Incorporation of Hydroxyapatite into Glass Ionomer Cement (GIC) Formulated Based on Alumino-Silicate-Fluoride Glass Ceramics from Waste Materials
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Materials
2.2. Preparation of CS and SLS Glass Powder
2.3. Synthesis of ASF Glass Ceramics Composition
2.4. Formulation of Control and Modified GIC Samples
2.5. Characterization of Control and Modified GIC Samples
2.5.1. Density
2.5.2. Compressive Strength Test
2.5.3. X-ray Diffraction (XRD)
2.5.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.5. Field Emission Scanning Electron Microscopy (FESEM)
2.5.6. Energy Dispersive X-ray (EDX)
2.5.7. Statistical Analysis
3. Results
3.1. Density Analysis
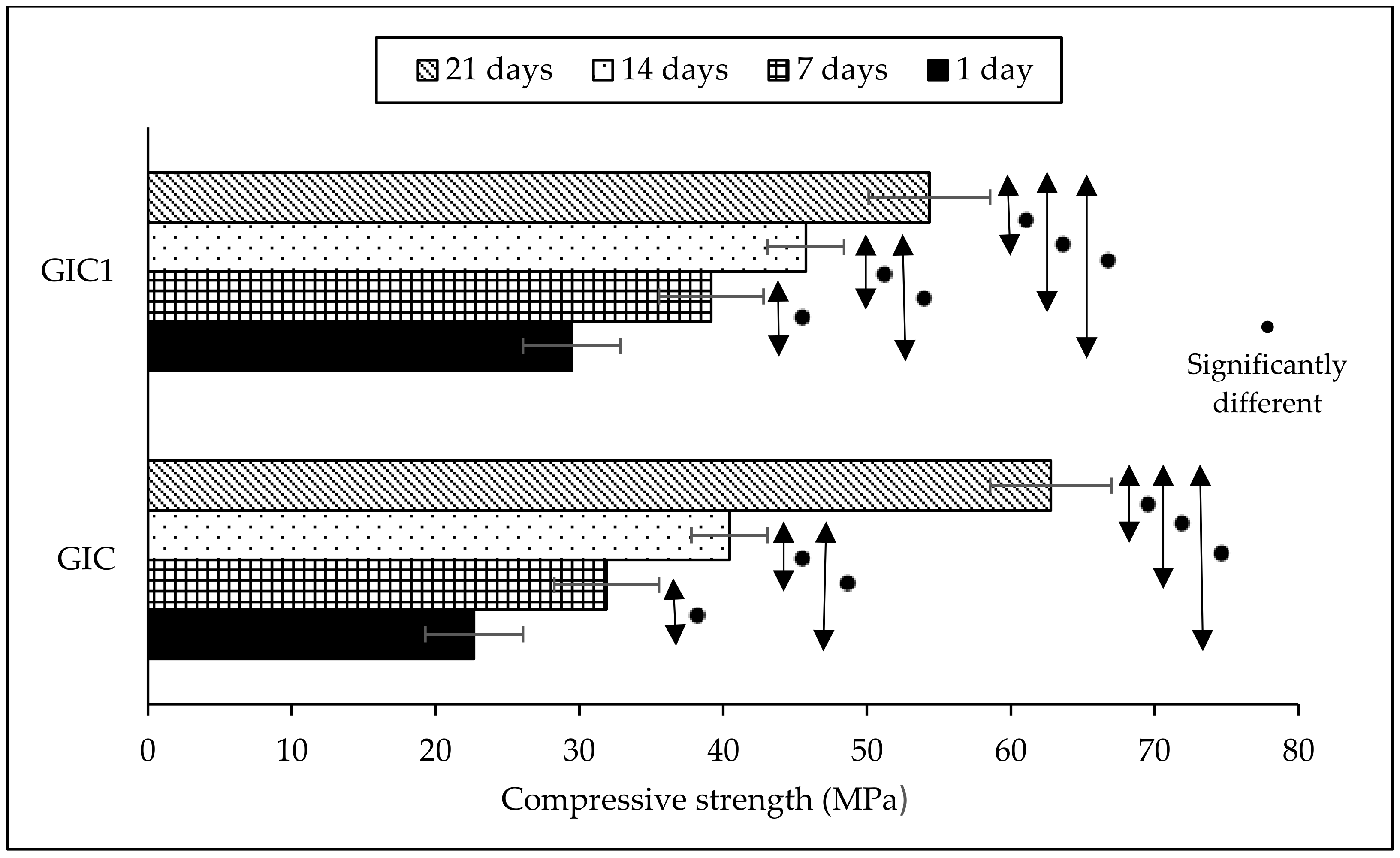
3.2. Compressive Strength Analysis
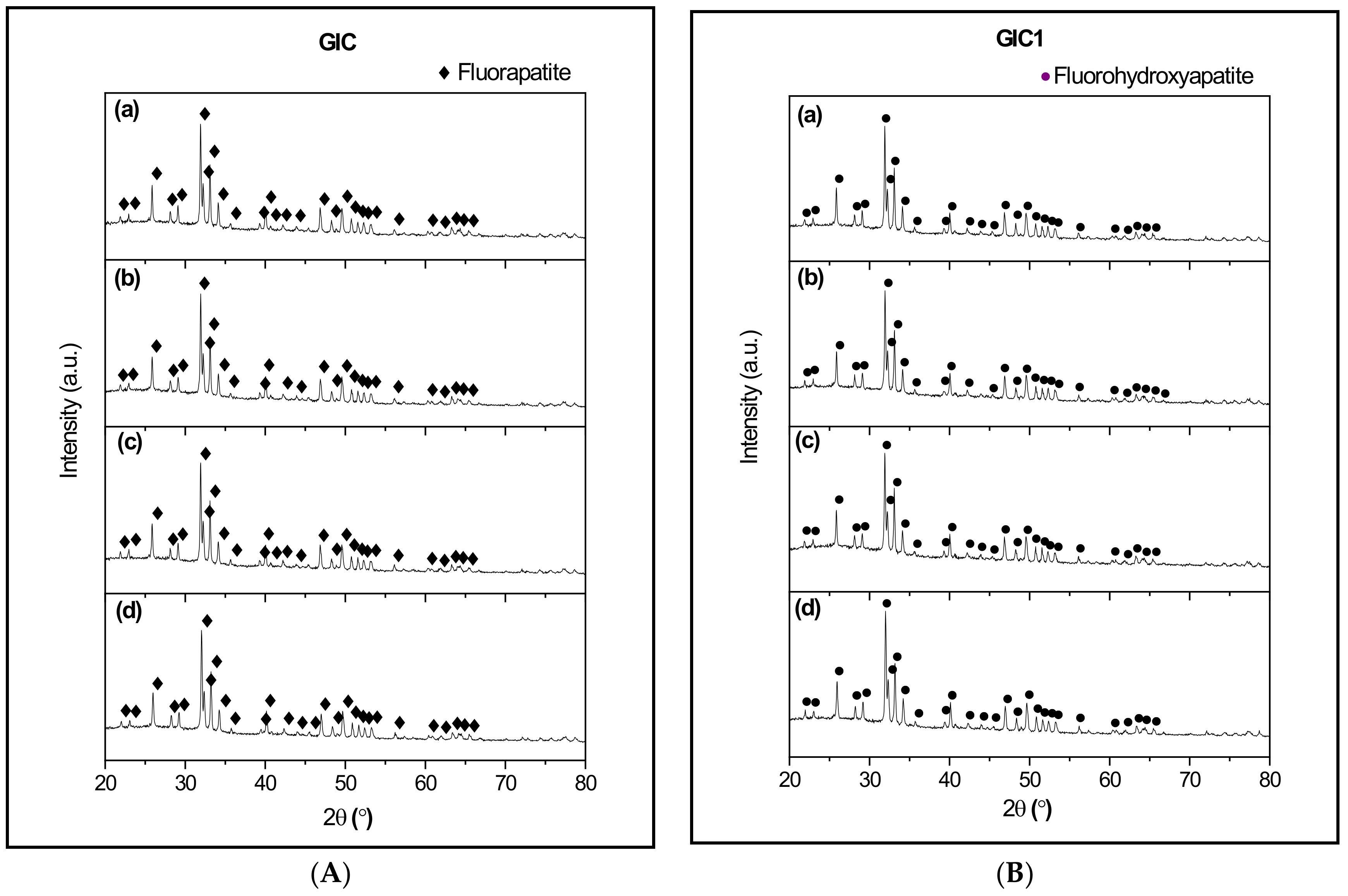
3.3. X-ray Diffraction (XRD) Analysis
3.4. Fourier Transform Infrared (FTIR) Analysis
3.5. Field Emission Scanning Electron Microscopy (FESEM) Analysis
3.6. Energy Dispersive X-ray (EDX) Analysis
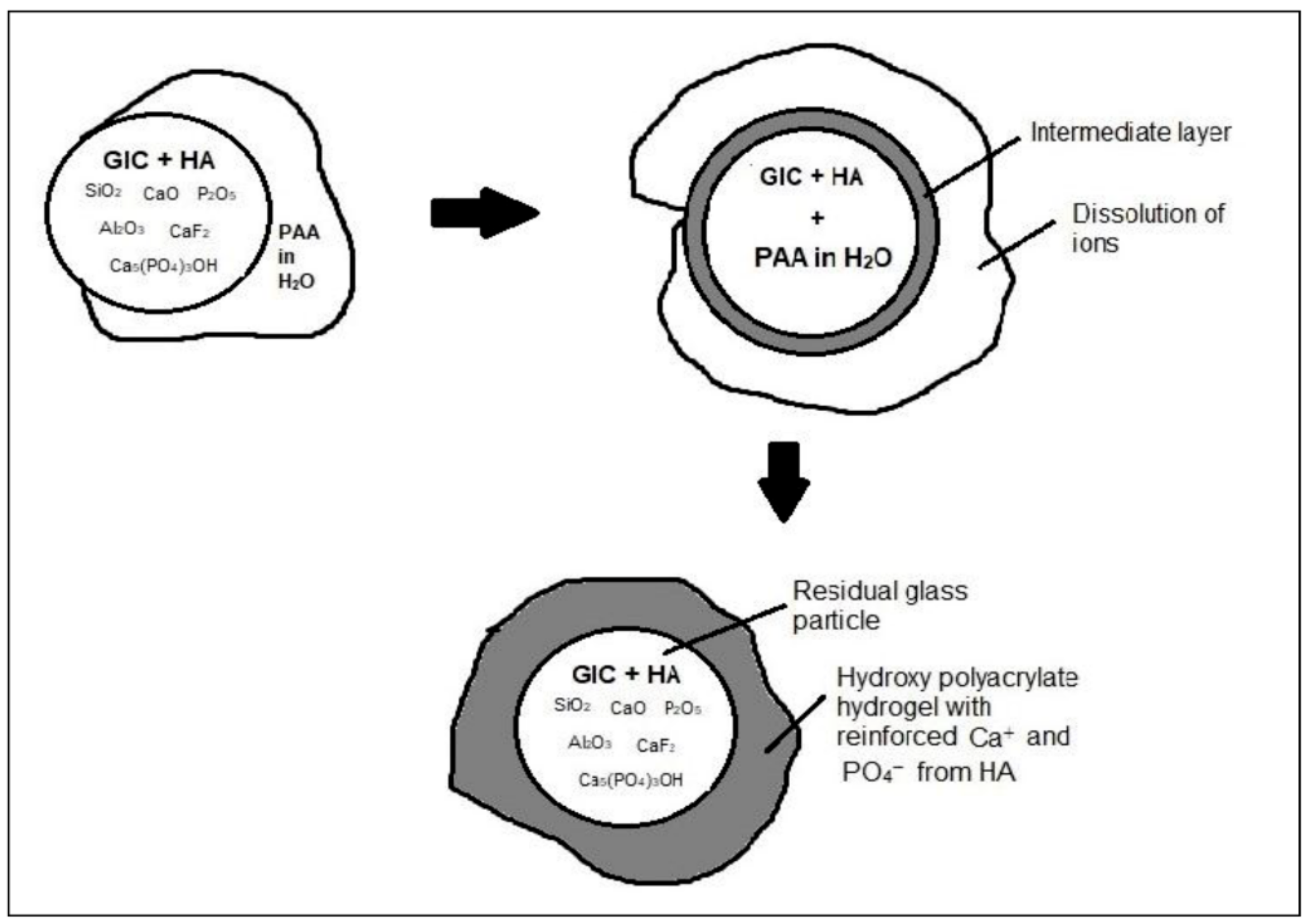
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry, the glass ionomer cement. J. Chem. Technol. Biotechnol. 1971, 21, 313. [Google Scholar] [CrossRef]
- McLean, J.W.; Gasser, O. Glass-cermet cements. Quintessence Int. 1985, 16, 333–343. [Google Scholar]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.; Schricker, S.R. A review of powder modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2011, 21, 1319–1328. [Google Scholar] [CrossRef]
