Graphene/MoS2 Nanohybrid for Biosensors
Abstract
1. Introduction
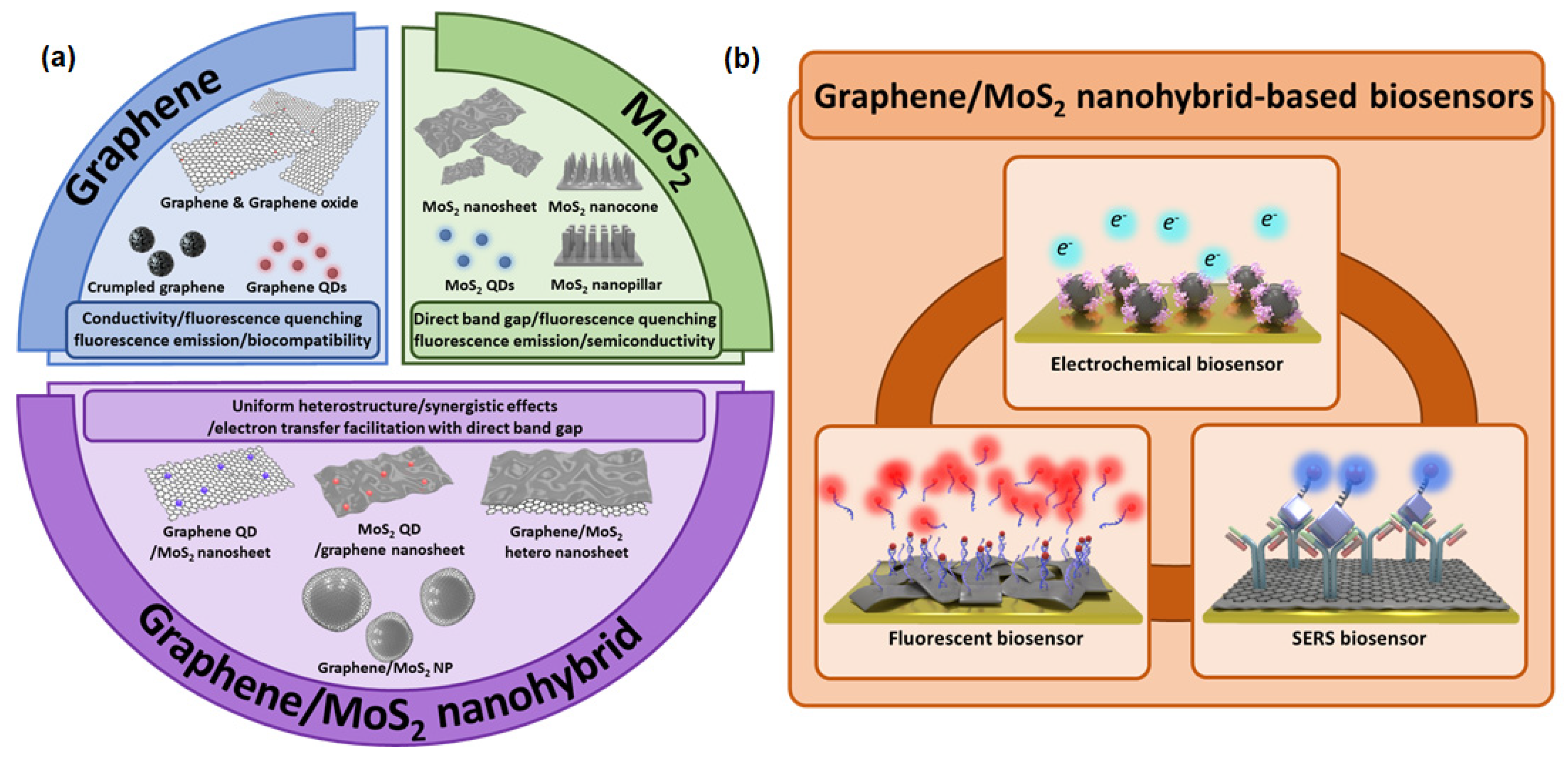
2. Graphene and MoS2
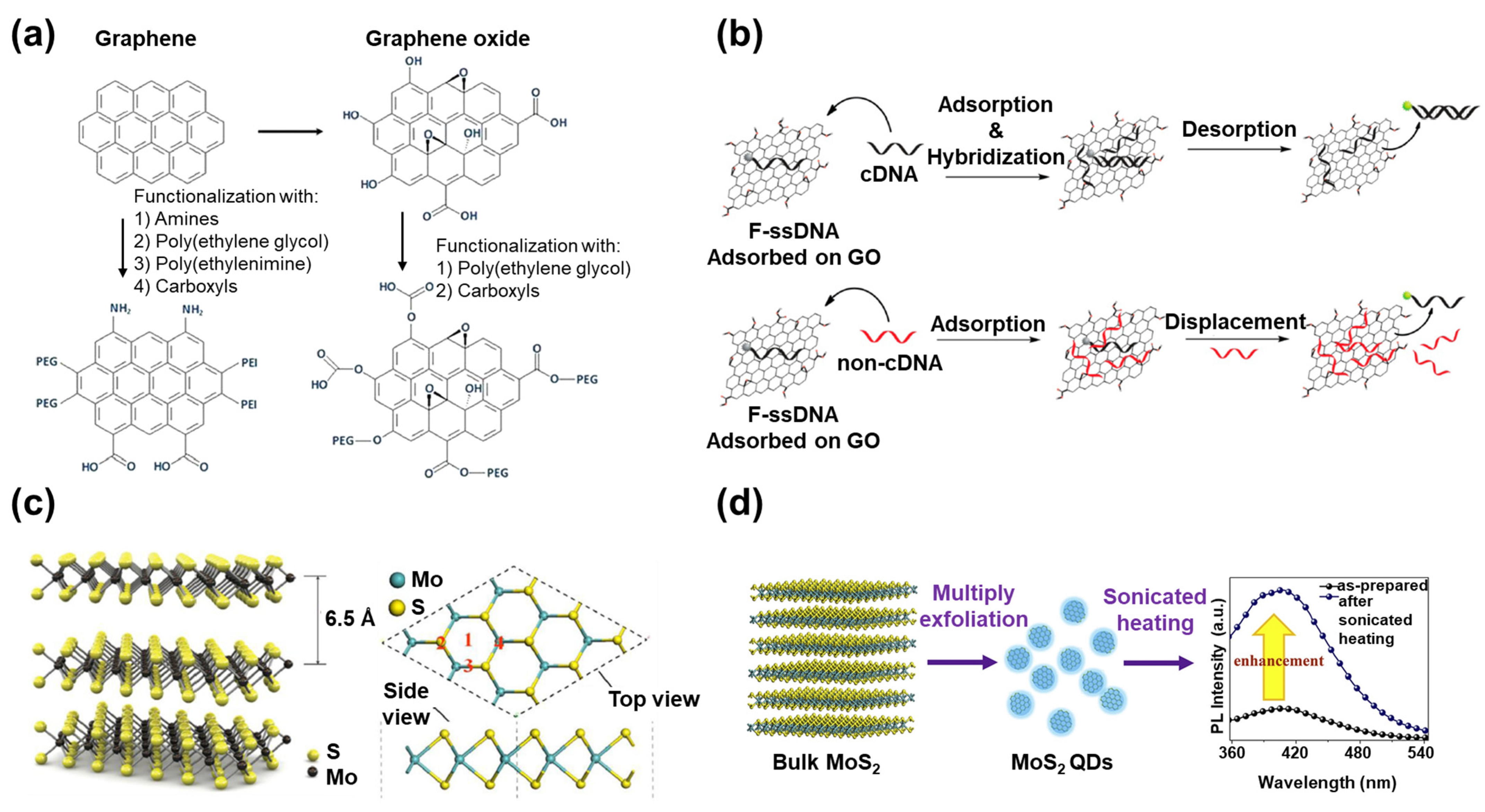
2.1. Graphene
2.2. MoS2
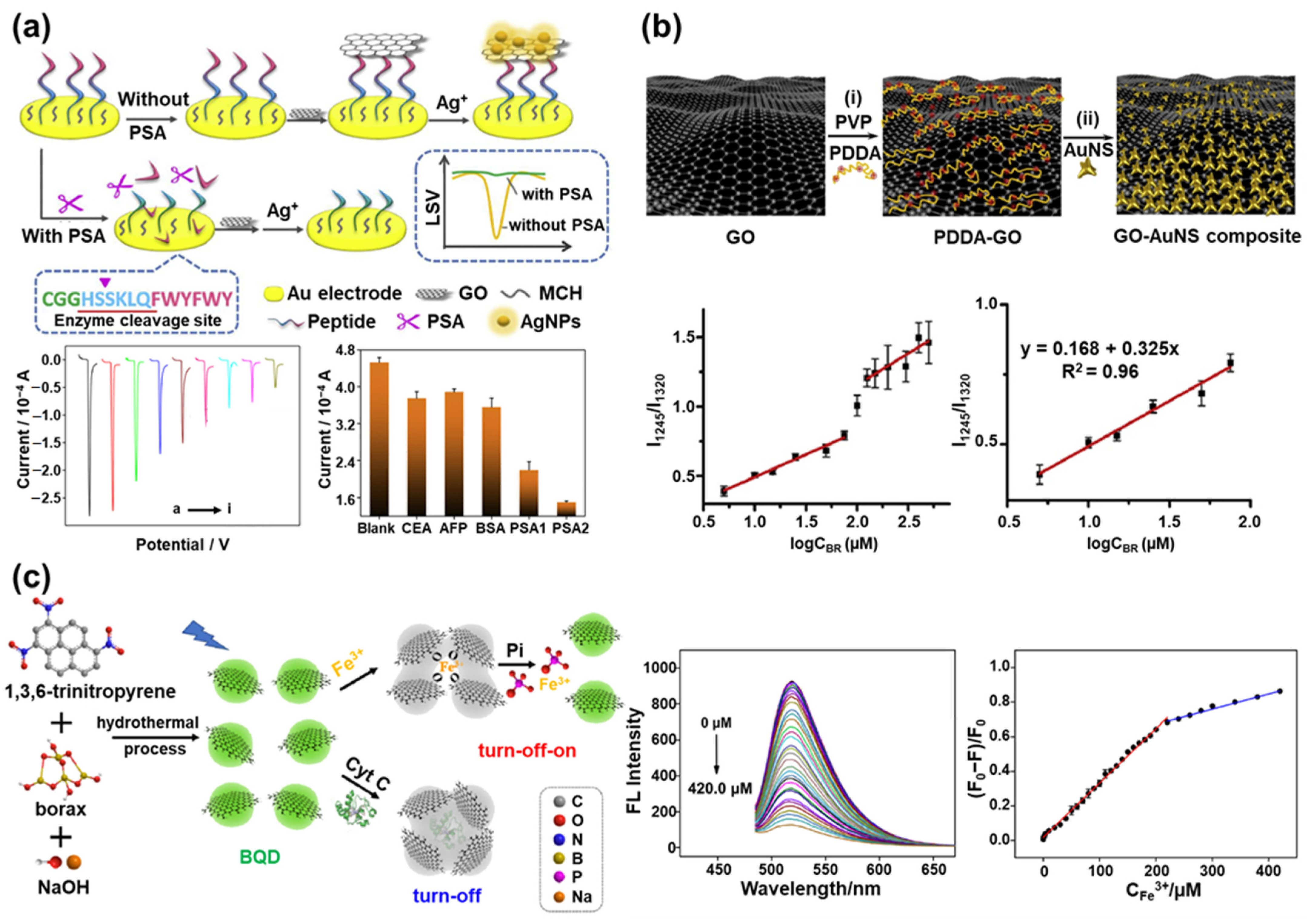
3. Graphene in Biosensors
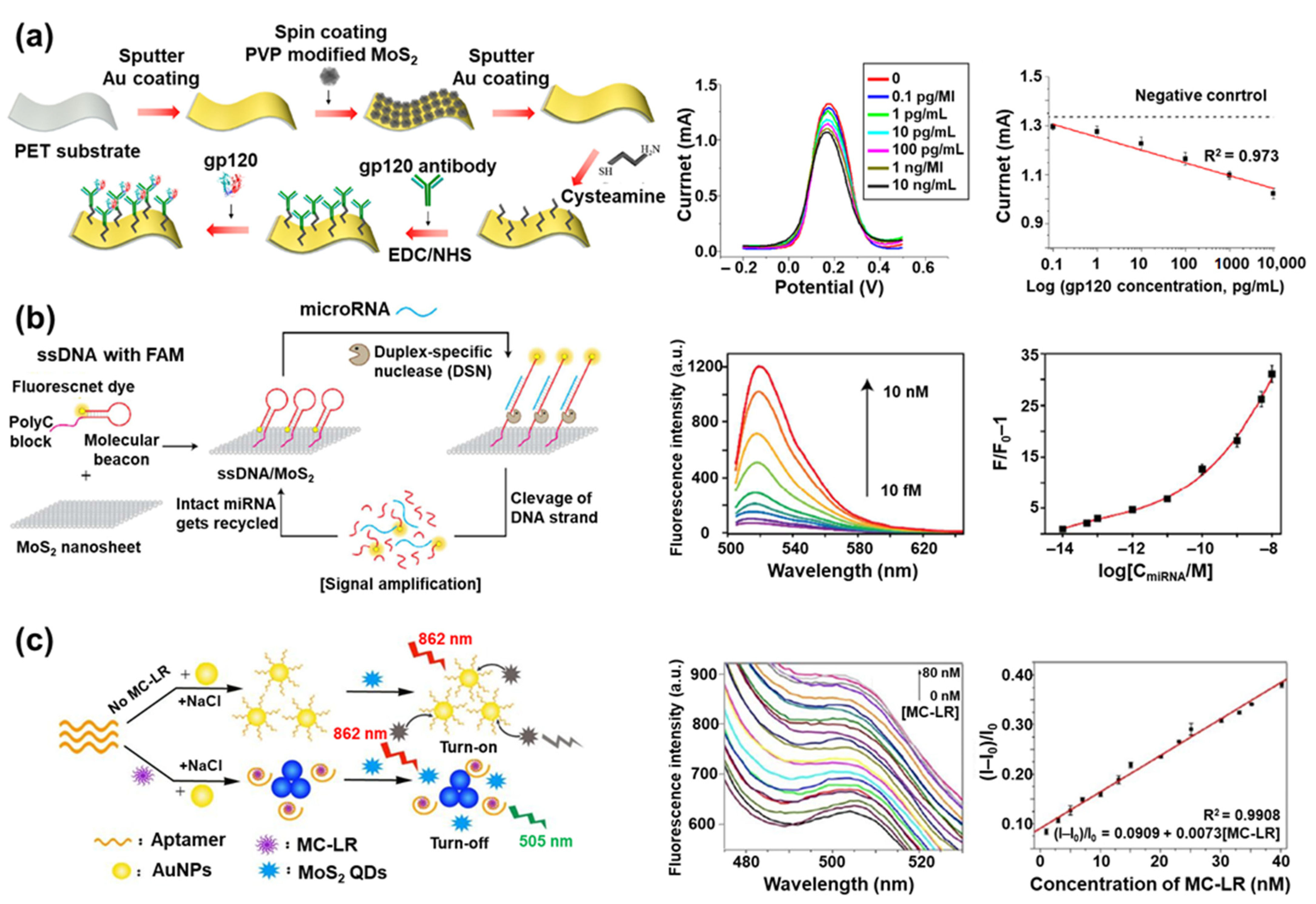
4. MoS2 in Biosensors
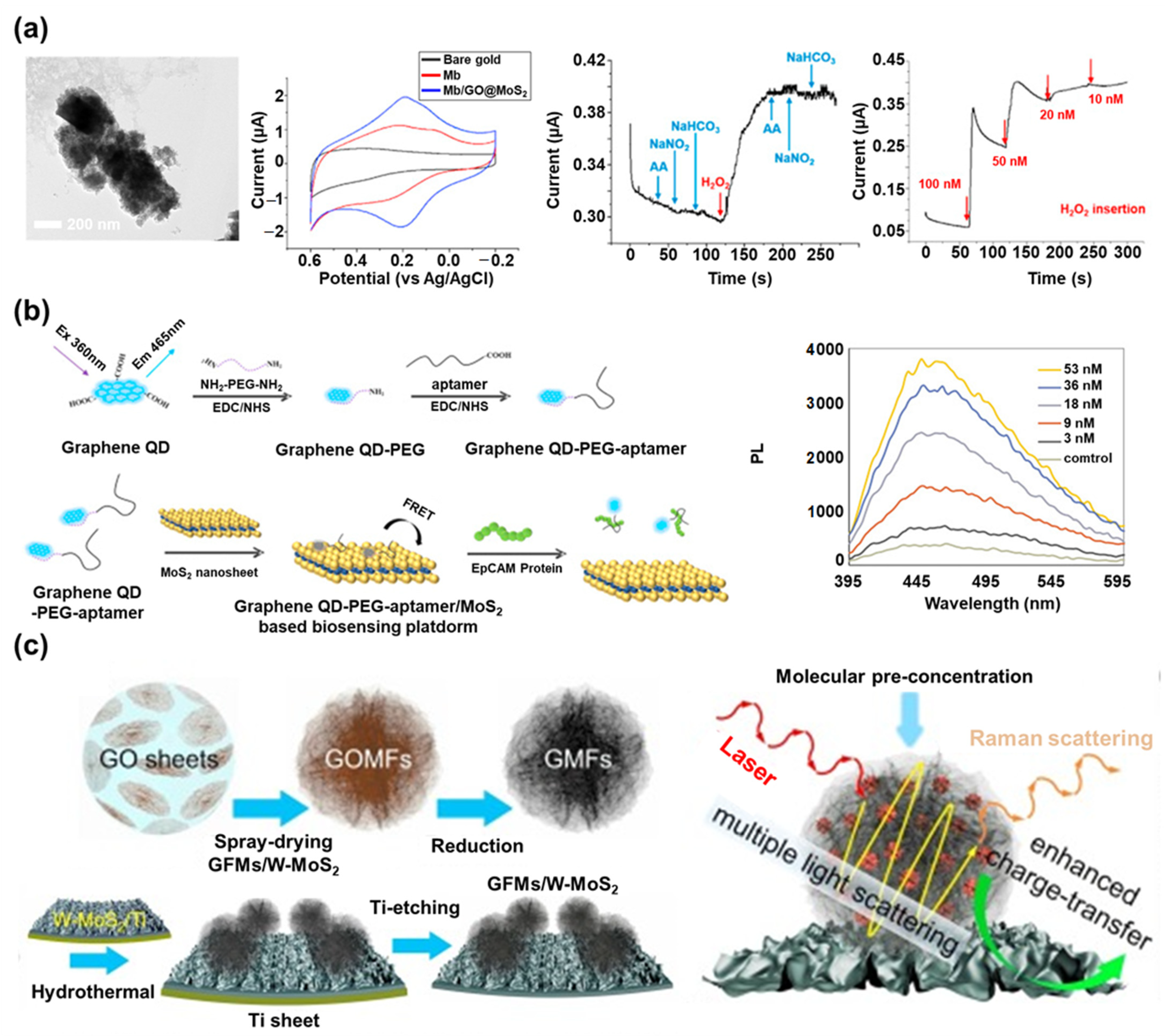
5. Graphene/MoS2 Nanohybrid in Biosensors
6. Conclusions and the Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Yoon, C.-H.; Cho, J.-H.; Oh, H.-I.; Kim, M.-J.; Lee, C.-W.; Choi, J.-W.; Paek, S.-H. Development of a membrane strip immunosensor utilizing ruthenium as an electro-chemiluminescent signal generator. Biosens. Bioelectron. 2003, 19, 289–296. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications, 1st ed.; World Scientific: Singapore, 2004. [Google Scholar]
- Li, Y.; Li, Z.; Chi, C.; Shan, H.; Zheng, L.; Fang, Z. Plasmonics of 2D nanomaterials: Properties and applications. Adv. Sci. 2017, 4, 1600430. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Wigginton, K.R. Nanomaterial enabled biosensors for pathogen monitoring—A review. Environ. Sci. Technol. 2010, 44, 3656–3669. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: Recent progress, applications, and future perspective. Chem. Rev. 2018, 119, 120–194. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Pehlivan, Z.S.; Torabfam, M.; Kurt, H.; Ow-Yang, C.; Hildebrandt, N.; Yüce, M. Aptamer and nanomaterial based FRET biosensors: A review on recent advances (2014–2019). Microchim. Acta 2019, 186, 563. [Google Scholar] [CrossRef]
- Cardinal, M.F.; Vander Ende, E.; Hackler, R.A.; McAnally, M.O.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Expanding applications of SERS through versatile nanomaterials engineering. Chem. Soc. Rev. 2017, 46, 3886–3903. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuator. A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Barua, S.; Dutta, H.S.; Gogoi, S.; Devi, R.; Khan, R. Nanostructured MoS2-based advanced biosensors: A review. ACS Appl. Nano Mater. 2017, 1, 2–25. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Tsang, W.; Henley, S.; Stolojan, V.; Silva, S. Negative differential conductance observed in electron field emission from band gap modulated amorphous-carbon nanolayers. Appl. Phys. Lett. 2006, 89, 193103. [Google Scholar] [CrossRef]
- Vairavapandian, D.; Vichchulada, P.; Lay, M.D. Preparation and modification of carbon nanotubes: Review of recent advances and applications in catalysis and sensing. Anal. Chim. Acta 2008, 626, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical application of graphene: From drug delivery, tumor therapy, to theranostics. Colloid. Surf. B Biointerfaces 2020, 185, 110596. [Google Scholar] [CrossRef]
- Li, X.; Zhi, L. Graphene hybridization for energy storage applications. Chem. Soc. Rev. 2018, 47, 3189–3216. [Google Scholar] [CrossRef]
- Wu, X.; Ding, S.-J.; Lin, K.; Su, J. A review on the biocompatibility and potential applications of graphene in inducing cell differentiation and tissue regeneration. J. Mater. Chem. B 2017, 5, 3084–3102. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Hui, D.; Feo, L.; Fraternali, F. Graphene as biomedical sensing element: State of art review and potential engineering applications. Compos. Part B Eng. 2018, 134, 193–206. [Google Scholar] [CrossRef]
- Huang, H.; Fan, X.; Singh, D.J.; Zheng, W.T. Recent progress of TMD nanomaterials: Phase transitions and applications. Nanoscale 2020, 12, 1247–1268. [Google Scholar] [CrossRef]
- Samadi, M.; Sarikhani, N.; Zirak, M.; Zhang, H.; Zhang, H.-L.; Moshfegh, A.Z. Group 6 transition metal dichalcogenide nanomaterials: Synthesis, applications and future perspectives. Nanoscale Horiz. 2018, 3, 90–204. [Google Scholar] [CrossRef]
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered Transition Metal Dichalcogenide-Based Nanomaterials for Electrochemical Energy Storage. Adv. Mater. 2020, 32, 1903826. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-processed two-dimensional MoS2 nanosheets: Preparation, hybridization, and applications. Angew. Chem. Int. Ed. 2016, 55, 8816–8838. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, J.; Wu, C.; Ye, C.; Zou, D.; Wang, S. Facile synthesis of colloidal stable MoS2 nanoparticles for combined tumor therapy. Chem. Eng. J. 2018, 351, 548–558. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y. MoS2 quantum dot growth induced by S vacancies in a ZnIn2S4 monolayer: Atomic-level heterostructure for photocatalytic hydrogen production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef]
