Impact of Chitosan on Water Stability and Wettability of Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
- ⚬
- pH measured in 1:2.5 soil:water suspension after 5 min continuous stirring;
- ⚬
- Particle size distribution determined for organic matter-depleted soil (H2O2) by sieving and the pipette method after chemical dispersion of soil sample in sodium pyrophosphate;
- ⚬
- Particle density, PD, measured by helium pycnometry using Quantum Crome Ultrapycnometer 1000 (Quantachrome, Boynton Beach, FL, USA);
- ⚬
2.2. Chitosans
- ⚬
- Total carbon and nitrogen content and particle density determined similarly to soil analysis;
- ⚬
- Degree of deacetylation (DD) calculated from the carbon/nitrogen ratio (C/N) using the following equation from Xu et al. [47]:
- ⚬
- Average molecular weight (M) determined from viscometric measurements performed for series of CS1 and CS2 solutions of decreasing concentrations in 0.02 Mol dm−3 acetic acid/0.02 Mol dm−3 NaCl, at 24 °C, using Hoppler rheo-viscometer. The intrinsic viscosity (ηint) was determined as follows:where η(c) is the viscosity of the chitosan solution at a given concentration c, ηs is the viscosity of the solvent and f is the w/w fraction of the chitosan in the solution.ηint = limc→0 [(η(c) − ηs)/(ηs × f)]
- ⚬
- ⚬
- Contact angle (θ) measured on the pressed chitosan pellets using a DSO 100 automatic drop shape analyzer (KRUSS, Hamburg, Germany).
2.3. Preparation of Soil–Chitosan Aggregates
2.4. Studies of Soil–Chitosan Aggregates
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- European Chitin Society. Available online: http://euchis.org (accessed on 2 November 2021).
- Morganti, P.; Stoller, M. Chitin and lignin: Natural ingredients from waste materials to make innovative and healthy products for humans and plant. Chem. Eng. Trans. 2017, 60, 319–324. [Google Scholar] [CrossRef]
- Majeti, N.V.; Kumar, R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Esyanti, R.R.; Dwivany, F.M.; Mahani, S.; Nugrahapraja, H.; Meitha, K. Foliar application of chitosan enhances growth and modulates expression of defense genes in chilli pepper (Capsicum annuum L.). Aust. J. Crop Sci. 2019, 13, 55–60. [Google Scholar] [CrossRef]
- Pandey, P.; Kumar Verma, M.; De, N. Chitosan in agricultural context-a review. Bull. Env. Pharmacol. Life Sci. 2018, 7, 87–96. [Google Scholar]
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The multifunctional role of chitosan in horticultural crops: A review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Cho, M.H.; No, H.K.; Prinyawiwatkul, W. Chitosan treatments affect growth and selected quality of sunflower sprouts. J. Food Sci. 2008, 73, 70–77. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Chitosan as soil amendment affects lettuce growth, photochemical efficiency, and gas exchange. Hort. Technol. 2008, 28, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Boukhlifi, F.; Mamouni, F.Z.; Razouk, R. Chitin/Chitosan’s bio-fertilizer: Usage in vegetative growth of wheat and potato crops. In Chitin-Chitosan-Myriad Functionalities in Science and Technology; Intechopen: London, UK, 2018; p. 75208. Available online: https://www.intechopen.com/chapters/61679 (accessed on 13 December 2021).
- Silva, W.O.; Stamford, N.P.; Silva, E.V.; Santos, C.E.; Freitas, A.D.S.; Silva, M.V. The impact of biofertilizers with diazotrophic bacteria and fungi chitosan on melon characteristics and nutrient uptake as an alternative for conventional fertilizers. Sci. Hortic. 2016, 209, 236–240. [Google Scholar] [CrossRef]
- Chen, Y.E.; Yuan, S.; Liu, H.M.; Chen, Z.Y.; Zhang, Y.H.; Zhang, H.Y. A combination of chitosan and chemical fertilizers improves growth and disease resistance in Begonia × hiemalis Fotsch. Hortic. Environ. Biotechnol. 2016, 57, 1725–1737. [Google Scholar] [CrossRef]
- Chookhongkha, N.; Miyagawa, S.; Jirakiattikul, Y.; Photchanachai, S. Chili growth and seed productivity as affected by chitosan. In Proceedings of the International Conference on Agriculture Technology and Food Sciences (ICATFS’2012), Manila, Philippines, 17–18 November 2012; pp. 146–149. Available online: http://psrcentre.org/images/extraimages/31.%201112041.pdf (accessed on 13 December 2021).