- Goenka, S.; Balu, R.; Kumar, T.S. Effects of nanocrystalline calcium deficient hydroxyapatite incorporation in glass ionomer cements. J. Mech. Behav. Biomed. Mater. 2012, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, R.A.; Rahman, A.I.; Luddin, N. Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol-gel synthesis and characterization. Ceram. Int. 2014, 40, 3165–3170. [Google Scholar] [CrossRef]
- Sharafeddin, F.; Feizi, N. Evaluation of the effect of adding micro-hydroxyapatite and nano-hydroxyapatite on the microleakage of conventional and resin-modified Glass-ionomer Cl V restorations. J. Clin. Exp. Dent. 2017, 9, e242–e248. [Google Scholar] [CrossRef]
- Noori, A.J.; Kareem, F.A. Setting time, mechanical and adhesive properties of magnesium oxide nanoparticles modified glass-ionomer cement. J. Mater. Res. 2019, 9, 1809–1818. [Google Scholar] [CrossRef]
- Rahman, I.A.; Ghazali, N.A.M.; Bakar, W.Z.W.; Masudi, S.A.M. Modification of glass ionomer cement by incorporating nanozirconia-hydroxyapatite-silica nano-powder composite by the one-pot technique for hardness and aesthetics improvement. Ceram. Int. 2017, 43, 13247–13253. [Google Scholar] [CrossRef]
- Ramsden, R.T.; Herdman, R.C.T.; Lye, R.H. Ionomeric bone cement in otoneurological surgery. J. Laryngol. Otol. 1992, 106, 949–953. [Google Scholar] [CrossRef]
- Abdul Jalil, R.; Amin Matori, K.; Mohd Zaid, M.H.; Zainuddin, N.; Ahmad Khiri, M.Z.; Abdul Rahman, N.A.; Wan Jusoh, W.N.; Kul, E. A study of fluoride-containing bioglass system for dental materials derived from clam shell and soda lime silica glass. J. Spectrosc. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Jusoh, W.N.W.; Matori, K.A.; Zaid, M.H.M.; Zainuddin, N.; Khiri, M.Z.A.; Rahman, N.A.A.; Jalil, R.A.; Kul, E. Effect of sintering temperature on physical and structural properties of Alumino-Silicate-Fluoride glass ceramics fabricated from clam shell and soda lime silicate glass. Results Phys. 2019, 12, 1909–1914. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Matori, K.A.; Zaid, M.H.M.; Zainuddin, N.; Ab Aziz, S.; Khiri, M.Z.A.; Jalil, R.A.; Jusoh, W.N.W. Fabrication of Alumino-Silicate-Fluoride based bioglass derived from waste clam shell and soda lime silica glasses. Results Phys. 2019, 12, 743–747. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Henao, J.; Poblano-Salas, C.; Monsalve, M.; Corona-Castuera, J.; Barceinas-Sanchez, O. Bio-active glass coatings manufactured by thermal spray: A status report. J. Mater. Res. Technol. 2019, 8, 4965–4984. [Google Scholar] [CrossRef]
- Bauccio, M. ASM Engineered Materials Reference Book; CRC Press LLC: Boca Raton, FL, USA, 1994. [Google Scholar]
- Pfaender, H.G. Schott Guide to Glass; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Awang-Hazmi, A.J.; Zuki, A.B.Z.; Noordin, M.M.; Jalila, A.; Norimah, Y. Mineral composition of the cockle (Anadara granosa) shells of west coast of Peninsular Malaysia and it’s potential as biomaterial for use in bone repair. J. Anim. Vet. Adv. 2007, 6, 591–594. [Google Scholar]
- Loyd, D. Physics Lab Manual; Cengage Learning: Orlando, FL, USA, 2007. [Google Scholar]
- Mandal, T.; Mishra, B.K.; Garg, A.; Chaira, D. Optimization of milling parameters for the mechanosynthesis of nanocrystalline hydroxyapatite. Powder Technol. 2014, 253, 650–656. [Google Scholar] [CrossRef]
