1. Introduction
The inspiring principles of sustainability are (i) balanced and lasting economic growth, (ii) social progress and improvement of the quality of life, and (iii) protection and enhancement of the environment.
During the twentieth century, in the world, the use of fossil fuels has grown 12 times, and the extraction of natural resources has grown 34 times [
1]. To preserve our planet Earth, help comes from the new circular economy approach. Within it, “the biological and technical nutrients” contribute to maintain the value of products, materials, and resources for the longest possible time, by returning them to the product cycle at the end of their use, thus minimizing the waste generation.
The new circular-economy action plan, in fact, illustrates new initiatives that affect all the product cycles, to modernize and transform our economy, safeguarding the environment. The plan has the ambition to do the following:
Contribute to the transition towards a regenerative growth model that gives the planet more than it takes, working towards maintaining the consumption of resources within the limits of the planet;
Double the use of circular materials in the next decade, thus reducing the footprint of European consumption;
Lead to a GDP growth of 0.5% by 2030, in addition to creating approximately 700,000 new jobs;
Strengthen the EU industrial base and encourage business creation and entrepreneurship among SMEs.
Since up to 80% of the environmental impact of the products is determined in the design phase, it is essential to transfer from the linear pattern of “take–make–use–dispose” to the circularity of production and the principles of sustainability:
Longer-lasting, reusable, upgradeable, and repairable products;
Greater quantity of recycled material in products;
High-quality remanufacturing and recycling;
Reduction of the environmental footprint;
Constraints on disposable products and premature obsolescence;
Prohibition of destruction of unsold durable goods;
Promotion of the “product as a service” model;
Digitization of product information;
Reward system based on product sustainability.
Therefore, from the point of view of materials, a circular economy should be achieved to improve efficiency in the use of resources and to prevent, or at least reduce, the negative impact linked to the generation and management of waste. Such improvement and innovation could be obtained through the recycling, as well as the reuse, of production and processing waste.
These actions are considered effective to reduce Europe’s dependence on the import of materials and to improve the overall environment and well-being of citizens. The actions and the increased awareness of citizens are driving cultural and economic changes, especially regarding recycled products. Indeed, recycled products are no longer perceived as “class B” assets of lower performance, but rather products of innovation and research, which the economy needs; moreover, they are preferred by most consumers culturally evolved. The European Commission’s circular economy action plan (11 March 2020) establishes the new roadmap until 2022, with strategic and cross-cutting actions to implement the circular economy. From this emerge the following:
The primary role of recycling, with expansion of the market for goods with recycled content;
Green Public Procurement, the driving factor to increase the demand for sustainable products (mandatory requirement for products with recycled content);
Avoid “facade ecology” and admit only certifications, with requirements of independence and objectivity.
The objectives described are pursued through both product and process sustainability, which can be assessed throughout the entire life cycle: raw material acquisition, production, distribution, use, disposal, or recycling (for a product); research and development, design, implementation, operation, dismantling, and site remediation (for a process).
In this research, we focused our attention particularly on some specific aspects: use of residues (in particular of the agro sector, post-consumer, and packaging glass cullet) mixed to a local raw material (red clay, km 0 concept). Such materials were hot-consolidated, comparing three different manufacturing technologies: (i) manual pelletization and flash heating in furnace, (ii) sintering of pressed powders, and (iii) impregnating open-cell natural marine sponges plus traditional firing in furnace. A porous clay ceramic which belongs to clay ceramics materials, a class of materials with a solid market and a significant environmental impact from their manufacture to their distribution, was chosen as the final product. As a matter of fact, some works have been just published by the authors on lightweight ceramic aggregates [
2,
3,
4] which belong to the aggregate materials and represent the second most consumed material after water [
5], equal to 2282 million tons in Europe in 2016 [
6]. The aggregate family is the main supplier of raw materials for the construction of infrastructures and buildings, as well as for industry and environmental protection, which confers to it a clearly strategic character [
5]. Based on the final structure and properties of aggregates, their use has been extended to different applications, ranging from light concrete and green roof to gardening (i.e., substrates in horticulture and hydroponic crops), or others, such as geotechnical applications, sidewalks, or filter media.
Commercial aggregates are produced by drying and sintering of pelletized clay or shale, exploiting the raw materials capability of being “bloated”. This phenomenon is caused by the gas release of the organic or mineral matter contained inside the abovementioned natural raw material during heating. A well-known commercial example is represented by LECA (Light Expanded Clay Aggregate), produced starting from natural clay, containing no harmful substances or organic materials, and certified by the Italian ANAB (National Association for Green Building Architecture)–ICEA (Ethical and Environmental Certification Institute), certification of bio building products according to UNI EN ISO 14,024 [
7] type I environmental labeling. By subjecting the clay to an appropriate firing cycle, it swells at 1000–1200 °C due to the action of gases generated within the mass [
8,
9] that cannot escape from the body (organic components of the clay, water vapor, and steam development). The result of the thermal expansion associated to a vitrification (clinkerization) process at temperatures higher than 1200 °C [
10] produces an internal light cellular structure and an external compact and resistant shell, and the whole offers an excellent weight/resistance ratio. The lightness of this product is due to the high percentage (up to 90% of the volume of the particles) of semi-closed porosity.
Most lightweight clay ceramics are obtained by extrusion [
11,
12] or agglomeration [
13,
14] by mixing the raw materials with a suitable amount of water in order to obtain a good plasticity for the forming.
In this study, three different manufacturing technologies, two of which are not usually applied for this type of products, were used and compared, namely pressing, pelletization, and “shell scaffold”.
The manufacturing process of lightweight aggregates (LWAs) has the same steps as the structural ceramics, with the substantial difference in the shaping processes, which occur in a pelletizer. Pelletization is a method of agglomeration that permits the particle-size enlargement, in which ceramic powders are processed into pellets or granules of dimensions between 0.5 and 2.0 mm [
15]. Pelletization is not a typical forming technique of traditional ceramics; it is used in a multitude of industries (pharmaceutical, food, fertilizer, etc.), to process thousands of materials from difficult-to-handle fine powders into easy-to-handle pellets. A specific advantage of producing LWAs via pelletization is that they are not so dense. Therefore, they are strong enough to hold up to handling and to the specific application, due to the sintering of raw materials during firing, but they can still release nutrients as needed. This is especially valuable when working with fertilizer or soil amendment products [
16]. This technique allows many advantages with respect to the manipulation of fine powders: (1) improve handling and application (pellets are easier to feed, due to improved and more consistent flowability) and (2) reduce dust loss [
14].
