Effects of Rapid Cooling on Properties of Aluminum-Steel Friction Stir Welded Joint
Abstract
1. Introduction
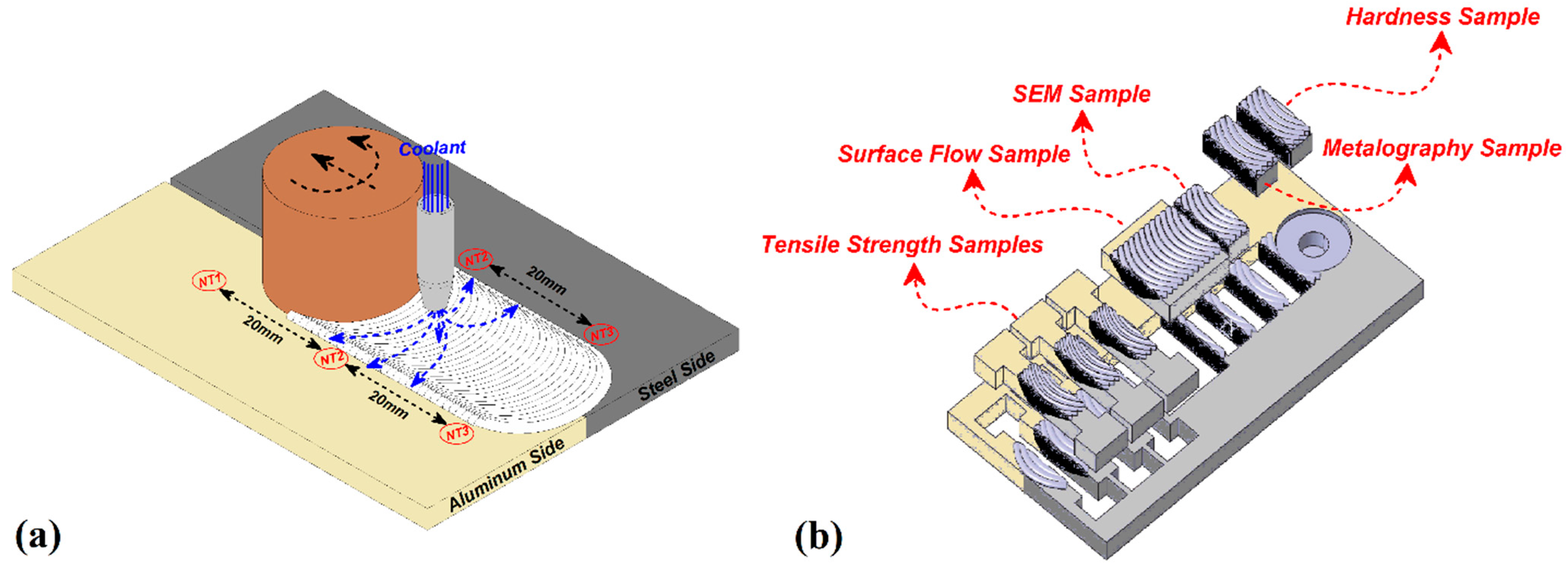
2. Experimental Procedure
3. Results and Discussion
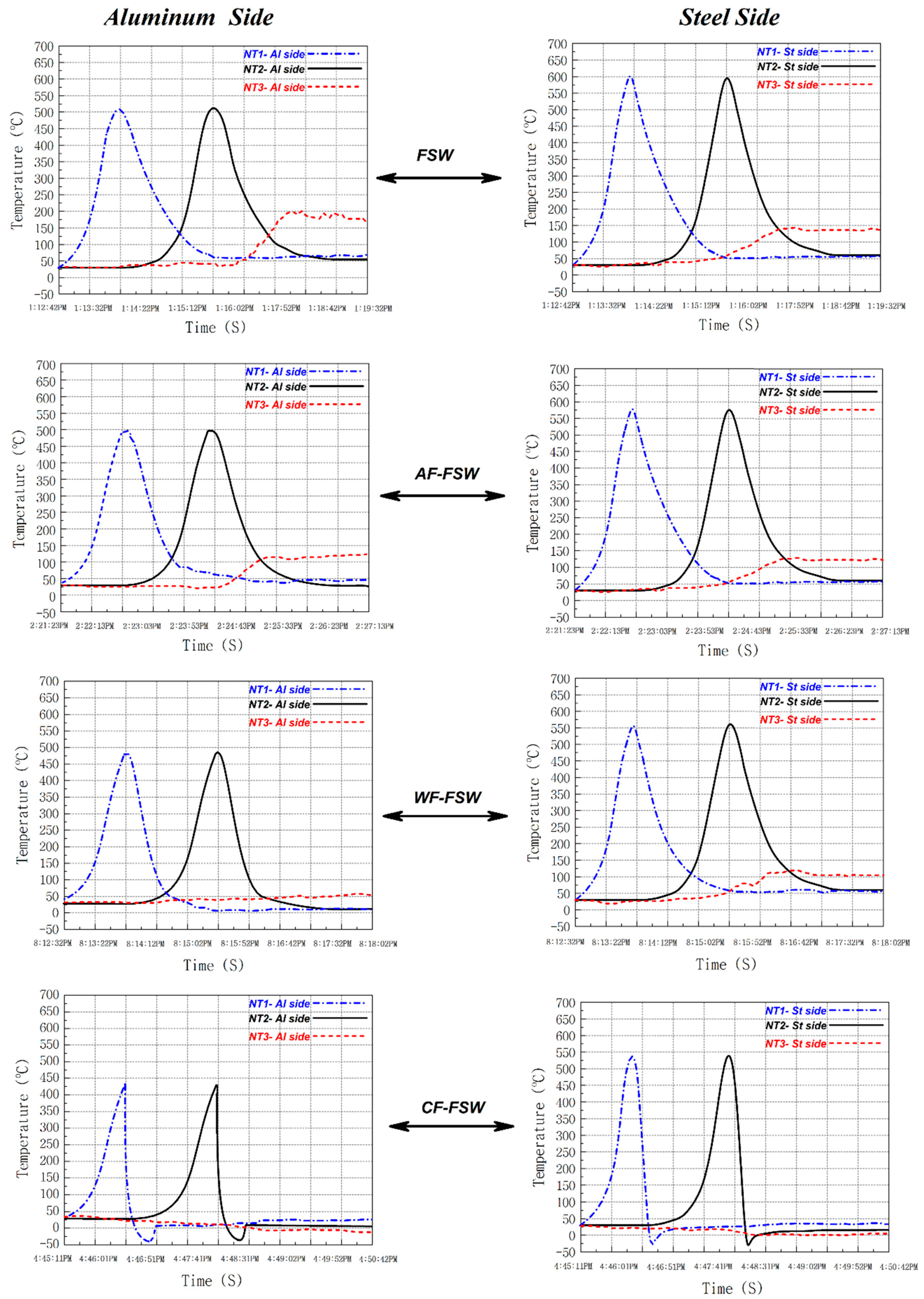
3.1. Thermal History
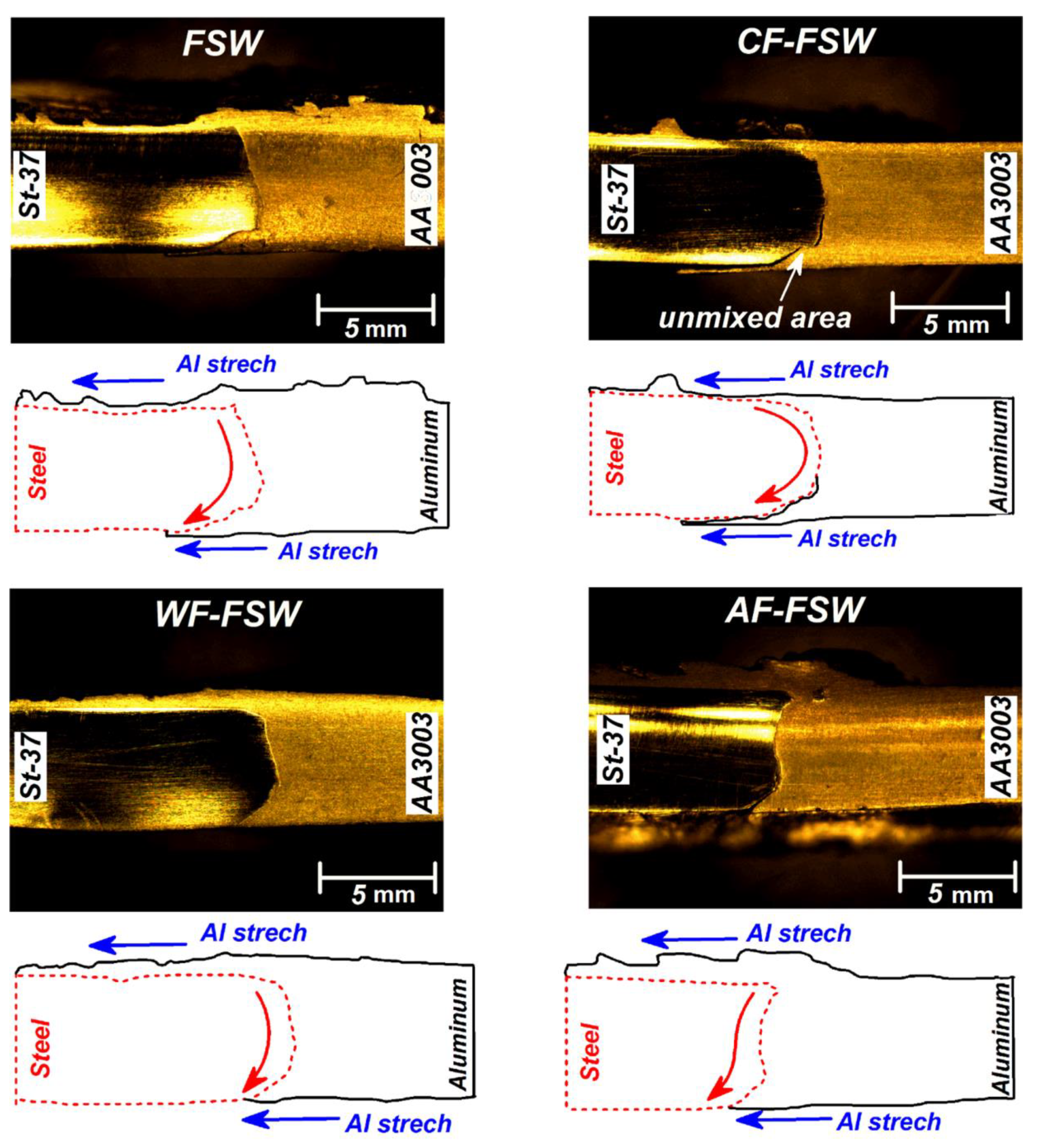
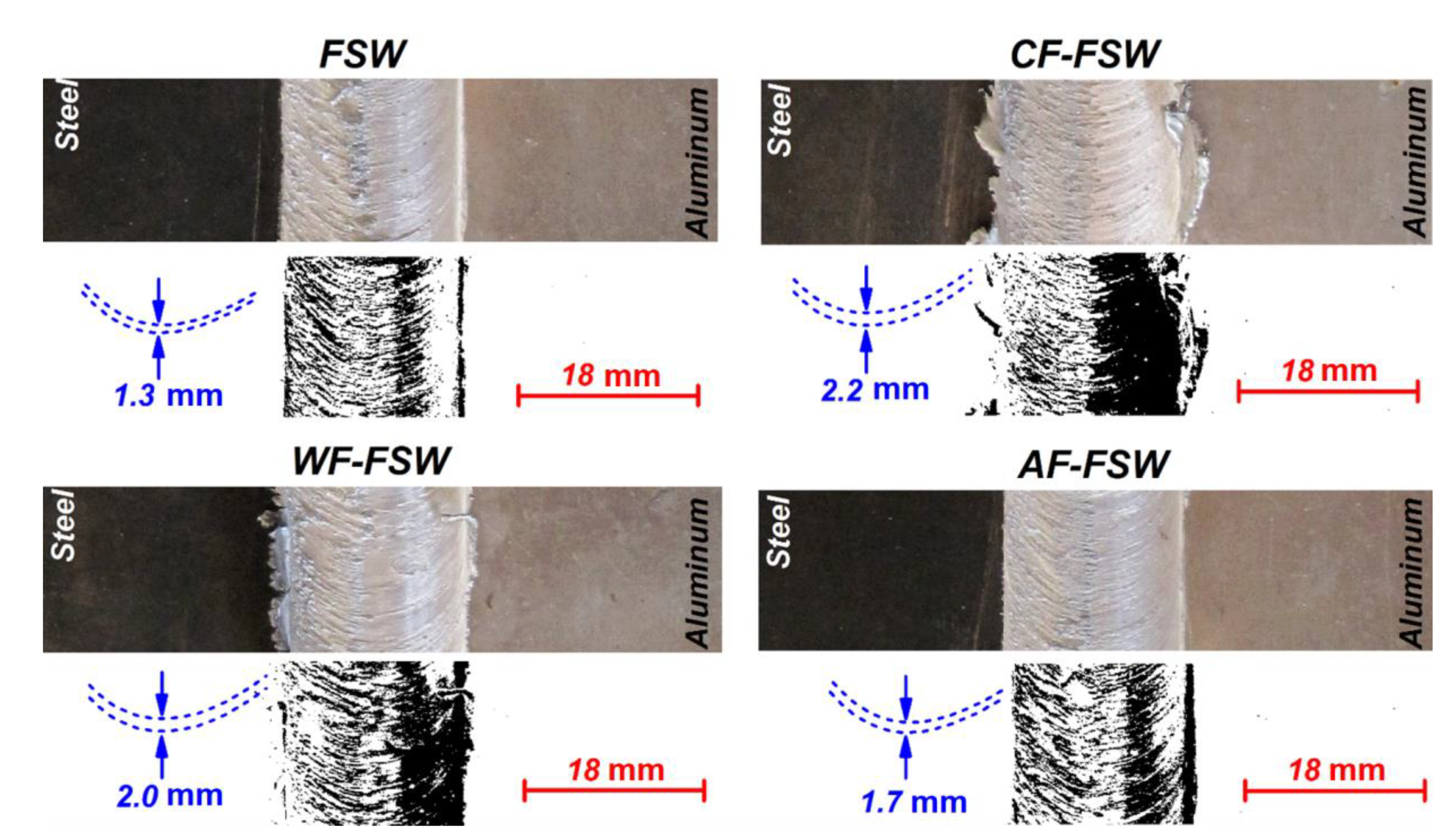
3.2. Material Flow
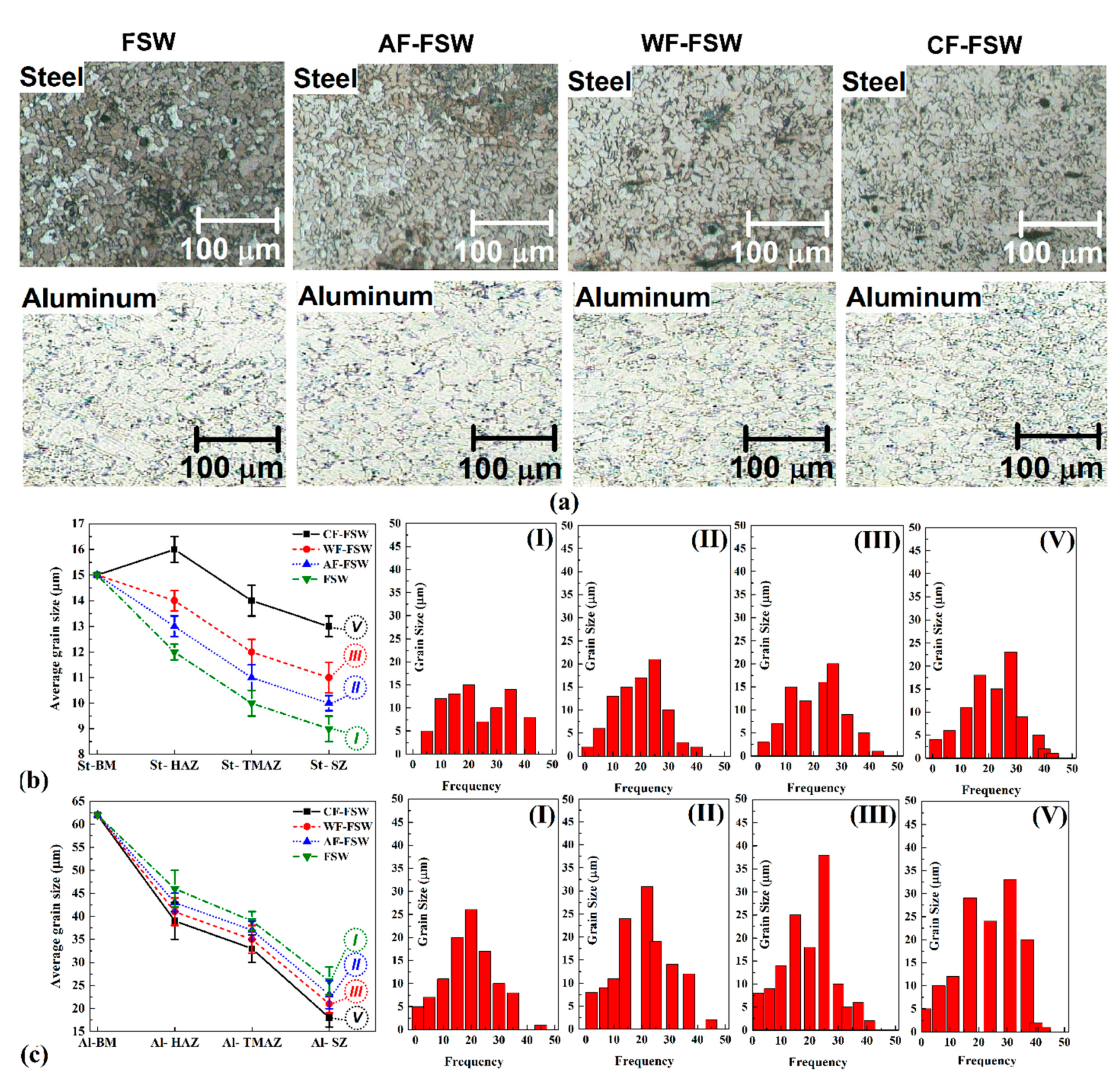
3.3. Micro Structure
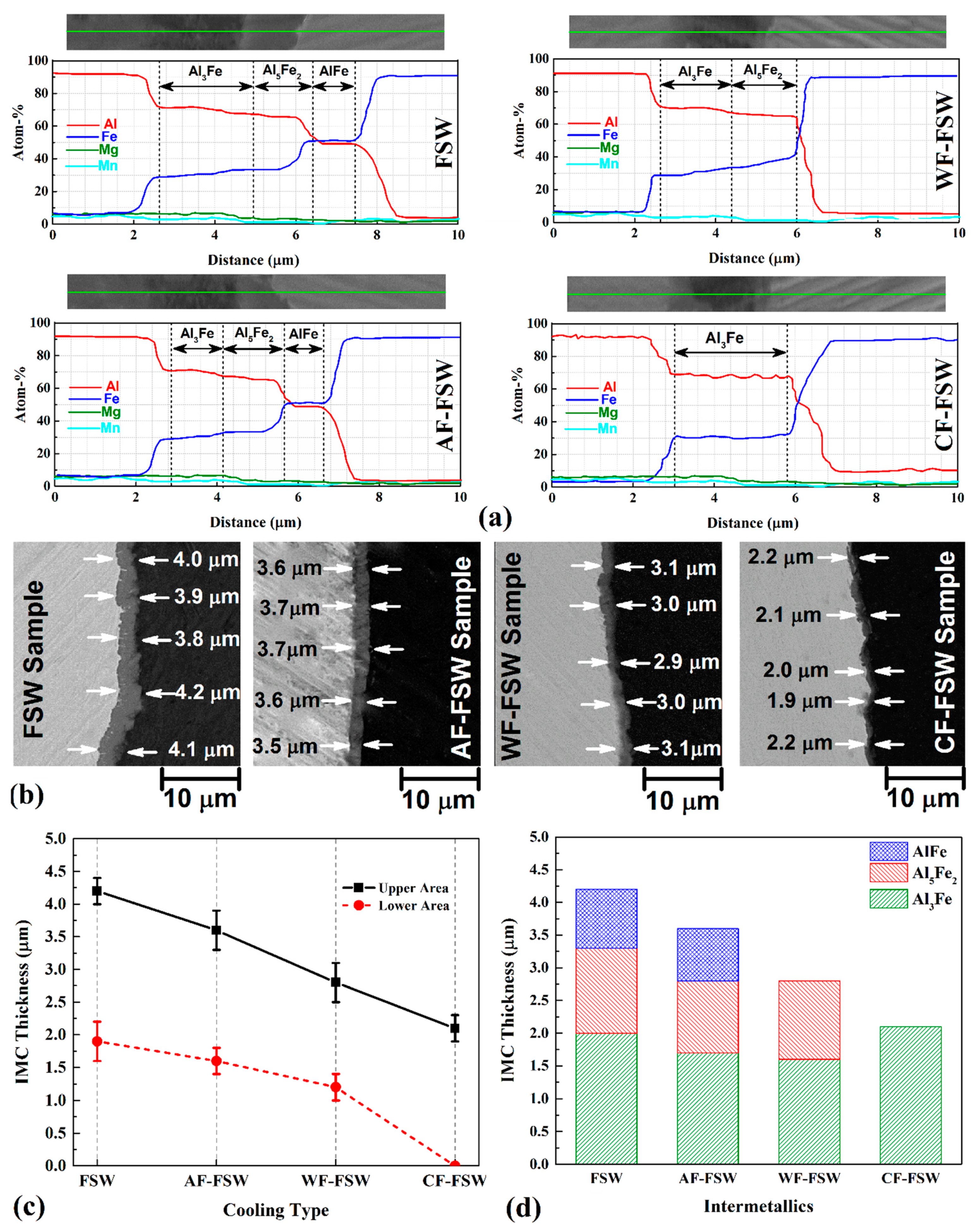
3.4. Intermetallic Compounds
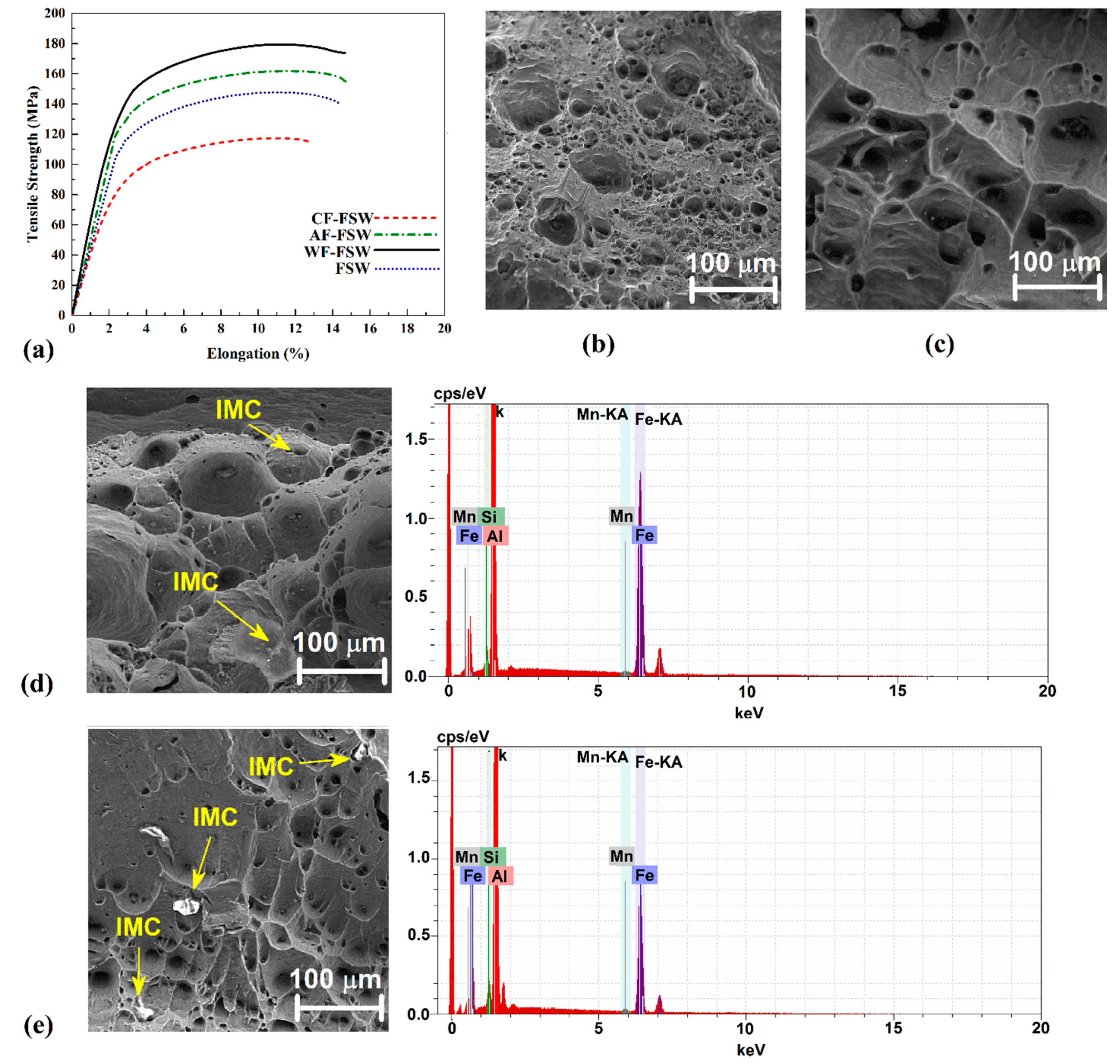
3.5. Tensile Strength and Fractography
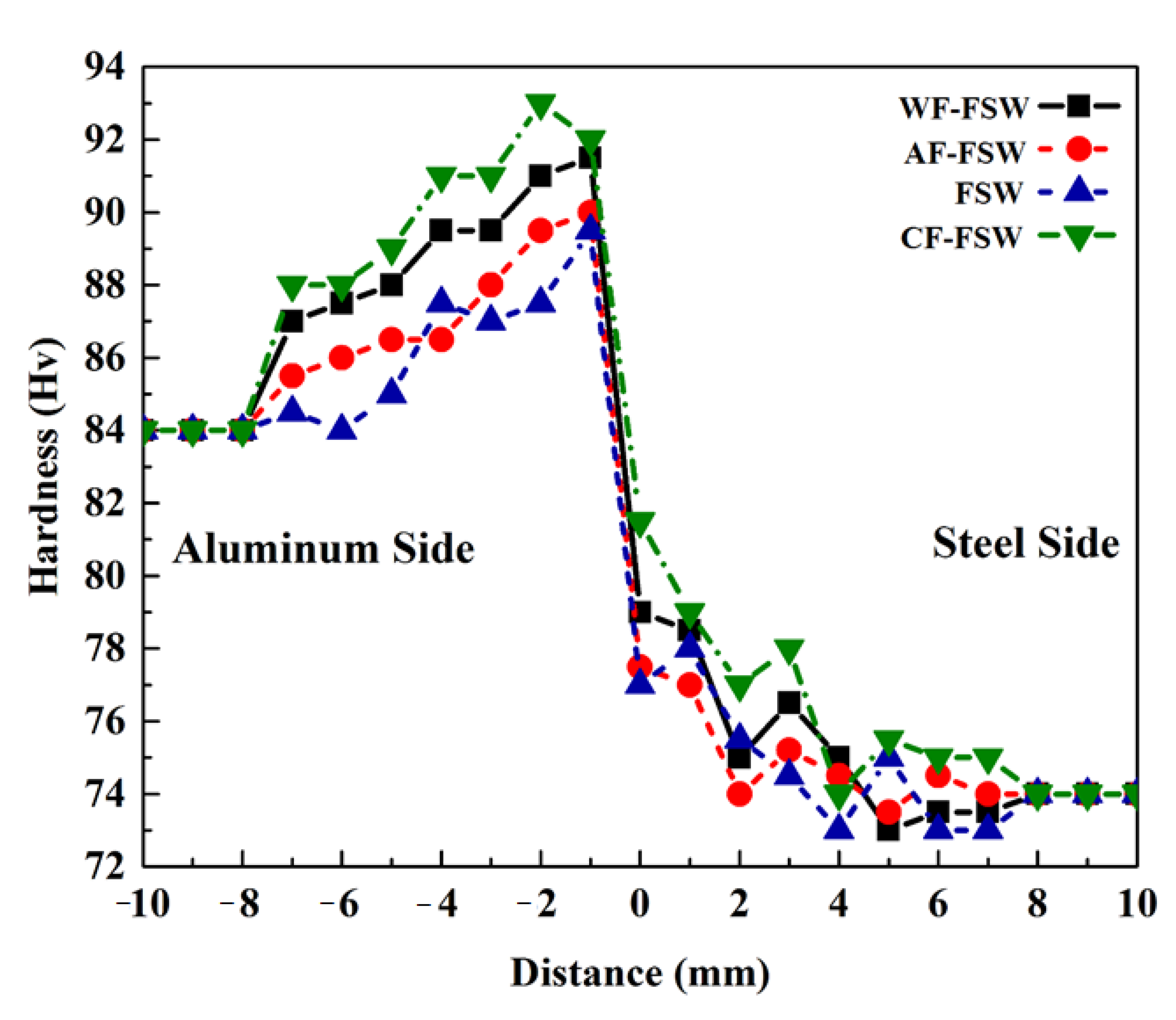
3.6. Hardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidarzadeh, A.; Mironov, S.; Kaibyshev, R.; Çam, G.; Simar, A.; Gerlich, A.; Khodabakhshi, F.; Mostafaei, A.; Field, D.P.; Robson, J.D.; et al. Friction stir welding/processing of metals and alloys: A comprehensive review on microstructural evolution. Prog. Mater. Sci. 2020, 100752. [Google Scholar] [CrossRef]
- Elyasi, M.; Derazkola, H.A.; Hosseinzadeh, M. Investigations of tool tilt angle on properties friction stir welding of A441 AISI to AA1100 aluminium. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2016, 230. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Khodabakhshi, F. Intermetallic compounds (IMCs) formation during dissimilar friction-stir welding of AA5005 aluminum alloy to St-52 steel: Numerical modeling and experimental study. Int. J. Adv. Manuf. Technol. 2019, 100, 2401–2422. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Khodabakhshi, F.; Gerlich, A.P. Fabrication of a nanostructured high strength steel tube by friction-forging tubular additive manufacturing (FFTAM) technology. J. Manuf. Process. 2020, 58, 724–735. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Simchi, A. A new procedure for the fabrication of dissimilar joints through injection of colloidal nanoparticles during friction stir processing: Proof concept for AA6062/PMMA joints. J. Manuf. Process. 2020, 49, 335–343. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Simchi, A. Processing and characterizations of polycarbonate/alumina nanocomposites by additive powder fed friction stir processing. Thin-Walled Struct. 2020, 157, 107086. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Khodabakhshi, F. A novel fed friction-stir (FFS) technology for nanocomposite joining. Sci. Technol. Weld. Join. 2020, 25. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Khodabakhshi, F. Development of fed friction-stir (FFS) process for dissimilar nanocomposite welding between AA2024 aluminum alloy and polycarbonate (PC). J. Manuf. Process. 2020, 54, 262–273. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Khodabakhshi, F.; Simchi, A. Evaluation of a polymer-steel laminated sheet composite structure produced by friction stir additive manufacturing (FSAM) technology. Polym. Test. 2020, 90, 106690. [Google Scholar] [CrossRef]
- Lambiase, F.; Derazkola, H.A.; Simchi, A. Friction Stir Welding and Friction Spot Stir Welding Processes of Polymers—State of the Art. Materials 2020, 13, 2291. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Khodabakhshi, F.; Gerlich, A.P. Friction-forging tubular additive manufacturing (FFTAM): A new route of solid-state layer-upon-layer metal deposition. J. Mater. Res. Technol. 2020, 9, 15273–15285. [Google Scholar] [CrossRef]
- Boumerzoug, Z.; Helal, Y. Friction Stir Welding of Dissimilar Materials Aluminum AL6061-T6 to Ultra Low Carbon Steel. Materials 2017, 7, 42. [Google Scholar] [CrossRef]
- Laska, A.; Szkodo, M. Manufacturing Parameters, Materials, and Welds Properties of Butt Friction Stir Welded Joints–Overview. Materials 2020, 13, 4940. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A. Recrystallization mechanisms and microstructure development in emerging metallic materials: A review. J. Sci. Adv. Mater. Devices 2019, 4, 19–33. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Kashiry Fard, R.; Khodabakhshi, F. Effects of processing parameters on the characteristics of dissimilar friction-stir-welded joints between AA5058 aluminum alloy and PMMA polymer. Weld. World 2018, 62. [Google Scholar] [CrossRef]
