The Impact of Ground Tire Rubber Oxidation with H2O2 and KMnO4 on the Structure and Performance of Flexible Polyurethane/Ground Tire Rubber Composite Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modifications of GTR
2.3. Preparation of Polyurethane/GTR Composite Foams
2.4. Measurements
3. Results and Discussion
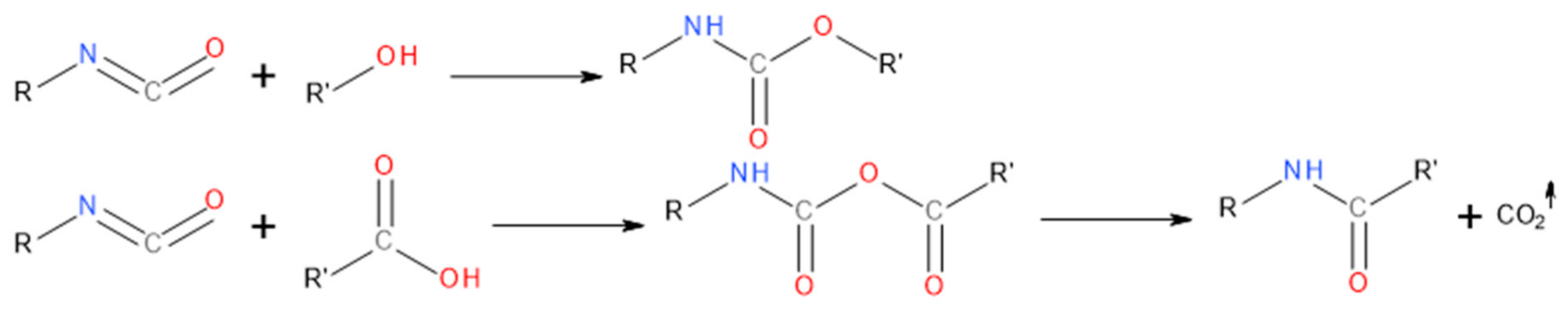
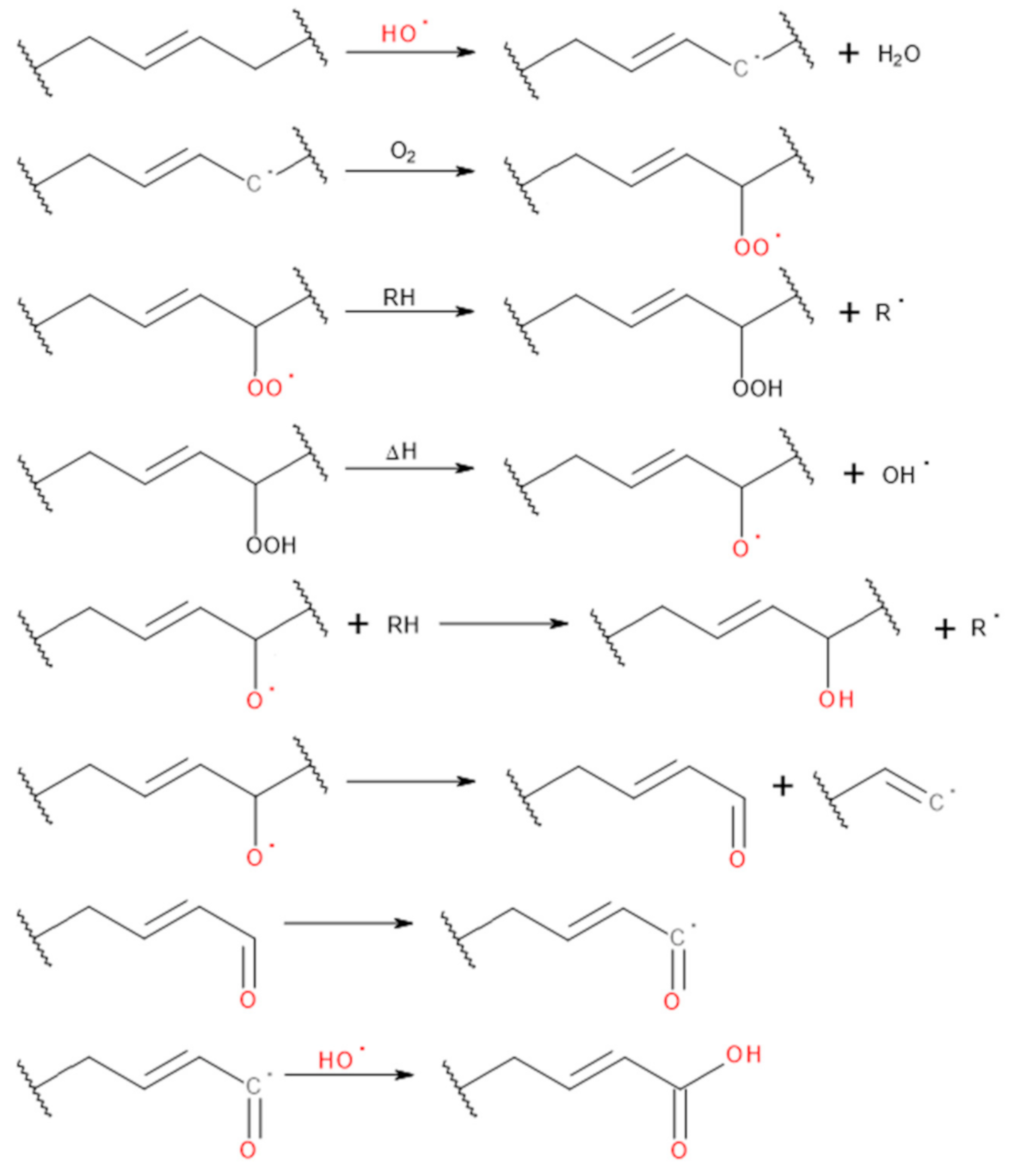
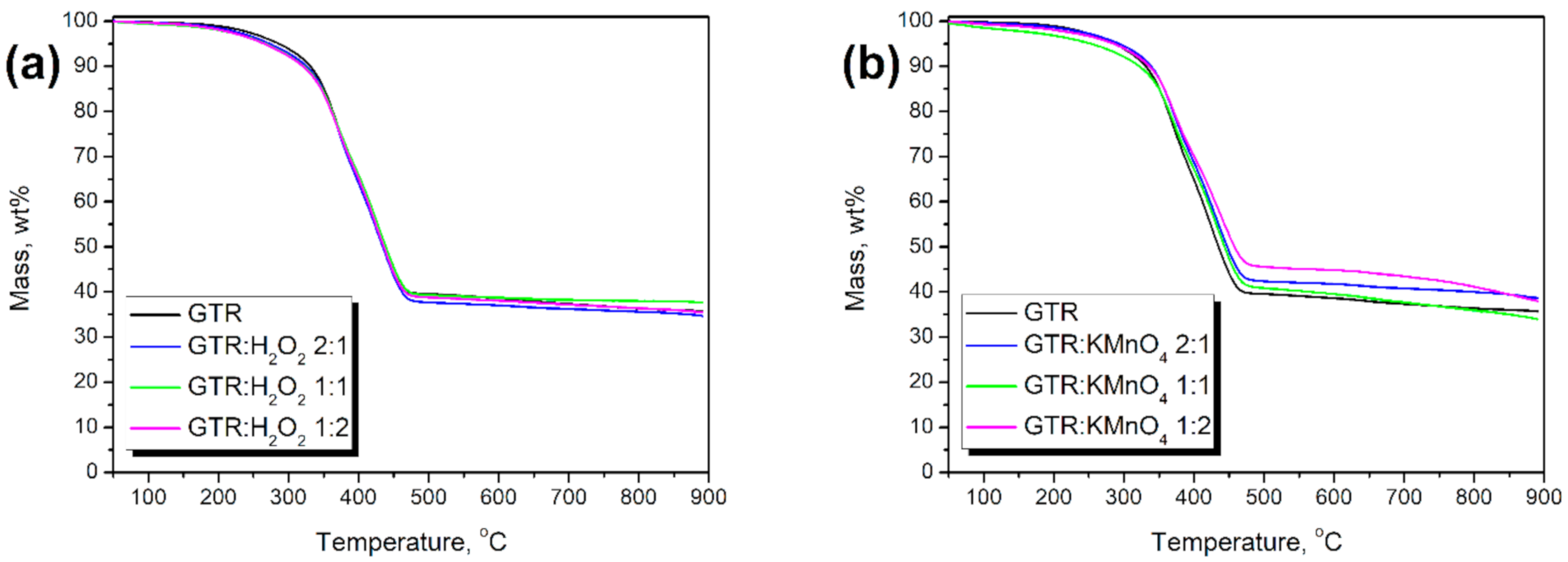
3.1. Changes in GTR Structure
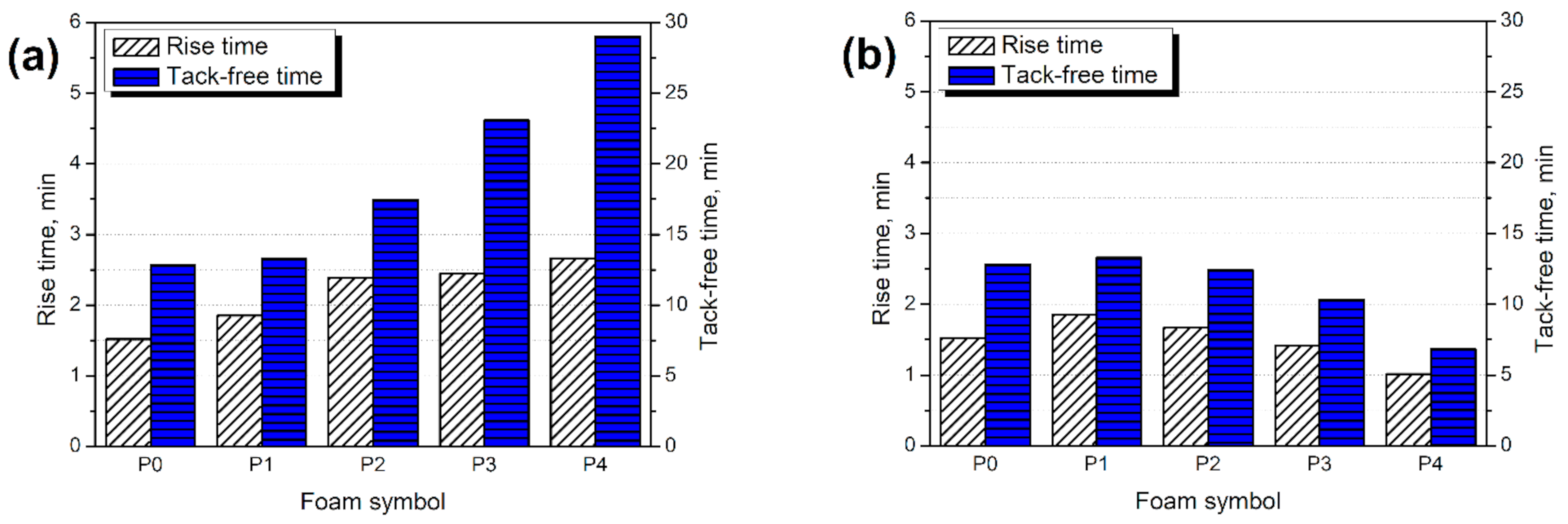
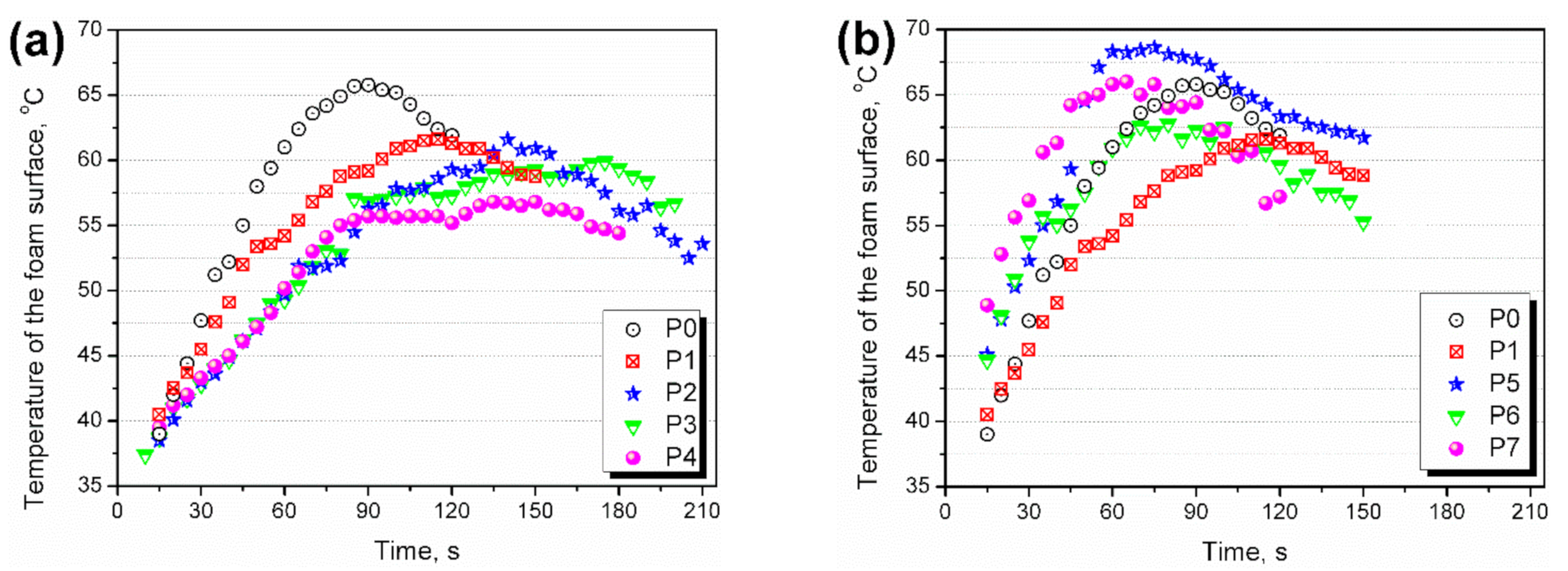
3.2. Kinetics of Foaming
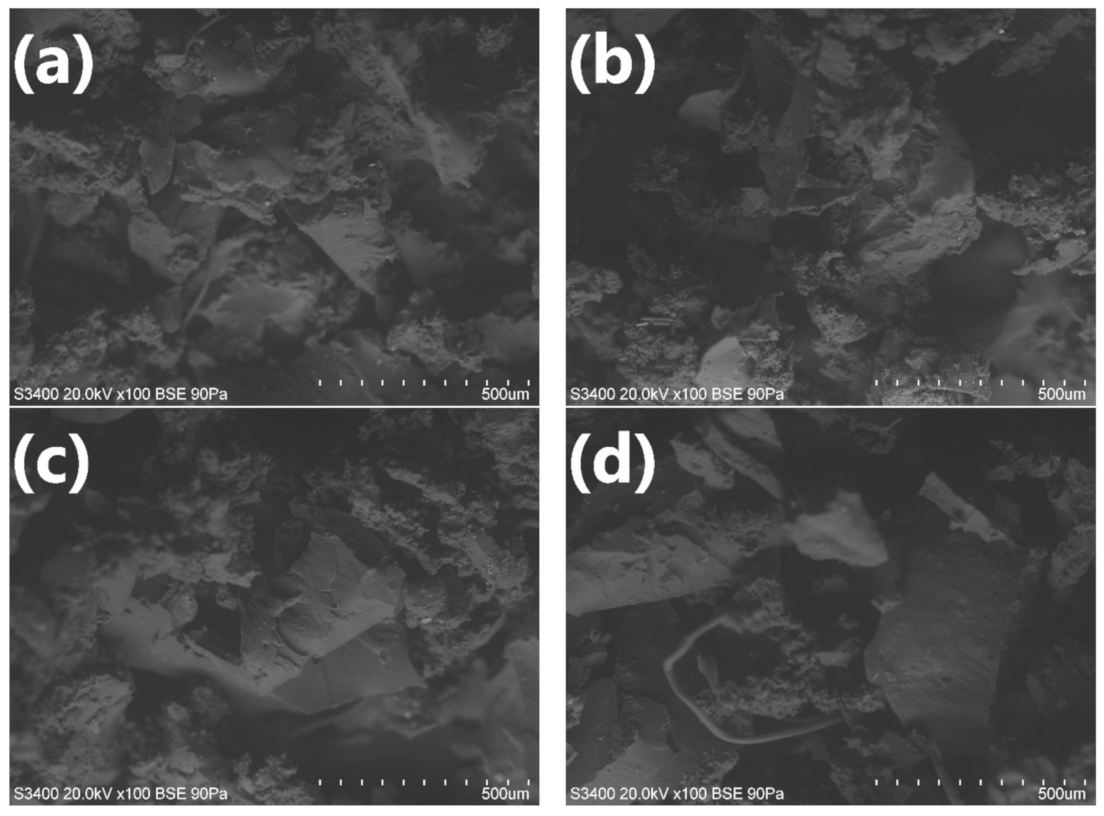
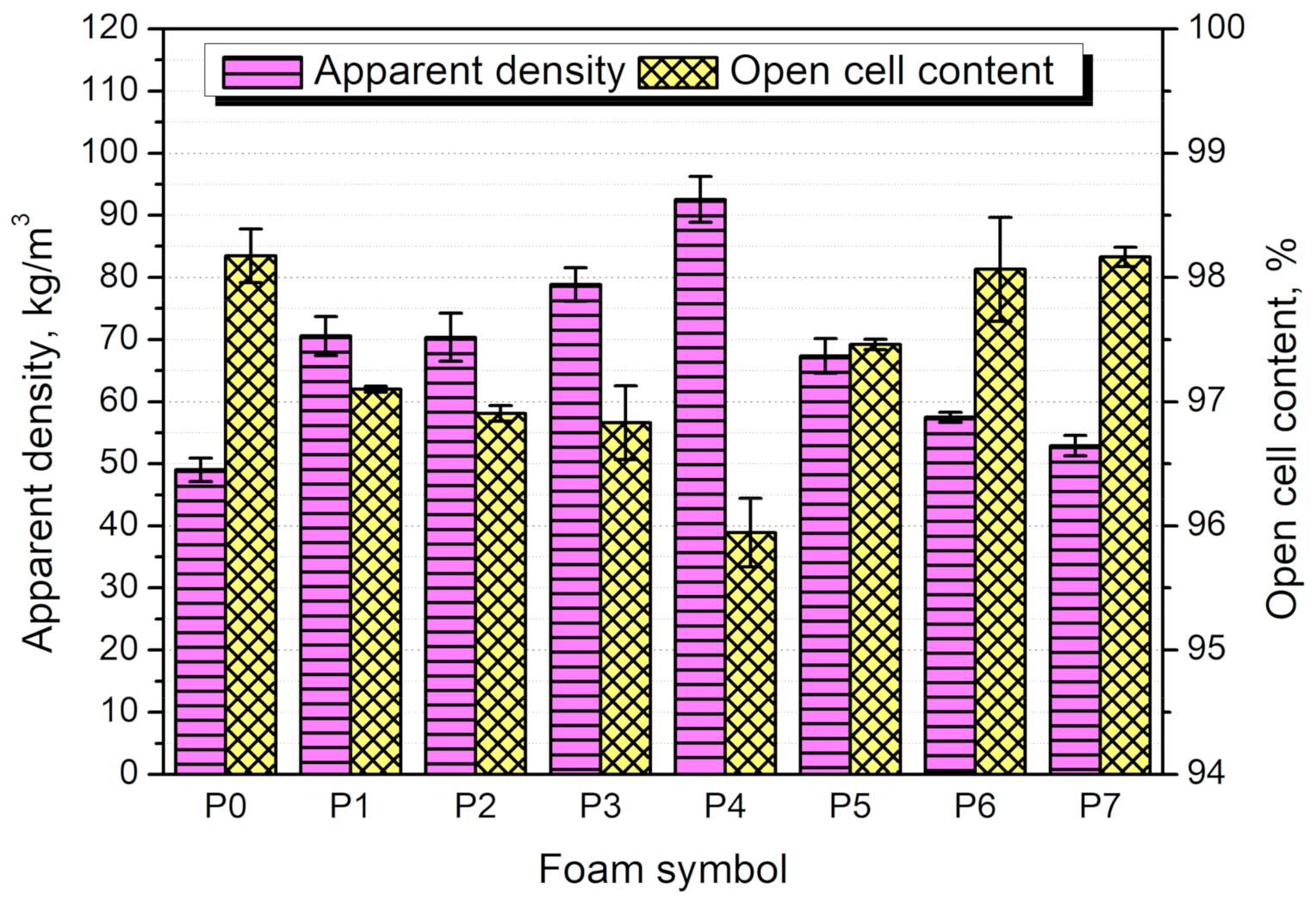
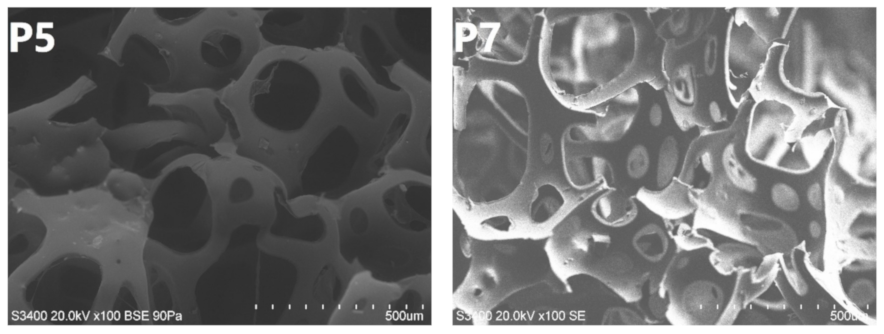
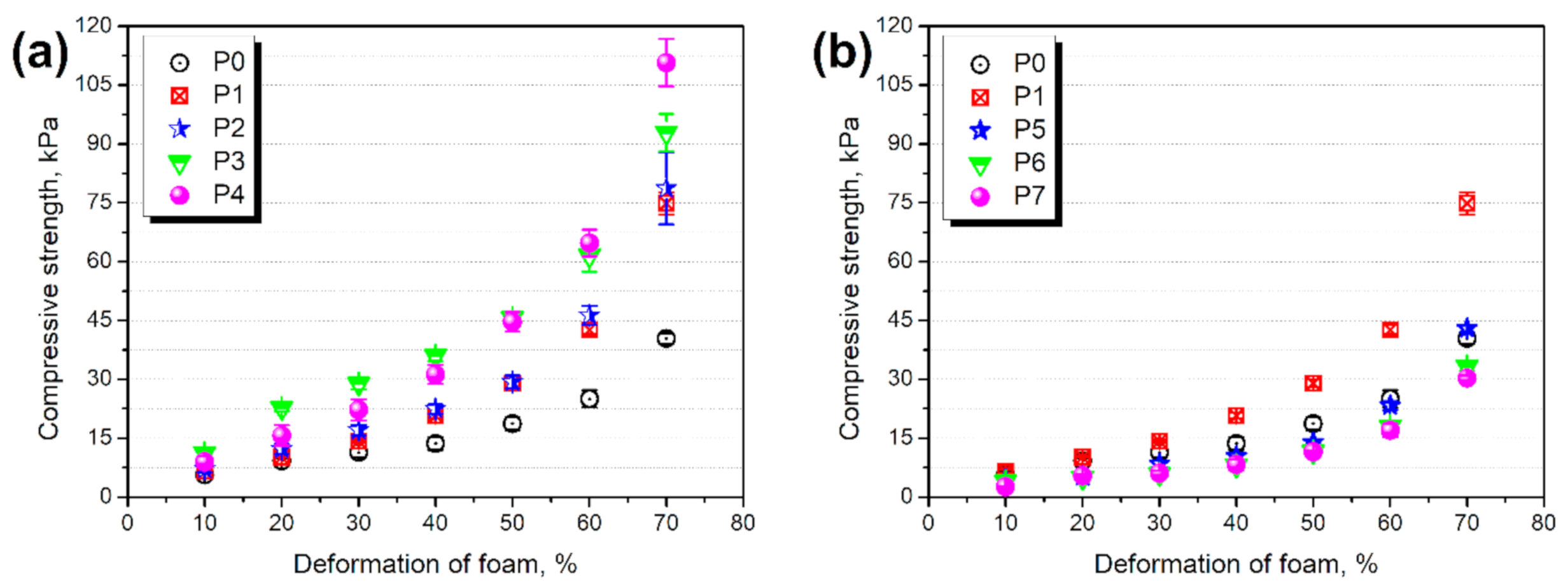
3.3. Structure and Properties of PU/GTR Composite Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papiński, J.; Żabski, L. Zrozumieć poliuretany. Mater. Bud. 2011, 1, 57–58. [Google Scholar]