- Park, H.; Han, G.; Lee, S.W.; Lee, H.; Jeong, S.H.; Naqi, M.; AlMutairi, A.; Kim, Y.J.; Lee, J.; Kim, W.-J. Label-free and recalibrated multilayer MoS2 biosensor for point-of-care diagnostics. ACS Appl. Mater. Interfaces 2017, 9, 43490–43497. [Google Scholar] [CrossRef]
- Arshad, M.M.; Gopinath, S.C.; Norhaimi, W.; Fathil, M. Current and future envision on developing biosensors aided by 2D molybdenum disulfide (MoS2) productions. Biosens. Bioelectron. 2019, 132, 248–264. [Google Scholar]
- Wang, C.; Jiang, J.; Ruan, Y.; Ao, X.; Ostrikov, K.; Zhang, W.; Lu, J.; Li, Y.Y. Construction of MoO2 quantum dot–graphene and MoS2 nanoparticle–graphene nanoarchitectures toward ultrahigh lithium storage capability. ACS Appl. Mater. Interfaces 2017, 9, 28441–28450. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, T.; Jo, J.; Oh, B.-K.; Choi, J.-W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 93, 14–20. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, J.-W.; Lim, J.; Mohammadniaei, M.; Bapurao, G.B.; Lee, T.; Choi, J.-W. Electrochemical nitric oxide biosensor based on amine-modified MoS2/graphene oxide/myoglobin hybrid. Colloid. Surf. B Biointerfaces 2017, 159, 729–736. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, B.; Lee, C. Progress of infrared guided-wave nanophotonic sensors and devices. Nano Converg. 2020, 7, 12. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1363–1375. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 27. [Google Scholar] [CrossRef]
- Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar]
- Zhao, F.; Wu, J.; Ying, Y.; She, Y.; Wang, J.; Ping, J. Carbon nanomaterial-enabled pesticide biosensors: Design strategy, biosensing mechanism, and practical application. TrAC Trends Anal. Chem. 2018, 106, 62–83. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Justino, C.I.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Omar, Y.M.; Maragliano, C.; Lai, C.-Y.; Iacono, F.L.; Bologna, N.; Diamanti, M.V.; Shah, T.; Al Ghaferi, A.; Chiesa, M. Multi-wall carbon nanostructured paper: Characterization and potential applications definition. Mater. Res. Express 2015, 2, 095601. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Chaudhary, V. Hybridized Graphene for Chemical Sensing. In Functionalized Graphene Nanocomposites and their Derivatives, 1st ed.; Jawaid, M., Bouhfid, R., Qaiss, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–338. [Google Scholar]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Pinto, A.M.; Goncalves, I.C.; Magalhaes, F.D. Graphene-based materials biocompatibility: A review. Colloid. Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Han, G.-C.; Xiao, H.; Chen, Z.; Fang, C. A novel 3D paper-based microfluidic electrochemical glucose biosensor based on rGO-TEPA/PB sensitive film. Anal. Chim. Acta 2020, 1096, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Li, G.; Liang, J.; Su, J.; Zhang, Y.; Chen, H.; Huang, Y.; Sui, W.; Zhao, Y. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2016, 82, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kasry, A.; Ardakani, A.A.; Tulevski, G.S.; Menges, B.; Copel, M.; Vyklicky, L. Highly efficient fluorescence quenching with graphene. J. Phys. Chem. C 2012, 116, 2858–2862. [Google Scholar] [CrossRef]
- Park, J.S.; Goo, N.-I.; Kim, D.-E. Mechanism of DNA adsorption and desorption on graphene oxide. Langmuir 2014, 30, 12587–12595. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Miao, X.; Ling, L. A novel fluorescent biosensor for sequence-specific recognition of double-stranded DNA with the platform of graphene oxide. Analyst 2011, 136, 2106–2110. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene quantum dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Q.; Xiao, F.; Duan, H. 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-dimensional nanomaterials for biomedical applications: Emerging trends and future prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zavabeti, A.; Quan, H.; Zhu, W.; Wei, H.; Chen, D.; Ou, J.Z. Recent advances in two-dimensional transition metal dichalcogenides for biological sensing. Biosens. Bioelectron. 2019, 142, 111573. [Google Scholar] [CrossRef]