Pressing is a forming technique widely used in the ceramic field, in which granular ceramic materials are made cohesive through mechanical densification. The mechanical features of the sample obtained after cold-forming (i.e., the “green body”) strongly influence the subsequent sintering process and then the mechanical properties of the final piece [
17]. Traditional ceramics, such as tiles, are pressed uniaxially, starting from powders with moisture content (5–7 wt.%). The pressures used vary according to the type of product; the more sintered ones (porcelain stoneware) require high pressure (in the order of 40–45 MPa), while for porous products (e.g., monoporosa), the applied pressure drops to about 25 MPa [
18]. Uniaxial pressing was applied in the study, as in the most common forming techniques used for traditional ceramics; the chosen pressure was 18 MPa, in order to avoid a too-high compaction level of the powders.
It is worth noting that the third technique was taken from the field of biomaterials and used for the first time in this context. Such an approach is inspired by the concept of translational research and cross-fertilization from other disciplines. Traditionally, scaffolds, i.e., highly porous structures used for bone regeneration, are produced by the classical replica technique, which uses a ceramic slurry to coat a polymeric sponge that is subsequently burned to obtain ceramic foams. A proper thermal treatment permits to burn out the sponge and, at the same time, to make the ceramic powders sinter. Scaffolds obtained by means of this technique show high porosity, which unfortunately results in poor mechanical properties. However, scaffolds should be porous enough and, simultaneously, should have sufficient mechanical strength to be handled. Regrettably, the requirements of porosity and adequate mechanical properties are interlinked and opposed, and this is the reason why the design of scaffolds is very complex but also intriguing. In recent years, an innovative technique for the production of scaffolds has been set up [
19,
20]. This new approach enabled the delivery of the so called “shell scaffolds”, i.e., more resistant structures, retaining the high porosity of the traditional scaffolds [
19,
20,
21,
22,
23,
24]. In fact, the produced samples were characterized by a compact and permeable surface (the shell), which provides both high permeability to fluids and mechanical support. Such a shell surrounds a highly porous internal network. For these reasons, the new protocol goes beyond the limits of the traditional replica method.
The “shell scaffold” technique was adapted here, for the first time, to produce lightweight clay ceramics as growing media/drainage layers in green roofs or hydroponic crops, by using the red clay and the phosphorus (P) and potassium (K) containing glass (instead of the bioactive glasses used up to now for the production of shell scaffolds for the biomedical field).
The objective of this study was (i) to contribute to the research on porous clay ceramics residues-containing and consolidated by different techniques and (ii) to create prototypes with good weight/resistance ratio, useful as growing media/drainage layers in green roofs or hydroponic crops, in order to generate the following:
- (a)
Porosity: Spent coffee grounds, as pore forming agents, were mixed together with a ferruginous red clay.
- (b)
Nutrient effect: Animal bone meal ash and K2CO3, suppliers of P and K respectively, were added, vitrified by packaging glass cullet.
2. Materials and Methods
2.1. Raw Materials Used
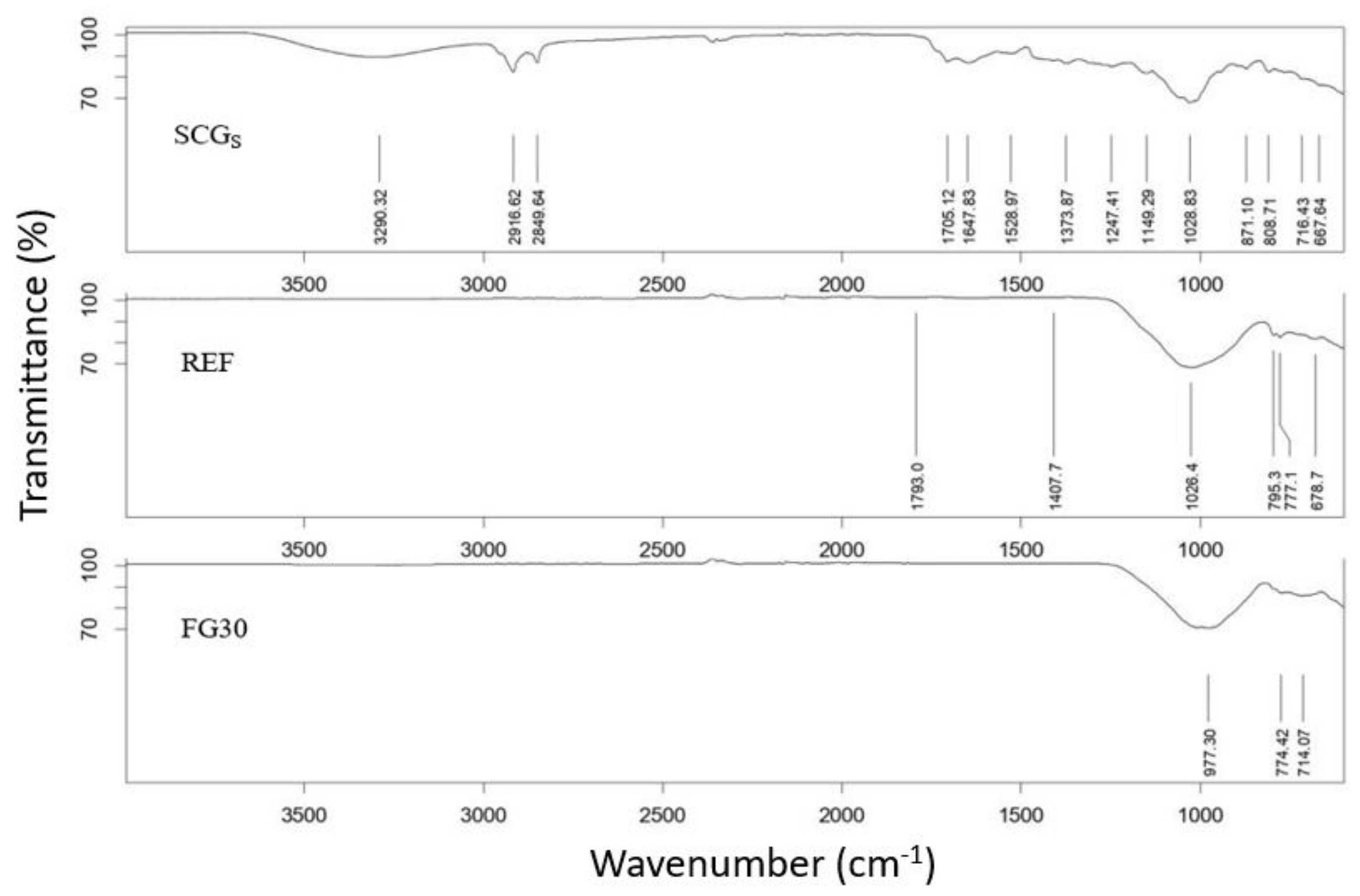
For the preparation of the specimens, a local ferruginous red clay (Zocca, Modena, Italy) and spent coffee grounds (SCGs) collected by the owner of a bar in Modena (Italy) were used. Due to the high humidity content (65% of water), the SCGs were dried by following a procedure optimized in a previous paper [
3]. The use of a pore-forming agent (SCGs) was necessary only in the materials prepared by manual pelletization and pressing, while for those prepared by shell scaffold, due to the use of a sacrificial skeleton that burned during firing, it was not necessary.