- Hamed Aghajani Derazkola, A.S. Experimental and thermomechanical analysis of the effect of tool pin profile on the friction stir welding of poly(methyl methacrylate) sheets. J. Manuf. Process. 2018, 34, 412–423. [Google Scholar] [CrossRef]
- Eyvazian, A.; Hamouda, A.M.; Aghajani Derazkola, H.; Elyasi, M. Study on the effects of tool tile angle, offset and plunge depth on friction stir welding of poly(methyl methacrylate) T-joint. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2019, 234, 773–787. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Simchi, A. Experimental and thermomechanical analysis of friction stir welding of poly(methyl methacrylate) sheets. Sci. Technol. Weld. Join. 2017. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Khodabakhshi, F.; Simchi, A. Friction-stir lap-joining of aluminium-magnesium/poly-methyl-methacrylate hybrid structures: Thermo-mechanical modelling and experimental feasibility study. Sci. Technol. Weld. Join. 2018, 23. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Simchi, A. An investigation on the dissimilar friction stir welding of T-joints between AA5754 aluminum alloy and poly(methyl methacrylate). Thin-Walled Struct. 2019, 135, 376–384. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Simchi, A. Effects of alumina nanoparticles on the microstructure, strength and wear resistance of poly(methyl methacrylate)-based nanocomposites prepared by friction stir processing. J. Mech. Behav. Biomed. Mater. 2018, 79. [Google Scholar] [CrossRef]
- Elyasi, M.; Derazkola, H.A. Experimental and thermomechanical study on FSW of PMMA polymer T-joint. Int. J. Adv. Manuf. Technol. 2018, 97, 1445–1456. [Google Scholar] [CrossRef]