- Swinarew, B. Poliuretany—Nowoczesne wszechstronne materiały. Część II—Pianki poliuretanowe. Przetw. Tworzyw 2015, 5, 428–434. [Google Scholar]
- Kurańska, M.; Prociak, A. Właściwości termoizolacyjne i mechaniczne spienionych kompozytów poliuretanowych z włóknami konopnymi. Chemik 2011, 65, 1055–1058. [Google Scholar]
- Auguścik-Królikowska, M.; Ryszkowska, J.; Szczepkowski, L.; Kwiatkowski, D.; Kołbuk-Konieczny, D.; Szymańska, J. Viscoelastic polyurethane foams with the addition of mint. Polimery 2020, 65, 196–207. [Google Scholar] [CrossRef]
- Kurańska, M.; Michałowski, S.; Radwańska, J.; Jurecka, M.; Zieleniewska, M.; Szczepkowski, L.; Ryszkowska, J.; Prociak, A. Bio-poliole z oleju rzepakowego jako surowce do kompozytów naturalnych z napełniaczami naturalnymi dla kosmetyki. Przem. Chem. 2016, 95, 256–262. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J. Kompozyty sztywnych pianek poliuretanowo-poliizocyjanurowych z korą dębu szypułkowego. Polimery 2017, 62, 666–672. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. Application of Walnut Shells-Derived Biopolyol in the Synthesis of Rigid Polyurethane Foams. Materials 2020, 13, 2687. [Google Scholar] [CrossRef]
- Barnat, W.; Miedzińska, D.; Niezgoda, T. Pianki poliuretanowe—Właściwości, zastosowania, recykling. Arch. Gospod. Odpadami Ochr. Sr. 2011, 13, 13–17. [Google Scholar]
- Tiuc, A.E.; Nemeş, O.; Vermesan, H.; Toma, A.C. New sound absorbent composite materials based on sawdust and polyurethane foam. Compos. Part B Eng. 2019, 165, 120–130. [Google Scholar] [CrossRef]
- El-Meligy, M.G.; Mohamed, S.H.; Mahani, R.M. Study mechanical, swelling and dielectric properties of prehydrolysed banana fiber—Waste polyurethane foam composites. Carbohydr. Polym. 2010, 80, 366–372. [Google Scholar] [CrossRef]
