Particle Image Velocimetry Method for Prediction Hydrodynamic Conditions during Leaching Process on the Basis of Sn–NaOH System
Abstract
1. Introduction
2. Testing Objects and Methodology
3. Results and Discussion
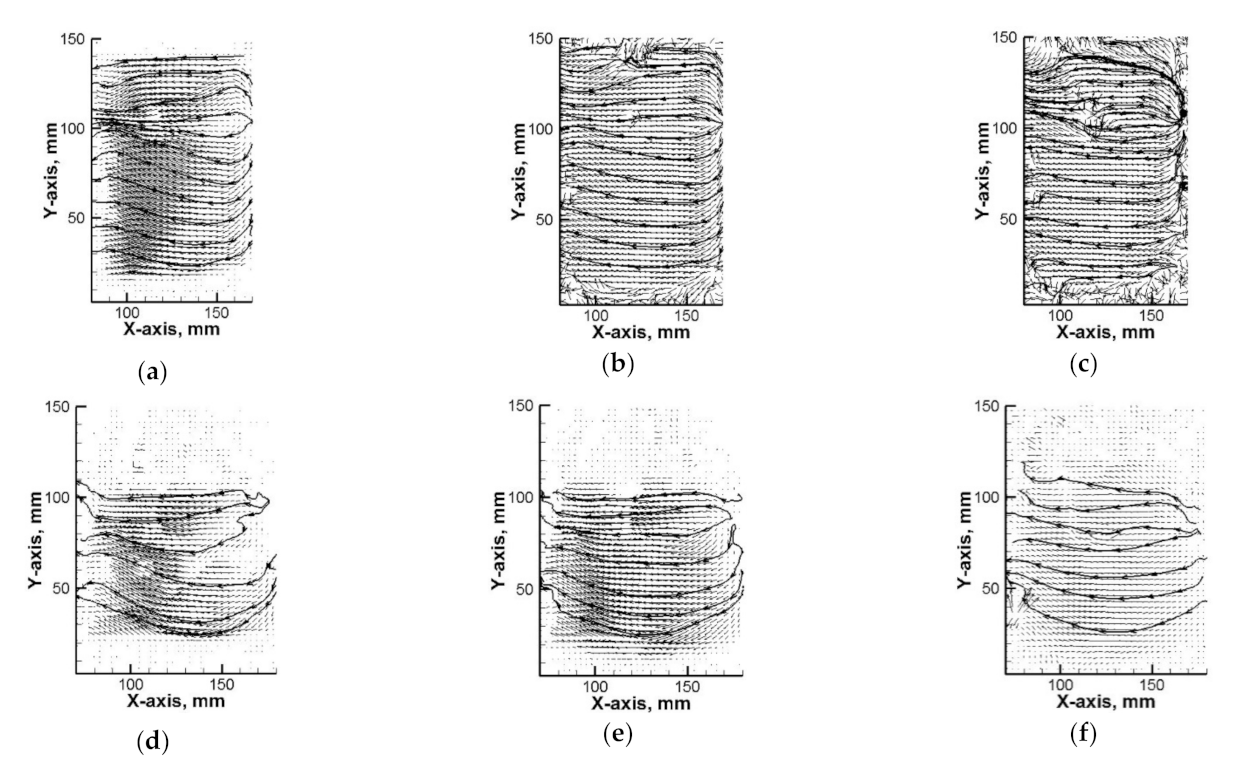
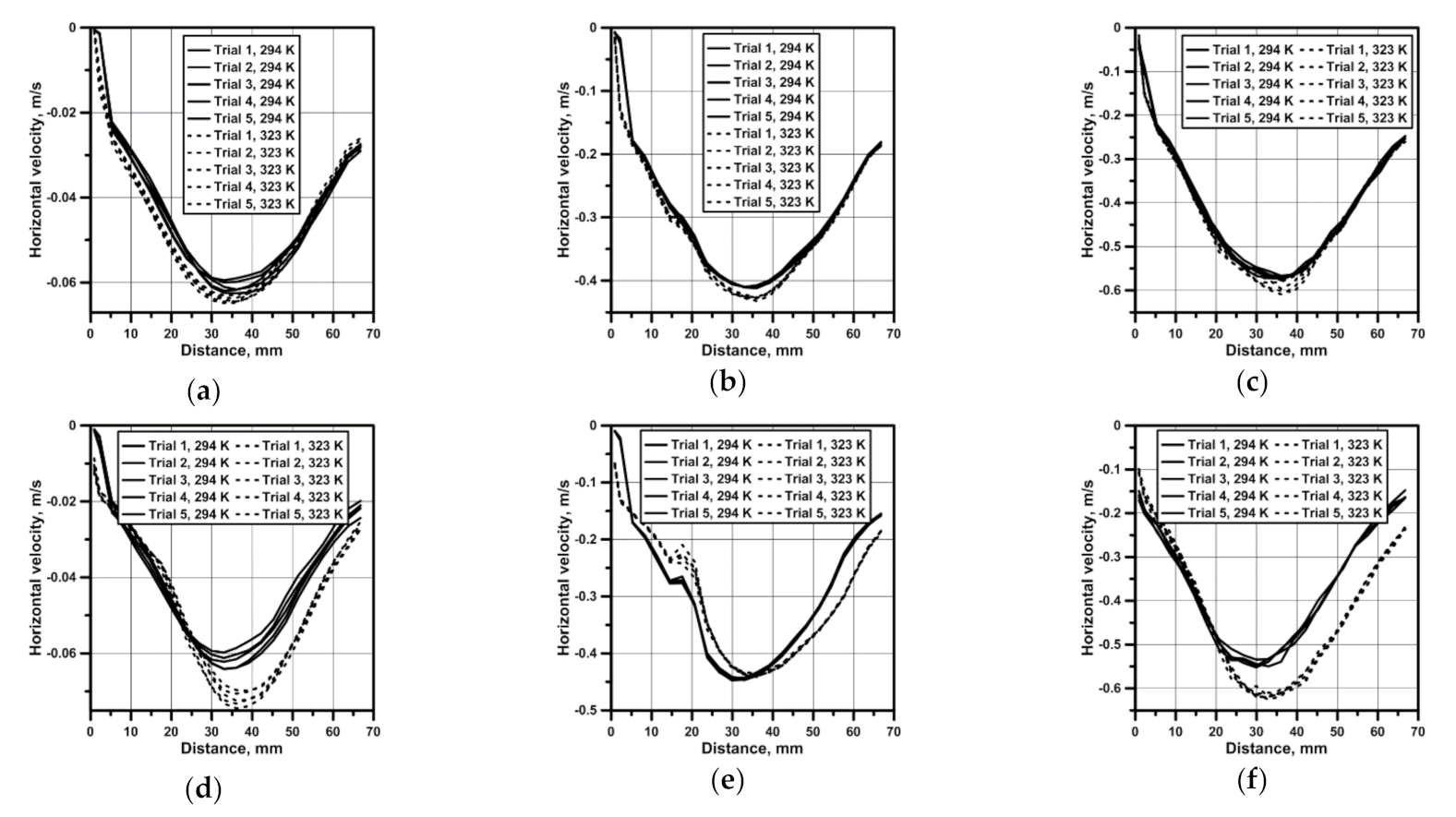
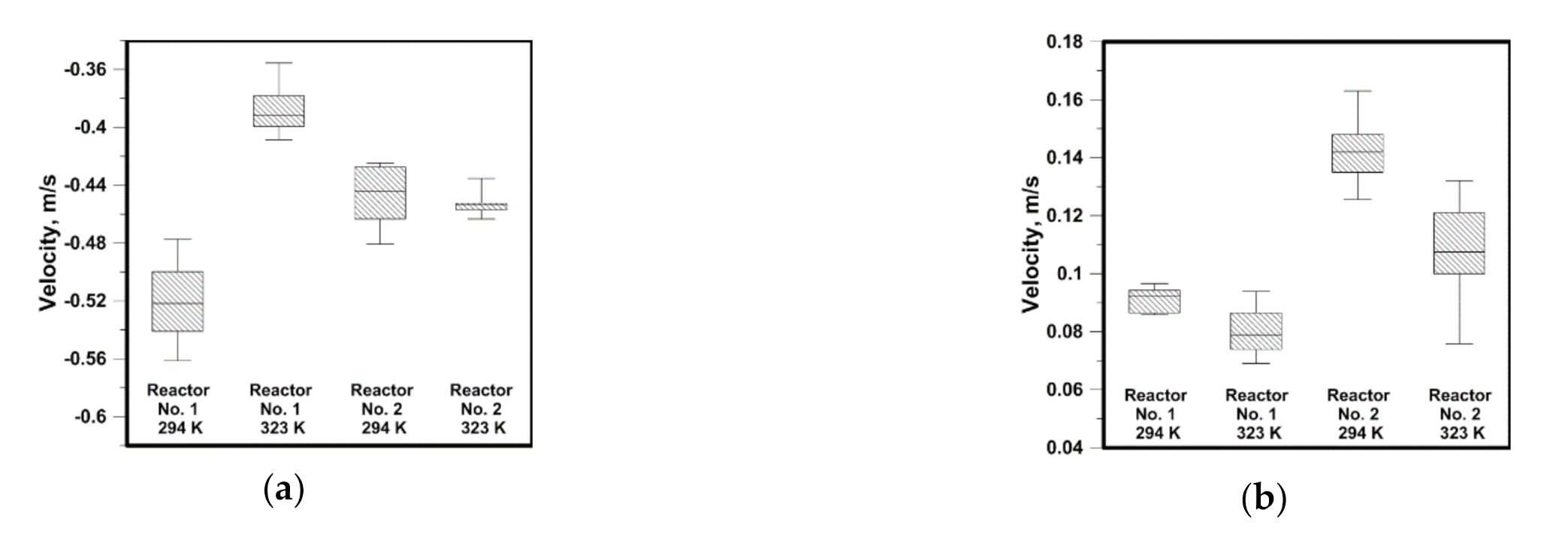
3.1. Hydrodynamics in the Water
3.2. Hydrodynamics in the NaOH Solution
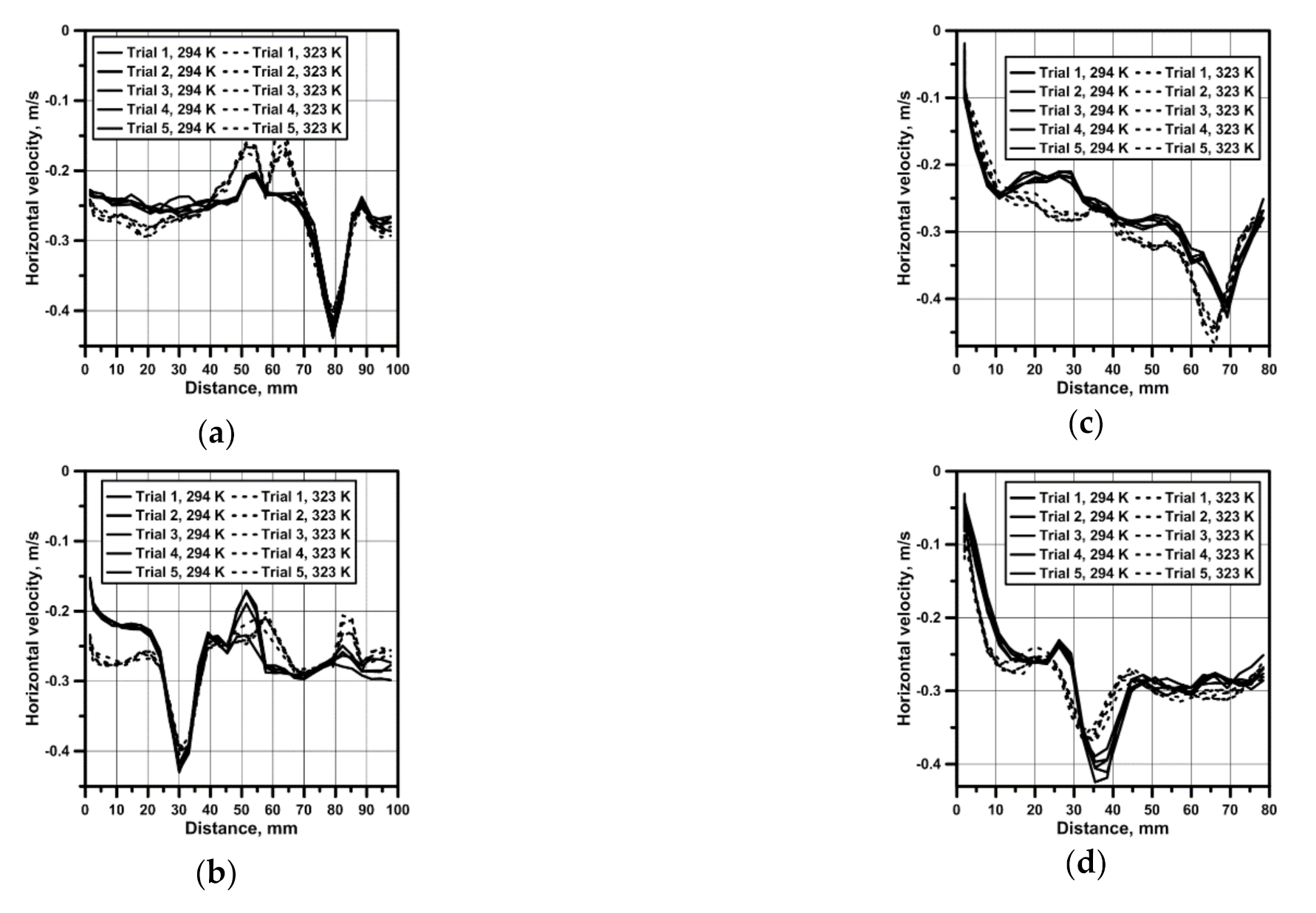
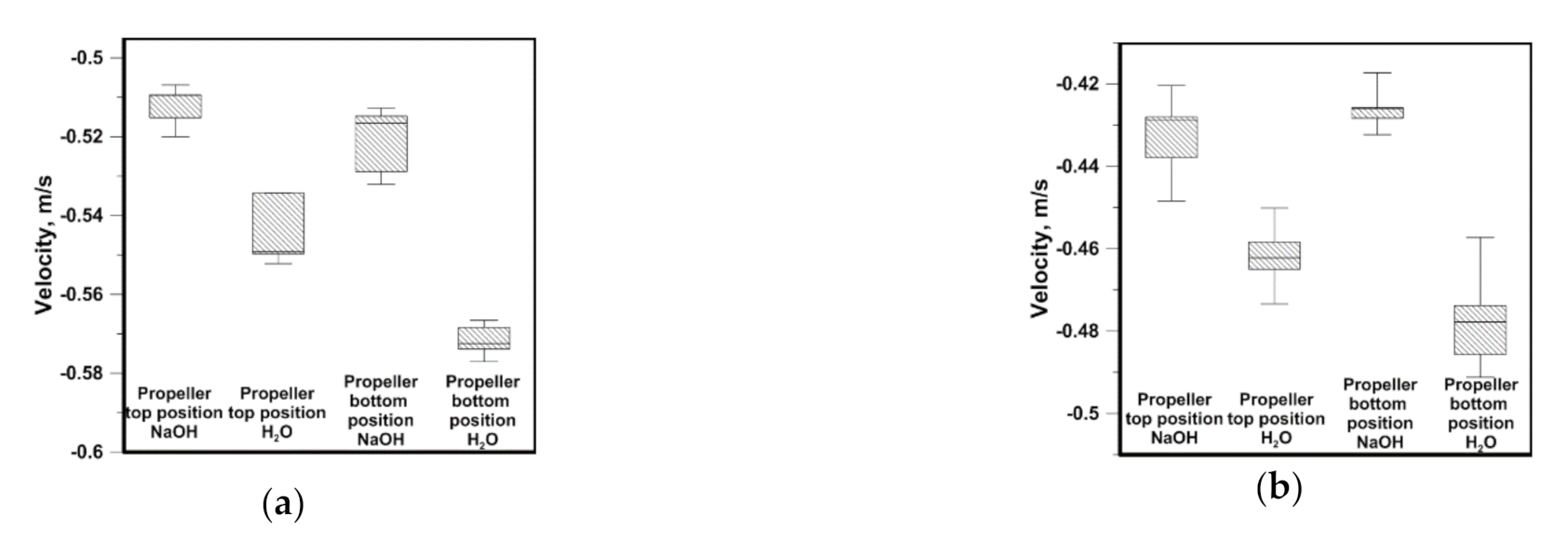
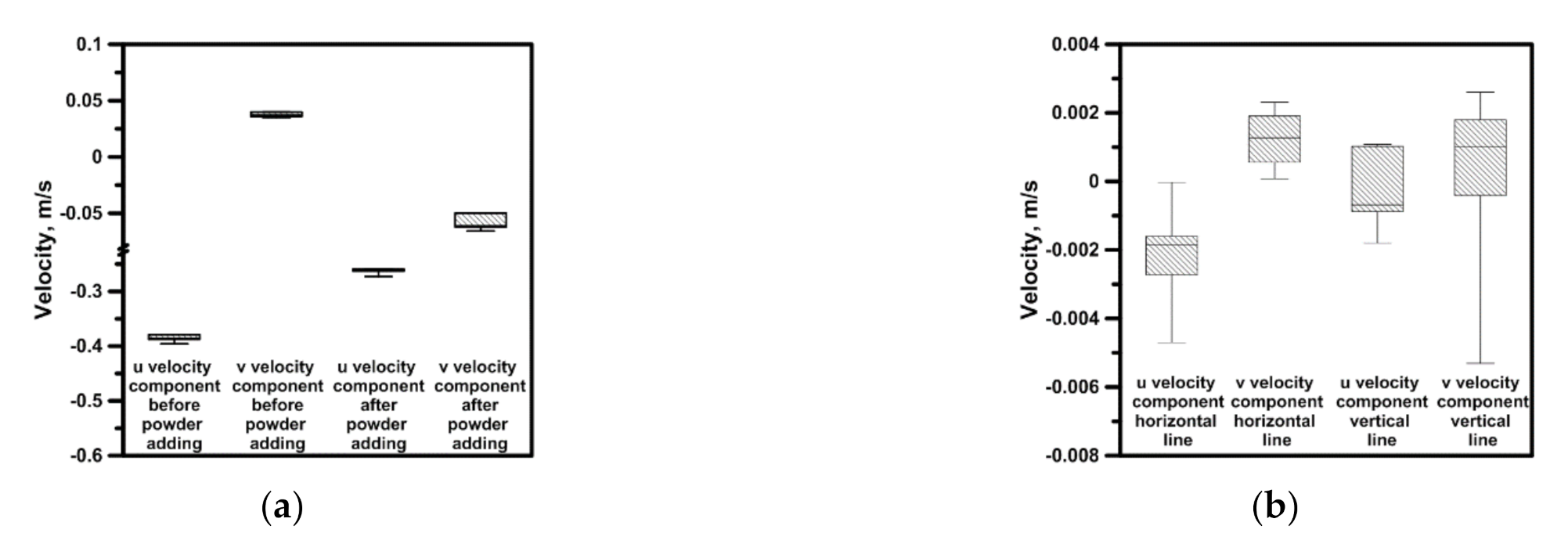
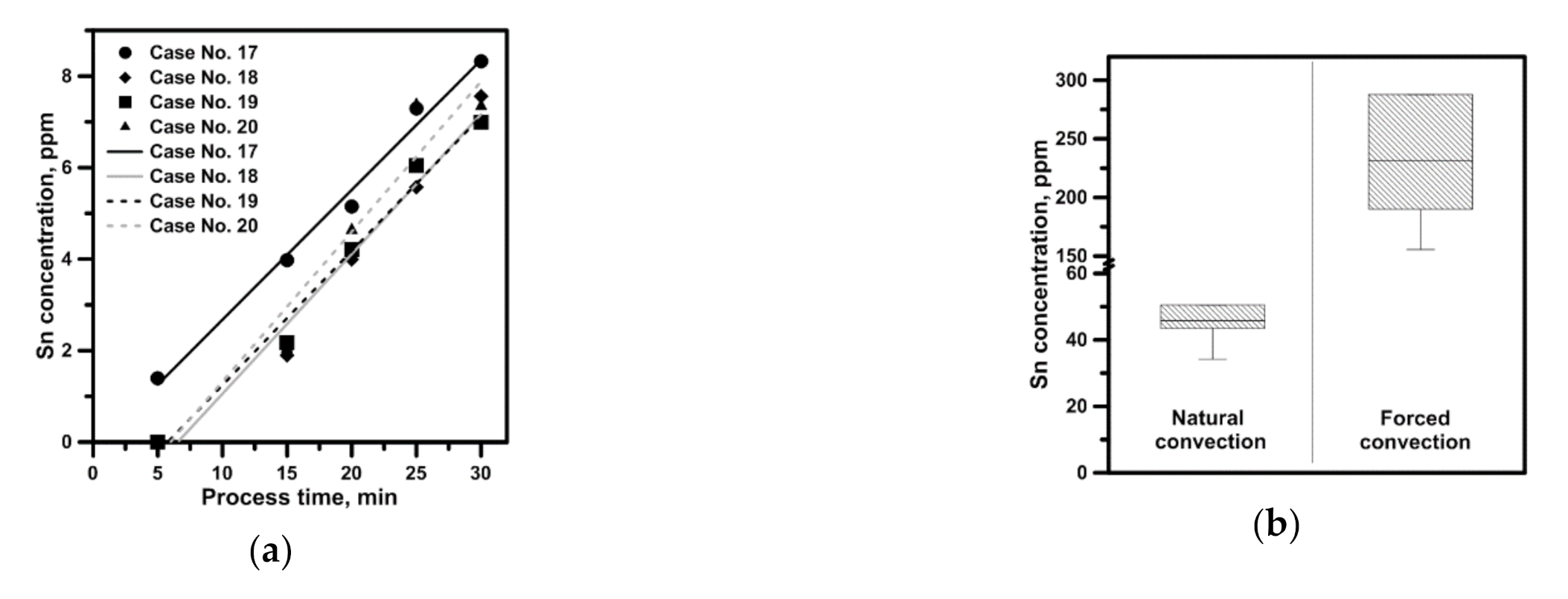
3.3. Hydrodynamics and Leaching Process in the Sn–NaOH System
4. Conclusions
- ▪
- The PIV method can be successfully applied to search for new solutions in the field of hydrometallurgy and to optimize the leaching processes, among others;
- ▪
- A parabolic velocity distribution developing in the considered reactors mixed by means of the mechanical propeller was detected. For the highest rotational speed of the propeller, nearly 50% reduction of fluid velocity was obtained in the zone of 70% distance from a propeller axis;
- ▪
- The hydrodynamic structures developing in the working space of a considered batch of reactors are affected by the type of continuous phase and the leached phase form. The difference in the maximal velocity between the distilled water system and NaOH-Sn powder system amounted to nearly 0.34 m/s for the same propeller speed, propeller blades position and reactor type;
