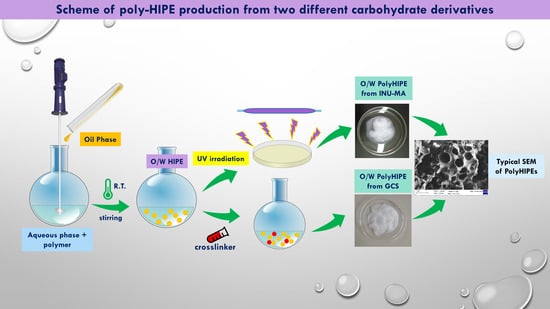
Inverse Poly-High Internal Phase Emulsions Poly(HIPEs) Materials from Natural and Biocompatible Polysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Methods
2.3.1. HIPE Preparation from GCS and INU-MA Polymers
2.3.2. PolyHIPE Preparation from GCS Polymer
2.3.3. PolyHIPE Preparation from INU-MA Polymer
2.3.4. Water Uptake Studies in Water
3. Results and Discussion
3.1. HIPE Feasibility Studies
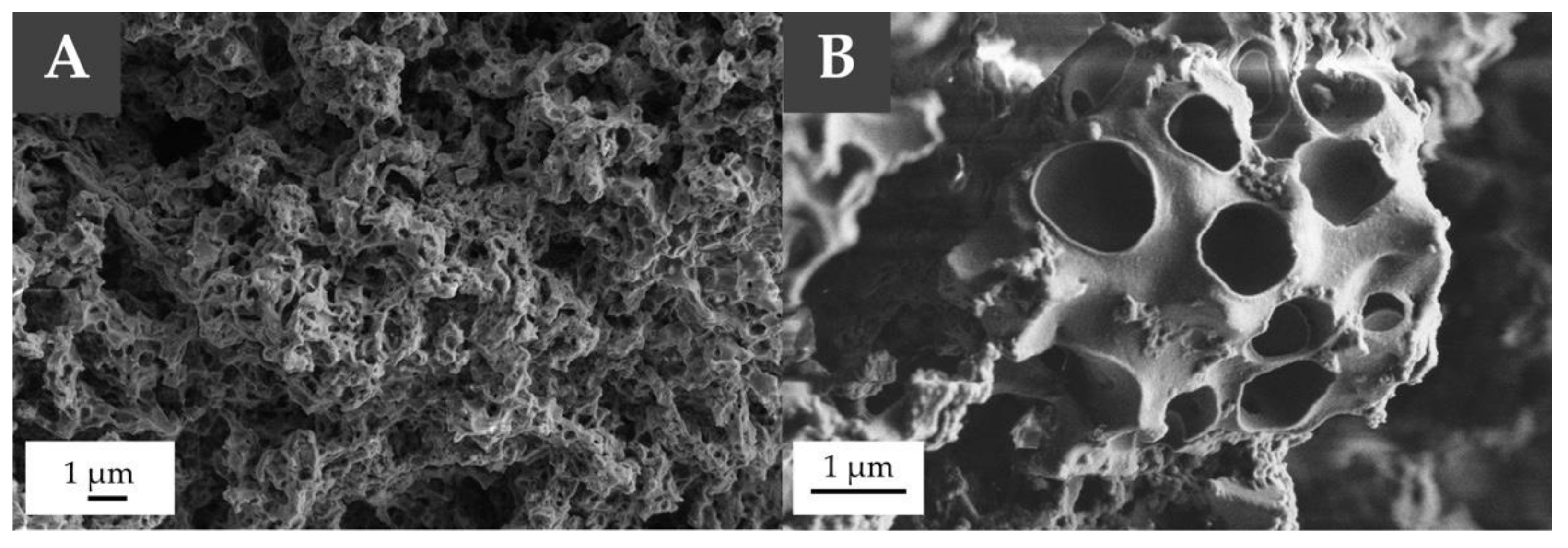
3.2. PolyHIPEs Preparation and Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Jafari, H.; Bernaerts, K.V.; Dodi, G.; Shavandi, A. Chitooligosaccharides for wound healing biomaterials engineering. Mater. Sci. Eng. C 2020, 117. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Claeyssens, F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef]
- Tripodo, G.; Marrubini, G.; Corti, M.; Brusotti, G.; Milanese, C.; Sorrenti, M.; Catenacci, L.; Massolini, G.; Calleri, E. Acrylate-based poly-high internal phase emulsions for effective enzyme immobilization and activity retention: From computationally-assisted synthesis to pharmaceutical applications. Polym. Chem. 2018, 9, 87–97. [Google Scholar] [CrossRef]
- Corti, M.; Calleri, E.; Perteghella, S.; Ferrara, A.; Tamma, R.; Milanese, C.; Mandracchia, D.; Brusotti, G.; Torre, M.L.; Ribatti, D.; et al. Polyacrylate/polyacrylate-PEG biomaterials obtained by high internal phase emulsions (HIPEs) with tailorable drug release and effective mechanical and biological properties. Mater. Sci. Eng. Biol. Appl. 2019, 105, 110060. [Google Scholar] [CrossRef]
- Hayward, A.S.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Acrylic-Acid-Functionalized PolyHIPE Scaffolds for Use in 3D Cell Culture. Macromol. Rapid Commun. 2013, 34, 1844–1849. [Google Scholar] [CrossRef]
- Xu, Y.T.; Yang, T.; Liu, L.L.; Tang, C.H. One-step fabrication of multifunctional high internal phase pickering emulsion gels solely stabilized by a softer globular protein nanoparticle: S-Ovalbumin. J. Colloid Interface Sci. 2020, 580, 515–527. [Google Scholar] [CrossRef]
- Miao, C.; Atifi, S.; Hamad, W.Y. Properties and stabilization mechanism of oil-in-water Pickering emulsions stabilized by cellulose filaments. Carbohydr. Polym. 2020, 248. [Google Scholar] [CrossRef]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Nalawade, A.C.; Ghorpade, R.V.; Shadbar, S.; Qureshi, M.S.; Chavan, N.N.; Khan, A.A.; Ponrathnam, S. Inverse high internal phase emulsion polymerization (i-HIPE) of GMMA, HEMA and GDMA for the preparation of superporous hydrogels as a tissue engineering scaffold. J. Mater. Chem. B 2016, 4, 450–460. [Google Scholar] [CrossRef]
- Yang, D.H.; Seo, D.I.; Lee, D.W.; Bhang, S.H.; Park, K.; Jang, G.; Kim, C.H.; Chun, H.J. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J. Ind. Eng. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Trapani, A.; Rosato, A.; Di Franco, C.; Tamma, R.; Trapani, G.; Ribatti, D.; Mandracchia, D. Hydrogels for biomedical applications from glycol chitosan and PEG diglycidyl ether exhibit pro-angiogenic and antibacterial activity. Carbohydr. Polym. 2018, 198, 124–130. [Google Scholar] [CrossRef]