- Tiuc, A.E.; Vermeşan, H.; Gabor, T.; Vasile, O. Improved Sound Absorption Properties of Polyurethane Foam Mixed with Textile Waste. Energy Procedia 2016, 85, 559–565. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and applicational evaluation of the rigid polyurethane foam composites with egg shell waste. Polym. Degrad. Stabil. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Cachaço, A.G.; Afonso, M.D.; Pinto, M.L. New applications for foam composites of polyurethane and recycled rubber. J. Appl. Polym. Sci. 2013, 129, 2873–2881. [Google Scholar] [CrossRef]
- Pianomat. Available online: http://www.pianomat.pl/en/ (accessed on 11 January 2021).
- Tiuc, A.E.; Moga, L. Improvement of acoustic and thermal comfort by turning waste into composite materials. Rom. J. Acoust. Vib. 2013, 10, 77–82. [Google Scholar]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Ichchou, M.N.; Salvia, M.; Chettah, A. Dynamic Damping Properties of Thermoplastic Elastomers Based on EVA and Recycled Ground Tire Rubber. J. Elastomers Plast. 2011, 43, 317–340. [Google Scholar] [CrossRef]
- Ubaidillah, H.; Yahya, I.; Kristiani, R.; Muqowi, E.; Mazlan, S.A. Perfect sound insulation property of reclaimed waste tire rubber. AIP Conf. Proc. 2016, 1717. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Tian, D.; Li, H.; Lu, C. Mechanochemical devulcanization of ground tire rubber and its application in acoustic absorbent polyurethane foamed composites. J. Appl. Polym. Sci. 2012, 127, 4006–4014. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Dell’Arciprete, G.A.; Aranguren, M.I.; Marcovich, N.E. Polyurethane Foams Obtained from Castor Oil-based Polyol and Filled with Wood Flour. J. Compos. Mater. 2009, 43, 3057–3072. [Google Scholar] [CrossRef]
- Formela, K.; Klein, M.; Colom, X.; Saeb, M.R. Investigating the combined impact of plasticizer and shear force on the efficiency of low temperature reclaiming of ground tire rubber (GTR). Polym. Degrad. Stab. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- ASTM D2527-83(2017) Standard Specification for Rubber Seals—Splice Strength. Available online: https://www.astm.org/Standards/D2527.htm (accessed on 11 January 2021).
- PN-EN ISO 845:2010—Wersja Polska—Tworzywa Sztuczne Porowate i Gumy—Oznaczanie Gęstości Pozornej. Available online: https://sklep.pkn.pl/pn-en-iso-845-2010p.html (accessed on 11 January 2021).
- ISO 604:2002 Plastics—Determination of Compressive Properties. Available online: https://www.iso.org/standard/31261.html (accessed on 11 January 2021).