- ▪
- The leaching is affected by the reactor geometry, leached phase form and the hydrodynamic structure developed in its working volume, especially when a convectional or diffusion mass transport decides the process efficiency. Hence appropriate preparation of the leached material, intensifying its dispersion in the leaching agent volume as well as mixer’s direct impact zones, is essential.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, M.S.B.; de Melo, R.A.C.; Lopes-Moriyama, A.L.; Souza, C.P. Electrochemical extraction of tin and copper from acid leachate of printed circuit boards using copper electrodes. J. Environ. Manag. 2019, 246, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Yanez, A.; Alonso, A.; Vengoechea-Pimienta, A.; Ramirez-Munoz, J. Indium and tin recovery from waste LCD panels using citrate as a complexing agent. Waste Manag. 2019, 96, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. Recovery of lead, tin and indium from alloy wire scrap. Hydrometallurgy 1998, 49, 63–73. [Google Scholar] [CrossRef]
- Havlik, T.; Orac, D.; Petranikowa, M.; Miskufova, A.; Kukurugya, F.; Tacacova, Z. Leaching of copper and Tin from used printed circuit boards after thermal treatment. J. Hazard. Mater. 2010, 183, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Grimes, S.M.; Yasri, N.G.; Chaudhary, A. Recovery of critical metals from dilute leach solutions—Separation of indium from tin and lead. Inorg. Chim. Acta. 2017, 461, 161–166. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A. Dissolution of ceramic monolith of spent catalytic converters by using hydrometallurgical methods. Arch. Metall. Mater. 2015, 60, 2945–2948. [Google Scholar] [CrossRef][Green Version]
- Flerus, B.; Friedrich, B. Recovery of Gallium from Smartphones—Part II: Oxidative Alkaline Pressure Leaching of Gallium from Pyrolysis Residue. Metals 2020, 10, 1565. [Google Scholar] [CrossRef]
- Boisvert, L.; Turgeon, K.; Boulanger, J.-F.; Bazin, C.; Houlachi, G. Recovery of Cobalt from the Residues of an Industrial Zinc Refinery. Metals 2020, 10, 1553. [Google Scholar] [CrossRef]
- Turan, M.D.; Sari, Z.A.; Demiraslan, A. Ultrasound-Assisted Leaching and Kinetic Study of Blended Copper Slag. Metall. Mater. Trans. B 2019, 50, 2413–2435. [Google Scholar] [CrossRef]
- Junwei, H.; Zhenyu, O.; Wei, L.; Fen, J.; Wenqing, Q. Recovery of antimony and bismuth from tin anode slime after soda roasting-alkaline leaching. Sep. Purif. Technol. 2020, 242, 116789. [Google Scholar]
- Souada, M.; Louage, C.; Doisy, J.-Y.; Meunier, L.; Benderrag, A.; Ouddane, B.; Bellayer, S.; Nuns, N.; Traisnel, M.; Maschke, U. Extraction of indium-tin oxide from end-of-life LCD panels using ultrasound assisted acid leaching. Ultrason. Sonochem. 