High-Temperature Oxidation Properties and Microstructural Evolution of Nanostructure Fe-Cr-Al ODS Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
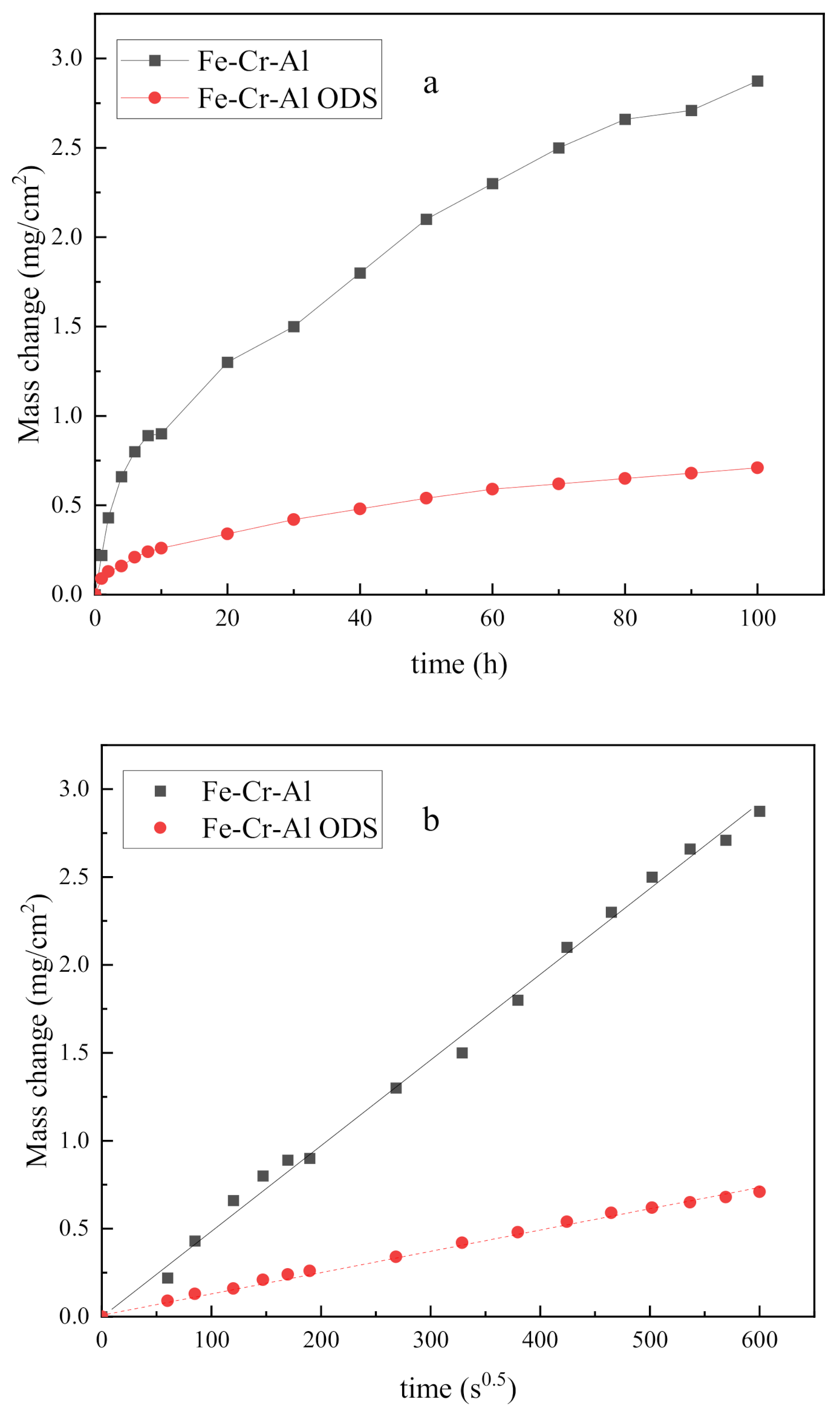
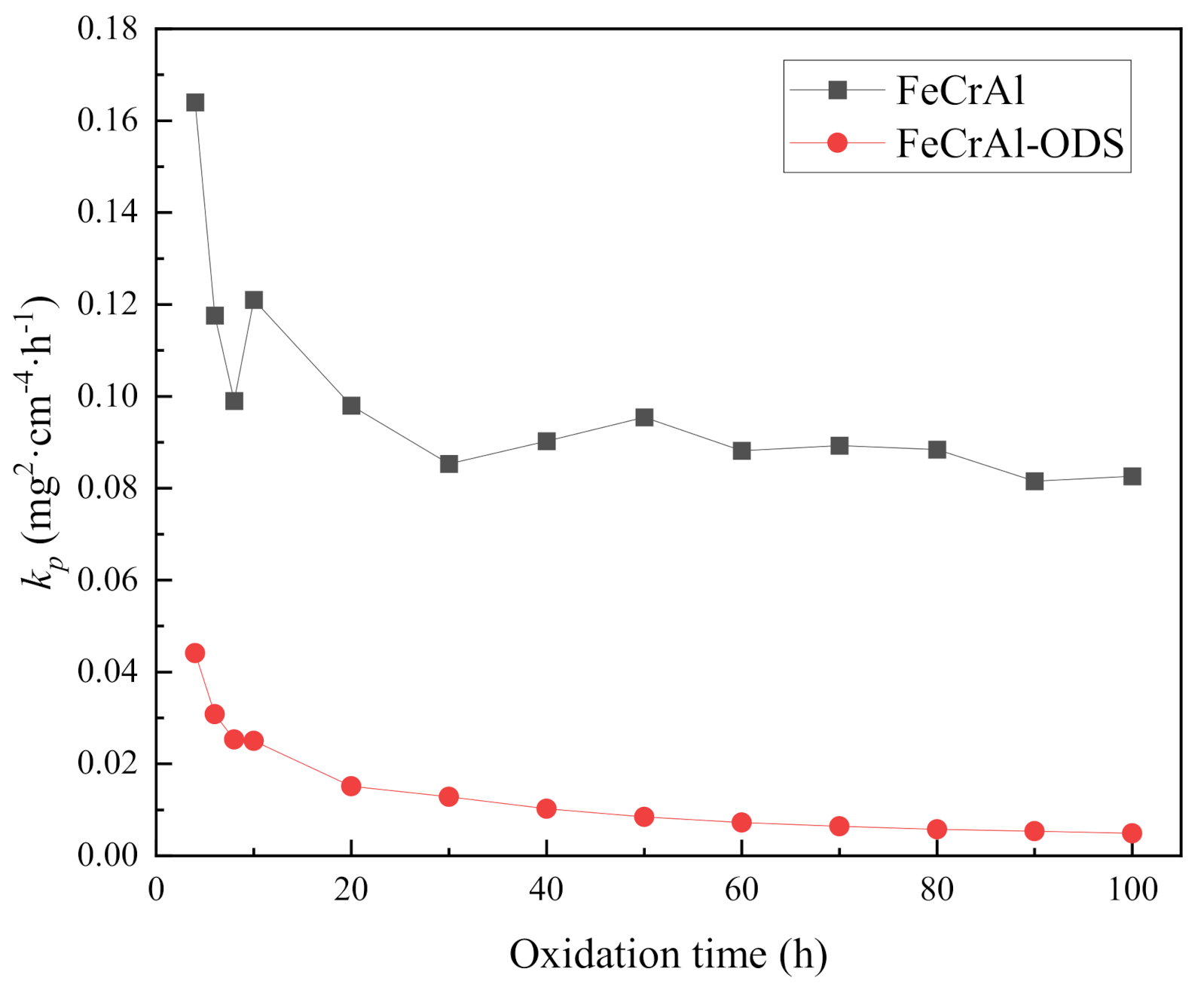
3.1. High-Temperature Oxidation
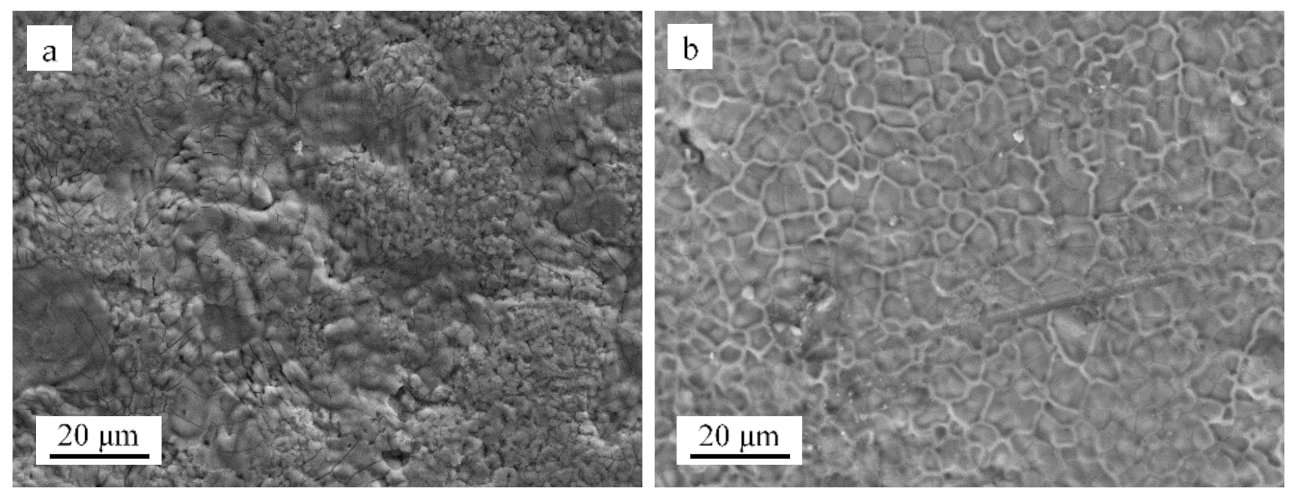
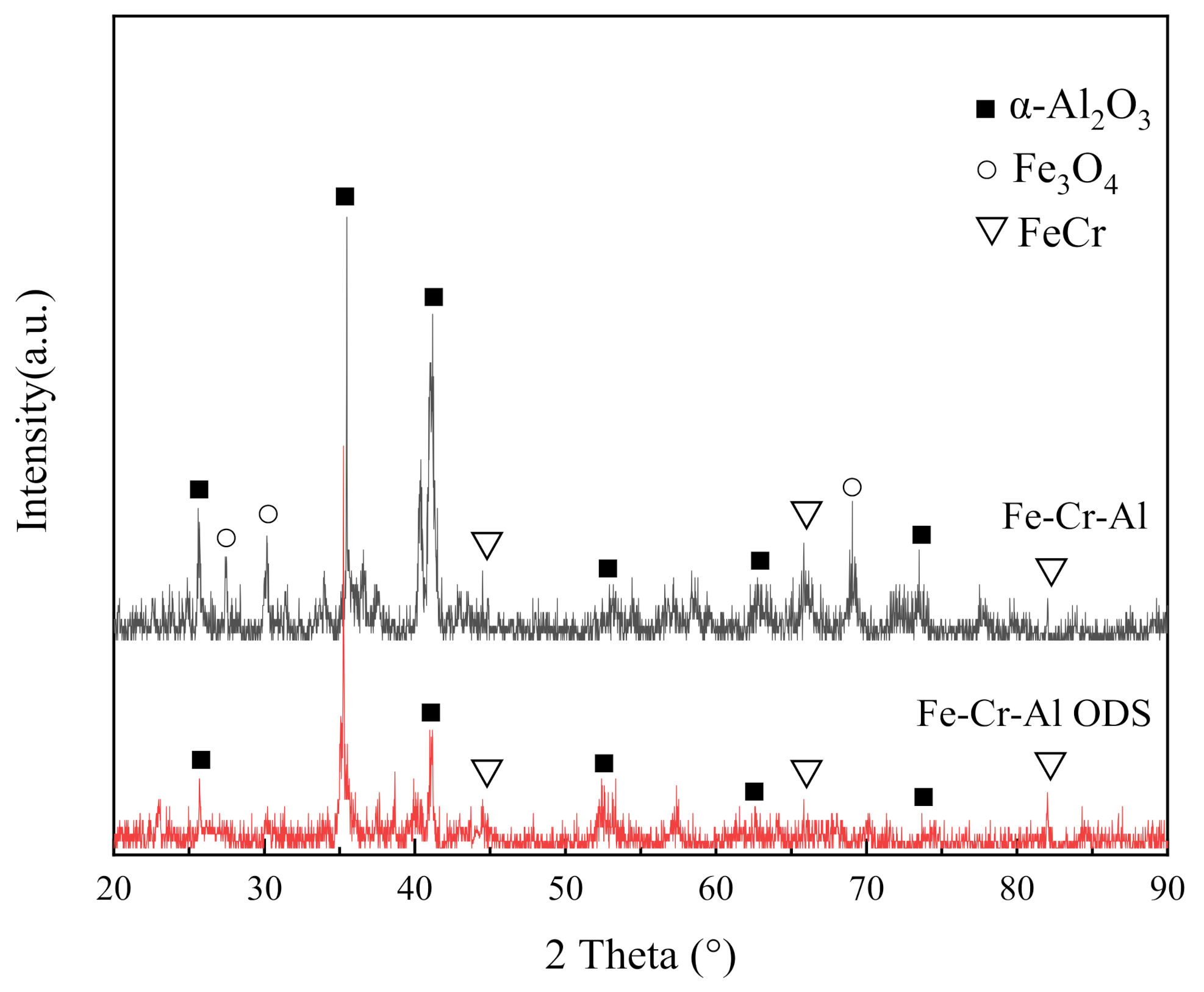
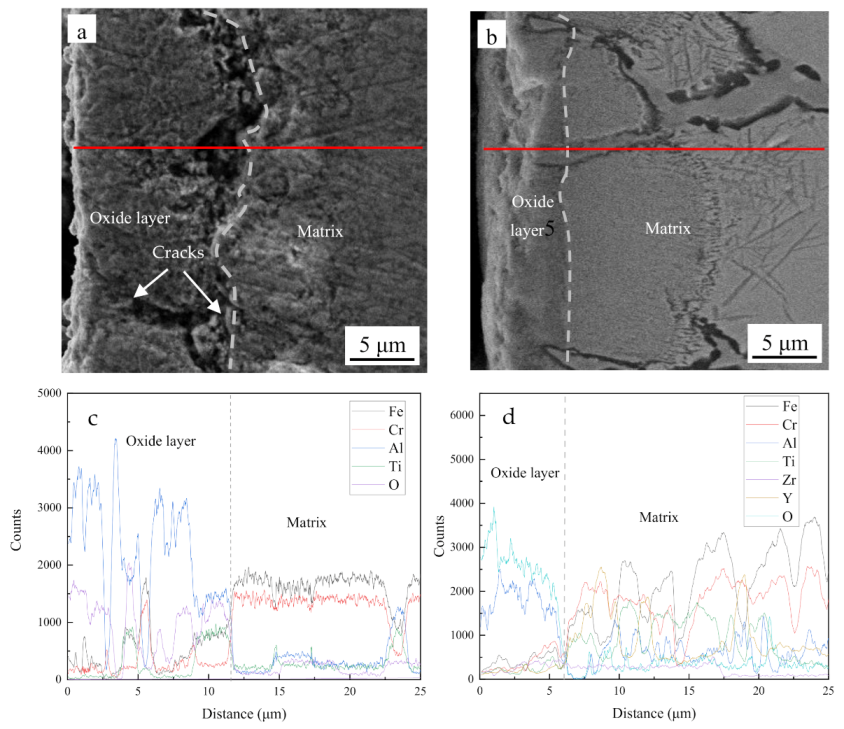
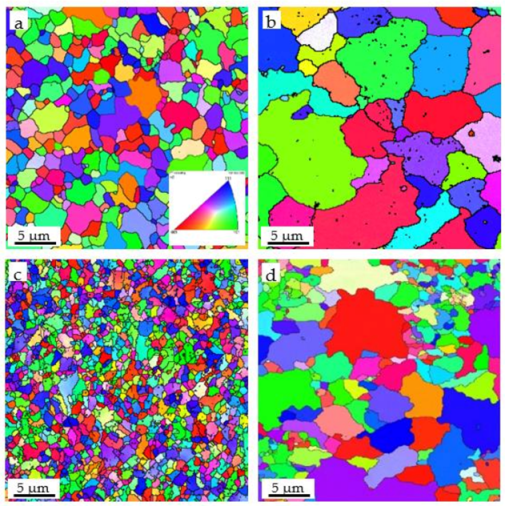
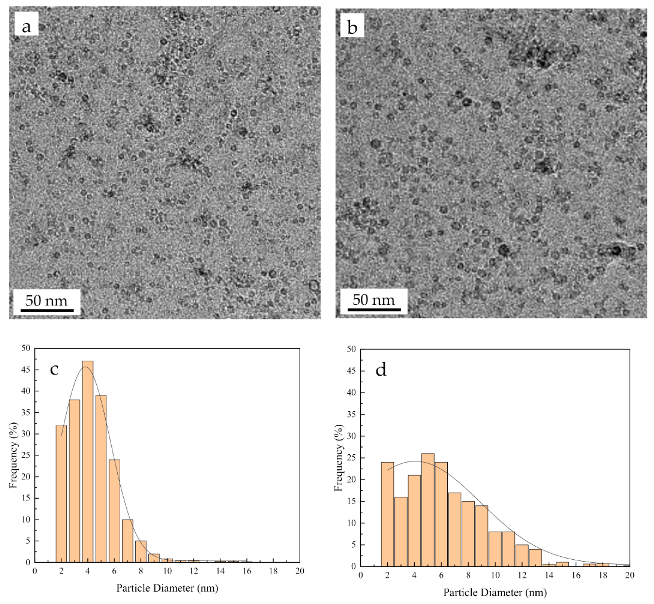
3.2. Microstructural Evolution
4. Discussion
5. Conclusions
- Although only one sample was measured, the oxidation rate of Fe-Cr-Al ODS alloys is obviously lower than that of Fe-Cr-Al alloys, and the oxide layer formed on the Fe-Cr-Al alloy appeared loose and cracked, whereas the oxide layer formed on the Fe-Cr-Al ODS alloys was adherent and flat.
- The main components of the oxide layers of the two alloys are Al2O3. However, due to the existence of voids and cracks in the oxide layer in the Fe-Cr-Al alloys, a small amount of iron oxide was contained in the oxide layer.
- Due to the pinning effect of nano-oxide, the Fe-Cr-Al ODS alloy exhibited excellent high-temperature stability. This shows that the formation of nano-oxides can not only increase the strength, but also improve the high-temperature oxidation performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar]