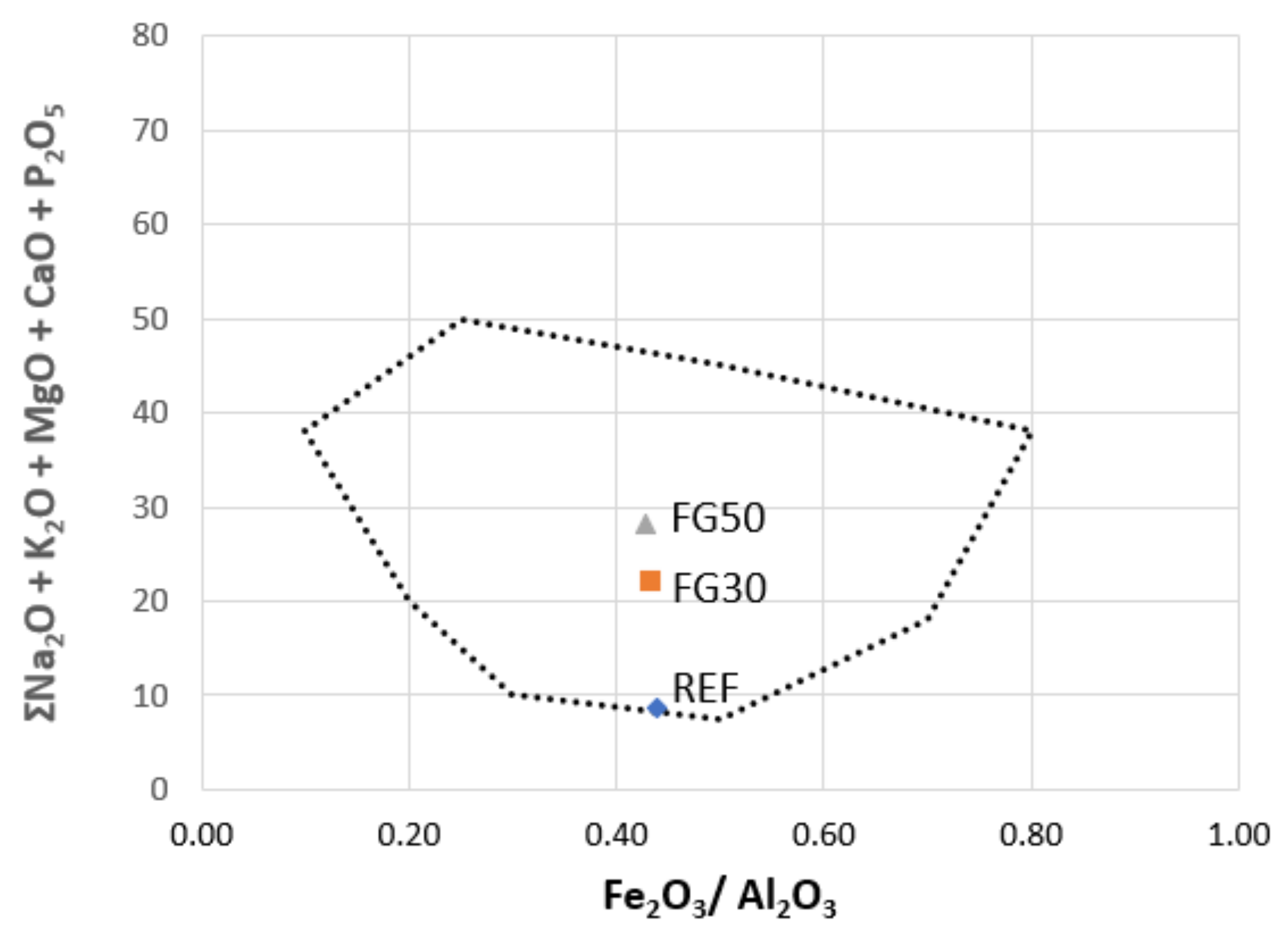
The porous clay ceramics were functionalized by phosphorus and potassium, as nutrient elements, added as vitrified by a so-called fertilizer glass (FG). The glass composition was properly designed by the authors, to achieve a controlled release, over time, of P and K [
2,
25]. A mix of glassy sand (a commercial product obtained by the second treatment of the fraction of packaging glass cullet not destined for glasswork), cattle bone flour ash (CBA) (i.e., phosphorous (P) intake), and potassium carbonate (i.e., potassium (K) intake) was melted at 1450 °C for 2 h. Subsequently, the clay and SCGs were ground and sieved (particle size < 1 mm), while fertilized glass was milled in an agate jar and sieved (particle size < 100 µm). The other raw materials did not need grinding and sieving before use.
All samples, described below, were codified by indicating the manufacturing technique used, i.e., pelletization (PE), pressing (PR), and scaffold shell (SS), followed by (a) REF for the starting matrix of 85 wt.% of clay and 15 wt.% of SCGs or (b) FG for the matrix added, with fertilizer glass indicating also the wt.% of this last (30, 50% with respect to clay plus SCGs). No SCGs were used in the case of shell scaffolds (see the
Section 2.4 for details).
2.2. Manual Pelletization
At a ceramic mixture of 85 wt.% of clay and 15 wt.% of SCGs, 30 or 50 wt.% (with respect to clay + SCGs) of fertilizer glass was added (see
Table 1). The powders were homogenized for 10 min by a slow ball mill and then moistened with proper water content (20–30% wt.%), to obtain the adequate plasticity needed for shaping. Thick cylinders (about 1 cm diameter and about 40 cm long) were manually formed from the well-blended paste; subsequently, they were cut into small cylinders, all of the same size. By hand-working the cylinders, small spheres of the same shape and size were made (1.5–2.0 g of weight and 1–1.5 cm of diameter). To avoid the presence of cracks on the external surface, a surface-smoothing operation was carried out: spraying distilled water on the surface and gently smoothing until the external surface was completely smooth and uniform. Then, samples were dried at 105 ± 5 °C (in a stove), to remove the free water from the body, to avoid cracking that can eventually occur during firing at 1000 °C, which took place in a static furnace (Lenton AWF13/12), as previously reported [
2]. Once the set temperature of 1000 °C was reached, the samples (in refractory crucibles) were fired for 1 h. Using this flash-firing process, the samples underwent thermal shock similar to that occurring during the industrial process at around 1300 °C. Then, samples were left to cool at room temperature for 24 h.