- Hamed Aghajani Derazkola, M.E. The influence of process parameters in friction stir welding of Al-Mg alloy and polycarbonate. J. Manuf. Process. 2018, 35, 88–98. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Simchi, A.; Lambiase, F. Friction stir welding of polycarbonate lap joints: Relationship between processing parameters and mechanical properties. Polym. Test. 2019, 79, 105999. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Eyvazian, A.; Simchi, A. Modeling and experimental validation of material flow during FSW of polycarbonate. Mater. Today Commun. 2020, 22, 100796. [Google Scholar] [CrossRef]
- Picot, F.; Gueydan, A.; Martinez, M.; Moisy, F.; Hug, E. A Correlation between the Ultimate Shear Stress and the Thickness Affected by Intermetallic Compounds in Friction Stir Welding of Dissimilar Aluminum Alloy–Stainless Steel Joints. Materials 2018, 8, 179. [Google Scholar] [CrossRef]
- Thomä, M.; Gester, A.; Wagner, G.; Fritzsche, M. Analysis of the Oscillation Behavior of Hybrid Aluminum/Steel Joints Realized by Ultrasound Enhanced Friction Stir Welding. Materials 2020, 10, 1079. [Google Scholar] [CrossRef]
- Kolnes, M.; Kübarsepp, J.; Sergejev, F.; Kolnes, M.; Tarraste, M.; Viljus, M. Performance of Ceramic-Metal Composites as Potential Tool Materials for Friction Stir Welding of Aluminium, Copper and Stainless Steel. Materials 2020, 13, 1994. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nezu, M.; Uchida, S.; Hirata, T. Mechanism of intermetallic compound formation during the dissimilar friction stir welding of aluminum and steel. J. Mater. Sci. 2020, 55, 3064–3072. [Google Scholar] [CrossRef]
- Tan, Y.B.; Wang, X.M.; Ma, M.; Zhang, J.X.; Liu, W.C.; Fu, R.D.; Xiang, S. A study on microstructure and mechanical properties of AA 3003 aluminum alloy joints by underwater friction stir welding. Mater. Charact. 2017, 127, 41–52. [Google Scholar] [CrossRef]
- Kaushik, P.; Dwivedi, D.K. Effect of tool geometry in dissimilar Al-Steel Friction Stir Welding. J. Manuf. Process. 2020. [Google Scholar] [CrossRef]