- Yamamoto, Y.; Pint, B.A.; Terrani, K.A.; Field, K.G.; Yang, Y.; Snead, L.L. Development and property evaluation of nuclear grade wrought FeCrAl fuel cladding for light water reactors. J. Nucl. Mater. 2015, 467, 703–716. [Google Scholar]
- Pint, B.A.; Terrani, K.A.; Yamamoto, Y.; Snead, L.L. Material Selection for Accident Tolerant Fuel Cladding. Metall. Mater. Trans. E 2015, 2, 190–196. [Google Scholar]
- Rebak, R.B.; Gupta, V.K.; Larsen, M. Oxidation Characteristics of Two FeCrAl Alloys in Air and Steam from 800 °C to 1300 °C. JOM 2018, 70, 1484–1492. [Google Scholar]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar]
- Cho, H.S.; Kimura, A.; Ukai, S.; Fujiwara, M. Corrosion properties of oxide dispersion strengthened steels in super-critical water environment. J. Nucl. Mater. 2004, 329, 387–391. [Google Scholar]
- Alamo, A.; Lambard, V.; Averty, X.; Mathon, M.H. Assessment of ODS-14%Cr ferritic alloy for high temperature applications. J. Nucl. Mater. 2004, 329, 333–337. [Google Scholar]
- Götlind, H.; Liu, F.; Svensson, J.E.; Halvarsson, M.; Johansson, L.G. The Effect of Water Vapor on the Initial Stages of Oxidation of the FeCrAl Alloy Kanthal AF at 900 °C. Oxid. Met. 2007, 67, 251–266. [Google Scholar]
