Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment
Abstract
1. Introduction
- i.
- Blast furnace (BF) iron slag, also known as a ground granulated blast furnace slag (GGBFS);
- ii.
- Electric arc furnace carbon or stainless steel slag (EAF-C/S);
- iii.
- Secondary metallurgical slag such as ladle furnace basic slag (LS), also called white slag;
- iv.
- Basic oxygen furnace slag (BOS);
- v.
- Others (e.g., desulphurization slag).
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation and Characterization of Alkali-Activated Mixture
2.3. Reactivity and Chemical Behavior of Slags and Alkali Activated Materials
3. Results and Discussion
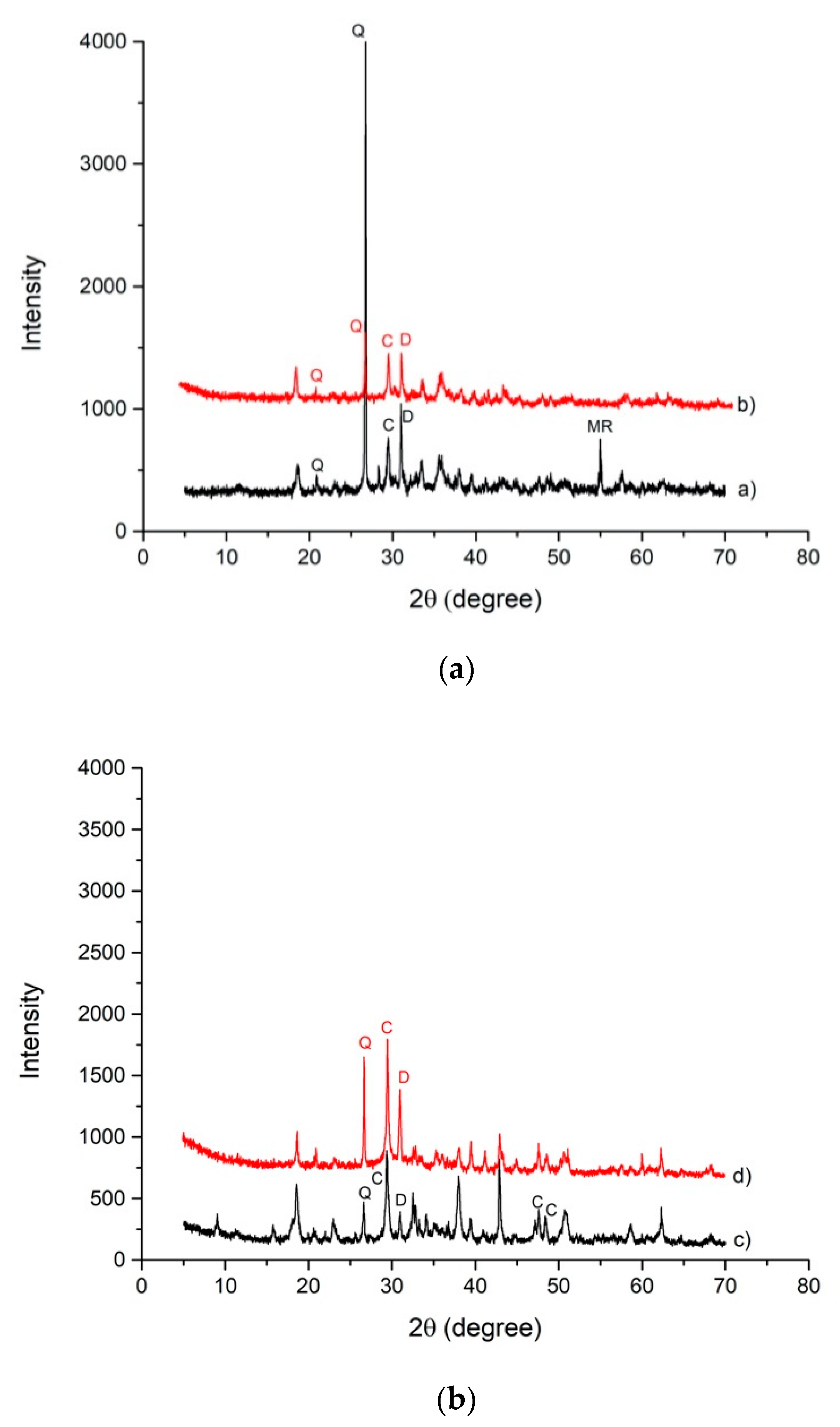
3.1. Slag A and Slag R Characterization
3.2. Alkali Activated Materials Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. Crucial insights on the mix design of alkali-activated cement-based binders (Book Chapter). In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 49–73. ISBN 978-178242288-4/978-178242276-1. [Google Scholar] [CrossRef]
- EUROSLAG. The European Association Representing Metallurgical Slag Producers and Processors. Available online: http://www.euroslag.com/products/eaf/ (accessed on 5 April 2019).
