Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes
Abstract
1. Introduction
2. Graphene Quantum Dots
2.1. Unmodified GQDs
2.2. Capped/Encapsulated/Coated GQDs
2.3. Functionalized GQDs
Nitrogen-Doped GQDs
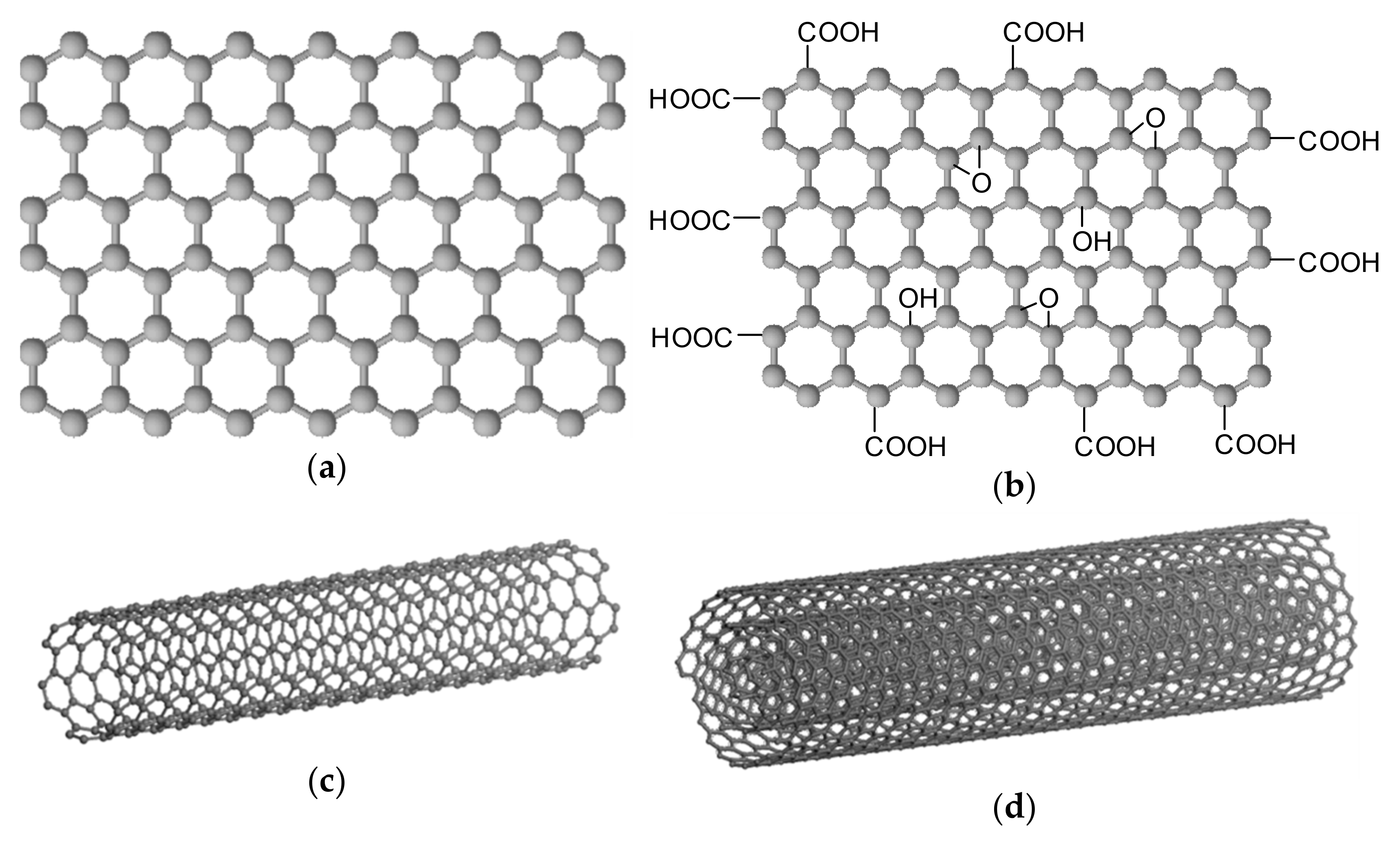
3. Graphene/Oxidized Graphene Nanoribbons and Nanoflakes
3.1. Graphene Nanoribbons
3.2. Oxidized Graphene Nanoribbons
3.3. Graphene/Graphene Oxide Nanoflakes
4. Graphene Oxide
4.1. Unmodified GO
4.2. Capped/Encapsulated GO
4.3. Coated GO
4.4. Functionalized GO
4.5. Magnetic GO
4.6. GO Used in Photoresponsive and Photothermal Therapy
4.7. Reduced GO
5. Carbon Nanotubes
5.1. Single-Walled Carbon Nanotubes
5.1.1. Unmodified SWCNTs
5.1.2. Capped/Encapsulated/Coated SWCNTs
5.1.3. Functionalized SWCNTs
5.1.4. Magnetic SWCNTs
5.2. Multi-Walled Carbon Nanotubes
5.2.1. Unmodified MWCNTs
5.2.2. Capped/Encapsulated/Coated MWCNTs
5.2.3. Functionalized MWCNTs
5.2.4. Magnetic MWCNTs
5.2.5. MWCNTs Used in Photoresponsive and Photothermal Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Roy, J. An Introduction to Pharmaceutical Sciences: Production, Chemistry, Techniques and Technology; Woodhead Publishing & Elsevier: Cambridge, UK, 2011. [Google Scholar]
- Tovey, G.D. Pharmaceutical Formulation: The Science and Technology of Dosage Forms; Royal Society of Chemistry: Croydon, UK, 2018. [Google Scholar]
- Buschmann, H.; Holenz, J.; Mannhold, R.; Bachhav, Y.G. Innovative Dosage Forms: Design and Development at Early Stage; Wiley-VCH: Wienheim, Germany, 2019. [Google Scholar]
- State Institute for Drug Control—About Drugs, Encyclopedia. 2021. Available online: www.olecich.cz (accessed on 21 January 2021). (In Czech).
- Tekade, R.K. Drug Delivery Systems; Academic Press & Elsevier: London, UK, 2019. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as potential nano-, micro- and macro-scale systems for controlled drug delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K.; Campos, E.V.R.; Fraceto, L.F. Bio-Based Nanoemulsion Formulations Applicable in Agriculture, Medicine and Food Industry. In Nanobiotechnology in Bioformulations; Prasad, R., Kumar, V., Kumar, M., Choudhary, D.K., Eds.; Springer: Cham, Switzerland, 2019; pp. 33–84. [Google Scholar]
- Jampilek, J.; Kralova, K. Application of Nanobioformulations For Controlled Release and Targeted Biodistribution of Drugs. In Nanobiomaterials: Applications in Drug Delivery; Sharma, A.K., Keservani, R.K., Kesharwani, R.K., Eds.; CRC Press: Warentown, NJ, USA, 2018; pp. 131–208. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanotechnology Based Formulations for Drug Targeting to Central Nervous System. In Nanoparticulate Drug Delivery Systems; Keservani, R.K., Sharma, A.K., Eds.; Apple Academic Press & CRC Press: Warentown, NJ, USA, 2019; pp. 151–220. [Google Scholar]
- Jampilek, J.; Kralova, K. Recent Advances in Lipid Nanocarriers Applicable in the Fight Against Cancer. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–294. [Google Scholar]
- Jampilek, J.; Kralova, K. Natural Biopolymeric Nanoformulations for Brain Drug Delivery. In Nanocarriers for Brain Targeting: Principles and Applications; Keservani, R.K., Sharma, A.K., Kesharwani, R.K., Eds.; Apple Academic Press & CRC Press: Warentown, NJ, USA, 2020; pp. 131–203. [Google Scholar]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; Van Zundert, I.; Masuhara, A.; Fron, E.; Mizuno, H.; Latterini, L.; Rocha, S.; et al. Polymeric engineering of nanoparticles for highly efficient multifunctional drug delivery systems. Sci. Rep. 2019, 9, 2666. [Google Scholar] [CrossRef]
- Singh, S.; Dhawan, A.; Karhana, S.; Bhat, A.; Dinda, A.K. Quantum dots: An emerging tool for point-of-care testing. Micromachines 2020, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.X.; Zhu, B.J. The research and applications of quantum dots as nano-carriers for targeted drug delivery and cancer therapy. Nanoscale Res. Lett. 2016, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Nano-Antimicrobials: Activity, Benefits and Weaknesses. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–54. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanoformulations—Valuable Tool in Therapy of Viral Diseases Attacking Humans and Animals. In Nanotheranostic—Applications and Limitations; Rai, M., Jamil, B., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 137–178. [Google Scholar]
- Jampilek, J.; Kralova, K. Impact of Nanoparticles on Toxigenic Fungi. In Nanomycotoxicology—Treating Mycotoxins in the Nano Way; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press & Elsevier: London, UK, 2020; pp. 309–348. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanocomposites: Synergistic Nanotools for Management Mycotoxigenic Fungi. In Nanomycotoxicology—Treating Mycotoxins in the Nano Way; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press & Elsevier: London, UK, 2020; pp. 349–383. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanoweapons against Tuberculosis. In Nanoformulations in Human Health—Challenges and Approaches; Talegaonkar, S., Rai, M., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 469–502. [Google Scholar]
- Pentak, D.; Kozik, V.; Bak, A.; Dybal, P.; Sochanik, A.; Jampilek, J. Methotrexate and cytarabine—Loaded nanocarriers for multidrug cancer therapy. Spectroscopic study. Molecules 2016, 21, 1689. [Google Scholar] [CrossRef]
- Kozik, V.; Bak, A.; Pentak, D.; Hachula, B.; Pytlakowska, K.; Rojkiewicz, M.; Jampilek, J.; Sieron, K.; Jazowiecka-Rakus, J.; Sochanik, A. Derivatives of graphene oxide as potential drug carriers. J. Nanosci. Nanotechnol. 2019, 19, 2489–2492. [Google Scholar] [CrossRef] [PubMed]
- Placha, D.; Jampilek, J. Graphenic materials for biomedical applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhou, Y.; Fan, T.; Lin, Y.; Zhang, H.; Mei, L. Inorganic nano-carriers based smart drug delivery systems for tumor therapy. Smart Mater. Med. 2020, 1, 32–47. [Google Scholar] [CrossRef]
- Dhas, N.; Parekh, K.; Pandey, A.; Kudarha, R.; Mutalik, S.; Mehta, T. Two dimensional carbon based nanocomposites as multimodal therapeutic and diagnostic platform: A biomedical and toxicological perspective. J. Control. Release 2019, 308, 130–161. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. From nanoengineering to nanomedicine: A facile route to enhance biocompatibility of graphene as a potential nano-carrier for targeted drug delivery using natural deep eutectic solvents. Chem. Eng. Sci. 2019, 195, 95–106. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Knizek, K.; Kubacki, J.; Goraus, J.; Goryczka, T.; Pietrasik, E.; Barsova, Z.; Jampilek, J.; Witkowska-Kita, B. Structure and properties of nano- and polycrystalline Mn-doped CuCr2Se4 obtained by ceramic method and grain reduction. Mater. Res. Bull. 2021, 137, 111174. [Google Scholar] [CrossRef]
- Vaculikova, E.; Grunwaldová, V.; Kral, V.; Dohnal, J.; Jampilek, J. Preparation of candesartan and atorvastatin nanoparticles by solvent evaporation. Molecules 2012, 17, 13221–13234. [Google Scholar] [CrossRef]
- Vaculikova, E.; Cernikova, A.; Placha, D.; Pisarcik, M.; Peikertova, P.; Dedkova, K.; Devinsky, F.; Jampilek, J. Preparation of hydrochlorothiazide nanoparticles for solubility enhancement. Molecules 2016, 21, 1005. [Google Scholar] [CrossRef]
- Jampilek, J.; Kos, J.; Kralova, K. Potential of nanomaterial applications in dietary supplements and foods for special medical purposes. Nanomaterials 2019, 9, 296. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Potential of nanonutraceuticals in increasing immunity. Nanomaterials 2020, 10, 2224. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics 2021, 13, 642019. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K.; Novak, P.; Novak, M. Nanobiotechnology in Neurodegenerative Diseases; Rai, M., Yadav, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 65–138. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Martinez, G.; Merinero, M.; Perez-Aranda, M.; Perez-Soriano, E.M.; Ortiz, T.; Begines, B.; Alcudia, A. Environmental impact of nanoparticles’ application as an emerging technology: A review. Materials 2021, 14, 166. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar]
- Clemons, T.D.; Singh, R.; Sorolla, A.; Chaudhari, N.; Hubbard, A.; Iyer, K.S. Distinction between active and passive targeting of nanoparticles dictate their overall therapeutic efficacy. Langmuir 2018, 34, 15343–15349. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Pandey, S.P.; Sudheesh, M.S. Site-Specific Drug Delivery, Targeting, and Gene Therapy. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 473–505. [Google Scholar]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhang, N.; Wang, Y. Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics. Clin. Transl. Med. 2021, 11, e292. [Google Scholar] [CrossRef]
- De Sousa, M.; de Luna, L.A.V.; Fonseca, L.C.; Giorgio, S.; Alves, O.L. Folic-acid-functionalized graphene oxide nanocarrier: Synthetic approaches, characterization, drug delivery study, and antitumor screening. ACS Appl. Nano Mater. 2018, 1, 922–932. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized graphene oxide for chemotherapeutic drug delivery and cancer treatment: A promising material in nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Han, X.M.; Zheng, K.W.; Wang, R.L.; Yue, S.F.; Chen, J.; Zhao, Z.W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-based nanomaterials for delivery of biologicals and therapeutics: A cutting-edge technology. C 2021, 7, 19. [Google Scholar]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Jamalipour Soufi, G.; Iravani, S. Carbon-based nanomaterials for targeted cancer nanotherapy: Recent trends and future prospects. J. Drug Target. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Barthelmy, D. Mineral Species Containing Carbon. In Mineralogy Database. 2021. Available online: http://webmineral.com/chem/Chem-C.shtml#.YAn3SxaLqM8 (accessed on 20 January 2021).