- Chen, H.-Z.; Li, B.-R.; Wen, B.; Ye, Q.; Zhang, N.-Q. Corrosion resistance of iron-chromium-aluminium steel in eutectic molten salts under thermal cycling conditions. Corrosion Sci. 2020, 173, 108798. [Google Scholar]
- Dömstedt, P.; Lundberg, M.; Szakálos, P. Corrosion studies of a low alloyed Fe-10Cr-4Al steel exposed in liquid Pb at very high temperatures. J. Nucl. Mater. 2020, 531, 152022. [Google Scholar]
- Pimentel, G.; Chao, J.; Capdevila, C. Recrystallization Process in Fe-Cr-Al Oxide Dispersion-Strengthened Alloy: Microstructural Evolution and Recrystallization Mechanism. JOM 2014, 66, 780–792. [Google Scholar]
- Massey, C.P.; Edmondson, P.D.; Unocic, K.A.; Yang, Y.; Dryepondt, S.N.; Kini, A.; Gault, B.; Terrani, K.A.; Zinkle, S.J. The effect of Zr on precipitation in oxide dispersion strengthened FeCrAl alloys. J. Nucl. Mater. 2020, 533, 152105. [Google Scholar]
- Unocic, K.A.; Pint, B.A.; Hoelzer, D.T. Advanced TEM characterization of oxide nanoparticles in ODS Fe-12Cr-5Al alloys. J. Mater. Sci. 2016, 51, 9190–9206. [Google Scholar]
- Ren, J.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Effects of Zr Addition on Strengthening Mechanisms of Al-Alloyed High-Cr ODS Steels. Materials 2018, 11, 118. [Google Scholar]