- Li, Y.; Duerloo, K.-A.N.; Wauson, K.; Reed, E.J. Structural semiconductor-to-semimetal phase transition in two-dimensional materials induced by electrostatic gating. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Susarla, S.; Kutana, A.; Hachtel, J.A.; Kochat, V.; Apte, A.; Vajtai, R.; Idrobo, J.C.; Yakobson, B.I.; Tiwary, C.S.; Ajayan, P.M. Quaternary 2D transition metal dichalcogenides (TMDs) with tunable bandgap. Adv. Mater. 2017, 29, 1702457. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 field-effect transistor for next-generation label-free biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Oh, B.-R.; Chen, P.; Yoon, J.S.; Wi, S.; Chen, M.; Kurabayashi, K.; Liang, X. Two different device physics principles for operating MoS2 transistor biosensors with femtomolar-level detection limits. Appl. Phys. Lett. 2015, 107, 012105. [Google Scholar] [CrossRef]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-dimensional transition metal dichalcogenide (TMD) nanosheets. Chem. Soc. Rev. 2015, 44, 2584–2586. [Google Scholar] [CrossRef]
- Escalera-López, D.; Griffin, R.; Isaacs, M.; Wilson, K.; Palmer, R.E.; Rees, N.V. MoS2 and WS2 nanocone arrays: Impact of surface topography on the hydrogen evolution electrocatalytic activity and mass transport. Appl. Mater. Today 2018, 11, 70–81. [Google Scholar] [CrossRef]
- Ning, L.; Jiang, T.; Shao, Z.; Ding, K.; Zhang, X.; Jie, J. Light-trapping enhanced ZnO–MoS2 core–shell nanopillar arrays for broadband ultraviolet-visible-near infrared photodetection. J. Mater. Chem. C 2018, 6, 7077–7084. [Google Scholar] [CrossRef]
- Cao, X.; Ding, C.; Zhang, C.; Gu, W.; Yan, Y.; Shi, X.; Xian, Y. Transition metal dichalcogenide quantum dots: Synthesis, photoluminescence and biological applications. J. Mater. Chem. B 2018, 6, 8011–8036. [Google Scholar] [CrossRef]
- Dong, H.; Tang, S.; Hao, Y.; Yu, H.; Dai, W.; Zhao, G.; Cao, Y.; Lu, H.; Zhang, X.; Ju, H. Fluorescent MoS2 quantum dots: Ultrasonic preparation, up-conversion and down-conversion bioimaging, and photodynamic therapy. ACS Appl. Mater. Interfaces 2016, 8, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Sinha, A.; Tan, B.; Huang, Y.; Zhao, H.; Dang, X.; Chen, J.; Jain, R. MoS2 nanostructures for electrochemical sensing of multidisciplinary targets: A review. TrAC Trends Anal. Chem. 2018, 102, 75–90. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Lee, H.S.; Min, S.-W.; Chang, Y.-G.; Park, M.K.; Nam, T.; Kim, H.; Kim, J.H.; Ryu, S.; Im, S. MoS2 nanosheet phototransistors with thickness-modulated optical energy gap. Nano Lett. 2012, 12, 3695–3700. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, W.; Zhao, D.; Hao, Q.; Li, J.; Huang, J.; Shi, J.; Chao, J.; Su, S.; Wang, L. Label-free electrochemical sensing platform for microRNA-21 detection using thionine and gold nanoparticles co-functionalized MoS2 nanosheet. ACS Appl. Mater. Interfaces 2017, 9, 35597–35603. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. Appl. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Jiang, K.; Wang, C.; Zhang, C. One-step synthesis of water-soluble and highly fluorescent MoS2 quantum dots for detection of hydrogen peroxide and glucose. Sens. Actuator. B Chem. 2017, 252, 183–190. [Google Scholar] [CrossRef]
- Ruan, L.; Zhao, Y.; Chen, Z.; Zeng, W.; Wang, S.; Liang, D.; Zhao, J. Ethylenediamine-assisted hydrothermal method to fabricate MoS2 quantum dots in aqueous solution as a fluorescent probe for Fe3+ ion detection. Appl. Surf. Sci. 2020, 528, 146811. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; John, S.A. Water-soluble MoS2 quantum dots as effective fluorescence probe for the determination of bilirubin in human fluids. Spectroc. Acta Part A Mol. Biomol. Spectr. 2019, 215, 290–296. [Google Scholar] [CrossRef]
- Ding, L.; Chang, Y.; Yang, P.; Gao, W.; Sun, M.; Bie, Y.; Yang, L.; Ma, X.; Guo, Y. Facile synthesis of biocompatible L-cysteine-modified MoS2 nanospheres with high photothermal conversion efficiency for photothermal therapy of tumor. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111371. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, J.; Meng, Z.; Li, Y.; Huang, Q.; Qi, Y.; Liu, Y.; Zhan, D.; Liu, X.Y. Enhanced exfoliation of biocompatible MoS2 nanosheets by wool keratin. ACS Appl. Nano Mater. 2018, 1, 5460–5469. [Google Scholar] [CrossRef]
- Wang, S.; Li, K.; Chen, Y.; Chen, H.; Ma, M.; Feng, J.; Zhao, Q.; Shi, J. Biocompatible PEGylated MoS2 nanosheets: Controllable bottom-up synthesis and highly efficient photothermal regression of tumor. Biomaterials 2015, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lyu, J.; Tian, F.; Yang, M. A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens. Bioelectron. 2017, 93, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wang, M.; Zhang, L.; Cao, M.; Ji, Y.; Kou, S.; Dou, J.; Sun, X.; Yang, Z. Wrinkled 2H-phase MoS2 sheet decorated with graphene-microflowers for ultrasensitive molecular sensing by plasmon-free SERS enhancement. Sens. Actuator. B Chem. 2020, 320, 128445. [Google Scholar] [CrossRef]