- Hirsch, A. The era of carbon allotropes. Nat. Mater 2010, 9, 868–871. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Allotropes of Carbon. Lumen Learning: Portland, OR, USA. Available online: https://courses.lumenlearning.com/introchem/chapter/allotropes-of-carbon/ (accessed on 20 January 2021).
- The Nobel Prize in Physics 2010. NobelPrize.org. Nobel Media AB. 2021. Available online: https://www.nobelprize.org/prizes/physics/2010/summary/ (accessed on 14 January 2021).
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Marconcini, P.; Macucci, M. The k.p method and its application to graphene, carbon nanotubes and graphene nanoribbons: The Dirac equation. Riv. Nuovo Cim. 2011, 34, 489–584. [Google Scholar]
- Nurunnabi, M.; McCarthy, J.R. Biomedical Applications of Graphene and 2D Nanomaterials (Micro and Nano Technologies); Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Ranjan, P.; Agrawal, S.; Sinha, A.; Rao, T.R.; Balakrishnan, J.; Thakur, A.D. A low-cost non-explosive synthesis of graphene oxide for scalable applications. Sci. Rep. 2018, 8, 12007. [Google Scholar] [CrossRef]
- Neustroev, E.P. Plasma Treatment of Graphene Oxide. In Graphene Oxide Applications and Opportunities; Kamble, G.S., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 7–24. [Google Scholar]
- Shabin, M.; Hanaa, H.; Ranwen, O.; Shasha, L.; Hongyu, M.; Xiaofang, C.; Tam, S.; Huanting, W. Effect of oxygen plasma treatment on the nanofiltration performance of reduced graphene oxide/cellulose nanofiber composite membranes. Green Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- Etching with Plasma. Diener Electronic, Plasma—Surface—Technology, Ebhausen, Germany. Available online: https://www.plasma.com/en/etching-with-plasma/?kampagne=1&gclid=Cj0KCQiAvbiBBhD-ARIsAGM48bzt1bCEbT4CqRUWwSqgJWCitReALhY-T5EBVf9B6c5AkWFWNdVxSxUaAkU0EALw_wcB (accessed on 18 February 2021).
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mat. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Joshi, S.; Siddiqui, R.; Sharma, P.; Kumar, R.; Verma, G.; Saini, A. Green synthesis of peptide functionalized reduced graphene oxide (rGO) nano bioconjugate with enhanced antibacterial activity. Sci. Rep. 2020, 10, 9441. [Google Scholar] [CrossRef]
- Kang, J.; Wei, Z.M.; Li, J.B. Graphyne and its family: Recent theoretical advances. ACS Appl. Mater. Interfaces 2019, 11, 2692–2706. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, H.B.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef] [PubMed]
- Nanowerk: Carbon Nanotubes—What They Are, How They Are Made, What They Are Used For. 2021. Available online: https://www.nanowerk.com/nanotechnology/introduction/introduction_to_nanotechnology_22.php (accessed on 21 January 2021).
- Foa Torres, L.E.F.; Roche, S.; Charlier, J.C. Introduction to Graphene-Based Nanomaterials, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Takai, K.; Tsujimura, S.; Kang, F.; Inagaki, M. Graphene: Preparations, Properties, Applications, and Prospects; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Dimiev, A.M.; Eigler, S. Graphene Oxide: Fundamentals and Applications; John Wiley and Sons: Chichester, UK, 2017. [Google Scholar]
- Tanaka, K.; Iijima, S. Carbon Nanotubes and Graphene, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, E3564. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.M.; Mou, X.Z.; Yang, K. Carbon-based nanomaterials for biomedical applications: A recent study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Sarkar, K. Biomedical applications of graphene nanomaterials and beyond. ACS Biomater. Sci. Eng. 2018, 4, 2653–2703. [Google Scholar] [CrossRef]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem. Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef]
- Jia, P.P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.B.; Cui, Z.S.; Wei, D.P.; Shi, H.F.; Pei, D.S. Nanotoxicity of different sizes of graphene (G) and graphene oxide (GO) in vitro and in vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef]
- Ameta, S.C.; Kodolov, V.I.; Vakhrushev, A.V.; Haghi, A.K. Carbon Nanotubes and Nanoparticles: Current and Potential Applications; Apple Academic Press & CRC Press: Palm Bay, FL, USA, 2019. [Google Scholar]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater. 2019, 12, e1904362. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Zhang, X.H.; Gomathi, T.; Wang, S.F.; Ansari, M.A.; Mydhili, G.; Nirmala, G.; Alzohairy, M.A.; Chung, I.M. Current use of carbon-based materials for biomedical applications—A prospective and review. Processes 2020, 8, 355. [Google Scholar] [CrossRef]
- Crista, M.A.; da Silva, J.C.G.E.; da Silva, L.P. Evaluation of different bottom-up routes for the fabrication of carbon dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.K. Pure and Functionalized Carbon Based Nanomaterials: Analytical, Biomedical, Civil and Environmental Engineering Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- European Union Observatory for Nanomaterials. 2021. Available online: https://euon.echa.europa.eu/medicine (accessed on 20 January 2021).
- Rahmati, M.; Mozafari, M. Biological response to carbon-family nanomaterials: Interactions at the nano-bio interface. Front. Bioeng. Biotechnol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Kitko, K.E.; Zhang, Q. Graphene-based nanomaterials: From production to integration with modern tools in neuroscience. Front. Syst. Neurosci. 2019, 13, 26. [Google Scholar] [CrossRef] [PubMed]
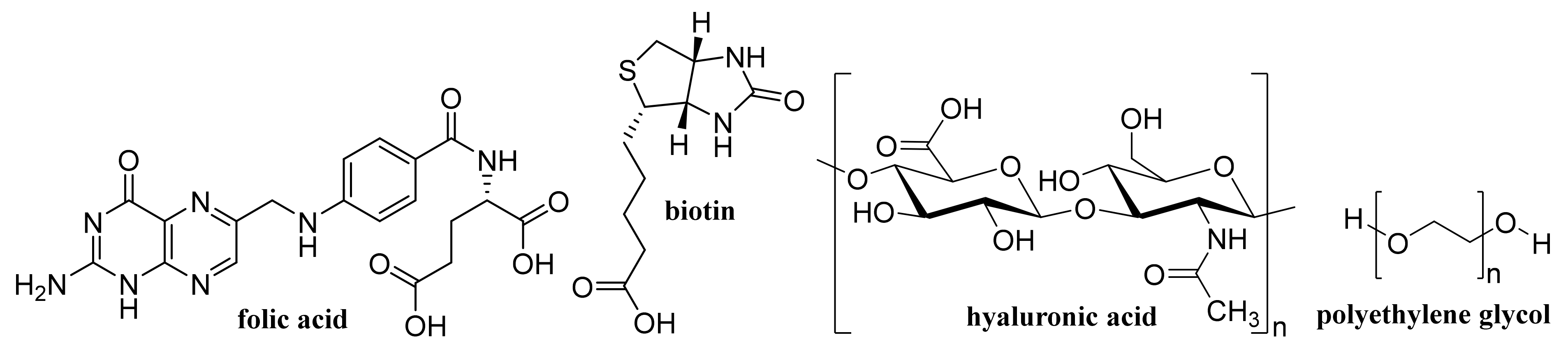
- Jun, S.W.; Manivasagan, P.; Kwon, J.; Nguyen, V.T.; Mondal, S.; Ly, C.D.; Lee, J.; Kang, Y.H.; Kim, C.S.; Oh, J. Folic acid-conjugated chitosan-functionalized graphene oxide for highly efficient photoacoustic imaging-guided tumor-targeted photothermal therapy. Int. J. Biol. Macromol. 2020, 155, 961–971. [Google Scholar] [CrossRef]
- Wong, X.Y.; Quesada-Gonzalez, D.; Manickam, S.; New, S.Y.; Muthoosamy, K.; Merkoci, A. Integrating gold nanoclusters, folic acid and reduced graphene oxide for nanosensing of glutathione based on “turn-off” fluorescence. Sci. Rep. 2021, 11, 2375. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon nanomaterials as versatile platforms for biosensing applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, J.; Park, R.; Jeong, J.; Shin, M.C.; Eom, S.U.; Park, J.; Hong, S.W. Graphene templated DNA arrays and biotin-streptavidin sensitive bio-transistors patterned by dynamic self-assembly of polymeric films confined within a roll-on-plate geometry. Nanomaterials 2020, 10, 1468. [Google Scholar] [CrossRef]
- Wang, S.; Hossain, M.Z.; Han, T.; Shinozuka, K.; Suzuki, T.; Kuwana, A.; Kobayashi, H. Avidin–biotin technology in gold nanoparticle-decorated graphene field effect transistors for detection of biotinylated macromolecules with ultrahigh sensitivity and specificity. ACS Omega 2020, 5, 30037–30046. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Zhao, X.J.; Jia, S.R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B Eng. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Cao, W.J.; He, L.; Cao, W.D.; Huang, X.B.; Jia, K.; Dai, J.Y. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene oxide-incorporated hydrogels for biomedical applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Gong, M.; Sun, J.; Liu, G.; Li, L.; Wu, S.; Xiang, Z. Graphene oxide–modified 3D acellular cartilage extracellular matrix scaffold for cartilage regeneration. Mat. Sci. Eng. C Mater. 2021, 119, 111603. [Google Scholar] [CrossRef]
- Luo, S.; Jin, S.; Yang, T.; Wu, B.; Xu, C.; Luo, L.; Chen, Y. Sustained release of tulobuterol from graphene oxide laden hydrogel to manage asthma. J. Biomater. Sci. Polym. Ed. 2021, in press. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- McDougall, G.J. The physical nature and manufacture of activated carbon. J. S. Afr. Inst. Min. Metal. 1991, 91, 109–120. [Google Scholar]
- Roy, G.M. Activated Carbon Applications in the Food and Pharmaceutical Industries; Technomic Publishing Company: Lancaster, PA, USA, 1995. [Google Scholar]
- Kerihuel, J.C. Effect of activated charcoal dressings on healing outcomes of chronic wounds. J. Wound Care 2010, 19, 208. [Google Scholar] [CrossRef]
- Afrin, M.R.; Arumugam, S.; Pitchaimani, V.; Karuppagounder, V.; Thandavarayan, R.A.; Harima, M.; Hossain, C.F.; Suzuki, K.; Sone, H.; Matsubayashi, Y.; et al. Le Carbone prevents liver damage in non-alcoholic steatohepatitis-hepatocellular carcinoma mouse model via AMPKα-SIRT1 signaling pathway activation. Heliyon 2021, 7, e05888. [Google Scholar] [CrossRef] [PubMed]
- Ramanayaka, S.; Vithanage, M.; Alessi, D.S.; Liu, W.J.; Jayasundera, A.C.A.; Ok, Y.S. Nanobiochar: Production, properties, and multifunctional applications. Environ. Sci. Nano 2020, 7, 3279–3302. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Potential of Nanoscale Carbon-Based Materials for Remediation of Pesticide-Contaminated Environment. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–399. [Google Scholar]
- Jampilek, J.; Kralova, K. Synthesis of Nanocomposite from Agricultural Waste. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-food and Ecosystems; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–98. [Google Scholar]
- Farjadian, F.; Abbaspour, S.; Sadatlu, M.A.A.; Mirkiani, S.; Ghasemi, A.; Hoseini-Ghahfarokhi, M.; Mozaffari, N.; Karimi, M.; Hamblin, M.R. Recent developments in graphene and graphene oxide: Properties, synthesis, and modifications: A review. ChemistrySelect 2020, 5, 10200–10219. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Huang, H.T.; Dong, Y.; Han, C.Y.; Sui, X.Y.; Jian, B.Y. Multi-walled carbon nanotube-based systems for improving the controlled release of insoluble drug dipyridamole. Exp. Ther. Med. 2019, 17, 4610–4616. [Google Scholar] [CrossRef]
- Jones, A.D.; Mi, G.; Webster, T.J. A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol. 2019, 37, 117–120. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Nanotechnology, US FDA. 2021. Available online: https://www.fda.gov/about-fda/nctr-research-focus-areas/nanotechnology (accessed on 20 January 2021).