- Unocic, K.A.; Essuman, E.; Dryepondt, S.; Pint, B.A. Effect of environment on the scale formed on oxide dispersion strengthened FeCrAl at 1050 °C and 1100 °C. Mater. High Temp. 2014, 29, 171–180. [Google Scholar]
- Unocic, K.A.; Yamamoto, Y.; Pint, B.A. Effect of Al and Cr Content on Air and Steam Oxidation of FeCrAl Alloys and Commercial APMT Alloy. Oxid. Met. 2017, 87, 431–441. [Google Scholar]
- Merceron, G.; Molins, R.; Strudel, J.-L. Oxidation behaviour and microstructural evolution of FeCrAl ODS alloys at high temperature. Mater. High Temp. 2014, 17, 149–157. [Google Scholar]
- Lipkina, K.; Hallatt, D.; Geiger, E.; Fitzpatrick, B.W.N.; Sakamoto, K.; Shibata, H.; Piro, M.H.A. A study of the oxidation behaviour of FeCrAl-ODS in air and steam environments up to 1400 °C. J. Nucl. Mater. 2020, 541, 152305. [Google Scholar]
- Maeda, T.; Ukai, S.; Hayashi, S.; Oono, N.; Shizukawa, Y.; Sakamoto, K. Effects of zirconium and oxygen on the oxidation of FeCrAl-ODS alloys under air and steam conditions up to 1500 °C. J. Nucl. Mater. 2019, 516, 317–326. [Google Scholar]
- Li, Z.; Chen, L.; Zhang, H.; Zhang, S.; Zhang, Z.; Liu, S. Effect of Hot Rolling on the Microstructure and Properties of Nanostructured 15Cr ODS Alloys with Al and Zr Addition. Materials 2020, 13, 3695. [Google Scholar]