- Firmiano, E.G.S.; Rabelo, A.C.; Dalmaschio, C.J.; Pinheiro, A.N.; Pereira, E.C.; Schreiner, W.H.; Leite, E.R. Supercapacitor Electrodes Obtained by Directly Bonding 2D MoS2 on Reduced Graphene Oxide. Adv. Energy Mater. 2014, 4, 1301380. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Kumar, A. Epoxy-graphene-MoS2 composites with improved tribological behavior under dry sliding contact. Tribol. Int. 2019, 130, 106–118. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Seo, M.H.; Hassan, F.M.; Hoque, M.A.; Chen, Z. Sulfur Atoms Bridging Few-Layered MoS2 with S-Doped Graphene Enable Highly Robust Anode for Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501106. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D. Stabilization and Band-Gap Tuning of the 1T-MoS2 Monolayer by Covalent Functionalization. Chem. Mater. 2015, 27, 3743–3748. [Google Scholar] [CrossRef]
- Shin, J.-H.; Lee, M.-J.; Choi, J.-H.; Song, J.-A.; Kim, T.-H.; Oh, B.-K. Electrochemical H2O2 biosensor based on horseradish peroxidase encapsulated protein nanoparticles with reduced graphene oxide-modified gold electrode. Nano Converg. 2020, 7, 39. [Google Scholar] [CrossRef]
- Zhao, K.; Yan, X.; Gu, Y.; Kang, Z.; Bai, Z.; Cao, S.; Liu, Y.; Zhang, X.; Zhang, Y. Self-Powered Photoelectrochemical Biosensor Based on CdS/RGO/ZnO Nanowire Array Heterostructure. Small 2016, 12, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; El-Said, W.A.; Abu-Zied, B.M.; Choi, J.-W. Nanosheet composed of gold nanoparticle/graphene/epoxy resin based on ultrasonic fabrication for flexible dopamine biosensor using surface-enhanced Raman spectroscopy. Nano Converg. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Xu, B.; Zhang, H.; Gao, Y.; Zhang, H.; Song, D. A novel surface plasmon resonance biosensor based on graphene oxide decorated with gold nanorod–antibody conjugates for determination of transferrin. Biosens. Bioelectron. 2013, 45, 230–236. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Palanisamy, S.; Chen, S.-M. A simple electrochemical approach to fabricate a glucose biosensor based on graphene–glucose oxidase biocomposite. Biosens. Bioelectron. 2013, 39, 70–75. [Google Scholar] [CrossRef]
- Meng, F.; Sun, H.; Huang, Y.; Tang, Y.; Chen, Q.; Miao, P. Peptide cleavage-based electrochemical biosensor coupling graphene oxide and silver nanoparticles. Anal. Chim. Acta 2019, 1047, 45–51. [Google Scholar] [CrossRef]
- Healy, D.A.; Hayes, C.J.; Leonard, P.; McKenna, L.; O’Kennedy, R. Biosensor developments: Application to prostate-specific antigen detection. Trends Biotechnol. 2007, 25, 125–131. [Google Scholar] [CrossRef]
- Wang, Y.; Brunsen, A.; Jonas, U.; Dostalek, J.; Knoll, W. Prostate specific antigen biosensor based on long range surface plasmon-enhanced fluorescence spectroscopy and dextran hydrogel binding matrix. Anal. Chem. 2009, 81, 9625–9632. [Google Scholar] [CrossRef]
- He, Y.; Xie, S.; Yang, X.; Yuan, R.; Chai, Y. Electrochemical peptide biosensor based on in situ silver deposition for detection of prostate specific antigen. ACS Appl. Mater. Interfaces 2015, 7, 13360–13366. [Google Scholar] [CrossRef]
- Wang, F.; Chu, Y.; Ai, Y.; Chen, L.; Gao, F. Graphene oxide with in-situ grown Prussian Blue as an electrochemical probe for microRNA-122. Microchim. Acta 2019, 186, 116. [Google Scholar] [CrossRef]
- Hashemzadeh, S.; Omidi, Y.; Rafii-Tabar, H. Amperometric lactate nanobiosensor based on reduced graphene oxide, carbon nanotube and gold nanoparticle nanocomposite. Microchim. Acta 2019, 186, 680. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, Y.; Wang, M.; Zhang, C.; Li, Z.; Huo, Y.; Li, Z.; Xu, S.; Man, B.; Jiang, S. A novel natural surface-enhanced Raman spectroscopy (SERS) substrate based on graphene oxide-Ag nanoparticles-Mytilus coruscus hybrid system. Sens. Actuators B Chem. 2018, 261, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Heng, H.; Lv, J.; Jiang, T.; Wang, Z.; Dai, Z. Graphene Oxide-Assisted and DNA-Modulated SERS of AuCu Alloy for the Fabrication of Apurinic/Apyrimidinic Endonuclease 1 Biosensor. Small 2019, 15, 1901506. [Google Scholar] [CrossRef]