- A Brief Review of FDA Approved Nano-Drugs, NBIC+, StatNano. 2021. Available online: https://statnano.com/news/61107/A-Brief-Review-of-FDA-Approved-Nano-drugs (accessed on 20 January 2021).
- Gustavsson, P.; Hedmer, M.; Rissler, J. Carbon Nanotubes—Exposure, Toxicology and Protective Measures in the Work Environment; Arbetsmiljöverket: Stockholm, Sweden, 2011; Available online: https://www.av.se/globalassets/filer/publikationer/kunskapssammanstallningar/carbon-nanotubes-knowledge-compliation-2011-1-eng.pdf (accessed on 20 January 2021).
- National Industrial Chemicals Notification and Assessment Scheme (NICNAS). Human Health Hazard Assessment and Classification of Carbon Nanotubes; Safe Work Australia: Canberra, Australia, 2012. Available online: https://www.safeworkaustralia.gov.au/system/files/documents/1702/human_health_hazard_assessment_and_classification_of_carbon_nanotubes.pdf (accessed on 20 January 2021).
- Garriga, R.; Herrero-Continente, T.; Palos, M.; Cebolla, V.L.; Osada, J.; Muñoz, E.; Rodríguez-Yoldi, M.J. Toxicity of Carbon Nanomaterials and Their Potential Application as Drug Delivery Systems: In Vitro Studies in Caco-2 and MCF-7 Cell Lines. Nanomaterials 2020, 10, 1617. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xue, Z.; Xie, J.; Dong, Y.; Ma, Z.; Sun, X.; Kebebe Borga, D.; Liu, Z.; Li, J. Toxicity of carbon nanotubes as anti-tumor drug carriers. Int. J. Nanomed. 2019, 14, 10179–10194. [Google Scholar] [CrossRef] [PubMed]
- Keservani, R.K.; Sharma, A.K. Nanoconjugate Nanocarriers for Drug Delivery; CRC Press: Warentown, NJ, USA, 2018. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Chemical Functionalization of Carbon Nanomaterials; CRC Press: Warentown, NJ, USA, 2018. [Google Scholar]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent advances on graphene quantum dots for bioimaging applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Henna, T.K.; Pramod, K. Graphene quantum dots redefine nanobiomedicine. Mater. Sci. Eng. C 2020, 110, 110651. [Google Scholar] [CrossRef] [PubMed]
- Kortel, M.; Mansuriya, B.D.; Vargas Santana, N.; Altintas, Z. Graphene quantum dots as flourishing nanomaterials for bio-imaging, therapy development, and micro-supercapacitors. Micromachines 2020, 11, 866. [Google Scholar] [CrossRef]
- Lesiak, A.; Drzozga, K.; Cabaj, J.; Banski, M.; Malecha, K.; Podhorodecki, A. Optical sensors based on II-VI quantum dots. Nanomaterials 2019, 9, 192. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Le, Q.V.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef]
- Zhang, M.; Bishop, B.P.; Thompson, N.L.; Hildahl, K.; Dang, B.; Mironchuk, O.; Chen, N.; Aoki, R.; Holmberg, V.C.; Nance, E. Quantum dot cellular uptake and toxicity in the developing brain: Implications for use as imaging probes. Nanoscale Adv. 2019, 1, 342–3442. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; Ciasca, G.; De Spirito, M.; Papi, M. Unravelling the potential of graphene quantum dots in biomedicine and neuroscience. Int. J. Mol. Sci. 2020, 21, 3712. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Mathur, P.; Ramteke, S.; Jain, N.K. Pharmaceutical potential of quantum dots. Artif. Cells Nanomed. Biotechnol. 2018, 46, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Chowdhury, P.P.; Nagpal, P. Quantum dot therapeutics: A new class of radical therapies. J. Biol. Eng. 2019, 13, 48. [Google Scholar] [CrossRef]
- Zhao, C.H.; Song, X.B.; Liu, Y.; Fu, Y.F.; Ye, L.L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M.; et al. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnology 2020, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.S.; Gharbi, S.; Jafarinejad-Farsangi, S.; Ansari-Asl, Z.; Dezfuli, A.S. Secondary toxic effect of graphene oxide and graphene quantum dots alters the expression of miR-21 and miR-29a in human cell lines. Toxicol. In Vitro 2020, 65, 104796. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Feng, B.; Dong, Y.Q.; Zhao, M.; Yang, X.D. Vanadium coordination compounds loaded on graphene quantum dots (GQDs) exhibit improved pharmaceutical properties and enhanced anti-diabetic effects. Nanoscale 2020, 12, 9219–9230. [Google Scholar] [CrossRef] [PubMed]
- Rakhshaei, R.; Namazi, H.; Hamishehkar, H.; Rahimi, M. Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties. Int. J. Biol. Macromol. 2020, 150, 1121–1129. [Google Scholar] [CrossRef]
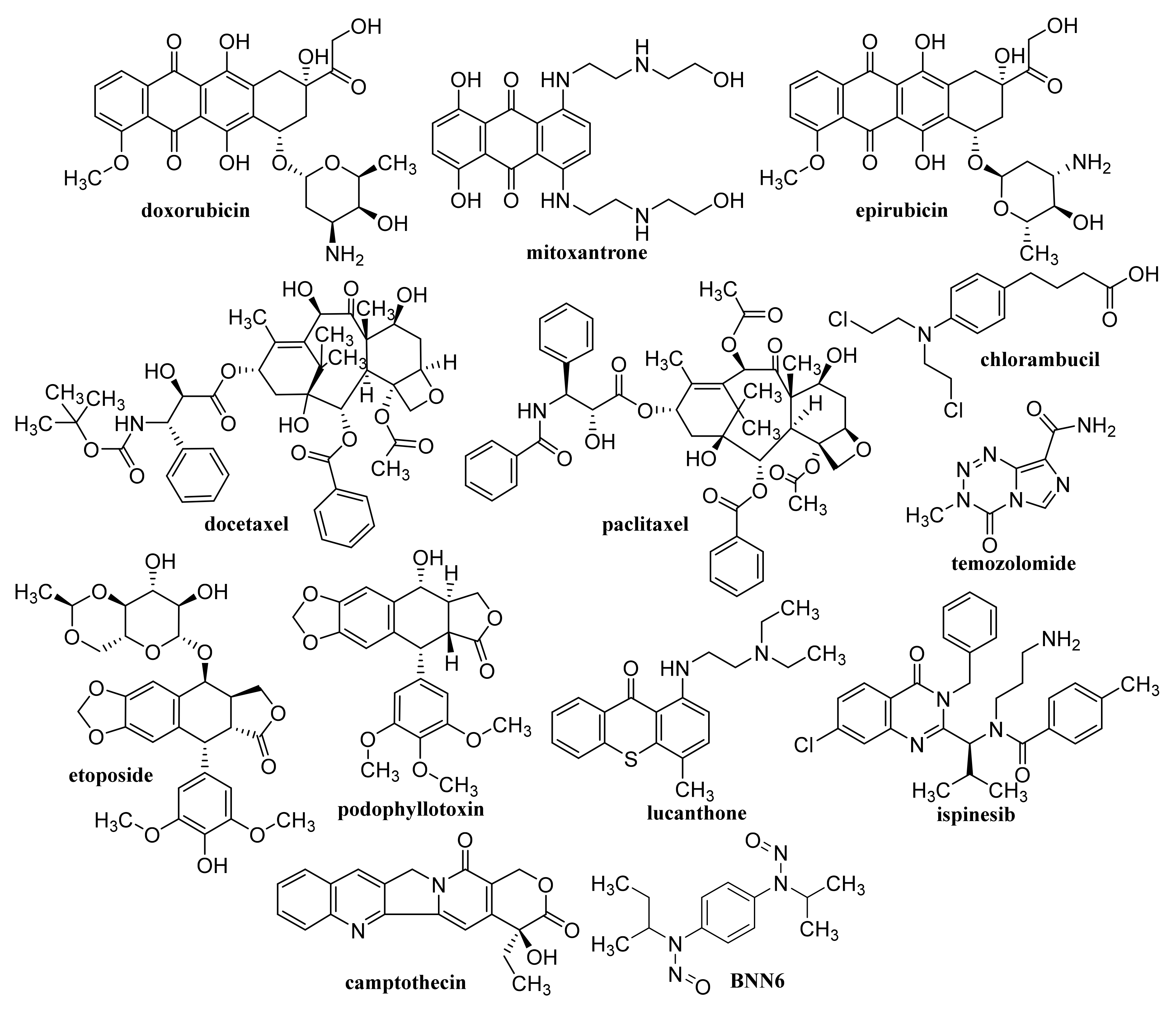
- Liang, J.L.; Huang, Q.W.; Hua, C.X.; Hu, J.H.; Chen, B.L.; Wan, J.M.; Hu, Z.W.; Wang, B. pH-Responsive nanoparticles loaded with graphene quantum dots and doxorubicin for intracellular imaging, drug delivery and efficient cancer therapy. ChemistrySelect 2019, 4, 6004–6012. [Google Scholar] [CrossRef]
- Sheng, Y.S.; Dai, W.; Gao, J.; Li, H.D.; Tan, W.S.; Wang, J.W.; Deng, L.H.; Kong, Y. pH-sensitive drug delivery based on chitosan wrapped graphene quantum dots with enhanced fluorescent stability. Mat. Sci. Eng. C Mater. 2020, 112, 110888. [Google Scholar] [CrossRef]
- Havanur, S.; Batish, I.; Cheruku, S.P.; Gourishetti, K.; JagadeeshBabu, P.E.; Kumar, N. Poly(N,N-diethyl acrylamide)/functionalized graphene quantum dots hydrogels loaded with doxorubicin as a nano-drug carrier for metastatic lung cancer in mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110094. [Google Scholar] [CrossRef]
- Nasrollahi, F.; Sana, B.; Paramelle, D.; Ahadian, S.; Khademhosseini, A.; Lim, S. Incorporation of graphene quantum dots, iron, and doxorubicin in/on ferritin nanocages for bimodal imaging and drug delivery. Adv. Ther. 2020, 3, 1900183. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Simple preparation of maltose-functionalized dendrimer/graphene quantum dots as a pH-sensitive biocompatible carrier for targeted delivery of doxorubicin. Int. J. Biol. Macromol. 2020, 156, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.X.; Niu, X.X.; Ma, K.X.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y.F. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small 2017, 13, 1602225. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhong, S.L.; Xu, L.F.; He, S.H.; Dou, Y.M.; Zhao, S.N.; Chen, P.; Cui, X.J. Mesoporous silica nanoparticles capped with graphene quantum dots as multifunctional drug carriers for photo-thermal and redox-responsive release. Microporous Mesoporous Mater. 2019, 278, 130–137. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.H.; Sun, S.A.; Guo, P.Y.; Wang, Y.; Qian, C.T.; Zhong, Y.Y.; Yang, D.Z. Wound therapy via a photo-responsively antibacterial nano-graphene quantum dots conjugate. J. Photochem. Photobiol. B 2020, 210, 111978. [Google Scholar] [CrossRef]
- Zheng, S.H.; Jin, Z.; Han, C.P.; Li, J.J.; Xu, H.; Park, S.; Park, J.O.; Choi, E.; Xu, K. Graphene quantum dots-decorated hollow copper sulfide nanoparticles for controlled intracellular drug release and enhanced photothermal-chemotherapy. J. Mater. Sci. 2020, 55, 1184–1197. [Google Scholar] [CrossRef]
- Yu, L.; Tian, X.; Gao, D.X.; Lang, Y.; Zhang, X.X.; Yang, C.; Gu, M.M.; Shi, J.M.; Zhou, P.K.; Shang, Z.F. Oral administration of hydroxylated-graphene quantum dots induces intestinal injury accompanying the loss of intestinal stem cells and proliferative progenitor cells. Nanotoxicology 2019, 13, 1409–1421. [Google Scholar] [CrossRef]
- Li, Z.; Fan, J.L.; Tong, C.Y.; Zhou, H.Y.; Wang, W.M.; Li, B.; Liu, B.; Wang, W. A smart drug-delivery nanosystem based on carboxylated graphene quantum dots for tumor-targeted chemotherapy. Nanomedicine 2019, 14, 2011–2025. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’Ascenzo, M.; Primiano, A.; Gervasoni, J.; De Maio, F.; De Spirito, M.; Papi, M. Enhanced chemotherapy for glioblastoma multiforme mediated by functionalized graphene quantum dots. Materials 2020, 13, 4139. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’Ascenzo, M.; Gervasoni, J.; Primiano, A.; Rinaldi, M.; Fioretti, D.; Prampolini, C.; Tiberio, F.; et al. Graphene quantum dots’ surface chemistry modulates the sensitivity of glioblastoma cells to chemotherapeutics. Int. J. Mol. Sci. 2020, 21, 6301. [Google Scholar] [CrossRef]
- Xue, Z.Y.; Sun, Q.; Zhang, L.; Kang, Z.Z.; Liang, L.J.; Wang, Q.; Shen, J.W. Graphene quantum dot assisted translocation of drugs into a cell membrane. Nanoscale 2019, 11, 4503–4514. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamo, M.; Galvagno, S.; Romeo, R.; Giofre, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef] [PubMed]