2.3. Powder Pressing
Two ceramic mixtures were prepared by this technique: the reference, i.e., 85 wt.% of clay and 15 wt.% of SCGs, and the reference added with 30 wt.% of fertilizer glass (
Table 1). Each batch composition was prepared homogenizing by slow ball mill for 10 min and then moistened with 7 wt.% of water content. The mixes were maintained in a hermetic plastic bag for 1 h, in order to homogenize the humidity, and again sieved below 2 mm, to obtain suitable press-powder. To obtain cylindrical samples (40 mm diameter and 5 mm thickness) from powders, a hydraulic press (Nannetti model S Mignon, Faenza, Italy) at 18 MPa of pressure was used. Subsequently, samples were sintered by using a static furnace (Lenton AWF13/12): (i) room temperature → 500 °C at 5 °C/min; (ii) 500 °C → 1000 °C at 10 °C/min. The final temperature (1000 °C) was kept constant for 1 h, to allow the total burning of the SCGs and the sintering. Afterward, the samples were allowed to cool down at room temperature.
2.4. Scaffolds
The “shell scaffold” technique was adapted to produce samples with red clay and fertilizer glass powder (see
Table 1 for compositions). This procedure represents an innovation with respect to the traditional replica method, where (i) the samples are usually squeezed before drying, in order to remove the exceeding slurry, and (ii) the samples are slowly dried. Instead, in the new protocol, the green bodies were kept fully loaded with the slurry during the retrieving and the drying steps.
A natural marine sponge (Spugnificio Rosenfeld, Muggia (TS), Italy), leftover from the industrial production of commercial marine sponges, was employed as a sacrificial template for the scaffolds. In fact, the pore size can be tailored by choosing different skeletons (i.e., sponges) for the process. The clay and fertilizer glass powders were dispersed in distilled water, to obtain the slurry. A polyvinylic binder was added to obtain a slurry with controlled viscosity and to promote the adhesion of powders to the sponge during immersion. Various weight ratios between distilled water, fertilizer glass and clay powders, and binder were examined, to achieve the optimization of slurry and of the technique. After some tests, the slurry was designed according to the following weight composition: 50 wt.% distilled water, 6 wt.% polyvinyl alcohol (PVA), 30.8 wt.% red clay, and 13.2 wt.% of glass.
The slurry was mixed under magnetic stirring. 50 wt.% distilled water and 6 wt.% polyvinyl alcohol were carefully mixed in a beaker, under vigorous stirring until PVA was completely dissolved. Then, 30.8 wt.% of red clay (powders diameter < 1 mm, Argilla Samone 1) and 13.2 wt.% of the fertilizer glass (powders diameter < 100 μm) were carefully added to the distilled water and PVA slurry under stirring and mixed until the obtainment of homogenous slurries. According to
Table 1, these samples were named as SS FG30 (the fertilizer glass was 30 wt.% and the clay 70 wt.% of the total powder, to which water and PVA were added)
Moreover, another composition (50 wt.% distilled water, 6 wt.% polyvinyl alcohol, 22 wt.% red clay and 22 wt.% of fertilizer glass) was prepared with the same procedure and named as SS FG50 (50 wt.% FG and 50 wt.% clay).
Subsequently the marine sponges, cut to the desired shape and dimensions (3 × 3 × 5 cm3), were manually immersed in the slurry and then extracted from the suspension; as mentioned, unlike the classical replica method, they were not squeezed but kept fully loaded with the slurry. The sponges were immediately dried, as quickly as possible, under a multidirectional vigorous air flux at 100 °C for 10 min. Moreover, the impregnated marine sponges were maintained in rotation into the air flux to keep them slurry-loaded and, at the same time, to guarantee an even distribution of the slurry within the sponges themselves. At the end of the drying process, the sponges resulted to be coated by a thin shell made of clay, glass and binder. Then, the impregnation/drying process was repeated in order to obtain an impregnation as much homogeneous as possible and a thicker outer shell. Finally, a post-forming heat treatment was performed to burn out the organic skeleton (i.e., marine sponge) and densify the network of clay/glass powders.
The samples were introduced into the furnace and were heat-treated, from room temperature to 500 °C, with a heating rate of 3 °C/min, and then subsequently up to 1000 °C at 10 °C/min. The final temperature (1000 °C) was kept constant for 2 h, to allow for the burning of the marine sponge and sintering of the composite structure. Subsequently, the highly porous structures were left to cool down at room temperature.
Additionally, for apparent density measurements and microscopy images, small samples (1 × 1 × 1 cm3) were prepared with the same procedure using a cubic sponge.
2.5. Samples Characterization
The spent coffee ground, as received, and the powder ceramic mixtures were analyzed by means of Fourier transform infrared (FTIR) analysis, to evaluate the modifications induced in SCGs after firing: (1) 85 wt.% of clay and 15 wt.% of SCGs; (2) 85 wt.% of clay and 15 wt.% of SCGs, added with 30 wt.% of fertilizer glass (with respect to clay + SCGs) after firing. The measure was conducted by using a Spectrophotometer FTIR VERTEX 70 (Leiderdorp, the Netherlands), detector MCT Mid-Band in a range 8000–600 cm−1. The analysis was performed by Software OPUS version 5.0.
The chemical analysis of raw materials, fertilizer glass, and waste/by products used was performed by X-ray Fluorescence (XRF), carried out by ARL-ADVANT‘XP+ (Thermo Fisher Scientific Inc., Milano, Italy), software Uniquant.
The physical properties for all the sintered materials were determined as follows: water absorption (WA%) capacity by immersion of samples in distilled water for 24 h. Then, the WA% was calculated by the following equation:
where ms is the water-saturated mass after 24 h, and md is the dry mass.
The apparent, ρ
a, and true, ρ
t, densities of all sintered samples were evaluated, and the results were used to determine the total porosity (TP):
where ρ
a was obtained by an envelope density analyzer (GeoPyc 1360, Micromeritics, Wyloway, GA, USA), using a dry medium, while ρ
t was determined by a gas (He) pycnometer (AccyPy1330, Micromeritic, Wyloway, GA, USA).
The chemical properties of the materials were assessed by measuring the pH and electrical conductivity. The pH was measured by using a pHmeter (XS Instruments, pH 6, Carpi (MO), Italy), according to UNI-EN 13037 (2012) standard [
26] and the electrical specific conductivity (ESC) was measured by an Oakton conductimeter (CON6/TDS6 (OAKTON Instruments P.O. Vernon Hills, IL, USA), according to UNI-EN 13038 (2012) standard [
27].