- Li, Z.; Lu, Z.; Xie, R.; Lu, C.; Liu, C. Effect of spark plasma sintering temperature on microstructure and mechanical properties of 14Cr-ODS ferritic steels. Mater. Sci. Eng. A 2016, 660, 52–60. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Zhang, H.; Zhang, S.; Zhang, Z. Effect of annealing temperature on microstructure and mechanical properties in oxide dispersion strengthened Fe-14Cr alloys prepared by spark plasma sintering. Mater. Res. Express 2019, 6, 126515. [Google Scholar] [CrossRef]
- Pieraggi, B. Calculations of parabolic reaction rate constants. Oxid. Met. 1987, 27, 177–185. [Google Scholar] [CrossRef]
- Fabrichnaya, O.; Seifert, H.J.; Ludwig, T.; Aldinger, F.; Navrotsky, A. The assessment of thermodynamic parameters in the Al 2O 3-Y 2O 3system and phase relations in the Y-Al-O system. Scand. J. Metall. 2002, 30, 175–183. [Google Scholar] [CrossRef]
- Dong, Q.; Hultquist, G.; Sproule, G.I.; Graham, M.J. Platinum-catalyzed high temperature oxidation of metals. Corrosion Sci. 2007, 49, 3348–3360. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, R.; Wang, H.; Lu, C.; Liu, Y. Formation and stability of oxide layer in FeCrAl fuel cladding material under high-temperature steam. J. Alloys Compd. 2016, 684, 549–555. [Google Scholar] [CrossRef]