- Zarifah, N.A.; Matori, K.A.; Sidek, H.A.A.; Wahab, Z.A.; Salleh, M.M.; Zainuddin, N.; Khiri, M.Z.A.; Farhana, N.S.; Omar, N.A.S. Effect of hydroxyapatite reinforced with 45S5 glass on physical, structural and mechanical properties. Procedia Chem. 2016, 19, 30–37. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Barandehfard, F.; Rad, M.K.; Hosseinnia, A.; Khoshroo, K.; Tahriri, M.; Jazayeri, H.E.; Moharamzadeh, K.; Tayebicgh, L. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016, 42, 17866–17875. [Google Scholar] [CrossRef]
- Alatawi, R.A.; Elsayed, N.H.; Mohamed, W.S. Influence of hydroxyapatite nanoparticles on the properties of glass ionomer cement. J. Mater. Res. Technol. 2019, 8, 344–349. [Google Scholar] [CrossRef]
- Khiri, M.Z.A.; Matori, K.A.; Zaid, M.H.M.; Abdullah, A.C.; Zainuddin, N.; Jusoh, W.N.W.; Jalil, R.A.; Rahman, N.A.A.; Kul, E.; Wahab, S.A.A.; et al. Soda lime silicate glass and clam shell act as precursor in synthesize calcium fluoroaluminosilicate glass to fabricate glass ionomer cement with different ageing time. J. Mater. Res. Technol. 2020, 9, 6125–6134. [Google Scholar] [CrossRef]
- Montazeri, N.; Jahandideh, R.; Biazar, E. Synthesis of fluorapatite-hydroxyapatite nanoparticles and toxicity investigations. Int. J. Nanomed. 2011, 6, 197–201. [Google Scholar]
- Jokanović, V.; Čolović, B.; Jović, N.; Babić-Stojić, B.; Jokanović, B. Mechanochemical and low-temperature synthesis of nanocrystalline fluorohydroxyapatite/fluorapatite. Int. J. Appl. Ceram. Technol. 2013, 10, 957–969. [Google Scholar] [CrossRef]
- Nicholson, J.W. Chemistry of glass-ionomer cements: A review. Biomaterials 1998, 19, 485–494. [Google Scholar] [CrossRef]
- Khaghani, M.; Alizadeh, S.; Doostmohammadi, A. Influence of incorporating fluoroapatite nanobioceramic on the compressive strength and bioactivity of glass ionomer cement. J. Dent. Biomater. 2016, 3, 276–283. [Google Scholar]