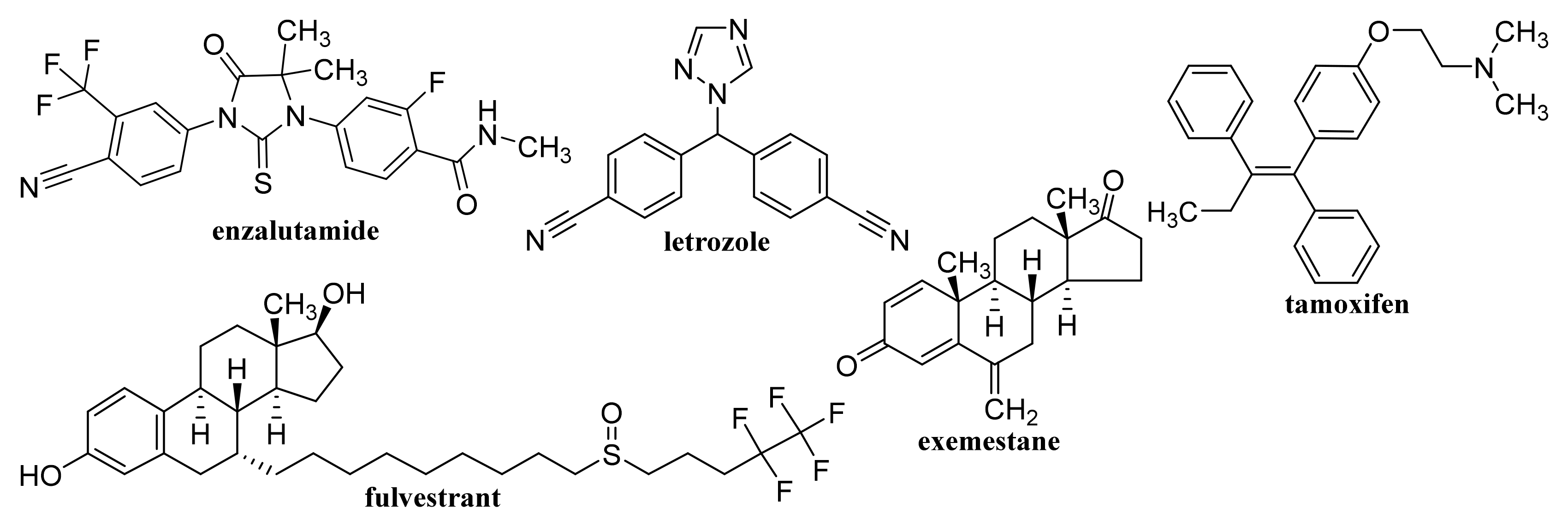
- Jiang, W.J.; Chen, J.Y.; Gong, C.A.; Wang, Y.Y.; Gao, Y.; Yuan, Y.F. Intravenous delivery of enzalutamide based on high drug loading multifunctional graphene oxide nanoparticles for castration-resistant prostate cancer therapy. J. Nanobiotechnology 2020, 18, 50. [Google Scholar] [CrossRef]
- Vatanparast, M.; Shariatinia, Z. Revealing the role of different nitrogen functionalities in the drug delivery performance of graphene quantum dots: A combined density functional theory and molecular dynamics approach. J. Mater. Chem. B 2019, 7, 6156–6171. [Google Scholar] [CrossRef] [PubMed]
- Senel, B.; Demir, N.; Buyukkoroglu, G.; Yildiz, M. Graphene quantum dots: Synthesis, characterization, cell viability, genotoxicity for biomedical applications. Saudi Pharm J. 2019, 27, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Lee, C.Y.; Doong, R.A.; Oon, C.E.; Thanh, N.T.K.; Lee, H.L. A titanium dioxide/nitrogen-doped graphene quantum dot nanocomposite to mitigate cytotoxicity: Synthesis, characterisation, and cell viability evaluation. RSC Adv. 2020, 10, 21795–21805. [Google Scholar] [CrossRef]
- Ahmadi-Kashani, M.; Dehghani, H.; Zarrabi, A. A biocompatible nanoplatform formed by MgAl-layered double hydroxide modified Mn3O4/N-graphene quantum dot conjugated-polyaniline for pH-triggered release of doxorubicin. Mat. Sci. Eng. C Mater. 2020, 114, 111055. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Augustine, S.; Prabhakar, B. A review on graphene nanoribbons for advanced biomedical applications. Carbon Lett. 2020, 30, 465–475. [Google Scholar] [CrossRef]
- Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Graphene nanoribbons: A promising nanomaterial for biomedical applications. J. Control. Release 2020, 325, 141–162. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Soroshnia, S.; Hashemi, S.A.; Babapoor, A.; Ghasemi, Y.; Savardashtaki, A.; Amani, A.M. Graphene nano-ribbon based high potential and efficiency for DNA, cancer therapy and drug delivery applications. Drug Metab. Rev. 2019, 51, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Janani, K.; Thiruvadigal, D.J. Density functional study on covalent functionalization of zigzag graphene nanoribbon through l-Phenylalanine and boron doping: Effective nanocarriers in drug delivery applications. Appl. Surf. Sci. 2018, 449, 815–822. [Google Scholar]
- Mari, E.; Mardente, S.; Morgante, E.; Tafani, M.; Lococo, E.; Fico, F.; Valentini, F.; Zicari, A. Graphene oxide nanoribbons induce autophagic vacuoles in neuroblastoma cell lines. Int. J. Mol. Sci. 2016, 17, 1995. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Zafar, S.; Tellez, V.; Sitharaman, B. Graphene nanoribbon-based platform for highly efficacious nuclear gene delivery. ACS Biomater. Sci. Eng. 2016, 2, 798–808. [Google Scholar] [CrossRef]
- Foreman, H.C.C.; Lalwani, G.; Kalra, J.; Krug, L.T.; Sitharaman, B. Gene delivery to mammalian cells using a graphene nanoribbon platform. J. Mater. Chem. B 2017, 5, 2347–2354. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Fang, J.; Sitharaman, B. Interaction of graphene nanoribbons with components of the blood vascular system. Future Sci. OA 2015, 1, FSO19. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Manepalli, P.; Sitharaman, B. Graphene nanoribbons elicit cell specific uptake and delivery via activation of epidermal growth factor receptor enhanced by human papillomavirus E5 protein. Acta Biomater. 2014, 10, 4494–4504. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Lalwani, G.; Zhang, K.; Yang, J.Y.; Neville, K.; Sitharaman, B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials 2013, 34, 283–293. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Surhland, C.; Sanchez, Z.; Chaudhary, P.; Kumar, M.A.S.; Lee, S.; Pena, L.A.; Waring, M.; Sitharaman, B.; Naidu, M. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine 2015, 11, 109–118. [Google Scholar] [CrossRef]
- Lu, Y.J.; Lin, C.W.; Yang, H.W.; Lin, K.J.; Wey, S.P.; Sun, C.L.; Wei, K.C.; Yen, T.C.; Lin, C.I.; Ma, C.C.M.; et al. Biodistribution of PEGylated graphene oxide nanoribbons and their application in cancer chemo-photothermal therapy. Carbon 2014, 74, 83–95. [Google Scholar] [CrossRef]
- Chng, E.L.K.; Chua, C.K.; Pumera, M. Graphene oxide nanoribbons exhibit significantly greater toxicity than graphene oxide nanoplatelets. Nanoscale 2014, 6, 10792–10797. [Google Scholar] [CrossRef] [PubMed]
- Peng, E.X.; Todorova, N.; Yarovsky, I. Effects of size and functionalization on the structure and properties of graphene oxide nanoflakes: An in silico investigation. ACS Omega 2018, 3, 11497–11503. [Google Scholar] [CrossRef]
- Duverger, E.; Picaud, F.; Stauffer, L.; Sonnet, P. Simulations of a graphene nanoflake as a nanovector to improve ZnPc phototherapy toxicity: From vacuum to cell membrane. ACS Appl. Mater. Interfaces 2017, 9, 37554–37562. [Google Scholar] [CrossRef]
- Lamb, J.; Fischer, E.; Rosillo-Lopez, M.; Salzmann, C.G.; Holland, J.P. Multi-functionalised graphene nanoflakes as tumour-targeting theranostic drug-delivery vehicles. Chem. Sci. 2019, 10, 8880–8888. [Google Scholar] [CrossRef] [PubMed]
- Yurt, F.; Ersoz, O.A.; Harputlu, E.; Ocakoglu, K. Preparation and evaluation of effect on Escherichia coli and Staphylococcus aureus of radiolabeled ampicillin-loaded graphene oxide nanoflakes. Chem. Biol. Drug Des. 2018, 91, 1094–1100. [Google Scholar] [CrossRef]
- Vovusha, H.; Sanyal, S.; Sanyal, B. Interaction of nucleobases and aromatic amino acids with graphene oxide and graphene flakes. J. Phys. Chem. Lett. 2013, 4, 3710–3718. [Google Scholar] [CrossRef]
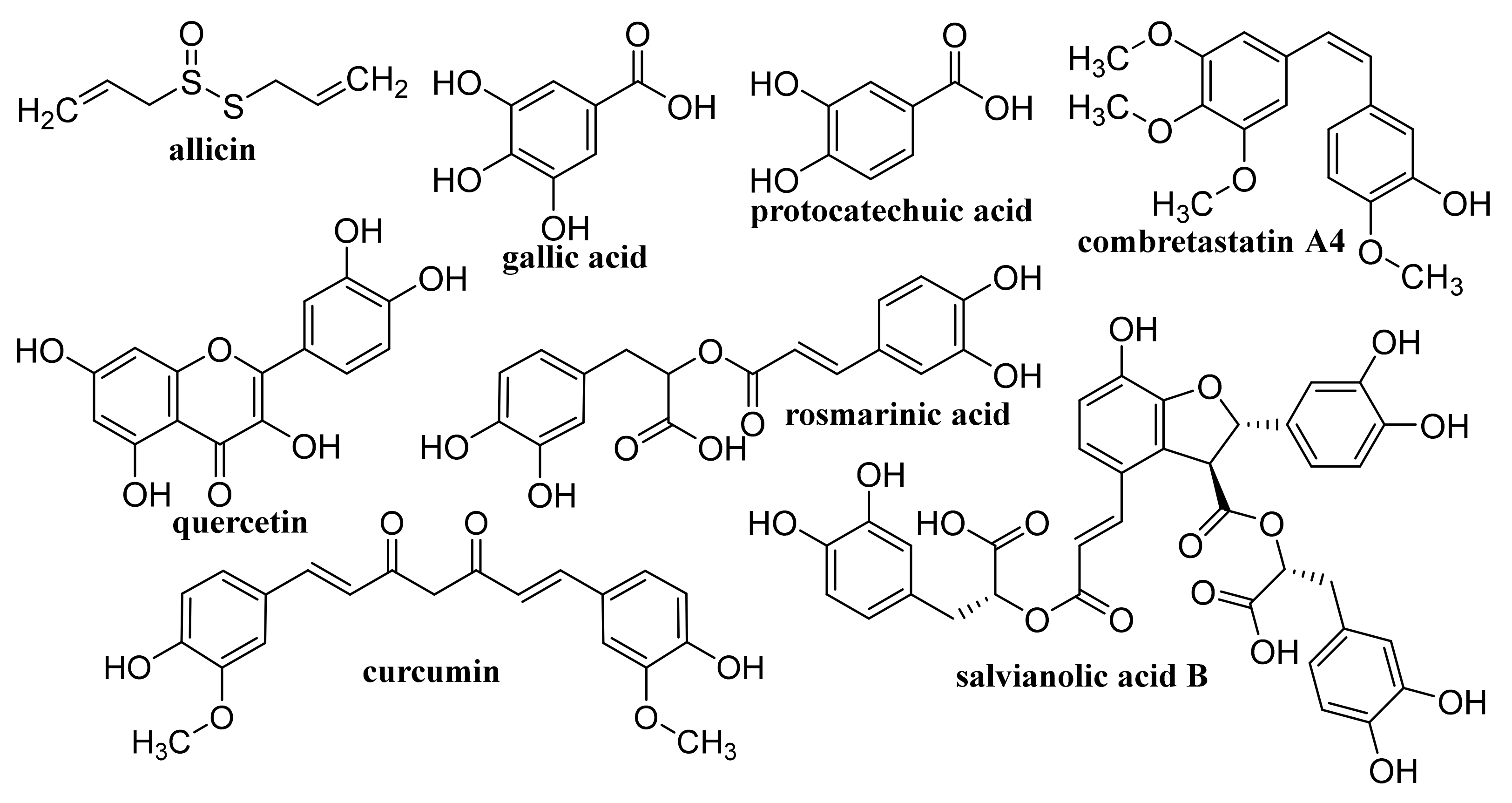
- Chhabra, P.; Chauhan, G.; Kumar, A. Augmented healing of full thickness chronic excision wound by rosmarinic acid loaded chitosan encapsulated graphene nanopockets. Drug Dev. Ind. Pharm. 2020, 46, 878–888. [Google Scholar] [CrossRef]
- Newman, L.; Jasim, D.A.; Prestat, E.; Lozano, N.; de Lazaro, I.; Nam, Y.; Assas, B.M.; Pennock, J.; Haigh, S.J.; Bussy, C.; et al. Splenic capture and in vivo intracellular biodegradation of biological-grade graphene oxide sheets. ACS Nano 2020, 14, 10168–10186. [Google Scholar] [CrossRef] [PubMed]
- Nizami, M.Z.I.; Takashiba, S.; Nishina, Y. Graphene oxide: A new direction in dentistry. Appl. Mater. Today 2020, 19, 100576. [Google Scholar] [CrossRef]
- Jagiello, J.; Chlanda, A.; Baran, M.; Gwiazda, M.; Lipinska, L. Synthesis and characterization of graphene oxide and reduced graphene oxide composites with inorganic nanoparticles for biomedical applications. Nanomaterials 2020, 10, 1846. [Google Scholar] [CrossRef]
- Malik, S.A.; Mohanta, Z.; Srivastava, C.; Atreya, H.S. Modulation of protein-graphene oxide interactions with varying degrees of oxidation. Nanoscale Adv. 2020, 2, 1904–1912. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yang, Y.K.; Xiang, Y.W.; Singh, P.; Feng, J.L.; Cui, S.F.; Carrier, A.; Oakes, K.; Luan, T.G.; Zhang, X. Multifunctional graphene-oxide-reinforced dissolvable polymeric microneedles for transdermal drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 352–360. [Google Scholar] [CrossRef]
- Gupta, N.; Bhagat, S.; Singh, M.; Jangid, A.K.; Bansal, V.; Singh, S.; Pooja, D.; Kulhari, H. Site-specific delivery of a natural chemotherapeutic agent to human lung cancer cells using biotinylated 2D rGO nanocarriers. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110884. [Google Scholar] [CrossRef] [PubMed]
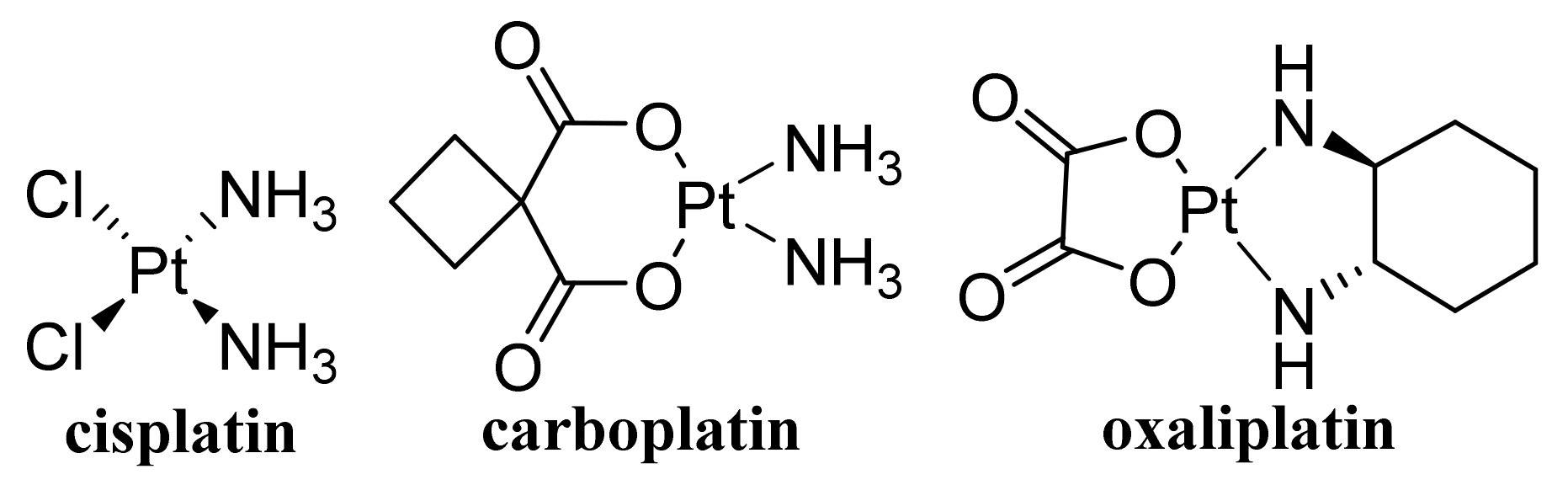
- Cuevas-Flores, M.D.; Bartolomei, M.; Garcia-Revilla, M.A.; Coletti, C. Interaction and reactivity of cisplatin physisorbed on graphene oxide nano-prototypes. Nanomaterials 2020, 10, 1074. [Google Scholar] [CrossRef]
- Shahabi, M.; Raissi, H. Payload delivery of anticancer drug Tegafur with the assistance of graphene oxide nanosheet during biomembrane penetration: Molecular dynamics simulation survey. Appl. Surf. Sci. 2020, 517, 146186. [Google Scholar] [CrossRef]
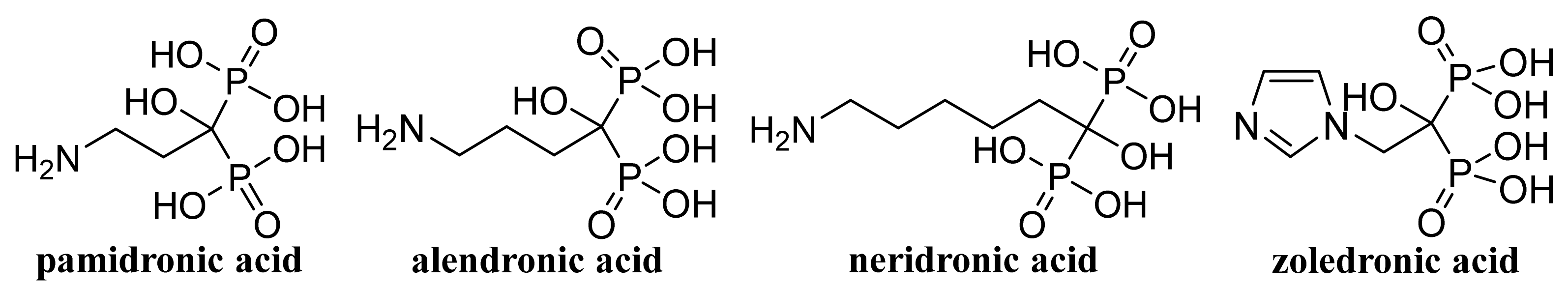
- Boran, G.; Tavakoli, S.; Dierking, I.; Kamali, A.R.; Ege, D. Synergistic effect of graphene oxide and zoledronic acid for osteoporosis and cancer treatment. Sci. Rep. 2020, 10, 7827. [Google Scholar] [CrossRef]