- Safeen, M.W.; Russo Spena, P. Main Issues in Quality of Friction Stir Welding Joints of Aluminum Alloy and Steel Sheets. Materials 2019, 9, 610. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Abd Elnabi, M.M.; Osman, T.A.; El Mokadem, A.; Elshalakany, A.B. Evaluation of the formation of intermetallic compounds at the intermixing lines and in the nugget of dissimilar steel/aluminum friction stir welds. J. Mater. Res. Technol. 2020, 9, 10209–10222. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H. Characteristics and Formation Mechanisms of Welding Defects in Underwater Friction Stir Welded Aluminum Alloy. Metallogr. Microstruct. Anal. 2012, 1, 269–281. [Google Scholar] [CrossRef]
- Godasu, A.K.; Kumar, A.; Mula, S. Influence of cryocooling on friction stir processing of Al-5083 alloy. Mater. Manuf. Process. 2020, 35, 202–213. [Google Scholar] [CrossRef]
- Patel, N.P.; Parlikar, P.; Singh Dhari, R.; Mehta, K.; Pandya, M. Numerical modelling on cooling assisted friction stir welding of dissimilar Al-Cu joint. J. Manuf. Process. 2019, 47, 98–109. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H. Mathematical model and optimization for underwater friction stir welding of a heat-treatable aluminum alloy. Mater. Des. 2013, 45, 206–211. [Google Scholar] [CrossRef]
- Derazkola, H.A.; Khodabakhshi, F. Underwater submerged dissimilar friction-stir welding of AA5083 aluminum alloy and A441 AISI steel. Int. J. Adv. Manuf. Technol. 2019, 102, 4383–4395. [Google Scholar] [CrossRef]
- Kar, A.; Vicharapu, B.; Morisada, Y.; Fujii, H. Elucidation of interfacial microstructure and properties in friction stir lap welding of aluminium alloy and mild steel. Mater. Charact. 2020, 168, 110572. [Google Scholar] [CrossRef]
- Xue, P.; Xiao, B.L.; Ni, D.R.; Ma, Z.Y. Enhanced mechanical properties of friction stir welded dissimilar Al-Cu joint by intermetallic compounds. Mater. Sci. Eng. A 2010, 527, 5723–5727. [Google Scholar] [CrossRef]
- Grong, Ø.; Sandnes, L.; Bergh, T.; Vullum, P.E.; Holmestad, R.; Berto, F. An analytical framework for modelling intermetallic compound (IMC) formation and optimising bond strength in aluminium-steel welds. Mater. Des. Process. Commun. 2019, 1, e57. [Google Scholar] [CrossRef]