- Arita, K.; Yamamoto, A.; Shinonaga, Y.; Harada, K.; Abe, Y.; Nakagawa, K.; Sugiyama, S. Hydroxyapatite particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glass ionomer cement. Dent. Mater. J. 2011, 30, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Bali, P.; Prabhakar, A.R.; Basappa, N. An invitro comparative evaluation of compressive strength and antibacterial activity of conventional GIC and hydroxyapatite reinforced GIC in different storage media. J. Clin. Diagn. Res. 2015, 9, ZC51–ZC55. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Pek, Y.S.; Kumar, R.A.; Cheang, P.; Khor, K.A. Experimental studies on a new bioactive material: HAIonomer cements. Biomaterials 2002, 23, 955–962. [Google Scholar] [CrossRef]
- Shinonaga, Y.; Arita, K.; Nishimura, T.; Chiu, S.Y.; Chiu, H.H.; Abe, Y.; Sonomoto, M.; Harada, K.; Nagaoka, N. Effects of porous-hydroxyapatite incorporated into glass-ionomer sealants. Dent. Mater. J. 2015, 34, 196–202. [Google Scholar] [CrossRef]
- Wei, M.; Evans, J.H.; Bostrom, T.; Grøndahl, L. Synthesis and characterization of hydroxyapatite, fluoride-substituted hydroxyapatite and fluorapatite. J. Mater. Sci. Mater. Med. 2003, 14, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and fluorapatite in conservative dentistry and oral implantology—A review. Materials 2019, 12, 2683–2699. [Google Scholar] [CrossRef]
- Eslamia, H.; Moztarzadeha, F.; Khoshroob, K.; Ashuria, M.; Tahriria, M. Synthesis and characterization of Ca5(PO4)3(OH) (0 ≤ x ≤ 1) nanopowders via pH-cycling method as bioceramics. In Proceedings of the 4th International Conference on Nanostructures (ICNS4), Kish Island, Iran, 12–14 March 2012. [Google Scholar]
