Sorption of Sulfamethoxazole on Inorganic Acid Solution-Etched Biochar Derived from Alfalfa
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Biomass
2.2. Modification of BCs (Sorbents)
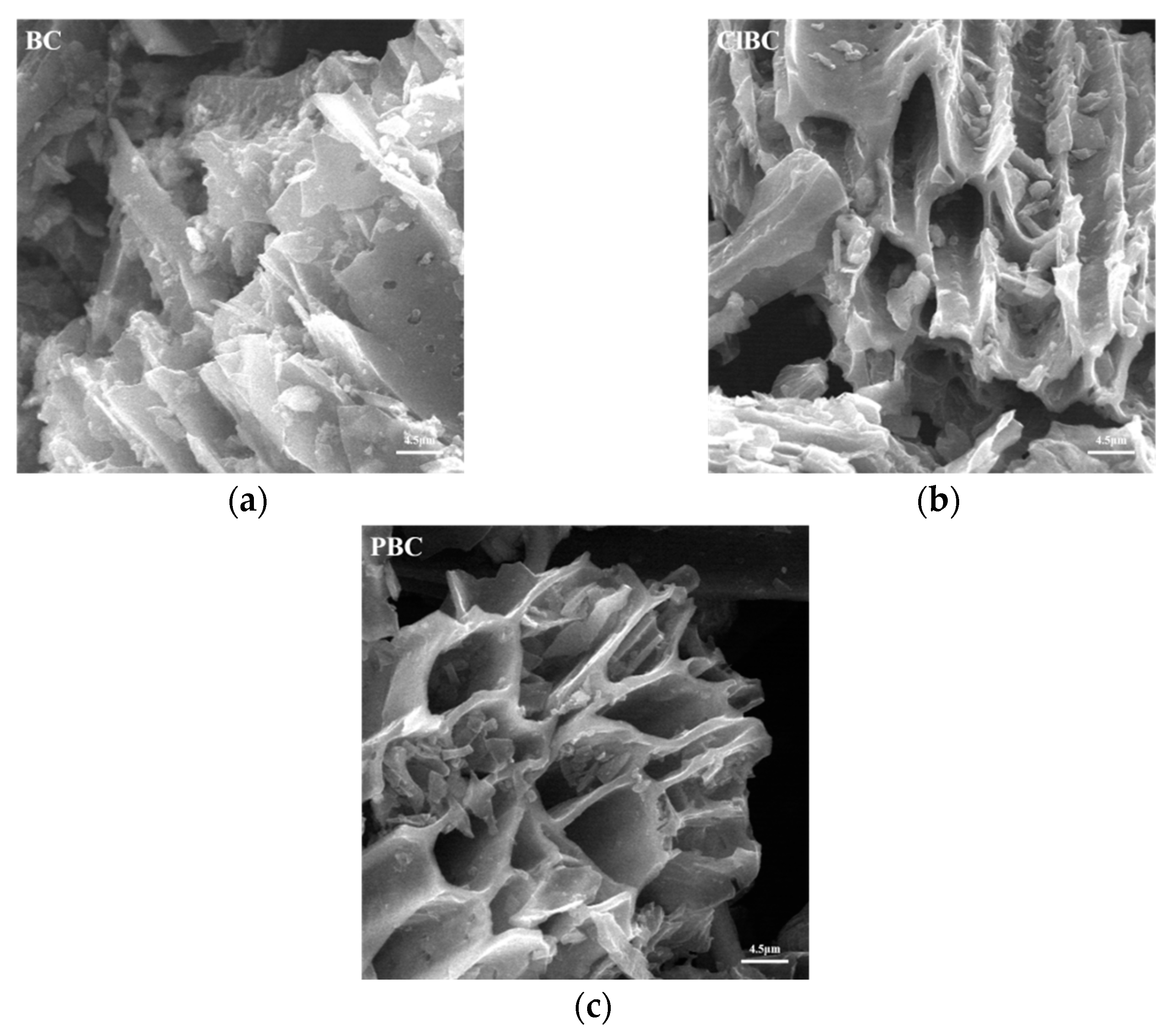
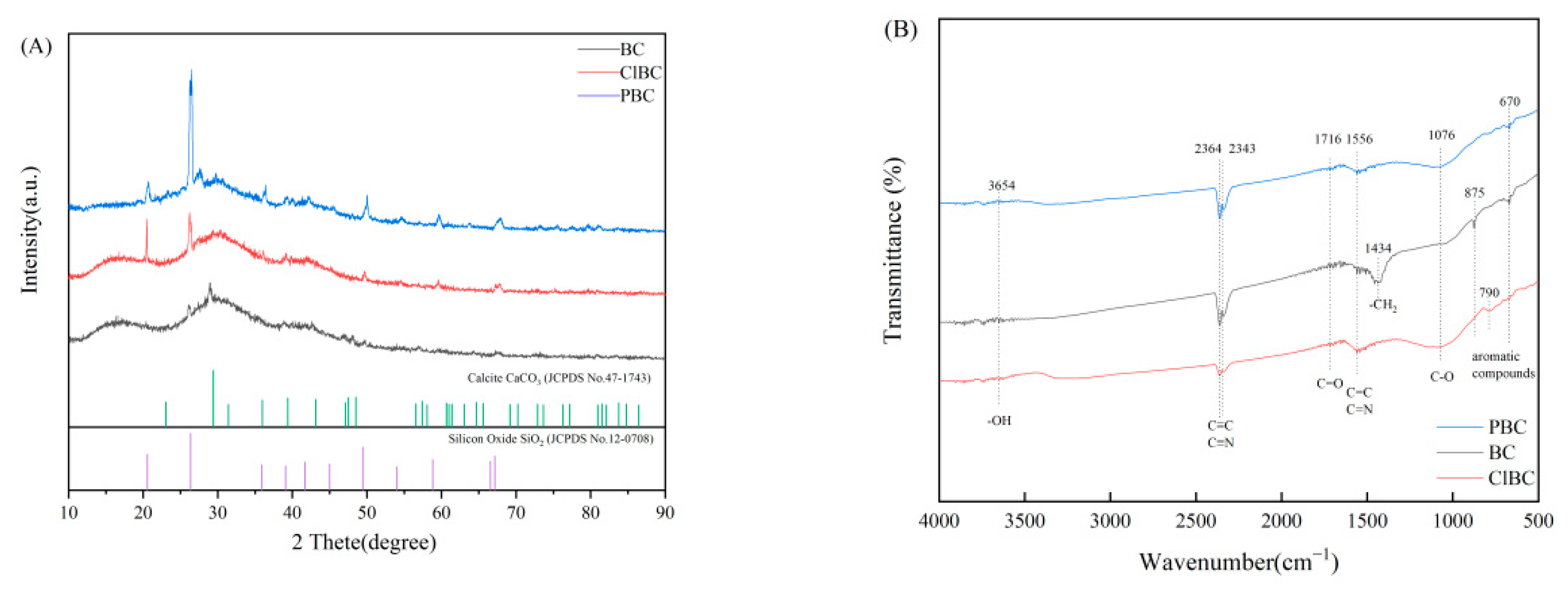
2.3. Characterization of BCs
2.4. Batch Adsorption Experiment
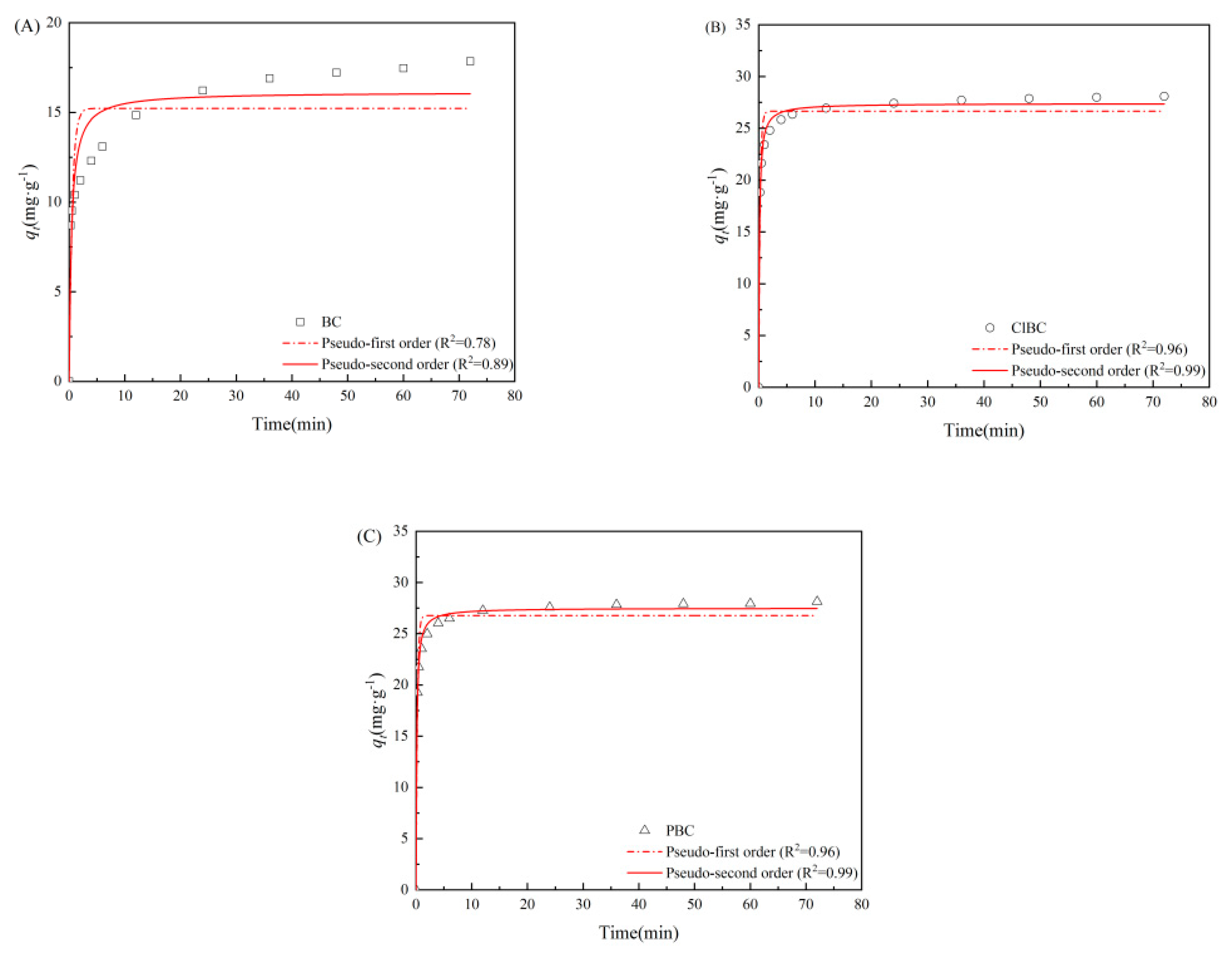
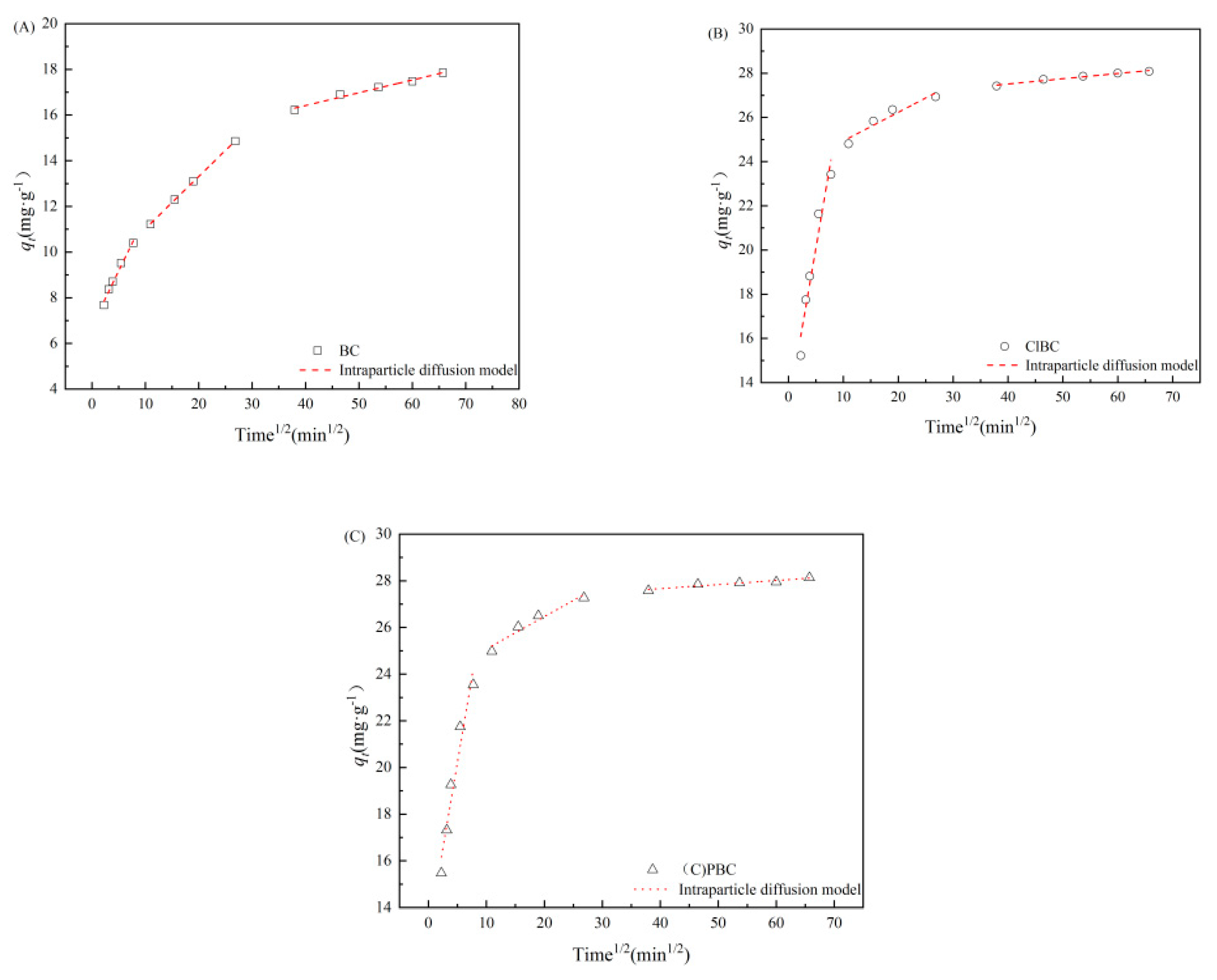
2.5. Data Analysis
3. Results and Discussion
3.1. Characterization
3.2. Adsorption Kinetics
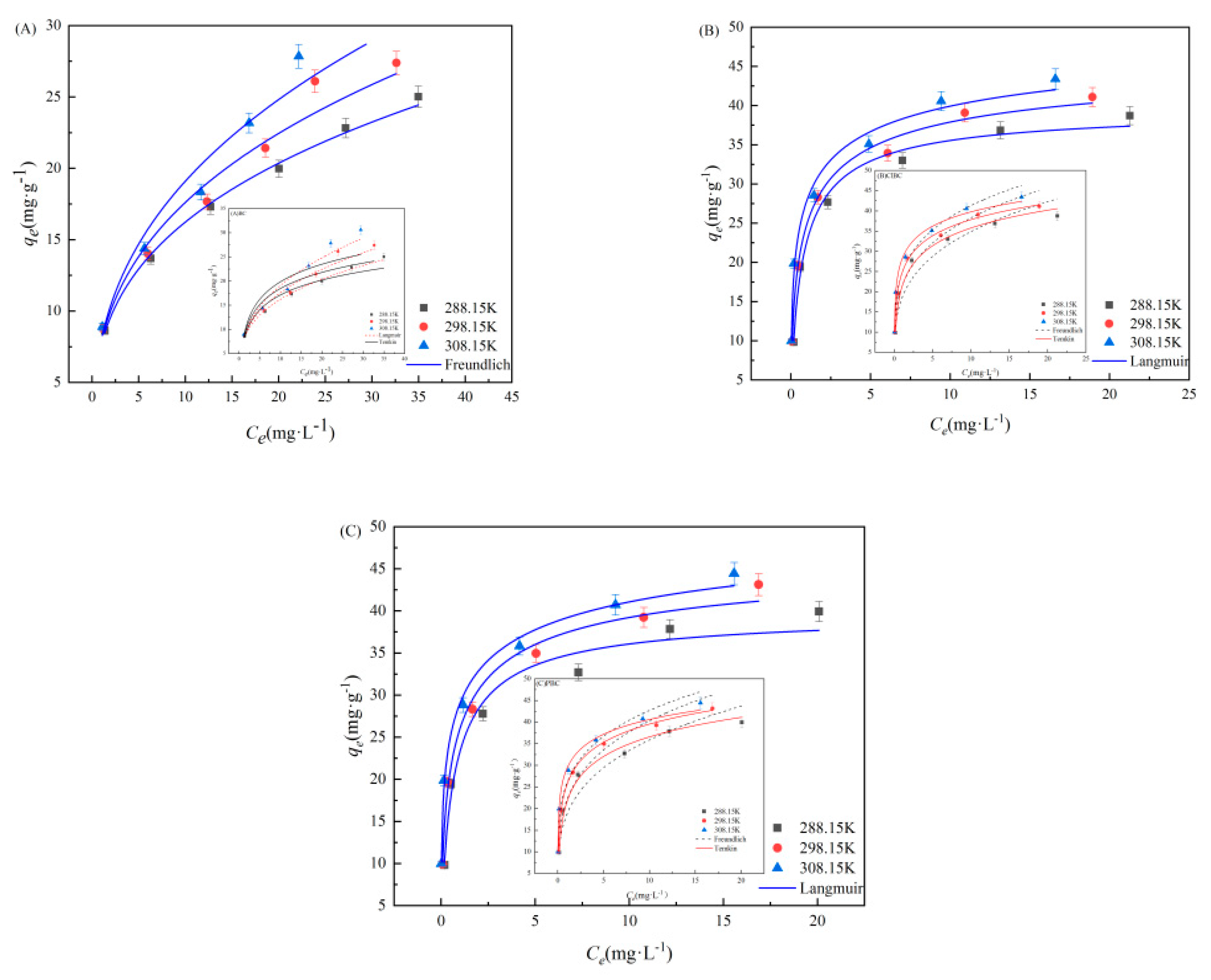
3.3. Adsorption Isotherms
3.4. Thermodynamic Analyses
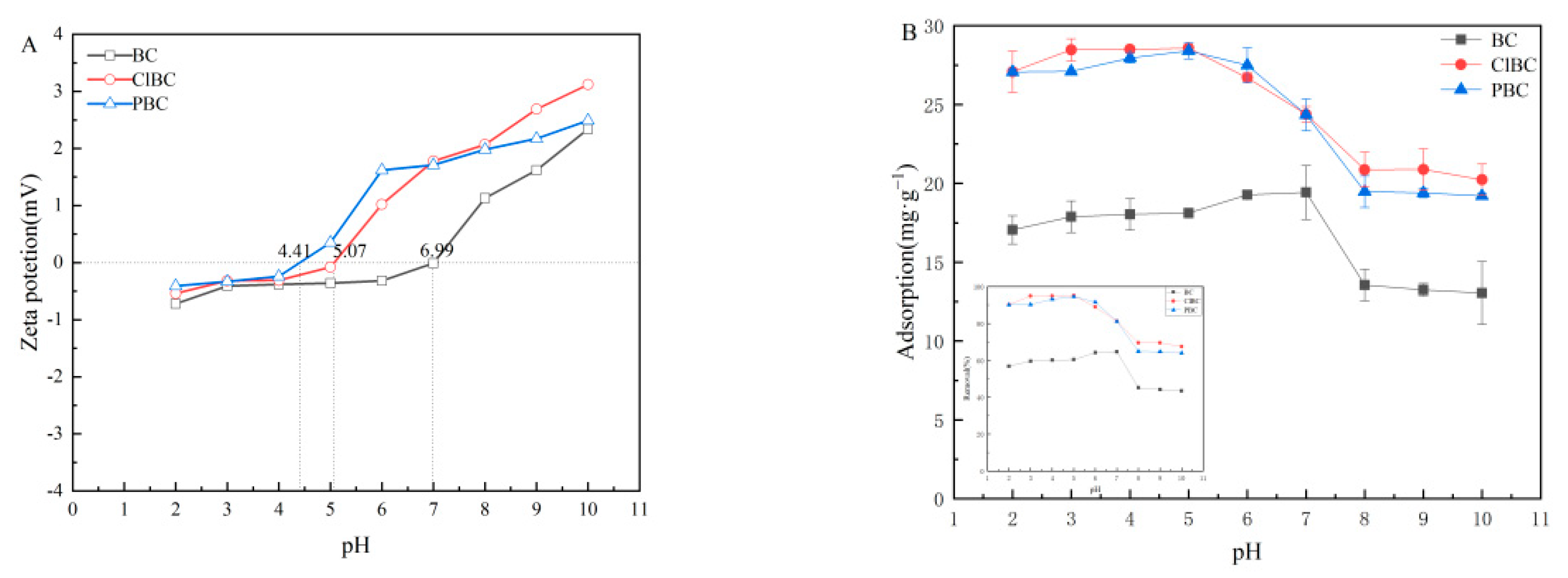
3.5. Influence of pHinitial of The Solution
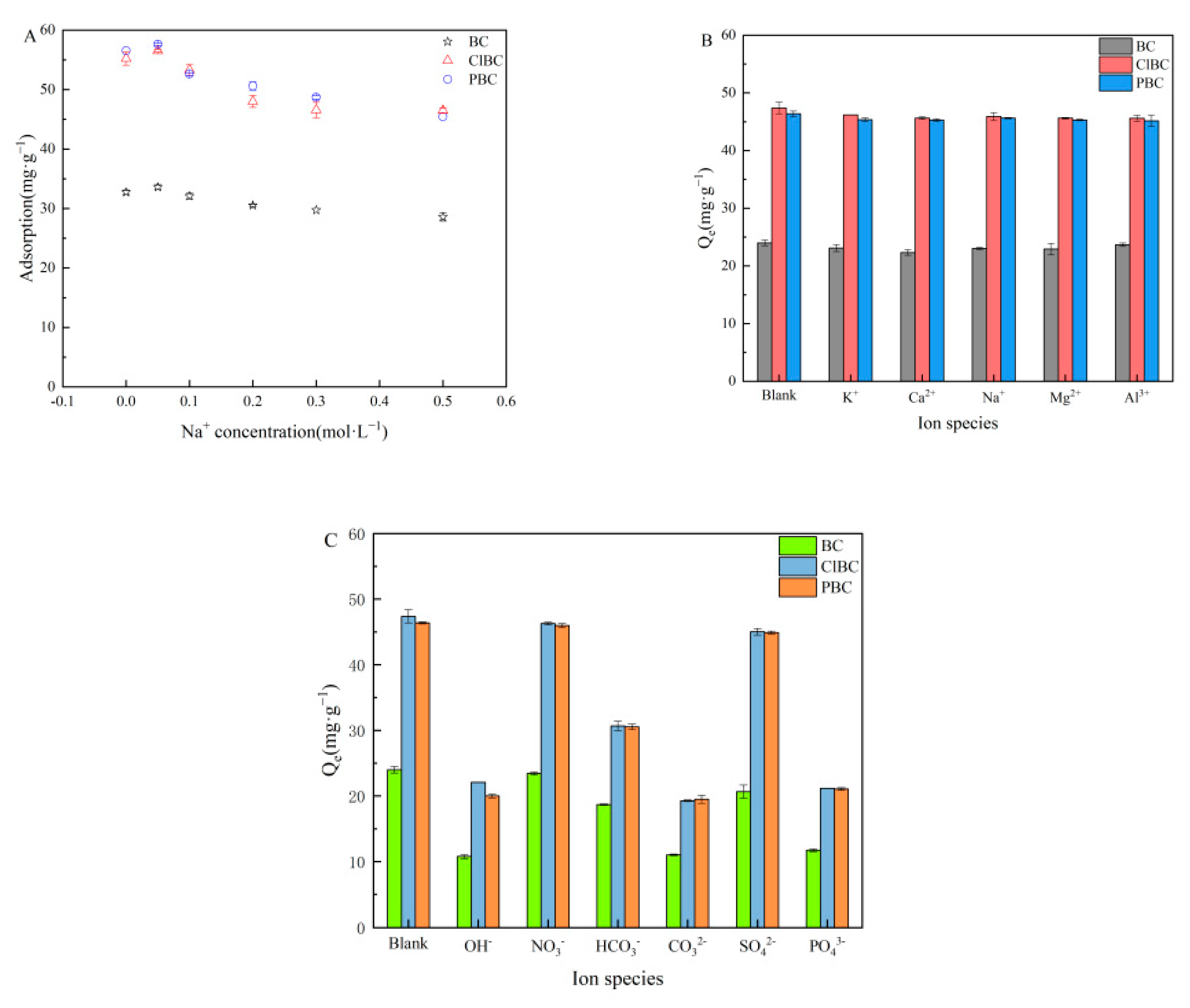
3.6. Effect of Ionic Strength and Types
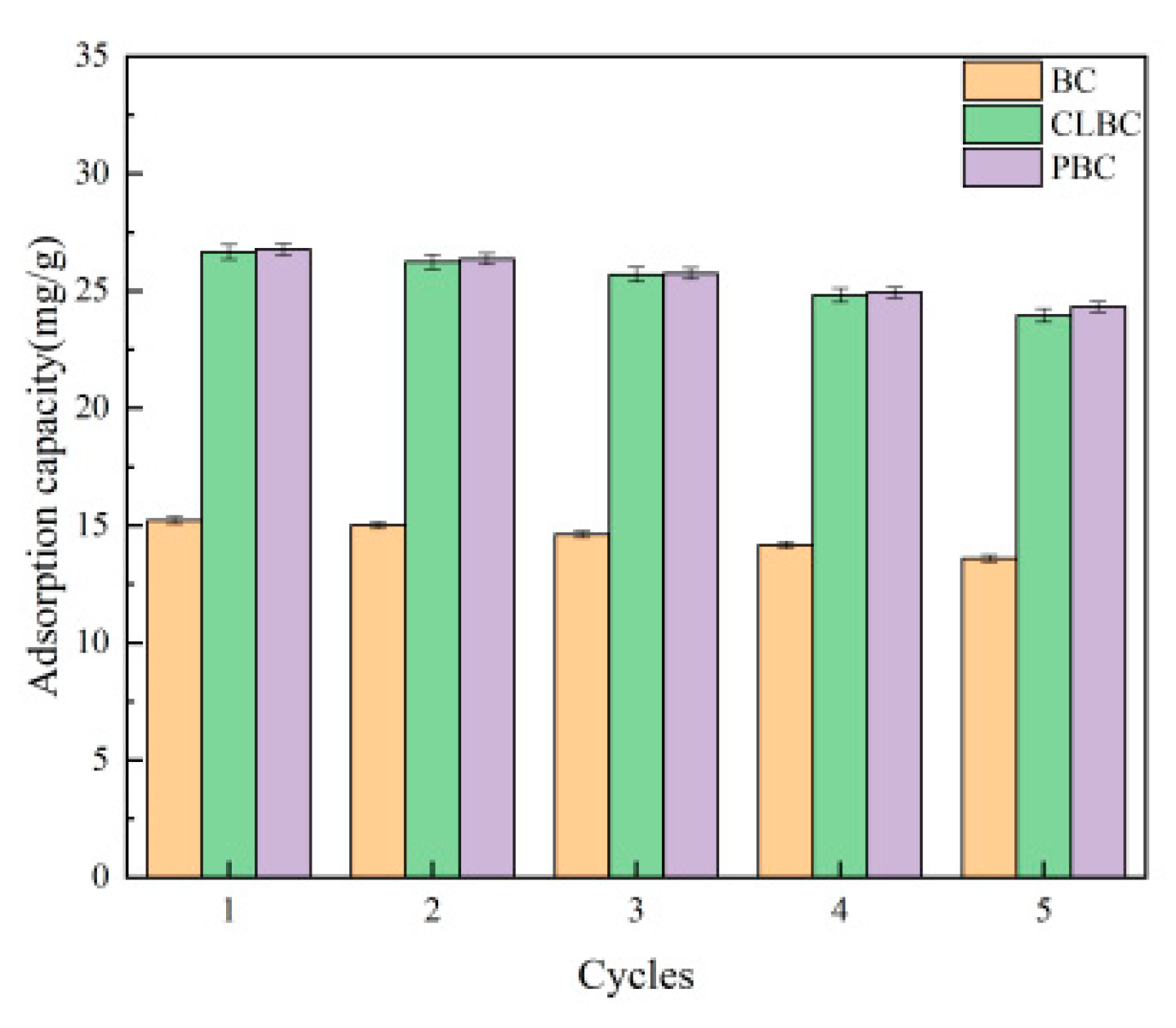
3.7. Regenerability and Stability
3.8. Comparative Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Lesnik, K.L.; Xu, S.; Wang, L.; Liu, H. Impact of tobramycin on the performance of microbial fuel cell. Microb. Cell Factories 2014, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Topp, E. Abundance of Antibiotic Resistance Genes in Bacteriophage following Soil Fertilization with Dairy Manure or Municipal Biosolids, and Evidence for Potential Transduction. Appl. Environ. Microbiol. 2015, 81, 7905–7913. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, P.; Kim, E.Y.; Lee, J.; Ryu, E.K.; Shin, S.Y.; Bang, J.K. Synthesis of Fmoc-Triazine Amino Acids and Its Application in the Synthesis of Short Antibacterial Peptidomimetics. Int. J. Mol. Sci. 2020, 21, 3602. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Sheng, H.; Shen, X.; Sun, X.; Yan, Y.; Wang, J.; Yuan, Q. Engineering probiotics as living diagnostics and therapeutics for improving human health. Microb. Cell Factories 2020, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Lillenberg, M.; Litvin, S.V.; Nei, L.; Roasto, M.; Sepp, K. Enrofloxacin and Ciprofloxacin Uptake by Plants from Soil. Agron. Res. 2010, 8, 807–814. [Google Scholar]
- Liu, P.; Liu, W.J.; Jiang, H.; Chen, J.J.; Li, W.W.; Yu, H.Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zheng, X.; Chen, B.; Ma, J.; Niu, X.; Zhang, D.; Lin, Z.; Fu, M.; Zhou, S. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar. J. Clean. Prod. 2020, 256, 120662. [Google Scholar] [CrossRef]
- Huang, D.; Wang, X.; Zhang, C.; Zeng, G.; Peng, Z.; Zhou, J.; Cheng, M.; Wang, R.; Hu, Z.; Qin, X. Sorptive removal of ionizable antibiotic sulfamethazine from aqueous solution by graphene oxide-coated biochar nanocomposites: Influencing factors and mechanism. Chemosphere 2017, 186, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, Y.; Meng, T.; Zhang, R.; Song, M.; Ren, J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018, 147, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, L.; Du, L.; Gao, P.; Liang, N.; Wu, S.; Minami, T.; Zang, L.; Yu, C.; Xu, X. Preparation of Polyaniline/Emulsion Microsphere Composite for Efficient Adsorption of Organic Dyes. Polymers 2020, 12, 167. [Google Scholar] [CrossRef]