- Aizawa, T.; Luangvaranunt, T.; Kondoh, K. Solid State Recycling of Recyclable Aluminum Wastes with In-Process Microstructure Control. Mater. Trans. 2002, 43, 315–321. [Google Scholar] [CrossRef]
- Mahto, R.P.; Gupta, C.; Kinjawadekar, M.; Meena, A.; Pal, S.K. Weldability of AA6061-T6 and AISI 304 by underwater friction stir welding. J. Manuf. Process. 2019, 38, 370–386. [Google Scholar] [CrossRef]
- Talebizadehsardari, P.; Musharavati, F.; Khan, A.; Sebaey, T.A.; Eyvaziana, A.; Derazkola, H.A. Underwater friction stir welding of Al-Mg alloy: Thermo-mechanical modeling and validation. Mater. Today Commun. 2021, 26, 101965. [Google Scholar] [CrossRef]
- Sabari, S.S.; Malarvizhi, S.; Balasubramanian, V. The effect of pin profiles on the microstructure and mechanical properties of underwater friction stir welded AA2519-T87 aluminium alloy. Int. J. Mech. Mater. Eng. 2016, 11, 5. [Google Scholar] [CrossRef]
- Sree Sabari, S.; Malarvizhi, S.; Balasubramanian, V.; Madusudhan Reddy, G. Experimental and numerical investigation on under-water friction stir welding of armour grade AA2519-T87 aluminium alloy. Def. Technol. 2016, 12, 324–333. [Google Scholar] [CrossRef]
- Chitturi, V.; Pedapati, S.R.; Awang, M. Effect of Tilt Angle and Pin Depth on Dissimilar Friction Stir Lap Welded Joints of Aluminum and Steel Alloys. Materails 2019, 12, 3901. [Google Scholar] [CrossRef]
- Heirani, F.; Abbasi, A.; Ardestani, M. Effects of processing parameters on microstructure and mechanical behaviors of underwater friction stir welding of Al5083 alloy. J. Manuf. Process. 2017, 25, 77–84. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.; Zhao, Y.; Yan, K.; Zhang, H. The adjustment strategy of welding parameters for spray formed 7055 aluminum alloy underwater friction stir welding joint. Mater. Des. 2015, 88, 1366–1376. [Google Scholar] [CrossRef]