- Azami, M.; Jalilifiroozinezhad, S.; Mozafari, M. Calcium fluoride/hydroxyfluorapatite nanocrystals as novel biphasic solid solution for tooth tissue engineering and regenerative dentistry. Key Eng. Mater. 2012, 493, 626–631. [Google Scholar] [CrossRef]
- Sasani, N.; Khadivi, A.H.; Zebarjad, S.M.; Vahdati, K.J. Characterization of rod-like high-purity fluorapatite nanopowders obtained by sol-gel method. J. Ultrafine Grained Nanostruct. Mater. 2013, 46, 31–37. [Google Scholar]
- Zandi, M.; Mirzadeh, H.; Mayer, C.; Urch, H.; Eslaminejad, M.B.; Bagheri, F.; Mivehchi, H. Biocompatibility evaluation of nano-rod hydroxyapatite/gelatin coated with nano-HAp as a novel scaffold using mesenchymal stem cells. J. Biomed. Mater. Res. A 2010, 92, 1244–1255. [Google Scholar] [CrossRef]
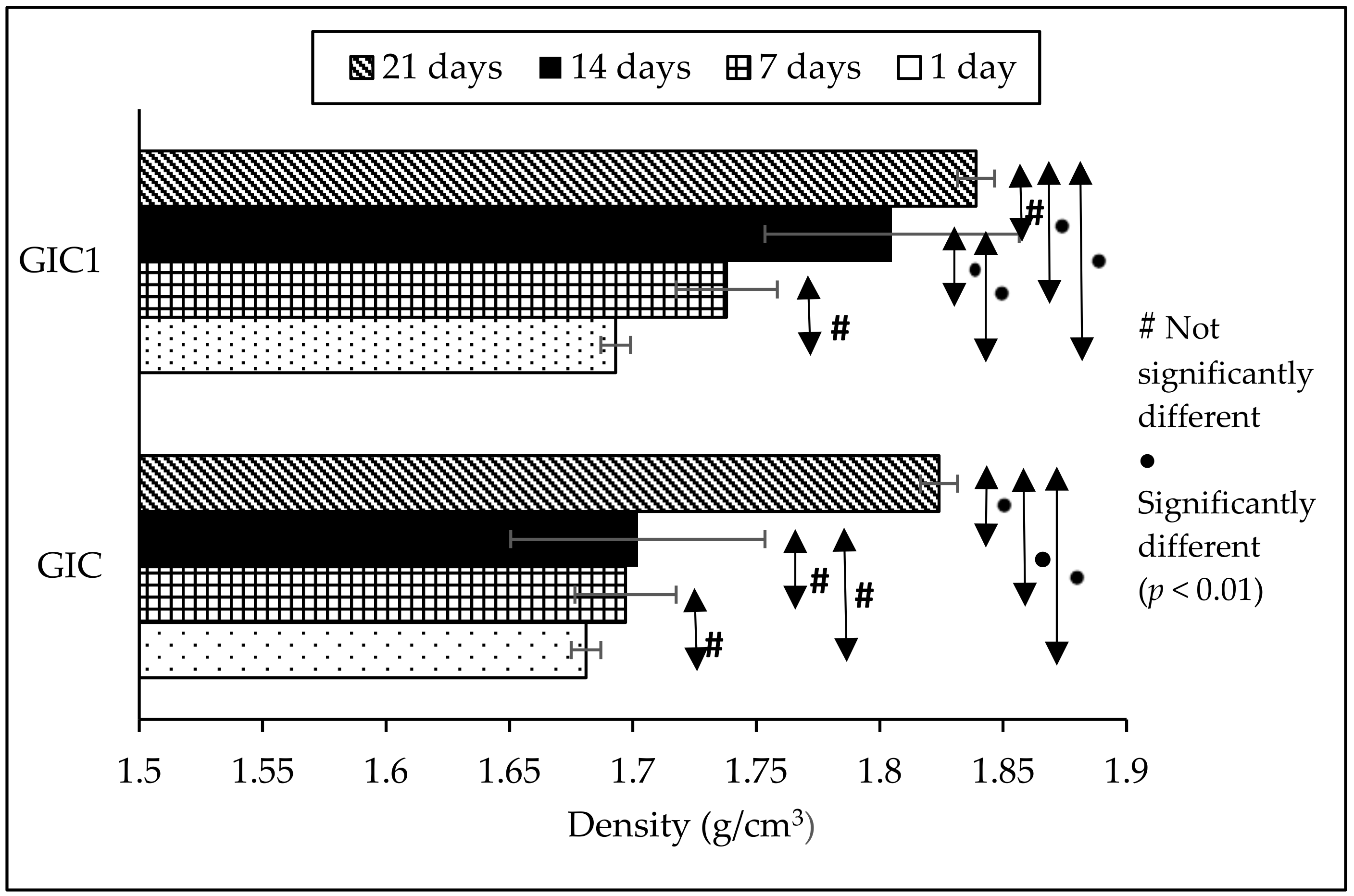


| Density of Sample (g/cm3) | Ageing Time | |||
|---|---|---|---|---|
| 1 Day | 7 Days | 14 Days | 21 Days | |
| GIC | 1.681 (0.011) a | 1.697 (0.006) a | 1.702 (0.015) a | 1.824 (0.043) b |
| GIC1 | 1.693 (0.015) a | 1.738 (0.033) a | 1.805 (0.007) b | 1.839 (0.016) b |
| Compressive Strength of Sample (MPa) | Ageing Time | |||
|---|---|---|---|---|
| 1 Day | 7 Days | 14 Days | 21 Days | |
| GIC | 22.68 (0.73) a | 31.89 (0.86) b | 40.44 (0.58) c | 62.78 (0.21) d |
| GIC1 | 29.47 (2.17) a | 39.17 (1.24) b | 45.75 (5.11) c | 54.34 (0.63) d |
| Wavenumber (cm−1) | Vibrational Mode | References |
|---|---|---|
| ~440 | v2 O–P–O bending | [19,20] |
| ~570, ~600 | v4 O–P–O bending | [4,21,22,23,24] |
| ~1020 | v3 asymmetric P‒O stretching | [19,20] |
| ~1460 | C–O vibration | [21] |
| ~1550 | Asymmetric COOH | [25] |
| ~3400 | OH vibration | [26,27] |
| ~3550 | OH–F vibration | [26,27] |
| Element | Weight Percentage (%) | |