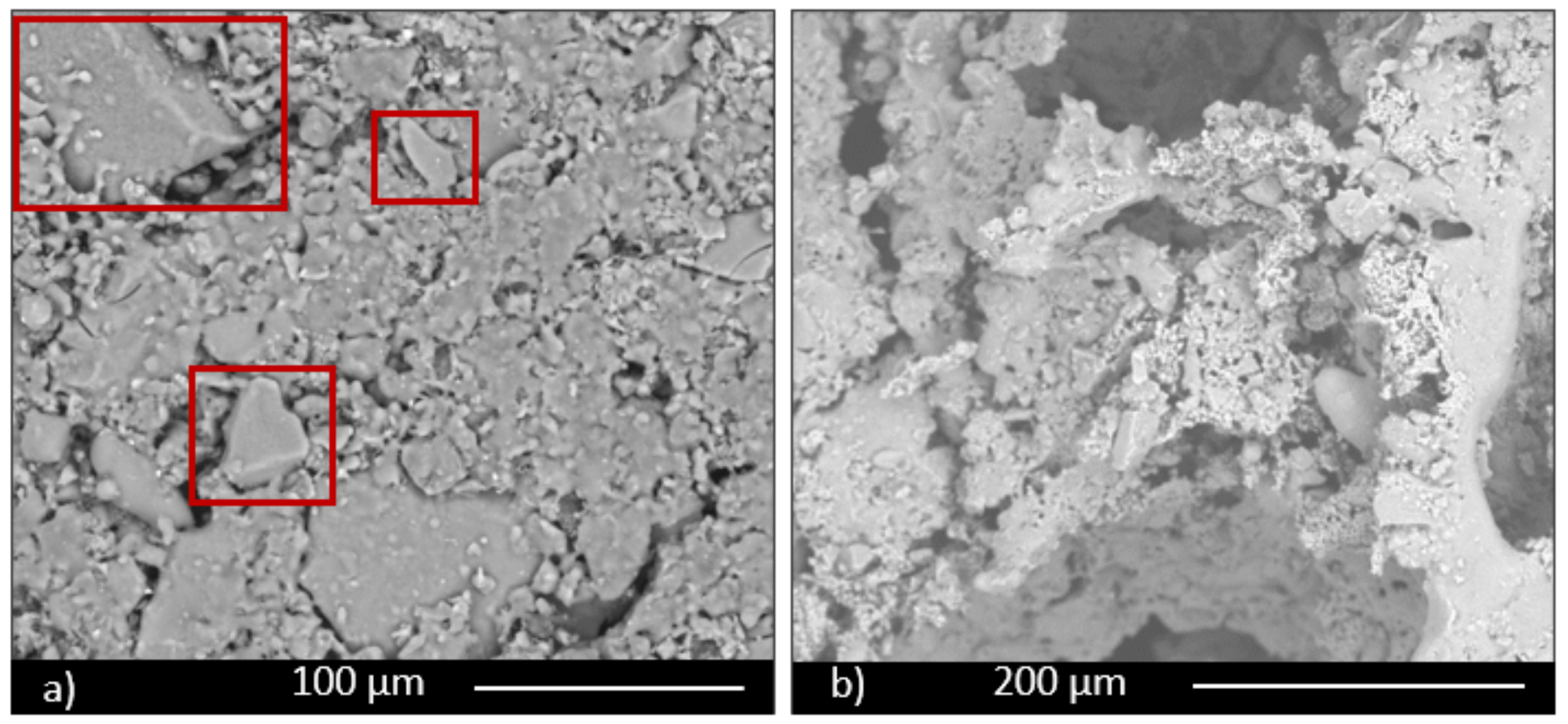
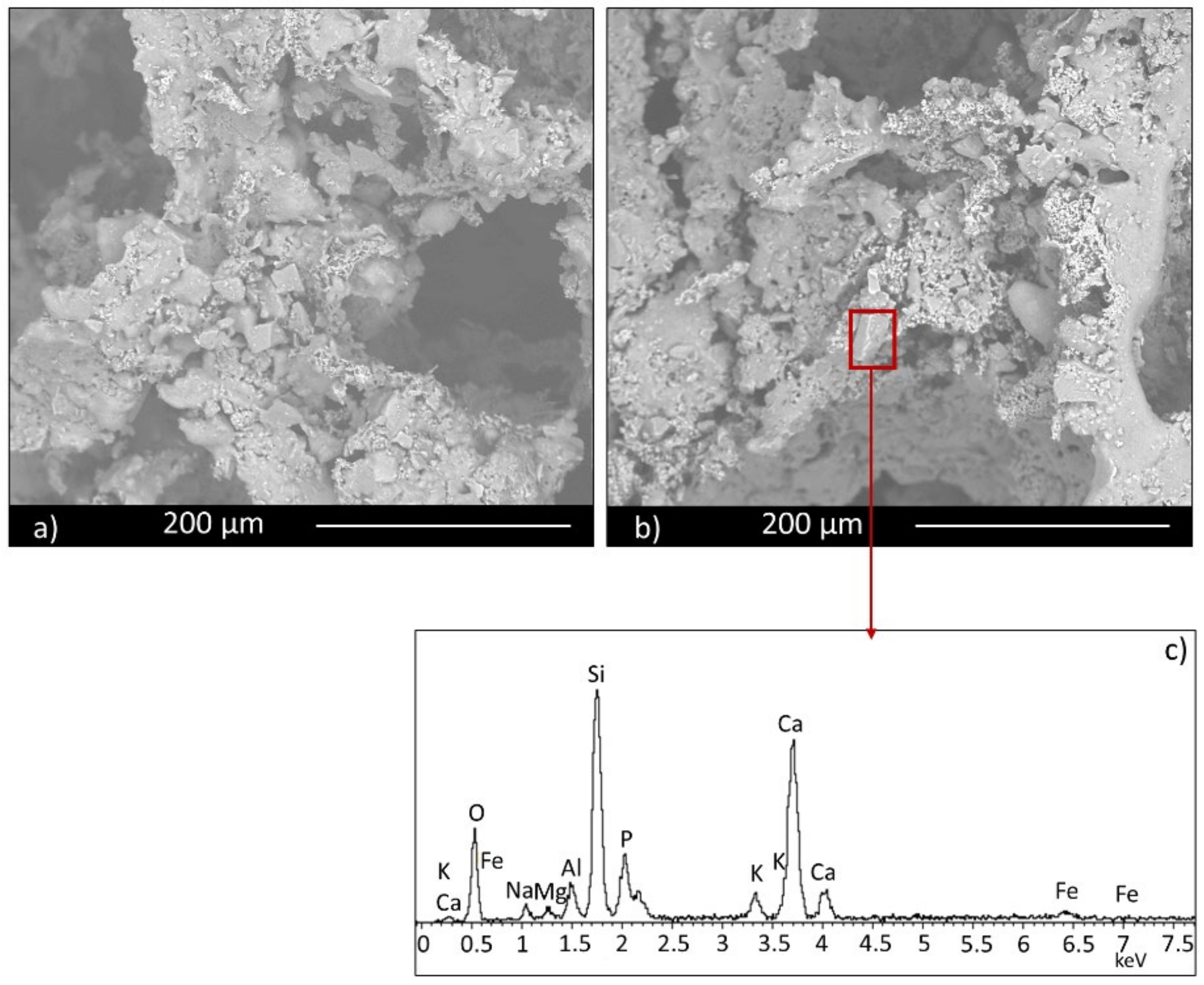
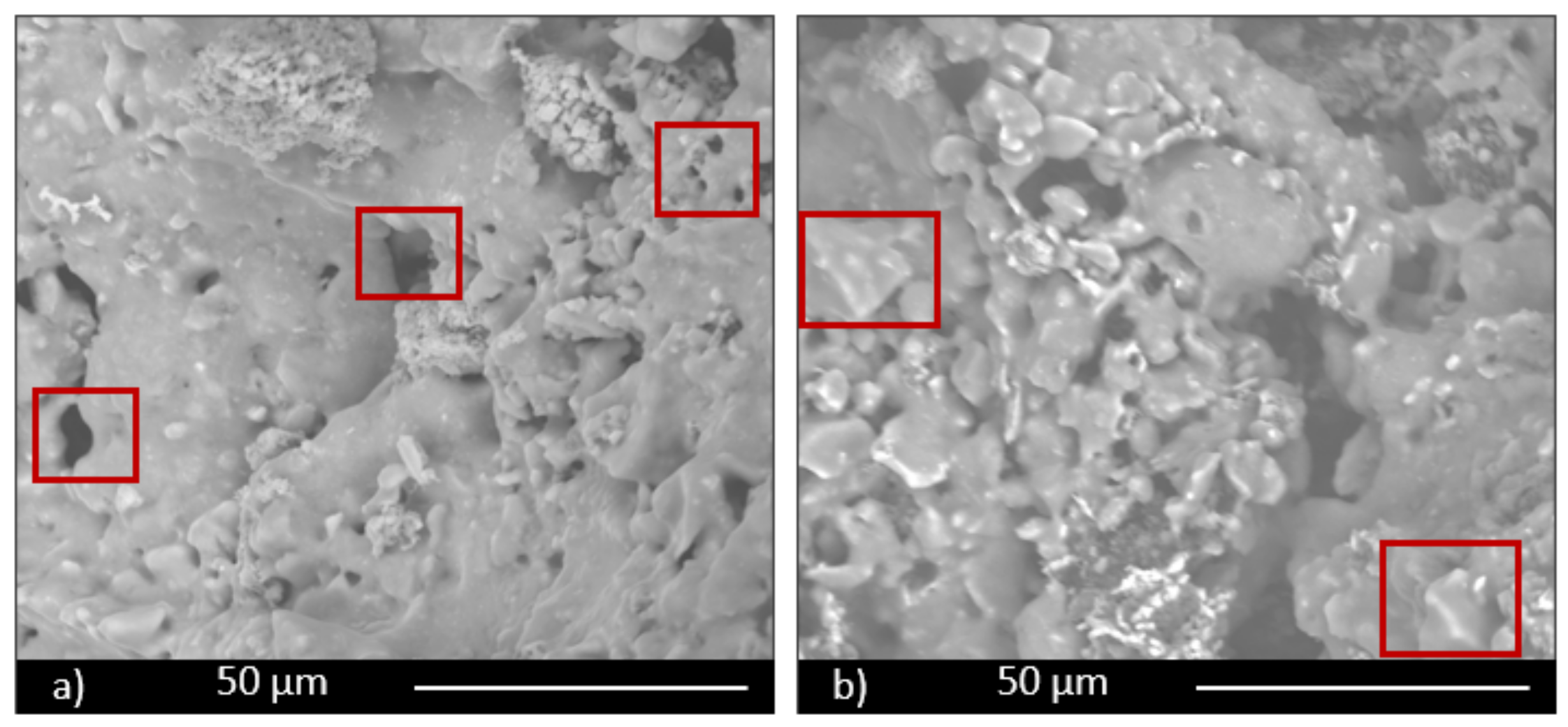
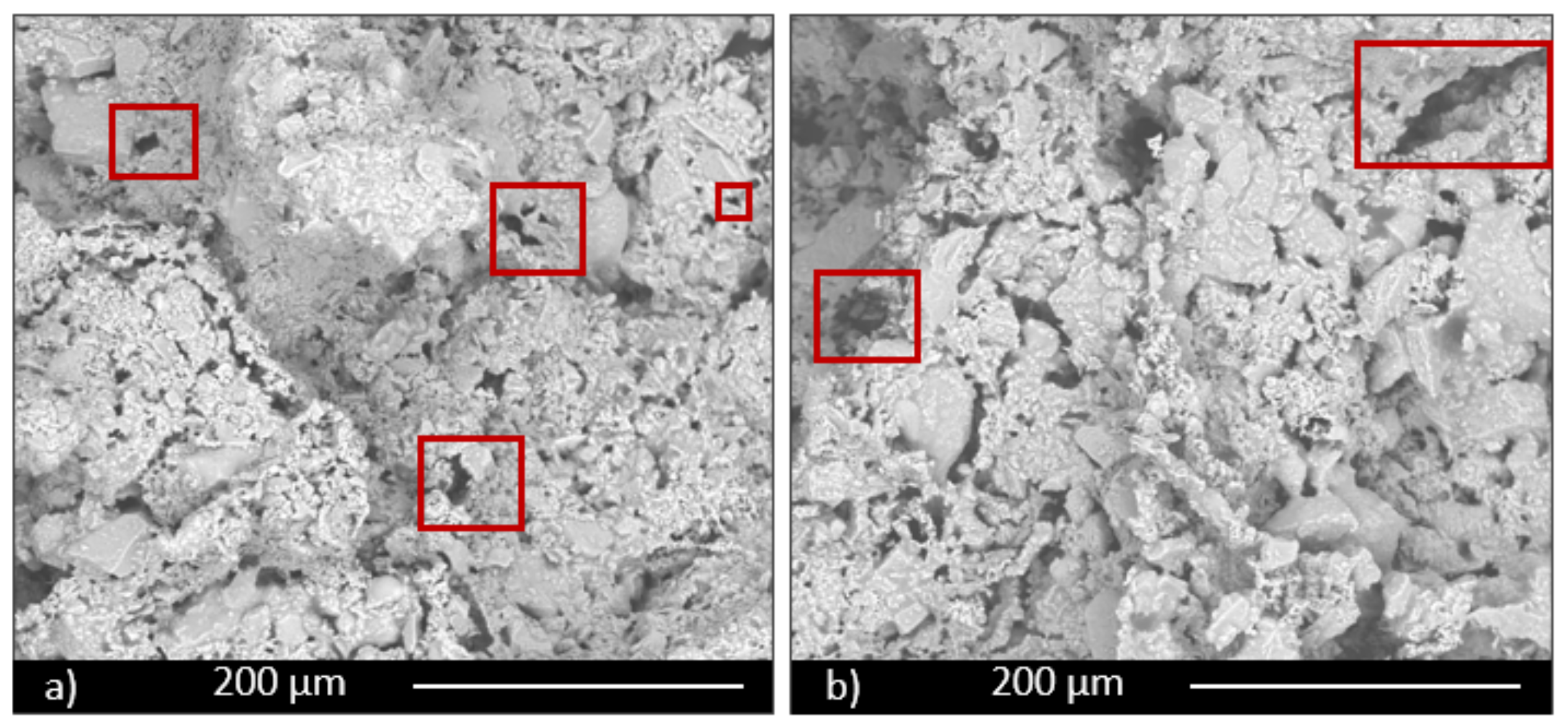
The morphological and microstructural characteristics of samples were investigated by scanning electron microscopy (ESEM, QUANTA200 FEI coupled with X-EDS Oxford INCA-350 for chemical analysis, Eindhoven, the Netherland).