- Matulewicz, K.; Kazmierski, L.; Wisniewski, M.; Roszkowski, S.; Roszkowski, K.; Kowalczyk, O.; Roy, A.; Tylkowski, B.; Bajek, A. Ciprofloxacin and graphene oxide combination—New face of a known drug. Materials 2020, 13, 4224. [Google Scholar] [CrossRef]
- Heo, J.; Tanum, J.; Park, S.; Choi, D.; Jeong, H.; Han, U.; Hong, J. Controlling physicochemical properties of graphene oxide for efficient cellular delivery. J. Ind. Eng. Chem. 2020, 88, 312–318. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Yang, D.T.; Zeng, K.; Li, D.R.; Qin, L.; Cai, Y.F.; Jin, J. Simultaneous delivery of antimiR-21 and doxorubicin by graphene oxide for reducing toxicity in cancer therapy. ACS Omega 2020, 5, 14437–14443. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yang, K.; Leng, X.Y.; Chen, M.S.; Novoselov, K.S.; Andreeva, D.V. Perspectives in the design and application of composites based on graphene derivatives and bio-based polymers. Polym. Int. 2020, 69, 1173–1186. [Google Scholar] [CrossRef]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Bares, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavailles, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110595. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Li, Y.C.; Yang, X.L. Hyaluronic acid and graphene oxide loaded silicon contact lens for corneal epithelial healing. J. Biomater. Sci. Polym. Ed. 2020. [Google Scholar] [CrossRef]
- Yun, Y.J.; Wu, H.W.; Gao, J.; Dai, W.; Deng, L.H.; Lv, O.; Kong, Y. Facile synthesis of Ca2+-crosslinked sodium alginate/graphene oxide hybrids as electro- and pH-responsive drug carrier. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Song, R.; Zhang, X.H.; Zhang, D.W. Enhanced antimicrobial activity and pH-responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin. Int. J. Biol. Macromol. 2020, 161, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, M.Q.; Zhang, Z.C.; Ren, G.H.; Liu, Y.J.; Wu, S.S.; Shen, J. Facile synthesis of ZnO QDs@GO-CS hydrogel for synergetic antibacterial applications and enhanced wound healing. Chem. Eng. J. 2019, 378, 122043. [Google Scholar] [CrossRef]
- Yu, C.H.; Chen, G.Y.; Xia, M.Y.; Xie, Y.; Chi, Y.Q.; He, Z.Y.; Zhang, C.L.; Zhang, T.; Chen, Q.M.; Peng, Q. Understanding the sheet size-antibacterial activity relationship of graphene oxide and the nano-bio interaction-based physical mechanisms. Colloids Surf. B Biointerfaces 2020, 191, 111009. [Google Scholar] [CrossRef] [PubMed]
- Rostami, F.; Tamjid, E.; Behmanesh, M. Drug-eluting PCL/graphene oxide nanocomposite scaffolds for enhanced osteogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111102. [Google Scholar] [CrossRef]
- Schneible, J.D.; Shi, K.H.; Young, A.T.; Ramesh, S.; He, N.F.; Dowdey, C.E.; Dubnansky, J.M.; Libya, R.L.; Gao, W.; Santiso, E.; et al. Modified graphene oxide (GO) particles in peptide hydrogels: A hybrid system enabling scheduled delivery of synergistic combinations of chemotherapeutics. J. Mater. Chem. B 2020, 8, 3852–3868. [Google Scholar] [CrossRef]
- Buskaran, K.; Hussein, M.Z.; Moklas, M.A.M.; Fakurazi, S. Morphological changes and cellular uptake of functionalized graphene oxide loaded with protocatechuic acid and folic acid in hepatocellular carcinoma cancer cell. Int. J. Mol. Sci. 2020, 21, 5874. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Amarasekara, Y.; Jayaweera, V.; Rajaphaksha, C.; Gunasekara, C.; Perera, I.C.; Amaratunga, G.A.J.; Weerasinghe, L. Graphene oxide-based manocomposite for sustained release of cephalexin. J. Pharm. Sci. 2020, 109, 1130–1135. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lv, X.G.; Xia, S.L.; Hao, B.J.; Huang, X.Y.; Shi, P. PEGylated graphene oxide as a nanocarrier of the disulfide prodrug of podophyllotoxin for cancer therapy. J. Nanoparticle Res. 2020, 22, 281. [Google Scholar] [CrossRef]
- Tas, A.; Cakmak, N.K. Synthesis of PEGylated nanographene oxide as a nanocarrier for docetaxel drugs and anticancer activity on prostate cancer cell lines. Hum. Exp. Toxicol. 2021, 40, 172–182. [Google Scholar] [CrossRef] [PubMed]
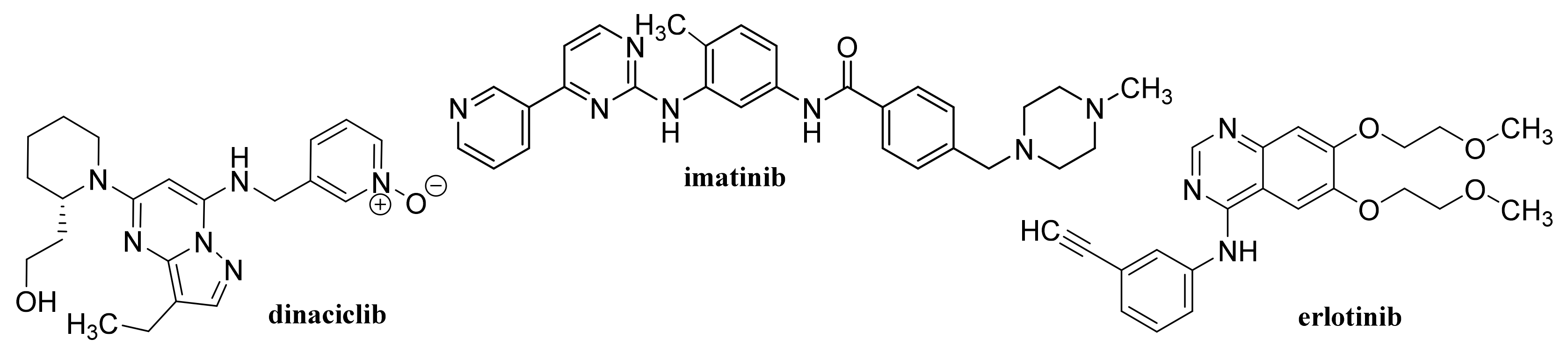
- Lan, M.Y.; Hsu, Y.B.; Lan, M.C.; Chen, J.P.; Lu, Y.J. Polyethylene glycol-boated graphene oxide loaded with erlotinib as an effective therapeutic agent for treating nasopharyngeal cancer cells. Int. J. Nanomed. 2020, 15, 7569–7582. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Hajinezhad, M.R.; Hamishekar, H.; Ghamkhari, A.; Kyzas, G.Z. Copolymer/graphene oxide nanocomposites as potential anticancer agents. Polym. Bull. 2020. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Zhou, M.R.; Chen, L.F.; Fang, H.M.; Liu, S.K.; Zhou, C.C.; Sun, J.M.; Wang, Z.X. Alendronate loaded graphene oxide functionalized collagen sponge for the dual effects of osteogenesis and anti-osteoclastogenesis in osteoporotic rats. Bioact. Mater. 2020, 5, 859–870. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Pesyan, N.N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef]
- Kheiltash, F.; Parivar, K.; Roodbari, N.H.; Sadeghi, B.; Badiei, A. Effects of 8-hydroxyquinoline-coated graphene oxide on cell death and apoptosis in MCF-7 and MCF-10 breast cell lines. Iran. J. Basic Med. Sci. 2020, 23, 871–878. [Google Scholar] [PubMed]
- Foroushani, M.S.; Shervedani, R.K.; Kefayat, A.; Torabi, M.; Ghahremani, F.; Yaghoobi, F. Near-infrared, light-triggered, on-demand anti-inflammatories and antibiotics folate-graphene chelate manganese nanoparticles as a theranostic system for colon cancer MR imaging and drug delivery: In-vivo examinations. J. Drug Deliv. Sci. Technol. 2019, 54, 101223. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Patel, D.K.; Maiti, P. Nanohybrid scaffold of chitosan and functionalized graphene oxide for controlled drug delivery and bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5139–5149. [Google Scholar] [CrossRef]
- Gholami, A.; Emadi, F.; Nazem, M.; Aghayi, R.; Khalvati, B.; Amini, A.; Ghasemi, Y. Expression of key apoptotic genes in hepatocellular carcinoma cell line treated with etoposide-loaded graphene oxide. J. Drug Deliv. Sci. Technol. 2020, 57, 101725. [Google Scholar] [CrossRef]
- Izadi, S.; Moslehi, A.; Kheiry, H.; Kiani, F.K.; Ahmadi, A.; Masjedi, A.; Ghani, S.; Rafiee, B.; Karpisheh, V.; Hajizadeh, F.; et al. Codelivery of HIF-1α siRNA and Dinaciclib by carboxylated graphene oxide-trimethyl chitosan-hyaluronate nanoparticles significantly suppresses cancer cell progression. Pharm. Res. 2020, 37, 196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; He, J.; Zhu, T.Y.; Hu, C.; Bo, R.N.; Wusiman, A.; Hu, Y.L.; Wang, D.Y. Lentinan-functionalized graphene oxide is an effective antigen delivery system that modulates innate immunity and improves adaptive immunity. ACS Appl. Mater. Interfaces 2020, 12, 39014–39023. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Liu, J.Y.; Sui, L.; Zhao, P.H.; Ma, H.D.; Wei, Z.; Wang, Y.L. Folate-modified graphene oxide as the drug delivery system to load temozolomide. Curr. Pharm. Biotechnol. 2020, 21, 1088–1098. [Google Scholar] [CrossRef]
- Assy, L.; Gemeay, A.; Gomaa, S.; Aldubayan, M.A.; Salem, M.L. Impact of graphene oxide nano sheets loaded with chemotherapeutic drug on tumor cells. J. Nanopart. Res. 2020, 22, 79. [Google Scholar] [CrossRef]
- Wang, Y.F.; Sun, G.P.; Gong, Y.Y.; Zhang, Y.Y.; Liang, X.F.; Yang, L.Q. Functionalized folate-modified graphene oxide/PEI siRNA nanocomplexes for targeted ovarian cancer gene therapy. Nanoscale Res. Lett. 2020, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.C.; Nong, Z.Z.; Wei, L.Y.; Wei, M.; Li, G.; Wu, N.N.; Liu, C.; Tang, B.L.; Qin, Q.X.; Li, X.H.; et al. Preparation and anti-cancer activity of transferrin/folic acid double-targeted graphene oxide drug delivery system. J. Biomater. Appl. 2020, 35, 15–27. [Google Scholar] [CrossRef]
- Wang, P.Y.; Wang, X.; Tang, Q.; Chen, H.; Zhang, Q.; Jiang, H.Y.; Wang, Z. Functionalized graphene oxide against U251 glioma cells and its molecular mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111187. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, N.; Mahjoub, A.R.; Shokrollahi, S.; Amiri, A.; Shahrnoy, A.A. Novel biocompatible amino acids-functionalized three-dimensional graphene foams: As the attractive and promising cisplatin carriers for sustained release goals. Int. J. Pharm. 2020, 589, 119857. [Google Scholar] [CrossRef]