- Ingerslev, F.; Halling-Sørensen, B. Biodegradability properties of sulfonamides in activated sludge. Environ. Toxicol. Chem. 2000, 19, 2467–2473. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Yin, R.-L.; Zhou, X.-J.; Du, J.-S.; Cao, H.-O.; Yang, S.-S.; Ren, N.-Q. Sulfamethoxazole degradation by ultrasound/ozone oxidation process in water: Kinetics, mechanisms, and pathways. Ultrason. Sonochem. 2015, 22, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Shimabuku, K.K.; Kearns, J.P.; Martinez, J.E.; Mahoney, R.B.; Moreno-Vasquez, L.; Summers, R.S. Biochar sorbents for sulfamethoxazole removal from surface water, stormwater, and wastewater effluent. Water Res. 2016, 96, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwang, Y.S.; Sharma, V.K. Adsorption of antibiotics and iopromide onto single-walled and multi-walled carbon nanotubes. Chem. Eng. J. 2014, 255, 23–27. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Ahmad, M.; Usman, A.R.; Sallam, A.S.; Hussain, Q.; Binyameen, R.B.; Shehu, M.R.; Ok, Y.S. Evaluating the efficiency of different natural clay sediments for the removal of chlortetracycline from aqueous solutions. J. Hazard. Mater. 2020, 384, 121500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, W.; Ngo, H.H.; Wen, H.; Li, N.; Wu, W. Performance evaluation of powdered activated carbon for removing 28 types of antibiotics from water. J. Environ. Manag. 2016, 172, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, P.; Wei, K.; Huang, X.; Zhang, X. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar. Chem. Eng. J. 2020, 385, 123921. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17 alpha-ethinylestradiol. Sci. Total Environ. 2018, 628, 722–730. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Li, P.; Yang, L.; Wu, L.; Gao, F.; Qi, X.; Zhang, Z. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour. Technol. 2021, 319, 124199. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, X.; Ge, C.; Müller, K.; Yu, H.; Huang, P.; Li, J.; Tsang, D.C.W.; Bolan, N.S.; Rinklebe, J.; et al. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour. Technol. 2018, 263, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shan, B.; Tang, W.; Zhu, Y. Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresour. Technol. 2017, 238, 352–360. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, H.; Huang, H.; Gao, H.; Wang, X.; Cao, R.; Li, J.; Xu, X.; Wang, X. Unexpected ultrafast and high adsorption capacity of oxygen vacancy-rich WOx/C nanowire networks for aqueous Pb2+ and methylene blue removal. J. Mater. Chem. A 2017, 5, 15913–15922. [Google Scholar] [CrossRef]
- Afrooz, A.N.; Boehm, A.B. Escherichia coli Removal in Biochar-Modified Biofilters: Effects of Biofilm. PLoS ONE 2016, 11, e0167489. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.F.; Song, X.M.; Huang, X.; Zhou, W.D.; Wu, J.L.; Zhu, Z.G.; Zheng, H.C.; Jiang, Y.Q. Effects of Alfalfa Meal on Growth Performance and Gastrointestinal Tract Development of Growing Ducks. Asian Australas. J. Anim. Sci. 2012, 25, 1445–1450. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Sun, L.S.; Su, S.; Xu, K.; He, L.M.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Askari, M.; Velashjerdi, M.; Arab, B. Phosphoric acid-treated Spent Tea Residue Biochar for Wastewater Decoloring: Batch Adsorption Study and Process Intensification using Multivariate Data-based Optimization. Chem. Eng. Process. Process Intensif. 2020, 158, 108170. [Google Scholar] [CrossRef]