2018, 40, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Gupta, B. Cyphos IL 104 assisted extraction of indium and recycling of indium, tin and zinc from discarded LCD screen. Sep. Purif. Technol. 2020, 237, 116407. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, L.; Xu, Z. Indium recovery from In-Sn-Cu-Al mixed system of waste liquid crystal display panels via acid leaching and two-step electrodeposition. J. Hazard. Mater. 2020, 381, 120973. [Google Scholar] [CrossRef] [PubMed]
- Padamata, S.K.; Yasinskiy, A.S.; Polyakov, P.V.; Pavlov, E.A.; Varyukhin, D.Y. Recovery of Noble Metals from Spent Catalysts: A Review. Metall. Mater. Trans. B 2020, 51, 2413–2435. [Google Scholar] [CrossRef]
- Lao, X.; Cheng, C.; Min, X.; Zhao, J.; Zhou, D.; Wang, L.; Li, X. Corrosion and leaching behaviors of Sn-based alloy in simulated soil solutions. Trans. Nonferrous Met. Soc. China 2016, 26, 581–588. [Google Scholar] [CrossRef]
- Han, J.; Liang, C.; Liu, W.; Qin, W.; Jiao, F.; Li, W. Pretreatment of Tin anode slime Rusing alkaline pressure oxidative leaching. Sep. Purif. Technol. 2017, 174, 389–395. [Google Scholar] [CrossRef]
- Jha, M.K.; Choubey, P.K.; Jha, A.K.; Kumari, A.; Lee, J.-C.; Kumar, V.; Jeong, J. Leaching studies for tin recovery from waste e-scarp. Waste Manag. 2012, 32, 1919–1925. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, P.; Liu, W.; Zhang, D. Recovery of tin from metal powders of waste printed circuit boards. Waste Manag. 2017, 68, 449–457. [Google Scholar]