- Vilar, W.D. Química e Tecnologia dos Poliuretanos, 2nd ed.; Vilar Consultoria Técnica Ltda.: Rio de Janeiro, Brazil, 1998. [Google Scholar]
- Zedler, Ł.; Kosmela, P.; Olszewski, A.; Burger, P.; Formela, K.; Hejna, A. Recycling of Waste Rubber by Thermo-Mechanical Treatment in a Twin-Screw Extruder. Proceedings 2020, in press. [Google Scholar] [CrossRef]
- Shatanawi, K.M.; Biro, S.; Naser, M.; Amirkhanian, S.N. Improving the rheological properties of crumb rubber modified binder using hydrogen peroxide. Road Mater. Pavement Des. 2013, 14, 723–734. [Google Scholar] [CrossRef]
- Yehia, A.A.; Mull, M.A.; Ismail, M.N.; Hefny, Y.A.; Abdel-Bary, E.M. Effect of chemically modified waste rubber powder as a filler in natural rubber vulcanizates. J. Appl. Polym. Sci. 2004, 93, 30–36. [Google Scholar] [CrossRef]
- Fournier, H.M. Transformation des alcools primaires saturès en acides monobasiques correspondants. Comptes Rendus Acad. Sci. 1907, 144, 331. [Google Scholar]
- Dash, S.; Patel, S.; Mishra, B.K. Oxidation by permanganate: Synthetic and mechanistic aspects. Tetrahedron 2009, 65, 707–739. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Saegebarth, K.A. The Mechanisms of Permanganate Oxidation. IV. Hydroxylation of Olefins and Related Reactions. J. Am. Chem. Soc. 1957, 79, 2822–2824. [Google Scholar] [CrossRef]
- Sonnier, R.; Leroy, E.; Clerc, L.; Bergeret, A.; Lopez-Cuesta, J.M. Polyethylene/ground tyre rubber blends: Influence of particle morphology and oxidation on mechanical properties. Polym. Test. 2007, 26, 274–281. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Wei, Y.; Zhang, X.; Wei, D.; Li, J. Microscopic Properties of Hydrogen Peroxide Activated Crumb Rubber and Its Influence on the Rheological Properties of Crumb Rubber Modified Asphalt. Materials 2019, 12, 1434. [Google Scholar] [CrossRef]
- Kleps, T.; Piaskiewicz, M.; Parasiewicz, W. The use of thermogravimetry in the study of rubber devulcanization. J. Therm. Anal. Calorim. 2000, 60, 271–277. [Google Scholar] [CrossRef]
- Scuracchio, C.H.; Waki, D.A.; Da Silva, M.L.C.P. Thermal analysis of ground tire rubber devulcanized by microwaves. J. Therm. Anal. Calorim. 2007, 87, 893–897. [Google Scholar] [CrossRef]
- Nadal Gisbert, A.; Crespo Amorós, J.E.; López Martínez, J.; Macias Garcia, A. Study of thermal degradation kinetics of elastomeric powder (ground tire rubber). Polym. Plast. Technol. Eng. 2007, 47, 36–39. [Google Scholar] [CrossRef]
- Zhang, X.; Saha, P.; Cao, L.; Li, H.; Kim, J. Devulcanization of waste rubber powder using thiobisphenols as novel reclaiming agent. Waste Manag. 2018, 78, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Piszczyk, L.; Hejna, A.; Formela, K.; Danowska, M.; Strankowski, M. Rigid Polyurethane Foams Modified with Ground Tire Rubber—Mechanical, Morphological and Thermal Studies. Cell. Polym. 2015, 34, 45–62. [Google Scholar] [CrossRef]
- Piszczyk, Ł.; Hejna, A.; Danowska, M.; Strankowski, M.; Formela, K. Polyurethane/ground tire rubber composite foams based on polyglycerol: Processing, mechanical and thermal properties. J. Reinf. Plast. Compos. 2015, 34, 708–717. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Przybysz, M.; Ryl, J.; Saeb, M.R.; Piszczyk, Ł. Structural, thermal and physico-mechanical properties of polyurethane/brewers’ spent grain composite foams modified with ground tire rubber. Ind. Crop. Prod. 2017, 108, 844–852. [Google Scholar] [CrossRef]
- Wu, J.W.; Sung, W.F.; Chu, H.S. Thermal conductivity of polyurethane foams. Int. J. Heat Mass Transf. 1999, 43, 2211–2217. [Google Scholar] [CrossRef]
- Piszczyk, Ł.; Hejna, A.; Formela, K.; Danowska, M.; Strankowski, M. Effect of ground tire rubber on structural, mechanical and thermal properties of flexible polyurethane foams. Iran. Polym. J. 2014, 24, 75–84. [Google Scholar] [CrossRef]
- Gayathri, R.; Vasanthakumari, R.; Padmanabhan, C. Sound absorption, thermal and mechanical behavior of polyurethane foam modified with nano silica, nano clay and crumb rubber fillers. Int. J. Sci. Eng. Res. 2013, 4, 301–308. [Google Scholar]