- Verde, V.; Longo, A.; Cucci, L.M.; Sanfilippo, V.; Magri, A.; Satriano, C.; Anfuso, C.D.; Lupo, G.; La Mendola, D. Anti-angiogenic and anti-proliferative graphene oxide nanosheets for tumor cell therapy. Int. J. Mol. Sci. 2020, 21, 5571. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Yang, C.; Jia, W.T.; Qi, X.; Liu, C.S.; Li, X.L. Delivery of salvianolic acid B for efficient osteogenesis and angiogenesis from silk fibroin combined with graphene oxide. ACS Biomater. Sci. Eng. 2020, 6, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Asgari, S.; Hosseini, S.H. Graphene oxide functionalized with oxygen -rich polymers as a pH -sensitive carrier for co -delivery of hydrophobic and hydrophilic drugs. J. Drug Deliv. Sci. Technol. 2020, 56 Pt A, 101542. [Google Scholar] [CrossRef]
- Kazempour, M.; Edjlali, L.; Akbarzadeh, A.; Davaran, S.; Farid, S.S. Synthesis and characterization of dual pH-and thermo-responsive graphene-based nanocarrier for effective anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101158. [Google Scholar] [CrossRef]
- Abdel-Bary, A.S.; Tolan, D.A.; Nassar, M.Y.; Taketsugu, T.; El-Nahas, A.M. Chitosan, magnetite, silicon dioxide, and graphene oxide nanocomposites: Synthesis, characterization, efficiency as cisplatin drug delivery, and DFT calculations. Int. J. Biol. Macromol. 2020, 154, 621–633. [Google Scholar] [CrossRef]
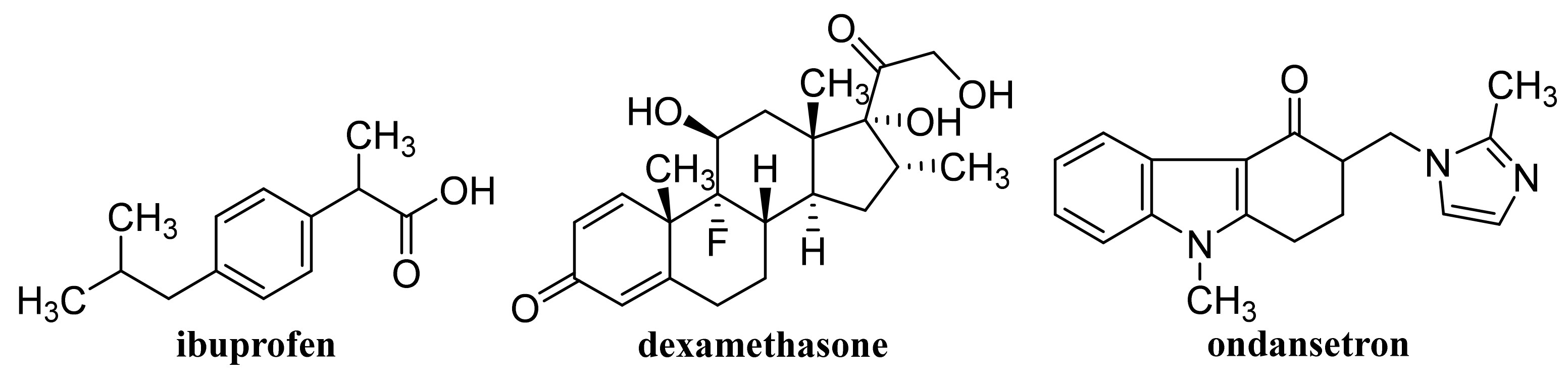
- Pooresmaeil, M.; Javanbakht, S.; Nia, S.B.; Namazi, H. Carboxymethyl cellulose/mesoporous magnetic graphene oxide as a safe and sustained ibuprofen delivery bio-system: Synthesis, characterization, and study of drug release kinetic. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124662. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kohestanian, M.; Yaghoubi, M. Poly(glycidyl methacrylate)-coated magnetic graphene oxide as a highly efficient nanocarrier: Preparation, characterization, and targeted DOX delivery. New J. Chem. 2019, 43, 18647–18656. [Google Scholar] [CrossRef]
- Qi, J.X.; Chen, Y.H.; Xue, T.T.; Lin, Y.; Huang, S.Y.; Cao, S.Y.; Wang, X.N.; Su, Y.; Lin, Z.K. Graphene oxide-based magnetic nanocomposites for the delivery of melittin to cervical cancer HeLa cells. Nanotechnology 2020, 31, 065102. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; Gemeay, A.; Gomaa, S.; Aldubayan, M.A.; Assy, L. Superparamagnetic graphene oxide/magnetite nanocomposite delivery system for doxorubicin-induced distinguished tumor cell cycle arrest and apoptosis. J. Nanoparticle Res. 2020, 22, 219. [Google Scholar] [CrossRef]
- Yang, Y.F.; Meng, F.Y.; Li, X.H.; Wu, N.N.; Deng, Y.H.; Wei, L.Y.; Zeng, X.P. Magnetic graphene oxide-Fe3O4-PANI nanoparticle adsorbed platinum drugs as drug delivery systems for cancer therapy. J. Nanosci. Nanotechnol. 2019, 19, 7517–7525. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Sui, L.; Zhao, P.H.; Ma, H.D.; Liu, J.Y.; Wei, Z.; Zhan, Z.J.; Wang, Y.L. A composite of graphene oxide and iron oxide nanoparticles for targeted drug delivery of temozolomide. Pharmazie 2020, 75, 313–317. [Google Scholar]
- Zhang, B.; Yu, Q.L.; Liu, Y. Alternating magnetic field controlled targeted drug delivery based on graphene oxide-grafted nanosupramolecules. Chem. Eur. J. 2020, 26, 13698–13703. [Google Scholar] [CrossRef]
- Xue, J.M.; Wang, X.C.; Wang, E.D.; Li, T.; Chang, J.; Wu, C.T. Bioinspired multifunctional biomaterials with hierarchical microstructure for wound dressing. Acta Biomater. 2019, 100, 270–279. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Shen, B.Q.; Shen, A.J.; Zhu, X.H. Cavitation-enhanced delivery of the nanomaterial graphene oxide-doxorubicin to hepatic tumors in nude mice using 20 kHz low-frequency ultrasound and microbubbles. J. Nanomater. 2020, 2020, 3136078. [Google Scholar] [CrossRef]
- Quagliarini, E.; Di Santo, R.; Pozzi, D.; Tentori, P.; Cardarelli, F.; Caracciolo, G. Mechanistic insights into the release of doxorubicin from graphene oxide in cancer cells. Nanomaterials 2020, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.X.; Donskyi, E.S.; Qiao, H.S.; Zhu, Z.L.; Unger, W.E.S.; Hackenberger, C.P.R.; Chen, W.; Adeli, M.; Haag, R. Graphene oxide-cyclic R10 peptide nuclear translocation nanoplatforms for the surmounting of multiple-drug resistance. Adv. Funct. Mater. 2020, 2000933. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Wu, W.; Yang, W.B.; Zhong, B.L.; Qing, X.C.; Shao, Z.W. Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo-photodynamic therapy triggers cell death in osteosarcoma. Acta Biomater. 2020, 109, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Alipour, N.; Namazi, H. Chelating ZnO-dopamine on the surface of graphene oxide and its application as pH-responsive and antibacterial nanohybrid delivery agent for doxorubicin. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110459. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.C.; Hu, J.; Li, Z.W.; Yang, X.X.; Hu, J.; You, Q.J.; Bai, S.; Mao, Y.; Hua, D.; Yin, J. Graphene oxide-based nanocomposite enabled highly efficient targeted synergistic therapy for colorectal cancer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124585. [Google Scholar] [CrossRef]
- Qi, Z.E.; Shi, J.; Zhang, Z.; Cao, Y.C.; Li, J.G.; Cao, S.K. PEGylated graphene oxide-capped gold nanorods/silica nanoparticles as multifunctional drug delivery platform with enhanced near-infrared responsiveness. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109889. [Google Scholar] [CrossRef]
- Qi, Z.E.; Shi, J.; Zhu, B.B.; Li, J.G.; Cao, S.K. Gold nanorods/graphene oxide nanosheets immobilized by polydopamine for efficient remotely triggered drug delivery. J. Mater. Sci. 2020, 55, 14530–14543. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Zarrabi, A.; Mirahmadi-Zare, S.Z.; Bidram, E. Hierarchical multifunctional graphene oxide cancer nanotheranostics agent for synchronous switchable fluorescence imaging and chemical therapy. Microchim. Acta 2020, 187, 553. [Google Scholar] [CrossRef]
- Gautam, M.; Gupta, B.; Soe, Z.C.; Poudel, K.; Maharjan, S.; Jeong, J.H.; Choi, H.G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Stealth polymer-coated graphene oxide decorated mesoporous titania nanoplatforms for in vivo chemo-photodynamic cancer therapy. Pharm. Res. 2020, 37, 162. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Liu, H.L.; Liao, K.D.; Hu, Q.Q.; Guo, R.; Deng, K.X. Functionalized GO nanovehicles with nitric oxide release and photothermal activity-based hydrogels for bacteria-infected wound healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [PubMed]
- Dhanavel, S.; Revathy, T.A.; Sivaranjani, T.; Sivakumar, K.; Palani, P.; Narayanan, V.; Stephen, A. 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines. Polym. Bull. 2020, 77, 213–233. [Google Scholar] [CrossRef]
- Dhanavel, S.; Praveena, P.; Narayanan, V.; Stephen, A. Chitosan/reduced graphene oxide/Pd nanocomposites for co-delivery of 5-fluorouracil and curcumin towards HT-29 colon cancer cells. Polym. Bull. 2020, 77, 5681–5696. [Google Scholar] [CrossRef]
- Palai, P.K.; Mondal, A.; Chakraborti, C.K.; Banerjee, I.; Pal, K.; Rathnam, V.S.S. Doxorubicin loaded green synthesized nanoceria decorated functionalized graphene nanocomposite for cancer-specific drug release. J. Clust. Sci. 2019, 30, 1565–1582. [Google Scholar] [CrossRef]
- Singh, G.; Nenavathu, B.P.; Imtiyaz, K.; Rizvi, M.M.A. Fabrication of chlorambucil loaded graphene-oxide nanocarrier and its application for improved antitumor activity. Biomed. Pharmacother. 2020, 129, 110443. [Google Scholar] [CrossRef]
- Tehrani, N.S.; Masoumi, M.; Chekin, F.; Baei, M.S. Nitrogen doped porous reduced graphene oxide hybrid as a nanocarrier of imatinib anticancer drug. Russ. J. Appl. Chem. 2020, 93, 1221–1228. [Google Scholar] [CrossRef]
- Lee, X.J.; Lim, H.N.; Gowthaman, N.S.K.; Rahman, M.B.A.; Abdullah, C.A.C.; Muthoosamy, K. In-situ surface functionalization of superparamagnetic reduced graphene oxide—Fe3O4 nanocomposite via Ganoderma lucidum extract for targeted cancer therapy application. Appl. Surf. Sci. 2020, 512, 145738. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.L.; Liu, C.L. Pluronic® F127 stabilized reduced graphene oxide hydrogel for transdermal delivery of ondansetron: Ex vivo and animal studies. Colloids Surf. B Biointerfaces 2020, 195, 111259. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, F.M.; Qi, X.X.; Wei, F.Q.; Chen, H.X.; Wang, T. Pluronic® F127 stabilized reduced graphene oxide hydrogel for the treatment of psoriasis: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 195, 111246. [Google Scholar] [CrossRef] [PubMed]