- Morgan, C.; Nigam, Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis 2013, 16, 493–502. [Google Scholar] [CrossRef]
- Flegg, J.A.; Menon, S.N.; Maini, P.K.; McElwain, D.L.S. On the mathematical modeling of wound healing angiogenesis in skin as a reaction-transport process. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef]
- Guerra, A.; Belinha, J.; Jorge, R.N. Modelling skin wound healing angiogenesis: A review. J. Theor. Biol. 2018, 459, 17. [Google Scholar] [CrossRef]
- Tripodo, G.; Pitarresi, G.; Palumbo, F.S.; Craparo, E.F.; Giammona, G. UV-photocrosslinking of inulin derivatives to produce hydrogels for drug delivery application. Macromol. Biosci. 2005, 5, 1074–1084. [Google Scholar] [CrossRef]
- Mandracchia, D.; Trapani, A.; Perteghella, S.; Di Franco, C.; Torre, M.L.; Calleri, E.; Tripodo, G. A Micellar-Hydrogel Nanogrid from a UV Crosslinked Inulin Derivative for the Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs. Pharmaceutics 2018, 10, 97. [Google Scholar] [CrossRef]
- Cha, X.; Han, S.; Yu, J.; Zhang, S.; Yu, S.; Fu, D.; Yao, M.; Zhang, L.; Feng, G. Inulin with a low degree of polymerization protects human umbilical vein endothelial cells from hypoxia/reoxygenation-induced injury. Carbohydr. Polym. 2019, 216, 97–106. [Google Scholar] [CrossRef]
- Mandracchia, D.; Tripodo, G.; Trapani, A.; Ruggieri, S.; Annese, T.; Chlapanidas, T.; Trapani, G.; Ribatti, D. Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. Eur. J. Pharm. Sci. 2016, 93, 141–146. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Dang, T.P.; Maibach, H.I. Comedogenicity in rabbit: Some cosmetic ingredients/vehicles. Cutan. Ocul. Toxicol. 2007, 26, 287–292. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Brusotti, G.; Calleri, E.; Milanese, C.; Catenacci, L.; Marrubini, G.; Sorrenti, M.; Girella, A.; Massolini, G.; Tripodo, G. Rational design of functionalized polyacrylate-based high internal phase emulsion materials for analytical and biomedical uses. Polym. Chem. 2016, 7, 7436–7445. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Y.; Zhang, S.; Chen, J. Porous Poly(Acrylic Acid) from High Internal Phase Emulsion: Effects of Emulsification Parameters on Porous Structure. In Proceedings of the MATEC Web of Conferences, Chiang Mai, Thailand, 20 August 2016. [Google Scholar]
- Silverstein, M.S. Emulsion-templated polymers: Contemporary contemplations. Polymer 2017, 126, 261–282. [Google Scholar] [CrossRef]
- Khodabandeh, A.; Dario Arrua, R.; Desire, C.T.; Rodemann, T.; Bon, S.A.F.; Thickett, S.C.; Hilder, E.F. Preparation of inverse polymerized high internal phase emulsions using an amphiphilic macro-RAFT agent as sole stabilizer. Polym. Chem. 2016, 7, 1803–1812. [Google Scholar] [CrossRef]
- Zhou, S.; Bismarck, A.; Steinke, J.H.G. Interconnected macroporous glycidyl methacrylate-grafted dextran hydrogels synthesised from hydroxyapatite nanoparticle stabilised high internal phase emulsion templates. J. Mater. Chem. 2012, 22, 18824–18829. [Google Scholar] [CrossRef]
