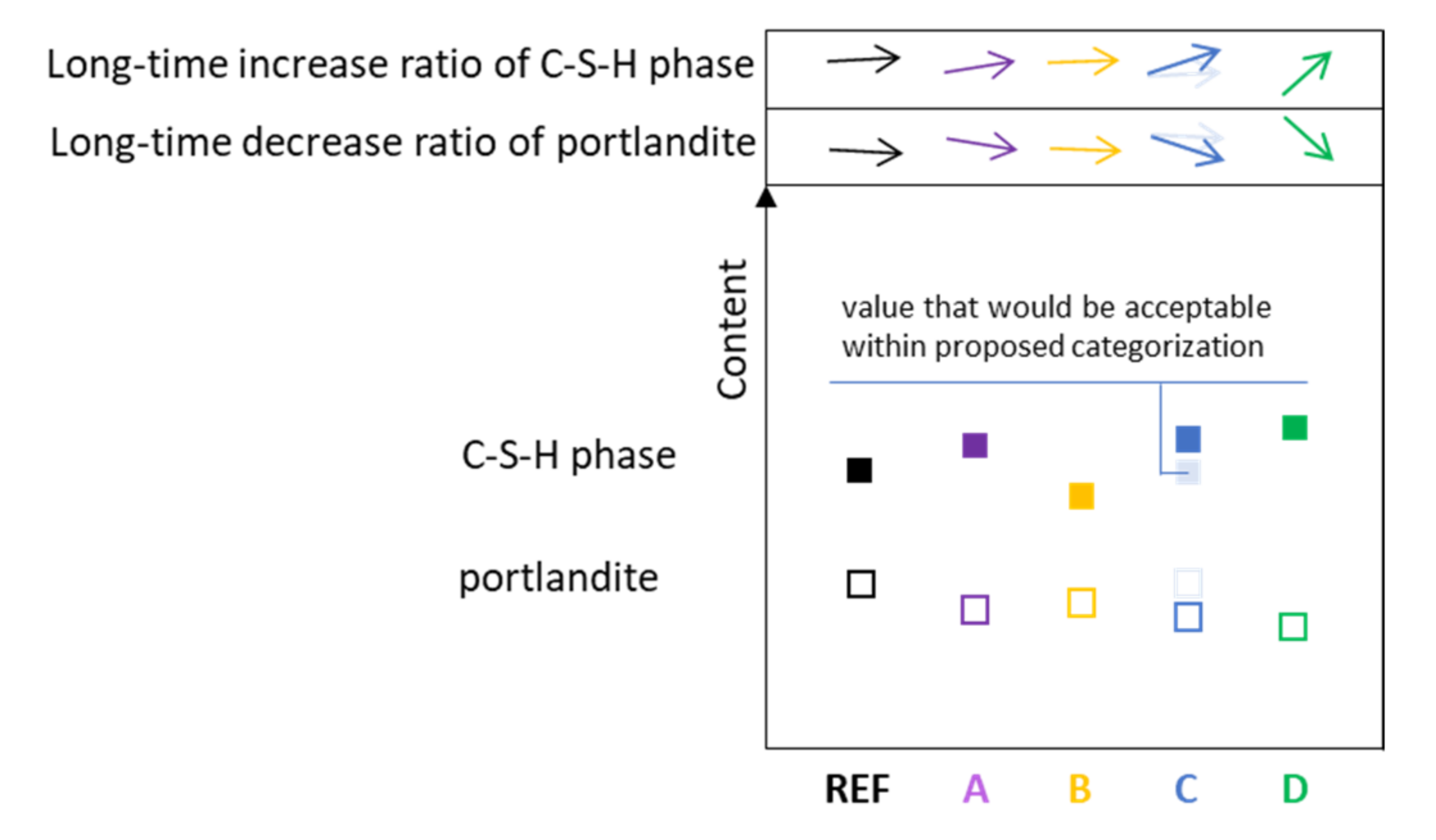
| Considered Assumption | Real Performance | |||
|---|---|---|---|---|
| Type | A | B | C, D | |
| Additive particle |  |  |  |  |
| Real binder in the w/b ratio | cem. + add. | cem. | cem. | cem. + add. |
| Relation to the designed w/b ratio | - | Higher | equal/lower | equal |
| Participation in hydration | Yes | no | no | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figmig, R.; Estokova, A.; Luptak, M. Concept of Evaluation of Mineral Additives’ Effect on Cement Pastes’ Durability and Environmental Suitability. Materials 2021, 14, 1448. https://doi.org/10.3390/ma14061448
Figmig R, Estokova A, Luptak M. Concept of Evaluation of Mineral Additives’ Effect on Cement Pastes’ Durability and Environmental Suitability. Materials. 2021; 14(6):1448. https://doi.org/10.3390/ma14061448
Chicago/Turabian StyleFigmig, Robert, Adriana Estokova, and Miloslav Luptak. 2021. "Concept of Evaluation of Mineral Additives’ Effect on Cement Pastes’ Durability and Environmental Suitability" Materials 14, no. 6: 1448. https://doi.org/10.3390/ma14061448
APA StyleFigmig, R., Estokova, A., & Luptak, M. (2021). Concept of Evaluation of Mineral Additives’ Effect on Cement Pastes’ Durability and Environmental Suitability. Materials, 14(6), 1448. https://doi.org/10.3390/ma14061448