|---|---|---|
| GIC | GIC1 | |
| O | 42.54 | 45.20 |
| C | 22.86 | 20.90 |
| Ca | 12.81 | 12.90 |
| Al | 7.18 | 7.11 |
| P | 5.11 | 5.16 |
| Si | 4.33 | 3.67 |
| F | 3.87 | 3.91 |
| Na | 1.30 | 1.15 |
| Total | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Jusoh, W.N.; Matori, K.A.; Mohd Zaid, M.H.; Zainuddin, N.; Ahmad Khiri, M.Z.; Abdul Rahman, N.A.; Abdul Jalil, R.; Kul, E. Incorporation of Hydroxyapatite into Glass Ionomer Cement (GIC) Formulated Based on Alumino-Silicate-Fluoride Glass Ceramics from Waste Materials. Materials 2021, 14, 954. https://doi.org/10.3390/ma14040954
Wan Jusoh WN, Matori KA, Mohd Zaid MH, Zainuddin N, Ahmad Khiri MZ, Abdul Rahman NA, Abdul Jalil R, Kul E. Incorporation of Hydroxyapatite into Glass Ionomer Cement (GIC) Formulated Based on Alumino-Silicate-Fluoride Glass Ceramics from Waste Materials. Materials. 2021; 14(4):954. https://doi.org/10.3390/ma14040954
Chicago/Turabian StyleWan Jusoh, Wan Nurshamimi, Khamirul Amin Matori, Mohd Hafiz Mohd Zaid, Norhazlin Zainuddin, Mohammad Zulhasif Ahmad Khiri, Nadia Asyikin Abdul Rahman, Rohaniah Abdul Jalil, and Esra Kul. 2021. "Incorporation of Hydroxyapatite into Glass Ionomer Cement (GIC) Formulated Based on Alumino-Silicate-Fluoride Glass Ceramics from Waste Materials" Materials 14, no. 4: 954. https://doi.org/10.3390/ma14040954
APA StyleWan Jusoh, W. N., Matori, K. A., Mohd Zaid, M. H., Zainuddin, N., Ahmad Khiri, M. Z., Abdul Rahman, N. A., Abdul Jalil, R., & Kul, E. (2021). Incorporation of Hydroxyapatite into Glass Ionomer Cement (GIC) Formulated Based on Alumino-Silicate-Fluoride Glass Ceramics from Waste Materials. Materials, 14(4), 954. https://doi.org/10.3390/ma14040954