- Karthika, V.; AlSalhi, M.S.; Devanesan, S.; Gopinath, K.; Arumugam, A.; Govindarajan, M. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci. Rep. 2020, 10, 18912. [Google Scholar] [CrossRef]
- Vinothini, K.; Rajendran, N.K.; Mariappan, R.; Andy, R.; Marraiki, N.; Elgorban, A.M. A magnetic nanoparticle functionalized reduced graphene oxide-based drug carrier system for a chemo-photodynamic cancer therapy. New J. Chem. 2020, 44, 5265–5277. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonca, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111294. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Zeinali, M.; Mohammadi-Sardoo, M.; Iranpour, M.; Behnam, B.; Mandegary, A. The effects of functionalization of carbon nanotubes on toxicological parameters in mice. Hum. Exp. Toxicol. 2020, 39, 1147–1167. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Dizaji, B.F.; Farboudi, A.; Rahbar, A.; Azarbaijan, M.H.; Asgary, M.R. The role of single- and multi-walled carbon nanotube in breast cancer treatment. Ther. Deliv. 2020, 11, 653–672. [Google Scholar] [CrossRef]
- Antonucci, A.; Kupis-Rozmyslowicz, J.; Boghossian, A.A. Noncovalent protein and peptide functionalization of single-walled carbon nanotubes for biodelivery and optical sensing applications. ACS Appl. Mater. Interfaces 2017, 9, 11321–11331. [Google Scholar] [CrossRef]
- Assali, M.; Zaid, A.N.; Kittana, N.; Hamad, D.; Amer, J. Covalent functionalization of SWCNT with combretastatin A4 for cancer therapy. Nanotechnology 2018, 29, 245101. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Kanchi, S.; Mandal, T.; Dasgupta, C.; Maiti, P.K. Translocation of bioactive molecules through carbon nanotubes embedded in the lipid membrane. ACS Appl. Mater. Interfaces 2018, 10, 6168–6179. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, G.T.; Li, J.C.; Liang, L.J.; Kong, Z.; Wang, H.B.; Jia, L.J.; Wang, X.P.; Zhang, W.; Shen, J.W. Molecular dynamics study on the configuration and arrangement of doxorubicin in carbon nanotubes. J. Mol. Liq. 2018, 262, 295–301. [Google Scholar] [CrossRef]
- Singh, N.; Sachdev, A.; Gopinath, P. Polysaccharide functionalized single walled carbon nanotubes as nanocarriers for delivery of curcumin in lung cancer cells. J. Nanosci. Nanotechnol. 2018, 18, 1534–1541. [Google Scholar] [CrossRef]
- Chegeni, M.; Rozbahani, Z.S.; Ghasemian, M.; Mehri, M. Synthesis and application of the calcium alginate/SWCNT-Gl as a bio-nanocomposite for the curcumin delivery. Int. J. Biol. Macromol. 2020, 156, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Ramezani, M.; Yazdian-Robati, R.; Behnam, B.; Azarkhiavi, K.R.; Nia, A.H.; Mokhtarzadeh, A.; Riahi, M.M.; Razavi, B.M.; Abnous, K. Acute toxicity of functionalized single wall carbon nanotubes: A biochemical, histopathologic and proteomics approach. Chem. Biol. Interact. 2017, 275, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Hashida, Y.; Yamashita, F.; Hashida, M. Development of novel drug and gene delivery carriers composed of single- walled carbon nanotubes and designed peptides with PEGylation. J. Pharm. Sci. 2016, 105, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Razzazan, A.; Atyabi, F.; Kazemi, B.; Dinarvand, R. In vivo drug delivery of gemcitabine with PEGylated single-walled carbon nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 614–625. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.X.; Huang, H.Y.; Chen, L.Q.; Cui, J.H.; Liu, Y.L.; Jin, H.H.; Lee, B.J.; Cao, Q.R. Effective deactivation of A549 tumor cells in vitro and in vivo by RGD-decorated chitosan-functionalized single-walled carbon nanotube loading docetaxel. Int. J. Pharm. 2018, 543, 8–20. [Google Scholar] [CrossRef]
- Karnati, K.R.; Wang, Y.X. Understanding the co-loading and releasing of doxorubicin and paclitaxel using chitosan functionalized single-walled carbon nanotubes by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2018, 20, 9389–9400. [Google Scholar] [CrossRef]
- Pinto, A.V.; Magalhaes, A.L. Intramolecular hydrogen bonds in tip-functionalized single-walled carbon nanotubes as pH-sensitive gates. J. Phys. Chem. A 2020, 124, 9542–9551. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Negi, S. Exploring the charge configuration of an armchair single walled carbon nanotube for drug delivery. Mater. Today Proc. 2020, 28, 185–187. [Google Scholar] [CrossRef]
- Gajewska, A.; Pawlowska, A.; Szwajca, A.; Da Ros, T.; Pluskota-Karwatka, D. Synthesis and structural characterization of single-walled carbon nanotubes functionalized with fluorinated phosphonate analogues of phenylglycine, as promising materials for synthetic and biomedical applications. J. Mol. Struct. 2020, 1210, 128027. [Google Scholar] [CrossRef]
- Ghadri, Z.; Raissi, H.; Shahabi, M.; Farzad, F. Molecular dynamics simulation study of Glycine tip-functionalisation of single-walled carbon nanotubes as emerging nanovectors for the delivery of anticancer drugs. Mol. Simul. 2020, 46, 111–120. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Wang, J.; Fan, L.; Zhu, W.Q.; Cai, D.F. Hyaluronic acid-coated single-walled carbon nanotubes loaded with doxorubicin for the treatment of breast cancer. Pharmazie 2019, 74, 83–90. [Google Scholar] [PubMed]
- Phan, Q.T.; Patil, M.P.; Tu, T.T.K.; Le, C.M.Q.; Kim, G.D.; Lim, K.T. Polyampholyte-grafted single walled carbon nanotubes prepared via a green process for anticancer drug delivery application. Polymer 2020, 193, 122340. [Google Scholar] [CrossRef]
- Tavakolifard, S.; Biazar, E.; Pourshamsian, K.; Moslemin, M.H. Synthesis and evaluation of single-wall carbon nanotube-paclitaxel-folic acid conjugate as an anti-cancer targeting agent. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1247–1253. [Google Scholar] [CrossRef]
- Gangrade, A.; Mandal, B.B. Injectable carbon nanotube impregnated silk based multifunctional hydrogel for localized targeted and on-demand anticancer drug delivery. ACS Biomater. Sci. Eng. 2019, 5, 2365–2381. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Xu, D.Q.; Liao, C.C.; Fang, Y.Q.; Guo, B.H. Development of a promising drug delivery for formononetin: Cyclodextrin-modified single-walled carbon nanotubes. J. Drug Deliv. Sci. Technol. 2018, 43, 461–468. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Lemos, J.A.; de Barros, A.L.B.; Geraldo, V.; da Silva, E.E.; Alisaraie, L.; Soares, D.C.F. Carboxylated versus bisphosphonate SWCNT: Functionalization effects on the biocompatibility and in vivo behaviors in tumor-bearing mice. J. Drug Deliv. Sci. Technol. 2019, 50, 266–277. [Google Scholar] [CrossRef]
- Ershadi, N.; Safaiee, R.; Golshan, M.M. Functionalized (4,0) or (8,0) SWCNT as novel carriers of the anticancer drug 5-FU; a first-principle investigation. Appl. Surf. Sci. 2021, 536, 147718. [Google Scholar] [CrossRef]
- Al Faraj, A.; Shaik, A.S.; Halwani, R.; Alfuraih, A. Magnetic targeting and delivery of drug-loaded SWCNTs theranostic nanoprobes to lung metastasis in breast cancer animal model: Noninvasive monitoring using magnetic resonance imaging. Mol. Imaging Biol. 2016, 18, 315–324. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.T.; Cui, Y.J.; Chu, X.H.; Sun, B.H.; Zhou, N.L.; Shen, J. Magnetofluorescent Fe3O4/carbon quantum dots coated single-walled carbon nanotubes as dual-modal targeted imaging and chemo/photodynamic/photothermal triple-modal therapeutic agents. Chem. Eng. J. 2018, 338, 526–538. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Naghinejad, M.; Kasaeian, A.; Lohrasbi, A.; Shahraeini, S.S.; Zomorodbakhsh, S. The applications of carbon nanotubes in the diagnosis and treatment of lung cancer: A critical review. Int. J. Nanomed. 2020, 15, 7063–7078. [Google Scholar] [CrossRef]
- Mehta, L.; Kumari, S.; Singh, R.P. Carbon nanotubes modulate activity of cytotoxic compounds via a Trojan horse mechanism. Chem. Res. Toxicol. 2020, 33, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Nahle, S.; Cassidy, H.; Leroux, M.M.; Mercier, R.; Ghanbaja, J.; Doumandji, Z.; Matallanas, D.; Rihn, B.H.; Joubert, O.; Ferrari, L. Genes expression profiling of alveolar macrophages exposed to non-functionalized, anionic and cationic multi-walled carbon nanotubes shows three different mechanisms of toxicity. J. Nanobiotechnol. 2020, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.; Mukaya, H.E.; Van Zyl, R.L.; Chen, C.T.; Zeevaart, R.J.; Mbianda, X.Y. Synthesis, characterization, kinetic drug release and anticancer activity of bisphosphonates multi-walled carbon nanotube conjugates. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109967. [Google Scholar] [CrossRef] [PubMed]