- Hejna, A.; Kopczyńska, M.; Kozłowska, U.; Klein, M.; Kosmela, P.; Piszczyk, Ł. Foamed Polyurethane Composites with Different Types of Ash—Morphological, Mechanical and Thermal Behavior Assessments. Cell. Polym. 2016, 35, 287–308. [Google Scholar] [CrossRef]
- Hejna, A.; Kosmela, P.; Kirpluks, M.; Cabulis, U.; Klein, M.; Haponiuk, J.; Piszczyk, Ł. Structure, Mechanical, Thermal and Fire Behavior Assessments of Environmentally Friendly Crude Glycerol-Based Rigid Polyisocyanurate Foams. J. Polym. Environ. 2017, 26, 1854–1868. [Google Scholar] [CrossRef]





| Component | Foam Symbol | |||||||
|---|---|---|---|---|---|---|---|---|
| P0 | P1 | P2 | P3 | P4 | P5 | P6 | P7 | |
| Component Content, wt.% | ||||||||
| Polyol | 58 | 47 | 47 | 47 | 47 | 47 | 47 | 47 |
| Isocyanate | 42 | 33 | 33 | 33 | 33 | 33 | 33 | 33 |
| GTR | - | 20 | - | - | - | - | - | - |
| GTR:H2O2 2:1 | - | - | 20 | - | - | - | - | - |
| GTR:H2O2 1:1 | - | - | - | 20 | - | - | - | - |
| GTR:H2O2 1:2 | - | - | - | - | 20 | - | - | - |
| GTR:KMnO4 2:1 | - | - | - | - | - | 20 | - | - |
| GTR:KMnO4 1:1 | - | - | - | - | - | - | 20 | - |
| GTR:KMnO4 1:2 | - | - | - | - | - | - | - | 20 |
| Sample | %NCO, % | ΔNCO, % | LOH mg KOH/g |
|---|---|---|---|
| GTR | 33.3 ± 1.1 | 9.4 ± 1.1 | 61.7 ± 3.0 |
| GTR:H2O2 2:1 | 37.0 ± 0.3 | 5.7 ± 0.3 | 36.4 ± 1.6 |
| GTR:H2O2 1:1 | 37.3 ± 1.1 | 5.4 ± 1.1 | 34.5 ± 2.4 |
| GTR:H2O2 1:2 | 37.9 ± 1.1 | 4.8 ± 1.1 | 32.1 ± 2.3 |
| GTR:KMnO4 2:1 | 12.1 ± 0.5 | 30.6 ± 0.5 | 205.9 ± 9.9 |
| GTR:KMnO4 1:1 | 7.4 ± 0.5 | 35.3 ± 0.5 | 226.3 ± 6.2 |
| GTR:KMnO4 1:2 | 3.7 ± 0.2 | 39.0 ± 0.2 | 248.9 ± 3.3 |
| GTR Type | T-2%, °C | T-5%, °C | T-10%, °C | T-50%, °C | Residue890 °C, % | Tmax1, °C | Tmax2, °C |
|---|---|---|---|---|---|---|---|
| GTR | 233.0 | 286.7 | 331.1 | 435.0 | 35.86 | 369.0 | 424.0 |
| GTR:H2O2 2:1 | 215.4 | 273.5 | 325.3 | 434.3 | 34.68 | 369.3 | 426.1 |
| GTR: H2O2 1:1 | 202.3 | 266.9 | 321.7 | 438.7 | 37.57 | 368.8 | 429.6 |
| GTR: H2O2 1:2 | 204.1 | 265.1 | 320.7 | 435.9 | 35.39 | 370.8 | 425.6 |
| GTR:KMnO4 2:1 | 227.9 | 293.6 | 338.9 | 447.3 | 38.56 | 369.5 | 432.0 |
| GTR:KMnO4 1:1 | 213.0 | 287.4 | 337.2 | 449.0 | 37.88 | 373.6 | 429.1 |
| GTR:KMnO4 1:2 | 204.0 | 285.3 | 336.6 | 457.8 | 37.85 | 369.2 | 438.7 |
| Foam Symbol | T-2%, °C | T-5%, °C | T-10%, °C | T-50%, °C | Residue890 °C, % | Tmax1, °C | Tmax2, °C | Tmax3, °C |
|---|---|---|---|---|---|---|---|---|
| P0 | 264.2 | 290.1 | 313.0 | 380.7 | 12.98 | - | - | 385.5 |
| P1 | 247.6 | 279.0 | 306.6 | 390.3 | 15.32 | - | 324.2 | 393.9 |
| P2 | 249.2 | 284.2 | 313.2 | 392.9 | 15.33 | 297.8 | - | 393.8 |
| P3 | 257.8 | 287.6 | 315.6 | 394.5 | 15.29 | 287.7 | 318.9 | 395.0 |
| P4 | 248.7 | 282.2 | 313.6 | 394.6 | 17.24 | 278.7 | 322.1 | 395.7 |
| P5 | 229.8 | 263.5 | 297.3 | 393.0 | 16.42 | 269.2 | - | 391.8 |
| P6 | 221.8 | 251.9 | 287.9 | 392.3 | 18.32 | 251.5 | - | 391.7 |
| P7 | 203.9 | 234.6 | 277.3 | 393.4 | 16.34 | 230.0 | 309.6 | 395.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejna, A.; Olszewski, A.; Zedler, Ł.; Kosmela, P.; Formela, K. The Impact of Ground Tire Rubber Oxidation with H2O2 and KMnO4 on the Structure and Performance of Flexible Polyurethane/Ground Tire Rubber Composite Foams. Materials 2021, 14, 499. https://doi.org/10.3390/ma14030499
Hejna A, Olszewski A, Zedler Ł, Kosmela P, Formela K. The Impact of Ground Tire Rubber Oxidation with H2O2 and KMnO4 on the Structure and Performance of Flexible Polyurethane/Ground Tire Rubber Composite Foams. Materials. 2021; 14(3):499. https://doi.org/10.3390/ma14030499
Chicago/Turabian StyleHejna, Aleksander, Adam Olszewski, Łukasz Zedler, Paulina Kosmela, and Krzysztof Formela. 2021. "The Impact of Ground Tire Rubber Oxidation with H2O2 and KMnO4 on the Structure and Performance of Flexible Polyurethane/Ground Tire Rubber Composite Foams" Materials 14, no. 3: 499. https://doi.org/10.3390/ma14030499
APA StyleHejna, A., Olszewski, A., Zedler, Ł., Kosmela, P., & Formela, K. (2021). The Impact of Ground Tire Rubber Oxidation with H2O2 and KMnO4 on the Structure and Performance of Flexible Polyurethane/Ground Tire Rubber Composite Foams. Materials, 14(3), 499. https://doi.org/10.3390/ma14030499