- Su, H.; Li, W.; Han, Y.; Liu, N. Magnetic carboxyl functional nanoporous polymer: Synthesis, characterization and its application for methylene blue adsorption. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Shen, J.X.; Peng, S.S.; Zhang, J.K.; Wu, J.; Liu, X.Q.; Sun, L.B. Enhancing oxidation resistance of Cu(I) by tailoring microenvironment in zeolites for efficient adsorptive desulfurization. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gayatri, S.L. Activated Tea Waste as a Potential Low-Cost Adsorbent for the Removal of p-Nitrophenol from Wastewater. J. Chem. Eng. Data 2010, 55, 4614–4623. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Li, J.; Ly, Q.V.; Nguyen, T.A.H.; Tran, V.S. Applying a new pomelo peel derived biochar in microbial fell cell for enhancing sulfonamide antibiotics removal in swine wastewater. Bioresour. Technol. 2020, 318, 123886. [Google Scholar] [CrossRef] [PubMed]
- Lykoudi, A.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of sulfamethoxazole with persulfate using spent coffee grounds biochar as activator. J. Environ. Manag. 2020, 271, 111022. [Google Scholar] [CrossRef]
- Hu, X.; Cao, J.; Yang, H.; Li, D.; Qiao, Y.; Zhao, J.; Zhang, Z.; Huang, L. Pb2+ biosorption from aqueous solutions by live and dead biosorbents of the hydrocarbon-degrading strain Rhodococcus sp. HX-2. PLoS ONE 2020, 15, e0226557. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ge, J.; Yang, C.; Wu, R.; Dang, Z.; Liu, S. Sorption behavior of tylosin and sulfamethazine on humic acid: Kinetic and thermodynamic studies. RSC Adv. 2015, 5, 58865–58872. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, X.; Yang, C.; Gao, L.; Hu, Y. An efficient method for tylosin removal from an aqueous solution by goethite modified straw mass. RSC Adv. 2016, 6, 95425–95434. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, X.; Peng, D. Iron and manganese oxides modified maize straw to remove tylosin from aqueous solutions. Chemosphere 2018, 205, 156–165. [Google Scholar] [CrossRef]
- Hu, X.; Du, X. Adsorption of Tea Polyphenols using Microporous Starch: A Study of Kinetics, Equilibrium and Thermodynamics. Molecules 2019, 24, 1449. [Google Scholar] [CrossRef]
- Channa, A.M.; Baytak, S.; Memon, S.Q.; Talpur, M.Y. Equilibrium, kinetic and thermodynamic studies of removal of phenol from aqueous solution using surface engineered chemistry. Heliyon 2019, 5, e01852. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Parasar, D.; Mondal, B.; Deb, P. Mesoporous magnetic secondary nanostructures as versatile adsorbent for efficient scavenging of heavy metals. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Lian, F.; Sun, B.; Song, Z.; Zhu, L.; Qi, X.; Xing, B. Physicochemical properties of herb-residue biochar and its sorption to ionizable antibiotic sulfamethoxazole. Chem. Eng. J. 2014, 248, 128–134. [Google Scholar] [CrossRef]
- Guo, X.; Yin, Y.; Yang, C.; Dang, Z. Maize straw decorated with sulfide for tylosin removal from the water. Ecotoxicol. Environ. Saf. 2018, 152, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Cao, Y.; Qi, F.F.; Li, X.Q.; Xu, Q. Atrazine adsorption removal with nylon6/polypyrrole core-shell nanofibers mat: Possible mechanism and characteristics. Nanoscale Res. Lett. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Lee, S.S.; Seo, D.C.; Tsang, D.C.; Ok, Y.S. Steam activation of biochars facilitates kinetics and pH-resilience of sulfamethazine sorption. J. Soils Sediments 2016, 16, 889–895. [Google Scholar] [CrossRef]
- Chiou, C.T.; Malcolm, R.L.; Brinton, T.I.; Kile, D.E. Water solubility enhancement of some organic pollutants and pesticides by dissolved humic and fulvic acids. Environ. Sci. Technol. 1986, 20, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Song, B.; Shao, Y.; Huang, W. Hybrid silicate-hydrochar composite for highly efficient removal of heavy metal and antibiotics: Coadsorption and mechanism. Chem. Eng. J. 2020, 387, 124097. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and sulfonamide antibiotics adsorption capacity of spent coffee grounds based biochar and hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 56. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liang, C.F.; Li, T.Q.; Wang, K.; Huang, H.G.; Yang, X.E. Simultaneous removal of cadmium and sulfamethoxazole from aqueous solution by rice straw biochar. J. Zhejiang Univ. Sci. B 2013, 14, 640–649. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, L.; Wu, L.; Li, P.; Qi, X.; He, L.; Cui, S.; Ding, Y.; Zhang, Z. Carbon nanotube supported sludge biochar as an efficient adsorbent for low concentrations of sulfamethoxazole removal. Sci. Total Environ. 2020, 718, 137299. [Google Scholar] [CrossRef] [PubMed]
| Adsorbents | Modification Methods | Qm(mg·g−1) | References |