- Fornalczyk, A.; Willner, J.; Gajda, B.; Sedlakova-Kadukova, J. Influence of H2O2 and O3 on PGM extraction from used car catalysts. Arch. Metall. Mater. 2018, 63, 963–968. [Google Scholar]
- Jun, W.S.; Yun, P.S.; Lee, E.C. Leaching behavior of tin from Sn-Fe alloys in sodium hydroxide solution. Hydrometallurgy 2004, 73, 71–80. [Google Scholar] [CrossRef]
- Lee, J.S.Y.; Lawson, F. The Leaching Rate of Tin Metal in Oxygenated Sodium Hydroxide Solutions. Hydrometallurgy 1989, 23, 23–35. [Google Scholar] [CrossRef]
- Free, M.L. Hydrometallurgy Fundamentals and Applications, 1st ed.; John Wiley & Sons—TMS: Hoboken, NJ, USA, 2013; ISBN 978-1-118-23077-0. [Google Scholar]
- Graedel, T.E.; Gunn, G.; Espinoza, L.T. Metal resources, use and criticality. In Critical Metals Handbook, 1st ed.; British Geological Survey: Keyworth, UK; American Geophysical Union and Wiley: Hoboken, NJ, USA, 2014; pp. 1–19. [Google Scholar]
- Cwudziński, A. PIV method and numerical computation for prediction of liquid steel flow structure in tundish. Arch. Metall. Mater. 2015, 60, 11–17. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Ye, X.; Wang, B. Study of Flow Characteristics of Tundish Based on Digital Image Velocimetry Technique. Metall. Mater. Trans. B 2016, 47, 3144–3157. [Google Scholar] [CrossRef]