- Pan, X.; Li, L.; Lin, H.; Tan, J.; Wang, H.; Liao, M.; Chen, C.; Shan, B.; Chen, Y.; Li, M. A graphene oxide-gold nanostar hybrid based-paper biosensor for label-free SERS detection of serum bilirubin for diagnosis of jaundice. Biosens. Bioelectron. 2019, 145, 111713. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, K.-S.; Park, D.-S.; Won, M.-S.; Shim, Y.-B. An amperometric bilirubin biosensor based on a conductive poly-terthiophene–Mn (II) complex. Biosens. Bioelectron. 2008, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yan, J.; Zhang, C. Piezoelectric detection of bilirubin based on bilirubin-imprinted titania film electrode. Anal. Biochem. 2012, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ji, D.; Meng, H.; Zhang, L.; Wang, J.; Huang, Z.; Chen, J.; Li, J.; Li, Z. A portable fluorescence biosensor for rapid and sensitive glutathione detection by using quantum dots-based lateral flow test strip. Sens. Actuators B Chem. 2018, 262, 486–492. [Google Scholar] [CrossRef]
- Lee, W.-I.; Shrivastava, S.; Duy, L.-T.; Kim, B.Y.; Son, Y.-M.; Lee, N.-E. A smartphone imaging-based label-free and dual-wavelength fluorescent biosensor with high sensitivity and accuracy. Biosens. Bioelectron. 2017, 94, 643–650. [Google Scholar] [CrossRef]
- Della Ventura, B.; Gelzo, M.; Battista, E.; Alabastri, A.; Schirato, A.; Castaldo, G.; Corso, G.; Gentile, F.; Velotta, R. Biosensor for point-of-care analysis of immunoglobulins in urine by metal enhanced fluorescence from gold nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 3753–3762. [Google Scholar] [CrossRef]
- Fan, Z.; Li, S.; Yuan, F.; Fan, L. Fluorescent graphene quantum dots for biosensing and bioimaging. RSC Adv. 2015, 5, 19773–19789. [Google Scholar] [CrossRef]
- Xie, R.; Wang, Z.; Zhou, W.; Liu, Y.; Fan, L.; Li, Y.; Li, X. Graphene quantum dots as smart probes for biosensing. Anal. Methods 2016, 8, 4001–4016. [Google Scholar] [CrossRef]
- Ge, S.; He, J.; Ma, C.; Liu, J.; Xi, F.; Dong, X. One-step synthesis of boron-doped graphene quantum dots for fluorescent sensors and biosensor. Talanta 2019, 199, 581–589. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, J.; Xin, J.H. A novel graphene oxide-based fluorescent nanosensor for selective detection of Fe3+ with a wide linear concentration and its application in logic gate. Biosens. Bioelectron. 2015, 70, 69–73. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahn, M.-S.; Hahn, Y.-B. ZnO nanorods array based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017, 498, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chan, C.; Pang, Y.; Ye, W.; Tian, F.; Lyu, J.; Zhang, Y.; Yang, M. A fluorescence resonance energy transfer (FRET) biosensor based on graphene quantum dots (GQDs) and gold nanoparticles (AuNPs) for the detection of mecA gene sequence of Staphylococcus aureus. Biosens. Bioelectron. 2015, 67, 595–600. [Google Scholar] [CrossRef]
- Liu, X.; Na, W.; Liu, Q.; Su, X. A novel label-free fluorescent sensor for highly sensitive detection of bleomycin based on nitrogen-doped graphene quantum dots. Anal. Chim. Acta 2018, 1028, 45–49. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Niu, C.-G.; Wang, X.-Y.; Huang, D.-W.; Zhang, L.; Zeng, G.-M. Magnetic separate “turn-on” fluorescent biosensor for Bisphenol A based on magnetic oxidation graphene. Talanta 2017, 168, 196–202. [Google Scholar] [CrossRef]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Ma, J.J.; Chen, J.R.; Feng, H. A universal fluorescence sensing strategy based on biocompatible graphene quantum dots and graphene oxide for the detection of DNA. Nanoscale 2014, 6, 5671–5674. [Google Scholar] [CrossRef]
- Afsahi, S.; Lerner, M.B.; Goldstein, J.M.; Lee, J.; Tang, X.; Bagarozzi, D.A., Jr.; Pan, D.; Locascio, L.; Walker, A.; Barron, F. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018, 100, 85–88. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Bagihalli, G.B.; Reddy, K.R.; Ravindranadh, K.; Reddy, C.V. A novel biosensor based on graphene oxide-nanoclay hybrid electrode for the detection of Theophylline for healthcare applications. Microchem. J. 2019, 149, 103985. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Wu, W. Graphene-based steganographic aptasensor for information computing and monitoring toxins of biofilm in food. Front. Microbiol. 2020, 10, 3139. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.-W.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 111343. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhang, X.; Zhang, Q.; Liang, Z.; Ma, Q.; Su, X. A novel high efficient electrochemiluminescence sensor based on reductive Cu (I) particles catalyzed Zn-doped MoS2 QDs for HPV 16 DNA determination. Biosens. Bioelectron. 2020, 160, 112217. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Feng, X.; Gao, T.; Liu, G.; Mao, Y.; Lin, J.; Yu, X.; Luo, X. Aptamer induced multicoloured Au NCs-MoS2 “switch on” fluorescence resonance energy transfer biosensor for dual color simultaneous detection of multiple tumor markers by single wavelength excitation. Anal. Chim. Acta 2017, 983, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.; Su, D.; Zhang, Y.; Lu, H.; Yan, X.; Bai, J.; Gao, Y.; Lu, G. The DNA controllable peroxidase mimetic activity of MoS2 nanosheets for constructing a robust colorimetric biosensor. Nanoscale 2020, 12, 19420–19428. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, X.J.; You, T.T.; Yang, N.; Wang, G.S.; Yin, P.G. Three-dimensional MoS2-NS@Au-NPs hybrids as SERS sensor for quantitative and ultrasensitive detection of melamine in milk. J. Raman Spectrosc. 2018, 49, 245–255. [Google Scholar] [CrossRef]
- Li, X.; Peng, K. Hydrothermal synthesis of MoS2 nanosheet/palygorskite nanofiber hybrid nanostructures for enhanced catalytic activity. Appl. Clay Sci. 2018, 162, 175–181. [Google Scholar] [CrossRef]
- Baghban, N.; Yilmaz, E.; Soylak, M. Nanodiamond/MoS2 nanorod composite as a novel sorbent for fast and effective vortex-assisted micro solid phase extraction of lead (II) and copper (II) for their flame atomic absorption spectrometric detection. J. Mol. Liq. 2017, 234, 260–267. [Google Scholar] [CrossRef]
- Sui, C.; Li, F.; Wu, H.; Yin, H.; Zhang, S.; Waterhouse, G.I.; Wang, J.; Zhu, L.; Ai, S. Photoelectrochemical biosensor for 5hmC detection based on the photocurrent inhibition effect of ZnO on MoS2/C3N4 heterojunction. Biosens. Bioelectron. 2019, 142, 111516. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Yoon, J.; Yi, C.; Lee, T.; Choi, J.-W. Flexible HIV-1 Biosensor Based on the Au/MoS2 Nanoparticles/Au Nanolayer on the PET Substrate. Nanomaterials 2019, 9, 1076. [Google Scholar] [CrossRef]
- Parra-Alfambra, A.M.; Casero, E.; Vázquez, L.; Quintana, C.; del Pozo, M.; Petit-Domínguez, M.D. MoS2 nanosheets for improving analytical performance of lactate biosensors. Sens. Actuators B Chem. 2018, 274, 310–317. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, Z.; Liu, X.; Yang, J. High-performance electrochemical sensing of circulating tumor DNA in peripheral blood based on poly-xanthurenic acid functionalized MoS2 nanosheets. Biosens. Bioelectron. 2018, 105, 116–120. [Google Scholar] [CrossRef]
- Xiao, M.; Chandrasekaran, A.R.; Ji, W.; Li, F.; Man, T.; Zhu, C.; Shen, X.; Pei, H.; Li, Q.; Li, L. Affinity-modulated molecular beacons on MoS2 nanosheets for microRNA detection. ACS Appl. Mater. Interfaces 2018, 10, 35794–35800. [Google Scholar] [CrossRef] [PubMed]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.W.; Li, L.; Wang, F.; Gu, Y.T. Effects of long non-coding RNA HOST2 on cell migration and invasion by regulating MicroRNA let-7b in breast cancer. J. Cell. Biochem. 2018, 119, 4570–4580. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Wu, Z.; Chen, Y.; Zhou, X.; Shen, A.; Hu, J. A novel biosensor based on single-layer MoS2 nanosheets for detection of Ag+. Talanta 2015, 132, 658–663. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, X.; Hu, W. A fluorescence aptasensor based on semiconductor quantum dots and MoS2 nanosheets for ochratoxin A detection. Sens. Actuators B Chem. 2017, 246, 61–67. [Google Scholar] [CrossRef]
- Cao, H.; Dong, W.; Wang, T.; Shi, W.; Fu, C.; Wu, Y. Aptasensor Based on MoS2 Quantum Dots with Upconversion Fluorescence for Microcystin-LR Detection via the Inner Filter Effect. ACS Sustain. Chem. Eng. 2020, 8, 10939–10946. [Google Scholar] [CrossRef]
- Han, C.; Doepke, A.; Cho, W.; Likodimos, V.; de la Cruz, A.A.; Back, T.; Heineman, W.R.; Halsall, H.B.; Shanov, V.N.; Schulz, M.J. A multiwalled-carbon-nanotube-based biosensor for monitoring microcystin-LR in sources of drinking water supplies. Adv. Funct. Mater. 2013, 23, 1807–1816. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Niu, X.-Y.; Chen, H.-L.; Zhao, S.-G.; Chen, X.-G. Switch-on fluorescence sensor for ascorbic acid detection based on MoS2 quantum dots-MnO2 nanosheets system and its application in fruit samples. Chin. Chem. Lett. 2017, 28, 338–344. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Zhang, L.; Su, X. Ratiometric fluorescence system for pH sensing and urea detection based on MoS2 quantum dots and 2, 3-diaminophenazine. Anal. Chim. Acta 2019, 1077, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, R.; Zhuo, J.; Zhu, Z.; Shao, Y.; Li, M. Direct detection of DNA below ppb level based on thionin-functionalized layered MoS2 electrochemical sensors. Anal. Chem. 2014, 86, 12064–12069. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, Y.; Wu, Z.; Li, S.; Zhang, Q.; Chen, Z.; Jiang, J.; Lin, S.; Zhu, L.; Li, C. Two-dimensional molybdenum disulfide (MoS2) with gold nanoparticles for biosensing of explosives by optical spectroscopy. Sens. Actuators B Chem. 2018, 261, 279–287. [Google Scholar] [CrossRef]