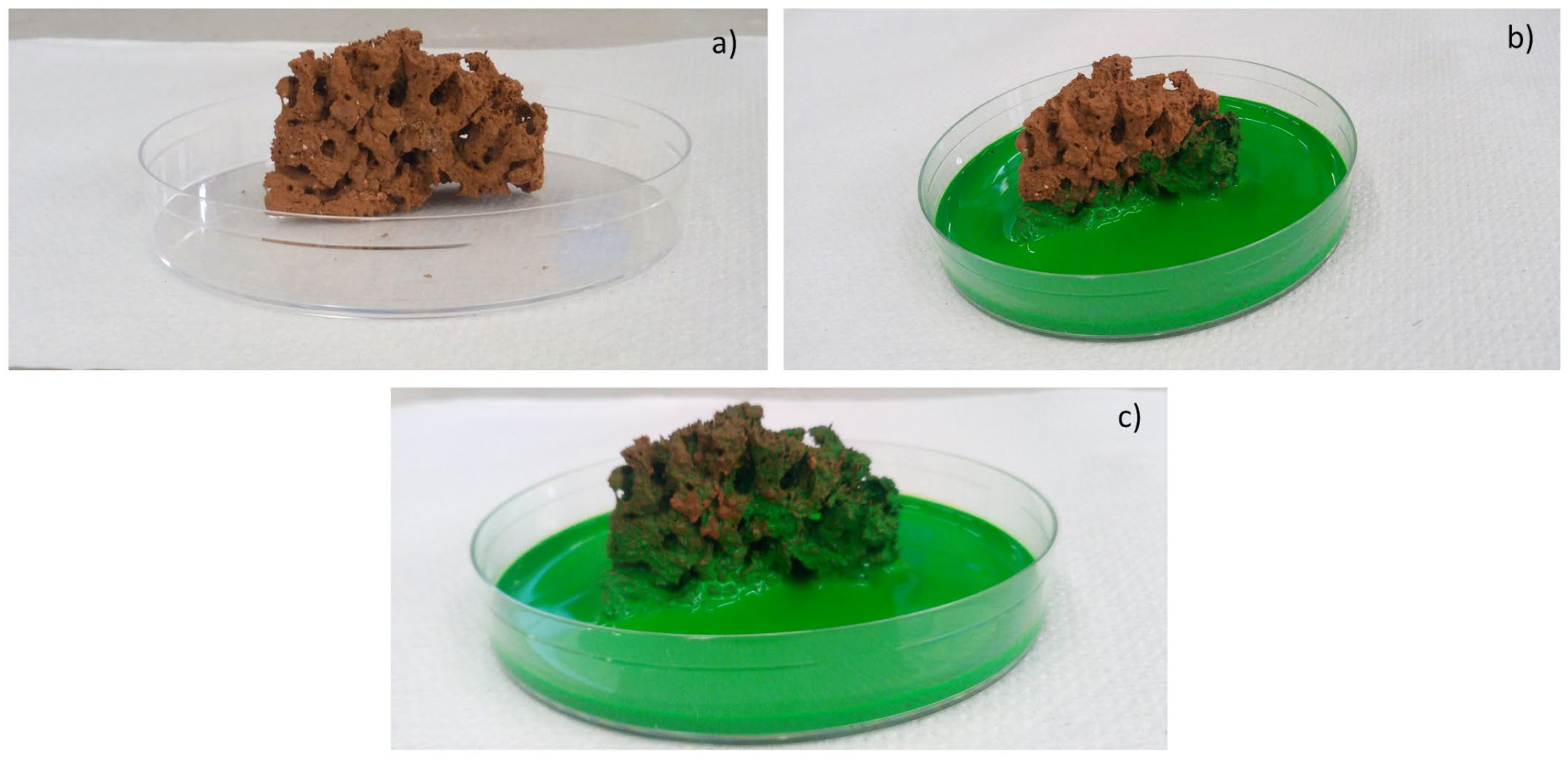
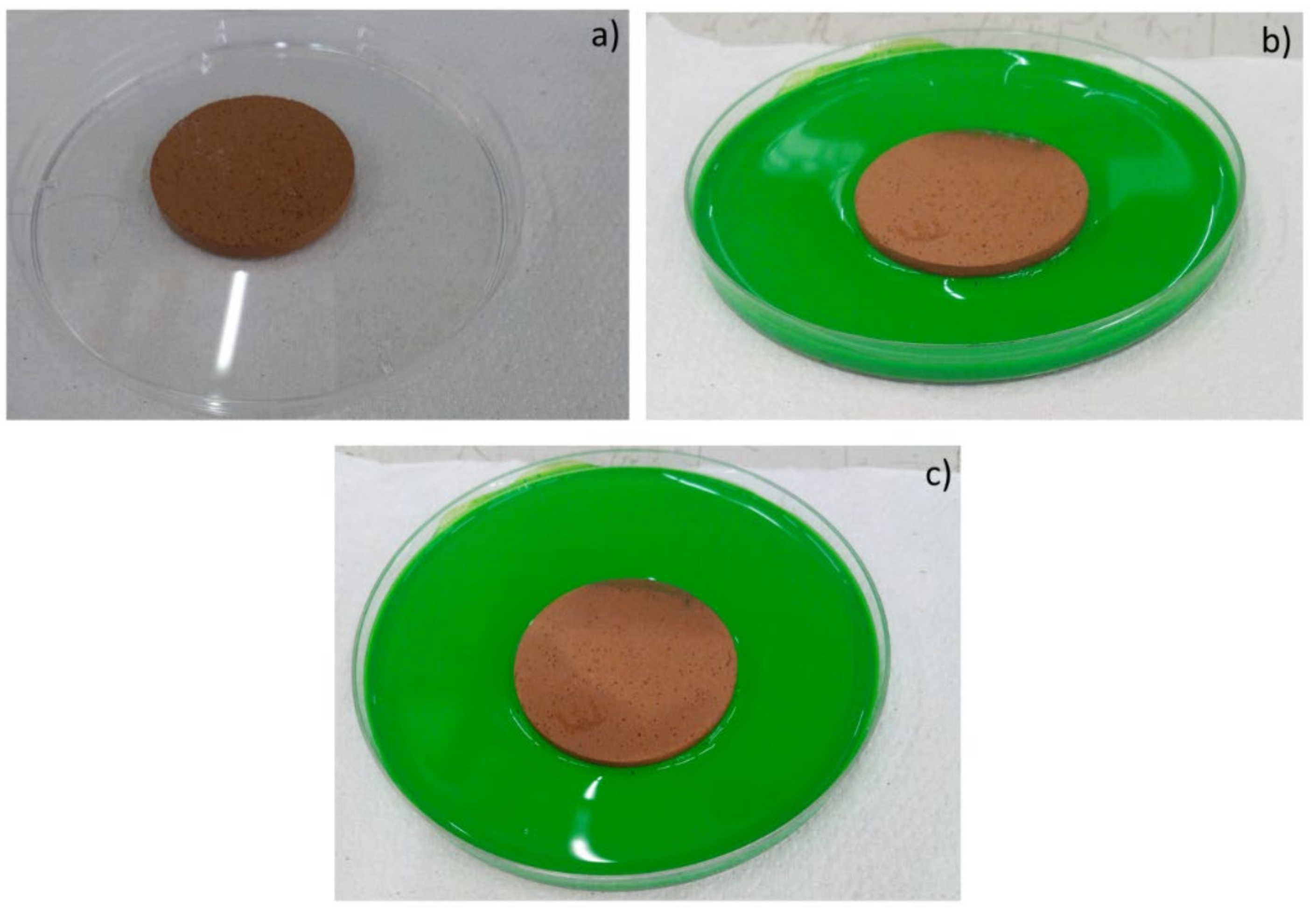
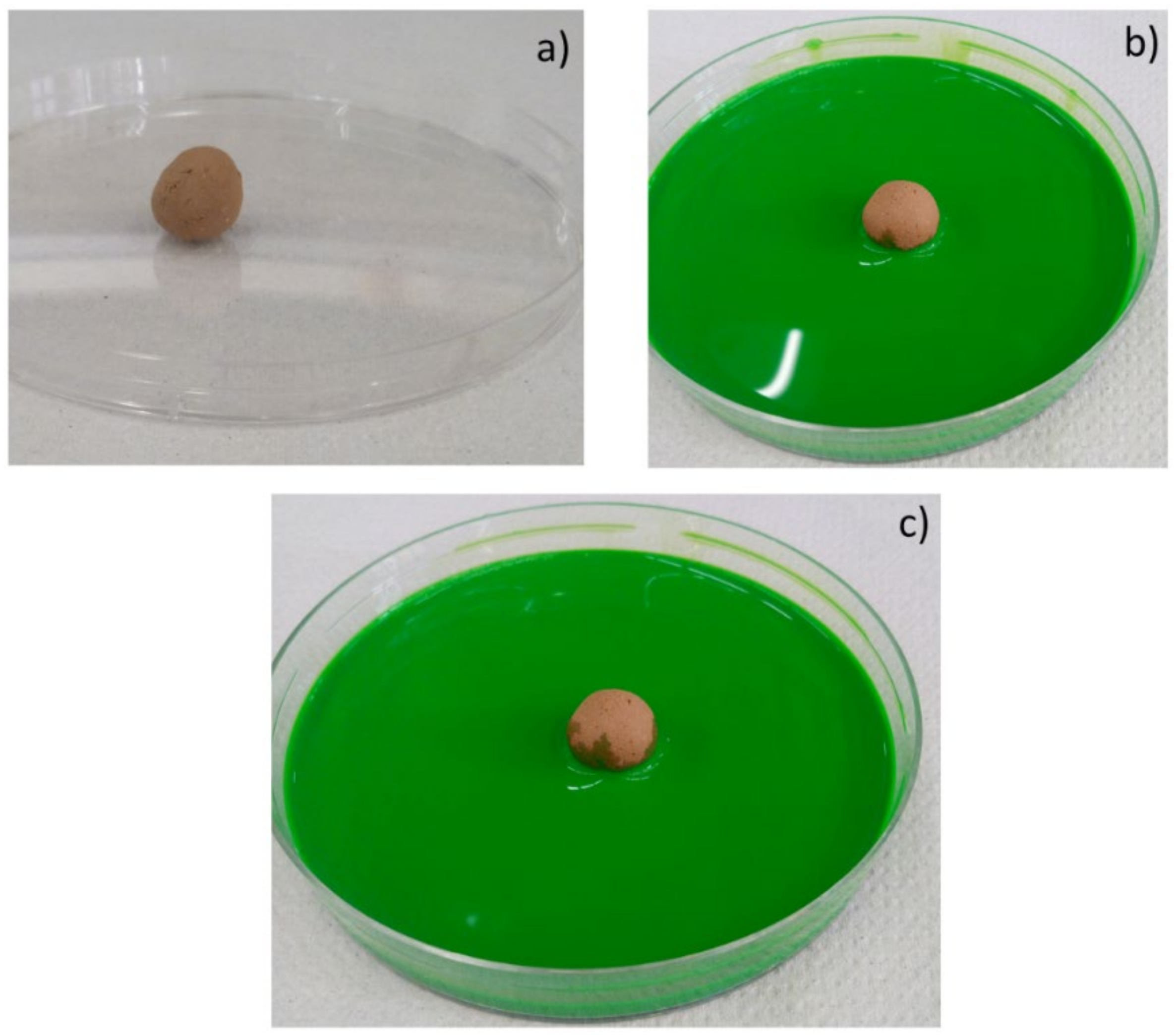
Moreover, the permeability of the scaffolds was qualitatively assessed through capillarity tests, in order to evaluate the porosity obtained with the three manufacturing techniques used. A solution with a viscosity similar to that used in green roofs or in agronomic applications (i.e., water plus nutrients) was employed. Green ink was dispersed into the solution, to easily observe the fluid infiltration within the porous material. A face of the sample was put into contact with the solution, and its infiltration driven by capillarity forces was immediately observed.
4. Conclusions
Three different techniques, namely manual pelletization, powder-pressing, and shell scaffold, were effectively employed to produce sustainable porous clay ceramics. All the manufacturing techniques resulted in being suitable for preparing the samples and thus can be considered feasible routes for the production of residue-based porous ceramics. The physical and chemical properties (such as density, porosity, pH, and electrical conductivity) resulted in being acceptable, with respect to the intended use, as per current regulations. In
Table 4, schematic summary of all the measured properties is proposed.
In details, comparing the different manufacturing techniques, the powder-pressing resulted in being the less suitable for obtaining lightweight materials, as expected, even if the applied pressure was low. On the other hand, pelletization permitted to obtain lightweight materials with good resistance, in order to support handling, storage, and transportation. It is expected that, while inserted into the soil, such materials can also break, to allow the release of nutrients.
Finally, the samples prepared by scaffold-shell technique showed the higher porosity; however, such a feature goes hand in hand with less resistance.
Thus, depending on the specific use, one technique can be preferable as compared to the others.
Future developments of this research will also explore other aspects, such as mechanical properties of the products, particularly the new shell scaffolds.