- Hua, C.; Wang, M.; Bao, Y. Effect of Nozzle Clogging on the Fluid Flow Pattern in a Billet Mold with Particle Image Velocimetry Technology. Metall. Mater. Trans. B 2020, 51, 2871–2881. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Sung, H.J.; Lee, S. Flow Oscillations and Meniscus Fluctuations in a Funnel-Type Water Mold Model. Metall. Mater. Trans. B 2010, 41, 121–130. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Oxfrod, UK, 2011; ISBN 978-0-08-096789-9. [Google Scholar]
- Vignes, A. Extractive Metallurgy 2. Metallurgical Reaction Processes, 1st ed.; John Wiley & Sons: London, UK, 2011; ISBN 978-0-08-096789-9. [Google Scholar]
- Tang, Z.; Kim, W.S.; Yu, T. Continuous synthesis of silver plates in a continuous stirring tank reactor (CSTR). J. Ind. Eng. Chem. 2018, 66, 411–418. [Google Scholar] [CrossRef]
- Zhang, D.M.; Teng, Q.; Zhang, D.; Jilani, G.; Ken, W.M.; Yang, Z.P.; Alam, T.; Ikram, M.; Iqbal, Z. Performance and microbial community dynamics in anaerobic continuously stirred tank reactor and sequencing batch reactor (CSTR-SBR) coupled with magnesium-ammonium-phosphate (MAP)-precipitation for treating swine wastewater. Bioresour. Technol. 2021, 320, 124336. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Zhao, X.; Zhou, J.; Du, G.; Chen, J. A conceptual air-lift reactor design for large scale animal cell cultivation in the context of in vitro meat production. Chem. Eng. Sci. 2020, 211, 115269. [Google Scholar] [CrossRef]