- Miras, J.; Vílchez, S.; Solans, C.; Tadros, T.; Esquena, J. Kinetics of chitosan hydrogel formation in high internal phase oil-in-water emulsions (HIPEs) using viscoelastic measurements. Soft Matter 2013, 9, 8678–8686. [Google Scholar] [CrossRef]
- Oh, B.H.L.; Bismarck, A.; Chan-Park, M.B. High Internal Phase Emulsion Templating with Self-Emulsifying and Thermoresponsive Chitosan-graft-PNIPAM-graft-Oligoproline. Biomacromolecules 2014, 15, 1777–1787. [Google Scholar] [CrossRef]
- Chambers, A.C.; Leaper, D.J. Role of oxygen in wound healing: A review of evidence. J. Wound Care 2011, 20, 160–164. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Yang, X. Characterizations of novel konjac glucomannan emulsion films incorporated with high internal phase Pickering emulsions. Food Hydrocoll. 2020, 109. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Z.; Zhao, T.; Liu, M.; Hu, M.; Li, J. Synthesis, characterization, and swelling behaviors of salt-sensitive maize bran-poly(acrylic acid) superabsorbent hydrogel. J. Agric. Food Chem. 2014, 62, 8867–8874. [Google Scholar] [CrossRef]
- Zhai, M.; Chen, Y.; Yi, M.; Ha, H. Swelling behaviour of a new kind of polyampholyte hydrogel composed of dimethylaminoethyl methacrylate and acrylic acid. Polym. Int. 2004, 53, 33–36. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Canal, T.; Peppas, N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]






| Surfactant/HLB/Mw | Surfactant % w/v | Emulsion Formation | Emulsion Stability Time |
|---|---|---|---|
| Tween 20/16.7/1228 Da | 10 | no | - |
| 15 | no | - | |
| 20 | no | - | |
| 25 | yes | 5 min | |
| 30 | yes | 15 min | |
| Lutrol F127/23/12600 Da | 10 | no | - |
| 15 | yes | >24 h | |
| 20 | yes | >24 h | |
| 25 | yes | >24 h | |
| 30 | yes | >24 h |
| Sample | HIPE Code | Polymer % w/v in Aqueous Phase | Lutrol F127 % w/v in Aqueous Phase | Crosslinking Agent |
|---|---|---|---|---|
| G-series | G1 | 1 | 15 | GLU * |
| G2 | 2 | 15 | GLU * | |
| I-series | I1 | 6 | 15 | UV light |
| I2 | 8 | 15 | UV light | |
| I3 | 10 | 15 | UV light |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripodo, G.; Calleri, E.; Franco, C.d.; Torre, M.L.; Memo, M.; Mandracchia, D. Inverse Poly-High Internal Phase Emulsions Poly(HIPEs) Materials from Natural and Biocompatible Polysaccharides. Materials 2020, 13, 5499. https://doi.org/10.3390/ma13235499
Tripodo G, Calleri E, Franco Cd, Torre ML, Memo M, Mandracchia D. Inverse Poly-High Internal Phase Emulsions Poly(HIPEs) Materials from Natural and Biocompatible Polysaccharides. Materials. 2020; 13(23):5499. https://doi.org/10.3390/ma13235499
Chicago/Turabian StyleTripodo, Giuseppe, Enrica Calleri, Cinzia di Franco, Maria Luisa Torre, Maurizio Memo, and Delia Mandracchia. 2020. "Inverse Poly-High Internal Phase Emulsions Poly(HIPEs) Materials from Natural and Biocompatible Polysaccharides" Materials 13, no. 23: 5499. https://doi.org/10.3390/ma13235499
APA StyleTripodo, G., Calleri, E., Franco, C. d., Torre, M. L., Memo, M., & Mandracchia, D. (2020). Inverse Poly-High Internal Phase Emulsions Poly(HIPEs) Materials from Natural and Biocompatible Polysaccharides. Materials, 13(23), 5499. https://doi.org/10.3390/ma13235499