- Prasad, R.D.; Chandrika, K.S.V.P.; Godbole, V. A novel chitosan biopolymer based Trichoderma delivery system: Storage stability, persistence and bio efficacy against seed and soil borne diseases of oilseed crops. Microbiol. Res. 2020, 237, 126487. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, F.; Liu, F.; Wang, Y.; Gao, Y. Structure and antimicrobial mechanism of -polylysine-chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 2014, 70, 427–434. [Google Scholar] [CrossRef]
- Buchovec, I.; Luksiene, Z. Novel approach to control microbial contamination of germinated wheat sprouts: Photoactivatedchlorophillin-chitosan complex. Int. J. Food Process. Technol. 2015, 2, 26–30. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Abscisic acid is involved in chitosan-induced resistance to tobacco necrosis virus (TNV). Plant Physiol. Biochem. 2008, 46, 1106–1111. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Chirkov, S.N.; Ilina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Prikl. Biokhim. Mikrobiol. 2006, 42, 224–228. [Google Scholar] [CrossRef]
- Fitza, K.N.E.; Payn, K.G.; Steenkamp, E.T.; Myburg, A.A.; Naidoo, S. Chitosan application improves resistance to Fusarium circinatum in Pinus patula. S. Afr. J. Bot. 2013, 85, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Jabnoun-Khiareddine, H.; El-Mohamedy, R.S.R.; Abdel-Kareem, F.; Abdallah, R.A.B.; Gueddes-Chahed, M.; Daami-Remadi, M. Variation in chitosan and salicylic acid efficacy towards soil-borne and air-borne fungi and their suppressive effect of tomato wilt severity. J. Plant Pathol. Microbiol. 2015, 6, 1000325. [Google Scholar] [CrossRef] [Green Version]
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibanez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. Front. Plant Sci. 2017, 8, 1415. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Fernando, W.J.N.; Uzir, M.H. Parametric evaluation using mechanistic model for release rate of phosphate ions from chitosan coated phosphorus fertiliser pellets. Biosyst. Eng. 2015, 129, 78–86. [Google Scholar] [CrossRef]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Lu, S.; Liu, M.; Ni, B. An injectable oxidized carboxymethylcellulose/N-succinyl-chitosan hydrogel system for protein delivery. Chem. Eng. J. 2010, 160, 779–787. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Basuki, K.T.; Swantomo, D.; Sanyoto, N.T. Characterization of chitosan-acrylamide hydrogels as soil conditioner. Adv. Mat. Res. 2015, 1112, 414–417. [Google Scholar] [CrossRef]
- Iftimea, M.M.; Ailieseia, G.L.; Ungureanub, E.; Marina, L. Designing chitosan based eco-friendly multifunctional soil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019, 223, 115040. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A mini-review on chitosan-based hydrogels with potential for sustainable agricultural applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Narayanan, A.; Dhamodharan, R. Super water-absorbing new material from chitosan, EDTA and urea. Carbohydr. Polym. 2015, 134, 337–343. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Martins, A.F.; Paulino, A.T.; Fajardo, A.R.; Guilherme, M.R.; Faria, M.G.I.; Linde, G.A.; Rubira, A.F.; Muniz, E.C. Recent advances in designing hydrogels from chitin and chitin derivatives and their Impact on environment and agriculture: A review. Rev. Virtual Quim. 2017, 9. [Google Scholar] [CrossRef]
- Ritonga, H.; Nurfadillah, A.; Rembon, F.S.; Ramadhan, L.O.A.N.; Nurdin, M. Preparation of chitosan-EDTA hydrogel as soil conditioner for soybean plant (Glycine max). Groundw. Sustain. Dev. 2019, 9, 100277. [Google Scholar] [CrossRef]
- Ritonga, H.; Basri, M.I.; Rembon, F.S.; Ramadhan, L.O.A.N.; Nurdin, M. High performance of chitosan-co-polyacrylamide-TiO2 crosslinked glutaraldehyde hydrogel as soil conditioner for soybean plant (Glycine max). Soil Sci. Annu. 2020, 71, 194–204. [Google Scholar] [CrossRef]
- Hataf, N.; Ghadir, P.; Ranjbar, N. Investigation of soil stabilization using chitosan biopolymer. J. Clean. Prod. 2018, 170, 1493–1500. [Google Scholar] [CrossRef]