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Wang, K.; Lemougna, P.N.; Tang, Q.; Li, W.; He, Y.; Cui, X. Low temperature depolymerization and polycondensation of a slag-based inorganic polymer. Ceram. Int. 2017, 43, 9067–9076. [Google Scholar] [CrossRef]
- Coppola, L.; Buoso, A.; Coffetti, D.; Kara, P.; Lorenzi, S. Electric arc furnace granulated slag for sustainable concrete. Constr. Build. Mater. 2016, 123, 115–119. [Google Scholar] [CrossRef]
- Arbi, K.; Palomo, A.; Fernández-Jiménez, A. Alkali-activated blends of calcium aluminate cement and slag/diatomite. Ceram. Int. 2013, 39, 9237–9245. [Google Scholar] [CrossRef]
- Muhmood, L.; Vitta, S.; Ventkateswaran, D. Cementious and pozzolanic behavior of electric arc furnace steel slags. Cem. Concr. Res. 2009, 39, 102–109. [Google Scholar] [CrossRef]
- Ozturk, M.; Bankir, M.B.; Bolukbasi, O.S.; Sevim, U.K. Alkali activation of electric arc furnace slag: Mechanical properties and micro analyzes. J. Build. Eng. 2019, 21, 97–105. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.; Peethamparan, S. Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2021, 288, 125584. [Google Scholar] [CrossRef]
- Borges Marinho, A.L.; Mol Santos, C.M.; Carvalho, J.M.F.; Mendes, J.C.; Brigolini, G.J.; Fiorotti Peixoto, R.A. Ladle furnace slag as binder forcement-based composites. J. Mater. Civ. Eng. 2017, 29, 04017207. [Google Scholar] [CrossRef]
- Shi, C. Characteristics and cementitious properties of ladle slagfines fromsteel production. Cem. Concr. Res. 2002, 32, 459–462. [Google Scholar] [CrossRef]
- Polanco, J.A.; Manso, J.M.; Setien, J.; Gonzalez, J.J. Strength and durability ofconcrete made with electric steelmaking slag. ACI Mater. J. 2011, 108, 196–203. [Google Scholar]
- Nguyen, H.; Carvelli, V.; Adesanya, E.; Kinnunen, P.; Illikainen, M. High performance cementitious composite from alkali-activated ladle slagreinforced with polypropylenefibers. Cem. Concr. Compos. 2018, 90, 150–160. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Alkali activation of ladle slag from steel-making process. J. Sustain. Metall. 2017, 3, 300–310. [Google Scholar] [CrossRef]
- Murri, A.N.; Rickard, W.D.A.; Bignozzi, M.C.; van Riessen, A. High tem-perature behaviour of ambient cured alkali-activated materials based on ladleslag. Cem. Concr. Res. 2013, 43, 51–61. [Google Scholar] [CrossRef]
- Da Fonseca, C.P.S.R.A.V.; Fernández-Jiménez, A.; Cristelo, N. Application of the response surface method to optimize alkali activated cements based on low-reactivity ladle furnace slag. Constr. Build. Mater. 2020, 264, 120271. [Google Scholar]
- Bignozzi, M.C.; Manzi, S.; Lancellotti, I.; Kamseu, E.; Barbieri, L.; Leonelli, C. Mix-design and characterization of alkali activated materials based on meta-kaolin and ladle slag. Appl. Clay Sci. 2013, 73, 78–85. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Use of ladle furnace slag containing heavy metals as a binding material in civil engineering. Sci. Total Environ. 2020, 705, 135854. [Google Scholar] [CrossRef]
- Cesnovar, M.; Traven, K.; Horvat, B.; Ducman, V. The potential of ladle slag and electric arc furnace slag use in synthesizing alkali activated materials; the influence of curing on mechanical properties. Materials 2019, 12, 1173, ISSN 1996-1944. [Google Scholar] [CrossRef]
- Češnovar, M.; Traven, K.; Ducman, V. Alkali activated foams from slag (FLOW). In Proceedings of the 6th International Slag Valorisation Symposium, Mechelen, Belgium, 1–5 April 2019; Science, innovation & entrepreneurship in pursuit of a sustainable world; Materials Engineering. Malfliet, A., Peys, A., Di Maria, A., Eds.; KU Leuven: Leuven, Belgium, 2019; pp. 237–240. [Google Scholar]