- Singha, S.S.; Mondal, S.; Bhattacharya, T.S.; Das, L.; Sen, K.; Satpati, B.; Das, K.; Singha, A. Au nanoparticles functionalized 3D-MoS2 nanoflower: An efficient SERS matrix for biomolecule sensing. Biosens. Bioelectron. 2018, 119, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shen, Q.; Xue, P.; Qi, H.; Wu, Y.; Teng, Y.; Zhang, Y.; Liu, Y.; Zhao, X.; Liu, X. A Highly Sensitive and Stable SERS Sensor for Malachite Green Detection Based on Ag Nanoparticles In Situ Generated on 3D MoS2 Nanoflowers. ChemistrySelect 2020, 5, 354–359. [Google Scholar] [CrossRef]
- Dou, X.; Zhao, L.; Li, X.; Qin, L.; Han, S.; Kang, S.-Z. Ag nanoparticles decorated mesh-like MoS2 hierarchical nanostructure fabricated on Ti foil: A highly sensitive SERS substrate for detection of trace malachite green in flowing water. Appl. Surf. Sci. 2020, 509, 145331. [Google Scholar] [CrossRef]
- Jiang, J.-W. Graphene versus MoS2: A short review. Front. Phys. 2015, 10, 287–302. [Google Scholar] [CrossRef]
- Zheng, G.; Zou, X.; Chen, Y.; Xu, L.; Rao, W. Fano resonance in graphene-MoS2 heterostructure-based surface plasmon resonance biosensor and its potential applications. Opt. Mater. 2017, 66, 171–178. [Google Scholar] [CrossRef]
- Yagati, A.K.; Lee, T.; Min, J.; Choi, J.-W. Electrochemical performance of gold nanoparticle–cytochrome c hybrid interface for H2O2 detection. Colloid. Surf. B Biointerfaces 2012, 92, 161–167. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M. A novel nonenzymatic hydrogen peroxide sensor based on the synthesized mesoporous carbon and silver nanoparticles nanohybrid. Sens. Actuator. B Chem. 2014, 203, 919–925. [Google Scholar] [CrossRef]
- Tajabadi, M.T.; Sookhakian, M.; Zalnezhad, E.; Yoon, G.H.; Hamouda, A.M.S.; Azarang, M.; Basirun, W.J.; Alias, Y. Electrodeposition of flower-like platinum on electrophoretically grown nitrogen-doped graphene as a highly sensitive electrochemical non-enzymatic biosensor for hydrogen peroxide detection. Appl. Surf. Sci. 2016, 386, 418–426. [Google Scholar] [CrossRef]
- Jeong, J.-M.; Yang, M.; Kim, D.S.; Lee, T.J.; Choi, B.G. High performance electrochemical glucose sensor based on three-dimensional MoS2/graphene aerogel. J. Colloid Interface Sci. 2017, 506, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Su, Z.; Wei, G. Three-dimensional porous reduced graphene oxide decorated with MoS2 quantum dots for electrochemical determination of hydrogen peroxide. Mater. Today Chem. 2018, 7, 76–83. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Marquette, C.A.; Dutta, P.; Sumana, G. Integrated graphene quantum dot decorated functionalized nanosheet biosensor for mycotoxin detection. Anal. Bioanal. Chem. 2020, 412, 7029–7041. [Google Scholar] [CrossRef]
- Schmetzer, O.; Moldenhauer, G.; Nicolaou, A.; Schlag, P.; Riesenberg, R.; Pezzutto, A. Detection of circulating tumor-associated antigen depends on the domains recognized by the monoclonal antibodies used: N-terminal trimmed EpCAM-levels are much higher than untrimmed forms. Immunol. Lett. 2012, 143, 184–192. [Google Scholar] [CrossRef]
- Seeber, A.; Martowicz, A.; Spizzo, G.; Buratti, T.; Obrist, P.; Fong, D.; Gastl, G.; Untergasser, G. Soluble EpCAM levels in ascites correlate with positive cytology and neutralize catumaxomab activity in vitro. BMC Cancer 2015, 15, 372. [Google Scholar] [CrossRef][Green Version]
- Jung, Y.K.; Woo, M.-A.; Soh, H.T.; Park, H.G. Aptamer-based cell imaging reagents capable of fluorescence switching. Chem. Commun. 2014, 50, 12329–12332. [Google Scholar] [CrossRef]
- Hassanzadeh, J.; Khataee, A. Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta 2018, 178, 992–1000. [Google Scholar] [CrossRef]
- Ahmadalinezhad, A.; Chen, A. High-performance electrochemical biosensor for the detection of total cholesterol. Biosens. Bioelectron. 2011, 26, 4508–4513. [Google Scholar] [CrossRef]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuator. B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Hong, L.; Liu, A.-L.; Li, G.-W.; Chen, W.; Lin, X.-H. Chemiluminescent cholesterol sensor based on peroxidase-like activity of cupric oxide nanoparticles. Biosens. Bioelectron. 2013, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Ji, J.; Sun, J.; Wang, J.; Wang, H.; Zhang, Y.; Ding, H.; Lu, Y.; Xu, D.; Sun, X. A novel magnetic fluorescent biosensor based on graphene quantum dots for rapid, efficient, and sensitive separation and detection of circulating tumor cells. Anal. Bioanal. Chem. 2019, 411, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Aksimsek, S.; Jussila, H.; Sun, Z. Graphene–MoS2–metal hybrid structures for plasmonic biosensors. Opt. Commun. 2018, 428, 233–239. [Google Scholar] [CrossRef]