- Beygi, R.; Zarezadeh Mehrizi, M.; Akhavan-Safar, A.; Safaei, S.; Loureiro, A.; da Silva, L.F.M. Design of friction stir welding for butt joining of aluminum to steel of dissimilar thickness: Heat treatment and fracture behavior. Int. J. Adv. Manuf. Technol. 2021, 112, 1951–1964. [Google Scholar] [CrossRef]
- Lü, N.C.; Hao, G.D.; Wang, Y.T. Weldablity of the Lap Joint between a 5182 Aluminum Alloy and a DP1180 Two-Phase Steel Sheet. Strength Mater. 2020, 52, 573–586. [Google Scholar] [CrossRef]
- Safeen, M.W.; Russo Spena, P.; Buffa, G.; Campanella, D.; Masnata, A.; Fratini, L. Effect of position and force tool control in friction stir welding of dissimilar aluminum-steel lap joints for automotive applications. Adv. Manuf. 2020, 8, 59–71. [Google Scholar] [CrossRef]
- Papahn, H.; Bahemmat, P.; Haghpanahi, M.; Sommitsch, C. Study on governing parameters of thermal history during underwater friction stir welding. Int. J. Adv. Manuf. Technol. 2015, 78, 1101–1111. [Google Scholar] [CrossRef]
- Eyvazian, A.; Hamouda, A.; Tarlochan, F.; Derazkola, H.A.; Khodabakhshi, F. Simulation and experimental study of underwater dissimilar friction-stir welding between aluminium and steel. J. Mater. Res. Technol. 2020, 9, 3767–3781. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Eyvazian, A.; Simchi, A. Submerged friction stir welding of dissimilar joints between an Al-Mg alloy and low carbon steel: Thermo-mechanical modeling, microstructural features, and mechanical properties. J. Manuf. Process. 2020, 50, 68–79. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, S.; Yang, S.; Lu, Z.; Yan, K. Influence of cooling conditions on joint properties and microstructures of aluminum and magnesium dissimilar alloys by friction stir welding. Int. J. Adv. Manuf. Technol. 2016, 83, 673–679. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhao, H.; Tong, H. Effect of Loading Methods on the Fatigue Properties of Dissimilar Al/Steel Keyhole-Free FSSW Joints. Materails 2020, 13, 4247. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Yao, X.; Xu, H.; Li, B. Investigation on dissimilar underwater friction stir lap welding of 6061-T6 aluminum alloy to pure copper. Mater. Des. 2014, 64, 74–80. [Google Scholar] [CrossRef]