- Kiventera, J.; Lancellotti, I.; Catauro, M.; Poggetto, F.D.; Leonelli, C.; Illikainen, M. Alkali activation as new option for gold mine tailings inertization. J. Clean. Prod. 2018, 187, 76–84. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Yang, K.; Mu, S.; Zhang, Z.; Magee, B.; Yang, C.; Basheer, M. Characterisation of temporal variations of alkali-activated slag cement property using microstructure features and electrical responses. Constr. Build. Mater. 2020, 261, 119884. [Google Scholar] [CrossRef]
- UNI EN 12457-2:2004. Caratterizzazione dei Rifiuti- Lisciviazione-Prova di Conformità per la Lisciviazione di Rifiuti Granulari e di Fanghi—Parte 2: Prova a Singolo Stadio, con un Rapporto Liquido/Solido di 10 l/kg, per Materiali con Particelle di Dimensioni Minori di 4 mm (con o Senza Riduzione Delle Dimensioni); UNI, Italian Organization for Standardization: Milan, Italy; Roma, Italy, 2004. [Google Scholar]
- Galiano, L.; Pereira, C.F.; Vale, J. Stabilization/solidification of a municipal solid waste incineration residue using fly-ash based geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali activation processes for incinerator residues management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Palomo, A. Quantitative determination of reactive SiO2 and Al2O3 in aluminosilicate materials. In Proceedings of the XIII International Congress on the Chemistry of Cement, Madrid, Spain, 3–8 July 2011. [Google Scholar]
- Fernàndez-Jiménez, A.; de la Torre, A.G.; Palomo, A.; Lòpez-Olmo, G.; Alonso, M.M.; Aranda, M.A.G. Quantitative determination of phases in the alkali activation of fly ash. Part I. Potential ash reactivity. Fuel 2006, 85, 625–634. [Google Scholar] [CrossRef]
- Davidovits, J. Chemistry of geopolymeric system. In Terminology, Geopolymere ’99, Geopolymer International Conference Proceedings; Davidovits, J., Davidovits, R., James, C., Eds.; Institute Geopolymere: Saint Quentin, France, 1999; pp. 9–39. [Google Scholar]
- Hong, S.-Y. Alkali Sorption by C-S-H and C-A-S-H gels: Part II. Role of Alumina. Cem. Concr. Res. 2002, 32, 1101–1111. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium Silicate-Based, Alkali-Activated Slag Mortars: Part I. Strength, Hydration and Microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Giorgetti, M.; Berrettoni, M.; Aquilanti, G.; Boldrini, G.; Lancellotti, I.; Leonelli, C. The coordination core and charge of chromium in Metakaolin-geopolymers as revealed by X-Ray absorption spectroscopy. Mater. Lett. 2020, 270, 127741. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H.; Fernandez-Pereira, C. Coal fly ash-slag-based geopolymers: Microstructure and metal leaching. J. Hazard. Mater. 2009, 166, 561–566. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.; Feng, D.; van Deventer, J.S.J. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vazquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesized from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, J.; Yliniemi, J.; Illikainen, M.; Kuokkanen, T.; Lassi, U. Stabilization/solidification of fly ash from fluidized bed combustion of recovered fuel and biofuel using alkali activation and cement addition. J. Environ. Chem. Eng. 2016, 4, 1759–1768. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic polymers from alkali activation of metakaolin: Effect of setting and curing on structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; de la Torre, A.G.; Palomo, A.; Lopez-Olmo, G.; Alonso, M.M.; Aranda, M.A.G. Quantitative determination of phases in the alkaline activation of fly ash. Part II: Degree of reaction. Fuel 2006, 85, 1960–1969. [Google Scholar] [CrossRef]
- Bobirică, C.; Shim, J.-H.; Park, J.Y. Leaching behavior of fly ash-waste glass and fly ash-slag-waste glass-based geopolymers. Ceram. Int. 2018, 44, 5886–5893. [Google Scholar] [CrossRef]
- US EPA Test Method 1311-TCLP; Toxicity Characteristic Leaching Procedure; United States Environmental Protection Agency: Washington, DC, USA, 1992.