- Coelho, F.E.B.; Balarini, J.C.; Arajuro, E.M.R.; Miranda, T.L.S.; Peres, A.E.C.; Martins, A.H.; Salum, A. A population balance approach to predict the performance of continuous leaching reactors: Model validation in a pilot plant using a roasted zinc concentrate. Hydrometallurgy 2020, 194, 105301. [Google Scholar] [CrossRef]
- Chen, Q.P.; Yan, H.J.; Ge, S.H.; Zhou, J.M. Experimental verification of mathematical model for multiphase flow in air-agitated seed precipitation tank. Trans. Nonferrous Met. Soc. China 2011, 21, 1680–1684. [Google Scholar] [CrossRef]
- Das, P.; Khan, M.M.K.; Rasul, M.G.; Wu, J.; Youn, I. Experimental investigation of hydrodynamic and heat transfer effects on scaling in an agitated tank. Chem. Eng. Proc. Proc. Inten. 2018, 128, 245–256. [Google Scholar] [CrossRef]


| Case No. | Temperature, K | Propeller Speed, Rpm | Propeller Blades Position | ||||
|---|---|---|---|---|---|---|---|
| 294 | 323 | 50 | 300 | 500 | Bottom | Top | |
| 1 | × | − | × | − | − | × | − |
| 2 | × | − | − | × | − | × | − |
| 3 | × | − | − | − | × | × | − |
| 4 | × | − | × | − | − | − | × |
| 5 | × | − | − | × | − | − | × |
| 6 | × | − | − | − | × | − | × |
| 7 | − | × | × | − | − | × | − |
| 8 | − | × | − | × | − | × | − |
| 9 | − | × | − | − | × | × | − |
| 10 | − | × | × | − | − | − | × |
| 11 | − | × | − | × | − | − | × |
| 12 | − | × | − | − | × | − | × |
| Case No. | Reactor No. | Sn Form | Temperature, K | Propeller Blades Position | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Powder | Drop | 294 | 323 | Bottom | Top | |