- Zomorodbakhsh, S.; Abbasian, Y.; Naghinejad, M.; Sheikhpour, M. The effects study of isoniazid conjugated multi-wall carbon nanotubes nanofluid on Mycobacterium tuberculosis. Int. J. Nanomed. 2020, 15, 5901–5909. [Google Scholar] [CrossRef]
- Badea, N.; Craciun, M.M.; Dragomir, A.S.; Balas, M.; Dinischiotu, A.; Nistor, C.; Gavan, C.; Ionita, D. Systems based on carbon nanotubes with potential in cancer therapy. Mater. Chem. Phys. 2020, 241, 122435. [Google Scholar] [CrossRef]
- Requardt, H.; Braun, A.; Steinberg, P.; Hampel, S.; Hansen, T. Surface defects reduce carbon nanotube toxicity in vitro. Toxicol. In Vitro 2019, 60, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Bibi, A.; Sadiq-ur-Rehman; Akhtar, T.; Akhtar, K.; Farooq, M.; Shahzad, M.I. Alginate-chitosan/MWCNTs nanocomposite: A novel approach for sustained release of Ibuprofen. J. Polym. Res. 2020, 27, 363. [Google Scholar] [CrossRef]
- Sharmeen, S.; Rahman, A.F.M.M.; Lubna, M.M.; Salem, K.S.; Islam, R.; Khan, M.A. Polyethylene glycol functionalized carbon nanotubes/gelatin-chitosan nanocomposite: An approach for significant drug release. Bioact. Mater. 2018, 3, 236–244. [Google Scholar] [CrossRef]
- Komane, P.P.; Kumar, P.; Marimuthu, T.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Dexamethasone-loaded, PEGylated, vertically aligned, multiwalled carbon nanotubes for potential ischemic stroke intervention. Molecules 2018, 23, 1406. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Naskar, S.; Kuotsu, K. Metronomic chemotherapy of carboplatin-loaded PEGylated MWCNTs: Synthesis, characterization and in vitro toxicity in human breast cancer. Carbon Lett. 2020, 30, 435–447. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Scala, A.; Sortino, G.; Zagami, R.; Zhu, Y.; Sciortino, M.T.; Pennisi, R.; Pizzo, M.M.; Neri, G.; Grassi, G.; et al. Intracellular trafficking and therapeutic outcome of multiwalled carbon nanotubes modified with cyclodextrins and polyethylenimine. Colloids Surf. B Biointerfaces 2018, 163, 55–63. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, A.G.; Luo, F.; Li, J.; Li, J.; Zhu, L.; Zhao, L.; Zhu, B.; Ling, F.; Wang, G.X. Application of virus targeting nanocarrier drug delivery system in virus-induced central nervous system disease treatment. ACS Appl. Mater. Interfaces 2019, 11, 19006–19016. [Google Scholar] [CrossRef] [PubMed]
- Nasari, M.; Semnani, D.; Hadjianfar, M.; Amanpour, S. Poly(ε-caprolactone)/poly(N-vinyl-2-pyrrolidone) core-shell nanofibers loaded by multi-walled carbon nanotubes and 5-fluorouracil: An anticancer drug delivery system. J. Mater. Sci. 2020, 55, 10185–10201. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Liu, Z.Q.; Luo, Y.L.; Xu, F.; Chen, Y.S. Tri-stimuli responsive carbon nanotubes covered by mesoporous silica graft copolymer multifunctional materials for intracellular drug delivery. J. Ind. Eng. Chem. 2019, 80, 431–443. [Google Scholar] [CrossRef]
- Karthika, V.; Kaleeswarran, P.; Gopinath, K.; Arumugam, A.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-anbr, M.N.; Benelli, G. Biocompatible properties of nano-drug carriers using TiO2-Au embedded on multiwall carbon nanotubes for targeted drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, A.; Kaur, J.; Khatri, M.; Puri, V.; Tuli, R.; Puri, S. Characterization of functionalized multiwalled carbon nanotubes and comparison of their cellular toxicity between HEK 293 cells and zebra fish in vivo. Heliyon 2019, 5, e02605. [Google Scholar] [CrossRef]
- Karimi, A.; Erfan, M.; Mortazavi, S.A.; Ghorbani-Bidkorbeh, F.; Landi, B.; Kobarfard, F.; Shirazi, F.H. The photothermal effect of targeted methotrexate-functionalized multi-walled carbon nanotubes on MCF7 cells. Iran. J. Pharm. Res. 2019, 18, 221–236. [Google Scholar] [PubMed]
- Kumar, M.; Sharma, G.; Misra, C.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. N-desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: A synergistic approach to overcome MDR in cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 274–282. [Google Scholar] [CrossRef]
- Badea, M.A.; Prodana, M.; Dinischiotu, A.; Crihana, C.; Ionita, D.; Balas, M. Cisplatin loaded multiwalled carbon nanotubes induce resistance in triple negative breast cancer cells. Pharmaceutics 2018, 10, 228. [Google Scholar] [CrossRef]
- Uttekar, P.S.; Lakade, S.H.; Beldar, V.K.; Harde, M.T. Facile synthesis of multi-walled carbon nanotube via folic acid grafted nanoparticle for precise delivery of doxorubicin. IET Nanobiotechnol. 2019, 13, 688–696. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, R.Z.; Hu, Y.; Sun, R.Y.; Song, T.; Shi, X.Y.; Yin, S.M. Stacking of doxorubicin on folic acid-targeted multiwalled carbon nanotubes for in vivo chemotherapy of tumors. Drug Deliv. 2018, 25, 1607–1616. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, A.; Shrivastava, C.; Jain, A.K. Hyaluronic acid conjugated multi-walled carbon nanotubes for colon cancer targeting. Int. J. Biol. Macromol. 2019, 123, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Singhai, N.J.; Maheshwari, R.; Ramteke, S. CD44 receptor targeted ‘smart’ multi-walled carbon nanotubes for synergistic therapy of triple-negative breast cancer. Colloid Interface Sci. Commun. 2020, 35, 100235. [Google Scholar] [CrossRef]
- Dong, Z.P.; Wang, Q.Y.; Huo, M.; Zhang, N.X.; Li, B.X.; Li, H.M.; Xu, Y.S.; Chen, M.; Hong, H.; Wang, Y. Mannose-modified multi-walled carbon nanotubes as a delivery nanovector optimizing the antigen presentation of dendritic cells. ChemistryOpen 2019, 8, 915–921. [Google Scholar] [CrossRef]
- Jain, S.; Dongave, S.M.; Date, T.; Kushwah, V.; Mahajan, R.R.; Pujara, N.; Kumeria, T.; Popat, A. Succinylated β-lactoglobuline-functionalized multiwalled carbon nanotubes with improved colloidal stability and biocompatibility. ACS Biomater. Sci. Eng. 2019, 5, 3361–3372. [Google Scholar] [CrossRef] [PubMed]
- Suo, N.; Wang, M.W.; Jin, Y.; Ding, J.; Gao, X.P.; Sun, X.L.; Zhang, H.Y.; Cui, M.; Zheng, J.L.; Li, N.L.; et al. Magnetic multiwalled carbon nanotubes with controlled release of epirubicin: An intravesical instillation system for bladder cancer. Int. J. Nanomed. 2019, 14, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Ghoderao, P.; Sahare, S.; Alegaonkar, P.; Kulkarni, A.A.; Bhave, T. Multiwalled carbon nanotubes decorated with Fe3O4 nanoparticles for efficacious doxycycline delivery. ACS Appl. Nano Mater. 2019, 2, 607–616. [Google Scholar] [CrossRef]
- Suo, X.B.; Eldridge, B.N.; Zhang, H.; Mao, C.Q.; Min, Y.Z.; Sun, Y.; Singh, R.; Ming, X. P-Glycoprotein-targeted photothermal therapy of drug-resistant cancer cells using antibody-conjugated carbon nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 33464–33473. [Google Scholar] [CrossRef]
- Karimi, A.; Erfan, M.; Mortazavi, S.A.; Ghorbani-Bidkorbeh, F.; Kobarfard, F.; Shirazi, F.H. Functionalisation of carbon nanotubes by methotrexate and study of synchronous photothermal effect of carbon nanotube and anticancer drug on cancer cell death. IET Nanobiotechnol. 2019, 13, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.H.; Zhang, P.; Hou, J.; Chen, W.P.; Bai, L.; Yoo, S.; Khalid, A.; Hou, X. Enhanced response of tamoxifen toward the cancer cells using a combination of chemotherapy and photothermal ablation induced by lentinan-functionalized multi-walled carbon nanotubes. Int. J. Biol. Macromol. Part B 2018, 120, 1525–1532. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. https://doi.org/10.3390/ma14051059
Jampilek J, Kralova K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials. 2021; 14(5):1059. https://doi.org/10.3390/ma14051059
Chicago/Turabian StyleJampilek, Josef, and Katarina Kralova. 2021. "Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes" Materials 14, no. 5: 1059. https://doi.org/10.3390/ma14051059
APA StyleJampilek, J., & Kralova, K. (2021). Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials, 14(5), 1059. https://doi.org/10.3390/ma14051059