| Oxides (wt.%) | Slag A | St.Dev | Slag R | St.Dev |
|---|---|---|---|---|
| (a) | ||||
| SiO2 | 21.05 | 0.02 | 13.80 | 0.15 |
| Al2O3 | 8.54 | 0.09 | 5.25 | 0.07 |
| Fe2O3 | 11.37 | 0.08 | 4.69 | 0.07 |
| CaO | 20.87 | 0.26 | 28.10 | 0.34 |
| MgO | 14.88 | 0.32 | 23.44 | 0.27 |
| Na2O | 0.13 | 0.01 | 0.30 | 0.03 |
| K2O | 0.18 | 0.01 | 0.15 | 0.01 |
| Cr2O3 | 3.76 | 0.02 | 0.19 | 0.01 |
| MnO | 2.24 | 0.03 | 0.62 | 0.01 |
| LOI | 14.15 | 0.01 | 20.47 | 0.01 |
| OTH | 1.3 | / | 2.1 | / |
| (b) | ||||
| P2O5 | 0.122 | 0.009 | 0.08 | 0.01 |
| SO3 | 0.23 | 0.02 | 1.229 | 0.006 |
| TiO2 | 0.40 | 0.02 | 0.190 | 0.003 |
| V2O5 | 0.0734 | 0.0002 | 0.032 | 0.003 |
| Co3O4 | 0.010 | 0.002 | <L.Q. | / |
| NiO | 0.1017 | 0.0006 | 0.026 | 0.005 |
| CuO | 0.026 | 0.001 | 0.010 | 0.001 |
| ZnO | 0.083 | 0.002 | 0.2 | 0.3 |
| As2O3 | 0.03 | 0.01 | 0.029 | 0.002 |
| SrO | 0.0297 | 0.0005 | 0.029 | 0.0001 |
| BaO | 0.03 | 0.01 | 0.024 | 0.009 |
| PbO | 0.017 | 0.003 | 0.008 | 0.003 |
| Mineralogical Phase | Slag A | Slag R |
|---|---|---|
| Quartz 00-046-1045 SiO2 | 6.5 | 12.9 |
| Wuestite FeO | 0.7 | 0.1 |
| Dolomite MgCa(CO3)2 | 8.6 | 19.4 |
| Chromite Cr2O3 | 6.8 | 0.1 |
| Calcite CaCO3 | 7.2 | 13.2 |
| Ankerite Ca(fe,Mg,Mn)(CO3)2 | 0.3 | 2.1 |
| Corundum Al2O3 | 1.5 | 1.2 |
| Merwinite Ca3Mg(SiO4)2 | 8.8 | 4.5 |
| Periclase MgO | 3.1 | 6.9 |
| Gehlenite Ca2Al(AlSi)O7 | 0.9 | / |
| Mayenite Ca12Al14O33 | / | 0.4 |
| Larnite Ca2SiO4 | / | 3.9 |
| Brucite Mg(OH)2 | / | 0.2 |
| Amorphous | 55.6 | 35.0 |
| Heavy Metals (mg/L) | Slag A | Slag R | Law Limit |
|---|---|---|---|
| As | <L.Q. | <L.Q. | 0.2 |
| Ba | 1.13 ± 0.34 | 1.1 ± 0.33 | 10 |
| Cd | <L.Q. | <L.Q. | 0.1 |
| Cr | <L.Q. | <L.Q. | 1 |
| Cu | 0.26 ± 0.08 | <L.Q. | 5 |
| Hg | <L.Q. | <L.Q. | 0.02 |
| Ni | <L.Q. | <L.Q. | 1 |
| Pb | <L.Q. | <L.Q. | 1 |
| Zn | 0.92 ± 0.28 | 0.59 ± 0.18 | 5 |
| Element (mg/L) | Slag A | Slag R |
|---|---|---|
| Al | 127 ± 38 | 78 ± 23 |
| Si | 236±71 | 142 ± 43 |
| Si/Al | 2.08 | 1.98 |
| As | 0.069 ± 0.021 | 0.104 ± 0.031 |