| 13 | × | − | − | − | × | − | × | − |
| 14 | × | − | − | − | × | − | − | × |
| 15 | − | × | − | − | × | − | × | − |
| 16 | − | × | − | − | × | − | − | × |
| 17 | × | − | − | × | × | − | × | − |
| 18 | × | − | − | × | − | × | × | − |
| 19 | − | × | − | × | × | − | × | − |
| 20 | − | × | − | × | − | × | × | − |
| 21 | × | − | × | − | − | × | − | − |
| 22 | × | − | × | − | − | × | × | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cwudziński, A.; Gajda, B. Particle Image Velocimetry Method for Prediction Hydrodynamic Conditions during Leaching Process on the Basis of Sn–NaOH System. Materials 2021, 14, 633. https://doi.org/10.3390/ma14030633
Cwudziński A, Gajda B. Particle Image Velocimetry Method for Prediction Hydrodynamic Conditions during Leaching Process on the Basis of Sn–NaOH System. Materials. 2021; 14(3):633. https://doi.org/10.3390/ma14030633
Chicago/Turabian StyleCwudziński, Adam, and Bernadeta Gajda. 2021. "Particle Image Velocimetry Method for Prediction Hydrodynamic Conditions during Leaching Process on the Basis of Sn–NaOH System" Materials 14, no. 3: 633. https://doi.org/10.3390/ma14030633
APA StyleCwudziński, A., & Gajda, B. (2021). Particle Image Velocimetry Method for Prediction Hydrodynamic Conditions during Leaching Process on the Basis of Sn–NaOH System. Materials, 14(3), 633. https://doi.org/10.3390/ma14030633