- Vahed, H.; Nadri, C. Sensitivity enhancement of SPR optical biosensor based on Graphene–MoS2 structure with nanocomposite layer. Opt. Mater. 2019, 88, 161–166. [Google Scholar] [CrossRef]
- Alamri, M.; Sakidja, R.; Goul, R.; Ghopry, S.; Wu, J.Z. Plasmonic Au nanoparticles on 2D MoS2/Graphene van der Waals heterostructures for high-sensitivity surface-enhanced Raman spectroscopy. ACS Appl. Nano Mater. 2019, 2, 1412–1420. [Google Scholar] [CrossRef]
- Rahman, M.S.; Anower, M.; Rahman, M.K.; Hasan, M.R.; Hossain, M.B.; Haque, M.I. Modeling of a highly sensitive MoS2-Graphene hybrid based fiber optic SPR biosensor for sensing DNA hybridization. Optik 2017, 140, 989–997. [Google Scholar] [CrossRef]
- Wu, L.; Jia, Y.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity improved SPR biosensor based on the MoS2/graphene–aluminum hybrid structure. J. Lightwave Technol. 2016, 35, 82–87. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Gao, X.; Man, P.; Sun, Y.; Liu, C.; Li, Z.; Xu, Y.; Man, B.; Yang, C. Formation of the AuNPs/GO@MoS2/AuNPs nanostructures for the SERS application. Sens. Actuators B Chem. 2019, 282, 809–817. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Hu, Y.; Sun, T.; Song, Z.; Zheng, Y.; Cao, Y.; Cai, Z.; Cao, M.; Peng, L.; et al. Raman enhancement on ultra-clean graphene quantum dots produced by quasi-equilibrium plasma-enhanced chemical vapor deposition. Nat. Commun. 2018, 9, 193. [Google Scholar] [CrossRef]
- Zheng, Z.; Cong, S.; Gong, W.; Xuan, J.; Li, G.; Lu, W.; Geng, F.; Zhao, Z. Semiconductor SERS enhancement enabled by oxygen incorporation. Nat. Commun. 2017, 8, 1993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, S.; Gui, M.; Asif, M.; Wang, W.; Wang, F.; Liu, H. Graphene paper supported MoS2 nanocrystals monolayer with Cu submicron-buds: High-performance flexible platform for sensing in sweat. Anal. Biochem. 2018, 543, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Cheng, Z.; Li, H.; Yang, L.; Zhu, J.; Zheng, X.; Chen, Y.; Liu, Z.; Zhu, H.; Cheng, H. Stretchable, ultrasensitive, and low-temperature NO2 sensors based on MoS2@rGO nanocomposites. Mater. Today Phys. 2020, 15, 100265. [Google Scholar] [CrossRef]
- Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Sharma, S.; Xuan, X.; Park, J.Y. MoS2-Decorated Laser-Induced Graphene for a Highly Sensitive, Hysteresis-free, and Reliable Piezoresistive Strain Sensor. ACS Appl. Mater. Interfaces 2019, 11, 22531–22542. [Google Scholar] [CrossRef] [PubMed]
| Biosensors Based on the Graphene, MoS2 and Graphene/MoS2 Nanohybrid for Biosensors | |||||
|---|---|---|---|---|---|
| Type | Composition | Sensing Probe | Target | Utilized Technique | Reference |
| Graphene-based biosensors | Peptide/GO/AgNP | Peptide | PSA | Linear sweep voltammetry | [88] |
| GO/AuNS | GO | Bilirubin | SERS | [96] | |
| BQD | BQD | Fe3+, Cyt C, Pi | Fluorescence | [104] | |
| MoS2-based biosensors | Au/MoS2/Au multilayer/gp120 antibody | Gp120 antibody | gp120 | Square wave voltammetry | [122] |
| MoS2 nanosheet/ssDNA with FAM/DSN | ssDNA with FAM | let-7b miRNA | Fluorescence | [125] | |
| MoS2 QD/aptamer-modified AuNP | Aptamer | MC–LR | Fluorescence | [130] | |
| Graphene/MoS2 nanohybrid-based biosensors | Mb/GO@MoS2 | Mb | H2O2 | Amperometry | [30] |
| graphene QD/aptamer/MoS2 nanosheet | EpCAM aptamer | EpCAM | Fluorescence | [76] | |
| W-MoS2/ GMFs/3D nickel | Raman signal itself from each target molecule | rhodamine B, methylene blue and adenosine | SERS | [77] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.; Lim, J.; Shin, M.; Lee, S.-N.; Choi, J.-W. Graphene/MoS2 Nanohybrid for Biosensors. Materials 2021, 14, 518. https://doi.org/10.3390/ma14030518
Yoon J, Lim J, Shin M, Lee S-N, Choi J-W. Graphene/MoS2 Nanohybrid for Biosensors. Materials. 2021; 14(3):518. https://doi.org/10.3390/ma14030518
Chicago/Turabian StyleYoon, Jinho, Joungpyo Lim, Minkyu Shin, Sang-Nam Lee, and Jeong-Woo Choi. 2021. "Graphene/MoS2 Nanohybrid for Biosensors" Materials 14, no. 3: 518. https://doi.org/10.3390/ma14030518
APA StyleYoon, J., Lim, J., Shin, M., Lee, S.-N., & Choi, J.-W. (2021). Graphene/MoS2 Nanohybrid for Biosensors. Materials, 14(3), 518. https://doi.org/10.3390/ma14030518