| Ba | 1.612 ± 0.484 | 0.573 ± 0.172 |
| Cd | <L.Q. | <L.Q. |
| Cr | 0.021 ± 0.007 | 0.034 ± 0.01 |
| Cu | 0.145 ± 0.044 | 0.117 ± 0.035 |
| Ni | 0.044 ± 0.013 | 0.011 ± 0.003 |
| Pb | 0.418 ± 0.125 | 0.165 ± 0.049 |
| Zn | 4.0 ± 1.2 | 10 ± 3 |
| Mo | 0.487 ± 0.146 | 0.104 ± 0.031 |
| Sb | 0.020 ± 0.006 | 0.012 ± 0.004 |
| Heavy Metals (mg/L) | A50NW | R50NW | Law Limit |
|---|---|---|---|
| As | 0.097 ± 0.029 | 0.163 ± 0.049 | 0.2 |
| Ba | 0.017 ± 0.005 | 0.018 ± 0.005 | 10 |
| Cd | <L.Q. | <L.Q. | 0.1 |
| Cr | 1.12 ± 0.336 | 0.196 ± 0.059 | 1 |
| Cu | 0.06 ± 0.018 | 0.04 ± 0.012 | 5 |
| Ni | 0.02 ± 0.006 | 0.061 ± 0.018 | 1 |
| Pb | 0.013 ± 0.004 | <L.Q. | 1 |
| Zn | 0.079 ± 0.024 | 0.075 ± 0.023 | 5 |
| Leaching in Distilled Water | A50NW | R50NW | Law Limit |
|---|---|---|---|
| Mo | 1.477 ± 0.443 | 1.004 ± 0.301 | 1 |
| Sb | 0.018 ± 0.005 | 0.021 ± 0.006 | 0.07 |
| As | 0.097 ± 0.029 | 0.163 ± 0.049 | 0.2 |
| Leaching in NaOH | Slag A | Slag R | Law Limit |
| Mo | 0.487 ± 0.146 | 0.104 ± 0.031 | 1 |
| Sb | 0.020 ± 0.006 | 0.012 ± 0.004 | 0.07 |
| As | 0.069 ± 0.021 | 0.104 ± 0.031 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lancellotti, I.; Piccolo, F.; Traven, K.; Češnovar, M.; Ducman, V.; Leonelli, C. Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment. Materials 2021, 14, 639. https://doi.org/10.3390/ma14030639
Lancellotti I, Piccolo F, Traven K, Češnovar M, Ducman V, Leonelli C. Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment. Materials. 2021; 14(3):639. https://doi.org/10.3390/ma14030639
Chicago/Turabian StyleLancellotti, Isabella, Federica Piccolo, Katja Traven, Mark Češnovar, Vilma Ducman, and Cristina Leonelli. 2021. "Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment" Materials 14, no. 3: 639. https://doi.org/10.3390/ma14030639
APA StyleLancellotti, I., Piccolo, F., Traven, K., Češnovar, M., Ducman, V., & Leonelli, C. (2021). Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment. Materials, 14(3), 639. https://doi.org/10.3390/ma14030639