|---|---|---|---|
| Chinese Medicine Residues | nHAP@biochars | 51.22 | [54] |
| Spent Coffee Grounds | Pyrolysis at 450 °C | 0.13 | [55] |
| Bagasse Powder | Pyrolysis at 800 °C | 35.43 | [8] |
| Giant Reed | Pyrolysis at 300 °C | 4.99 | [56] |
| Hickory Chips | Ball Milled | 24.30 | [57] |
| Pomelo Peel | Modified by KOH and HNO3 | 0.832 | [38] |
| Rice Straw | Pyrolysis at 400 °C | 1.828 | [58] |
| Pinus Radiata Sawdust | Modified by NaOH | 17.49 | [49] |
| Sludge | Modified by HCl | 5.43 | [59] |
| Alfalfa | Pyrolysis at 800 °C | 35.40 | Present Work |
| Alfalfa | Modified by HCl | 48.85 | |
| Alfalfa | Modified by H3PO4 | 51.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yu, W.; Guo, L.; Wang, Y.; Zhao, S.; Zhou, L.; Jiang, X. Sorption of Sulfamethoxazole on Inorganic Acid Solution-Etched Biochar Derived from Alfalfa. Materials 2021, 14, 1033. https://doi.org/10.3390/ma14041033
Li Q, Yu W, Guo L, Wang Y, Zhao S, Zhou L, Jiang X. Sorption of Sulfamethoxazole on Inorganic Acid Solution-Etched Biochar Derived from Alfalfa. Materials. 2021; 14(4):1033. https://doi.org/10.3390/ma14041033
Chicago/Turabian StyleLi, Qi, Wei Yu, Linwen Guo, Yuhang Wang, Siyu Zhao, Li Zhou, and Xiaohui Jiang. 2021. "Sorption of Sulfamethoxazole on Inorganic Acid Solution-Etched Biochar Derived from Alfalfa" Materials 14, no. 4: 1033. https://doi.org/10.3390/ma14041033
APA StyleLi, Q., Yu, W., Guo, L., Wang, Y., Zhao, S., Zhou, L., & Jiang, X. (2021). Sorption of Sulfamethoxazole on Inorganic Acid Solution-Etched Biochar Derived from Alfalfa. Materials, 14(4), 1033. https://doi.org/10.3390/ma14041033