- Ali, A.F.; Gorton, J.P.; Brown, N.R.; Terrani, K.A.; Jensen, C.B.; Lee, Y.; Blandford, E.D. Surface wettability and pool boiling Critical Heat Flux of Accident Tolerant Fuel cladding-FeCrAl alloys. Nucl. Eng. Des. 2018, 338, 218–231. [Google Scholar] [CrossRef]
- Evans, H.E. Stress effects in high temperature oxidation of metals. Int. Mater. Rev. 2013, 40, 1–40. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, P.; Qi, W.; Du, S.; Liu, Z.; Meng, F.; Zhang, X.; Wang, K.; Li, Q.; Yao, Z.; et al. Mechanism of Al on FeCrAl steam oxidation behavior and molecular dynamics simulations. J. Alloys Compd. 2020, 828, 154310. [Google Scholar] [CrossRef]
- Kaito, T.; Narita, T.; Ukai, S.; Matsuda, Y. High temperature oxidation behavior of ODS steels. J. Nucl. Mater. 2004, 329–333, 1388–1392. [Google Scholar] [CrossRef]
- Ju, J.; Yang, C.; Ma, S.; Kang, M.; Wang, K.; Li, J.; Fu, H.; Wang, J. Effect of temperature on oxidation resistance and isothermal oxidation mechanism of novel wear-resistant Fe-Cr-B-Al-C-Mn-Si alloy. Corrosion Sci. 2020, 170, 108620. [Google Scholar] [CrossRef]

| Material | Fe | Cr | Al | Ti | Zr | Y2O3 |
|---|---|---|---|---|---|---|
| Fe-Cr-Al | Bal. | 14.6 | 4.3 | 0.27 | - | - |
| Fe-Cr-Al ODS | Bal. | 14.8 | 4.2 | 0.28 | 0.28 | 0.31 |