| Parameters | A441 AISI Steel | AA3003 Aluminum Alloy |
|---|---|---|
| Density, kg/m3 | 7800 | 2730 |
| Melting Point, °C | 1400 | 644 |
| Thermal conductivity, W/m·k | 42.7 | 154 |
| Specific heat capacity, J/g-°C | 0.477 | 0.893 |
| Yielding strength, MPa | 344 | 186 |
| Ultimate tensile strength, MPa | 580 | 200 |
| Shear strength, MPa | 380 | 110 |
| Elongation, % | 15 | 10 |
| Vickers hardness | 355 | 90 |
| AA3003 Aluminum Alloy | ||||||
| Al | Fe | Si | Zn | Mn | Cu | |
| 97.1 | 0.7 | 0.6 | 0.2 | 1.2 | 0.20 | |
| A441 AISI Steel | ||||||
| S | P | C | Mn | Cu | Si | Fe |
| 0.05 | 0.04 | 0.22 | 1.00 | 0.20 | 0.40 | 97.00 |
| Parameter | Tool Rotational Speed | Tool Traveling Speed | Tool Tilt Angle | Tool Plunge Depth | Tool Offset |
|---|---|---|---|---|---|
| Value | 800 rpm | 40 mm/min | 2 | 0.2 mm | 1.3 mm in Al side |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghajani Derazkola, H.; García, E.; Eyvazian, A.; Aberoumand, M. Effects of Rapid Cooling on Properties of Aluminum-Steel Friction Stir Welded Joint. Materials 2021, 14, 908. https://doi.org/10.3390/ma14040908
Aghajani Derazkola H, García E, Eyvazian A, Aberoumand M. Effects of Rapid Cooling on Properties of Aluminum-Steel Friction Stir Welded Joint. Materials. 2021; 14(4):908. https://doi.org/10.3390/ma14040908
Chicago/Turabian StyleAghajani Derazkola, Hamed, Eduardo García, Arameh Eyvazian, and Mohammad Aberoumand. 2021. "Effects of Rapid Cooling on Properties of Aluminum-Steel Friction Stir Welded Joint" Materials 14, no. 4: 908. https://doi.org/10.3390/ma14040908
APA StyleAghajani Derazkola, H., García, E., Eyvazian, A., & Aberoumand, M. (2021). Effects of Rapid Cooling on Properties of Aluminum-Steel Friction Stir Welded Joint. Materials, 14(4), 908. https://doi.org/10.3390/ma14040908