| Material | Average Grain Size/μm | Number Density of Particles/m−3 | Average Particle Diameter/nm | Volume Fraction of Particles/% |
|---|---|---|---|---|
| Fe-Cr-Al (before oxidation) | 1.03 | - | - | - |
| Fe-Cr-Al (after oxidation) | 10.16 | - | - | - |
| Fe-Cr-Al ODS (before oxidation) | 0.33 | 3.9 × 1022 | 6.7 | 0.73 |
| Fe-Cr-Al ODS (after oxidation) | 4.42 | 0.66 × 1022 | 11.3 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, L.; Zhang, H.; Liu, S. High-Temperature Oxidation Properties and Microstructural Evolution of Nanostructure Fe-Cr-Al ODS Alloys. Materials 2021, 14, 526. https://doi.org/10.3390/ma14030526
Li Z, Chen L, Zhang H, Liu S. High-Temperature Oxidation Properties and Microstructural Evolution of Nanostructure Fe-Cr-Al ODS Alloys. Materials. 2021; 14(3):526. https://doi.org/10.3390/ma14030526
Chicago/Turabian StyleLi, Zhengyuan, Lijia Chen, Haoyu Zhang, and Siyu Liu. 2021. "High-Temperature Oxidation Properties and Microstructural Evolution of Nanostructure Fe-Cr-Al ODS Alloys" Materials 14, no. 3: 526. https://doi.org/10.3390/ma14030526
APA StyleLi, Z., Chen, L., Zhang, H., & Liu, S. (2021). High-Temperature Oxidation Properties and Microstructural Evolution of Nanostructure Fe-Cr-Al ODS Alloys. Materials, 14(3), 526. https://doi.org/10.3390/ma14030526