- Kamari, A.; Pulford, I.D.; Hargreaves, J.S.J. Chitosan as a potential amendment to remediate metal contaminated soil—A characterisation study. Colloids Surf. B. 2011, 82, 71–80. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, P. β-Cyclodextrin-chitosan biocomposites for synergistic removal of imazethapyr and imazamox from soils: Fabrication, performance and mechanisms. Sci. Total Environ. 2020, 710, 135659. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, H.; Fu, X.; Li, X.; Guo, Q.; Yang, T.; Li, X. Immobilization of esterase SulE in cross-linked gelatin/chitosan and its application in remediating soils polluted with tribenuron-methyl and metsulfuron-methyl. Process Biochem. 2020, 98, 217–223. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, X.; Yu, B.; Ding, N.; Liu, X.; Ma, Q.; Yang, S.; Yilihamu, A.; Yang, S.T. Carboxylated graphene oxide-chitosan spheres immobilize Cu2+ in soil and reduce its bioaccumulation in wheat plants. Environ. Int. 2019, 133, 105208. [Google Scholar] [CrossRef]
- Amezketa, E. Soil Aggregate Stability: A Review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Boix-Fayos, C.; Calvo-Cases, A.; Imeson, A.C.; Soriano-Soto, M.D. Influence of soil properties on the aggregation of some Mediterranean soils and the use of aggregate size and stability as land degradation indicators. Catena 2001, 44, 47–67. [Google Scholar] [CrossRef]
- Canton, Y.; Sole-Benet, A.; Asensio, C.; Chamizo, S.; Puigdefabregas, J. Aggregate stability in range sandy loam soils relationships with runoff and erosion. Catena 2009, 77, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Kodesova, R.; Rohoskova, M.; Zigova, A. Comparison of aggregate stability within six soil profiles under conventional tillage using various laboratory tests. Biologia 2009, 64, 550–554. [Google Scholar] [CrossRef]
- Filcheva, E.; Tsadilas, C. Influence of cliniptilolite and compost on soil properties. Commun. Soil Sci. Plan. 2002, 33, 595–607. [Google Scholar] [CrossRef]
- Kononova, M. Soil Organic Matter, 2nd ed.; Pergammon Press, Inc.: Oxford, UK, 1966; Available online: https://books.google.pl/books?hl=pl&lr=&id=ExjLBAAAQBAJ&oi=fnd&pg=PP1&dq=Kononova+M.+(1966) (accessed on 13 December 2021).
- Xu, J.; McCarthy, S.P.; Gross, R.A.; Kaplan, D.L. Chitosan Film Acylation and Effects on Biodegradability. Macromolecules 1996, 29, 3436–3440. [Google Scholar] [CrossRef]
- Tanford, C. Physical Chemistry of Macromolecules; Chapter 6; Wiley: New York, NY, USA, 1961; pp. 390–412. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/pol.1962.1206217338 (accessed on 13 December 2021).
- Varum, K.M.; Smidsrod, O. Structure—Property relationships in chitosan. In Polysaccharides Structural Diversity and Functional Versatility, 2nd ed.; chapter 26; Dumitru, S., Ed.; Taylor & Francis: New York, NY, USA, 2004; pp. 625–642. [Google Scholar] [CrossRef]
- Kasaai, M.R. Calculation of Mark-Houwink-Sakurada (MHS) equation viscometric constants for chitosan in any solvent-temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Galed, G.; Diaz, E.; Goycoolea, F.M.; Heras, A. Influence of N-Deacetylation conditions on chitosan production from α-Chitin. Nat. Prod. Commun. 2008, 3, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Kofuji, K.; Qian, C.J.; Nishimura, M.; Sugiyama, I.; Murata, Y.; Kawashima, S. Relationship between physicochemical characteristics and functional properties of chitosan. Eur. Polym. J. 2005, 41, 2784–2791. [Google Scholar] [CrossRef]
- Gierszewska-Druzynska, M.; Ostrowska-Czubenko, J.; Kwiatkowska, A. Effect of Ionic Crosslinking on Density of Hydrogel Chitosan Membranes. Prog. Chem. Appl. Chitin Deriv. 2013, 18, 48–57. Available online: http://psjd.icm.edu.pl/psjd/element/bwmeta1.element.psjd-2f27cc06-7e9b-499b-912e-8399f3783474 (accessed on 13 December 2021).
- Jozefaciuk, G.; Adamczuk, A.; Skic, K.; Boguta, P. New method for quantifying water stability of soil aggregates from air bubbling after immersion. Measurement 2020, 155, 107569. [Google Scholar] [CrossRef]
- Dekker, L.W.; Doerr, S.H.; Oostindie, K.; Ziogas, A.K.; Ritsema, C.J. Water repellency and critical soil water content in a dune sand. Soil Sci. Soc. Am. J. 2001, 65, 1667–1674. [Google Scholar] [CrossRef]
- Mucha, M.; Ludwiczak, S.; Kawinska, M. Kinetics of water sorption by chitosan and its blends with poly(vinyl alcohol). Carbohydr. Polym. 2005, 62, 42–49. [Google Scholar] [CrossRef]
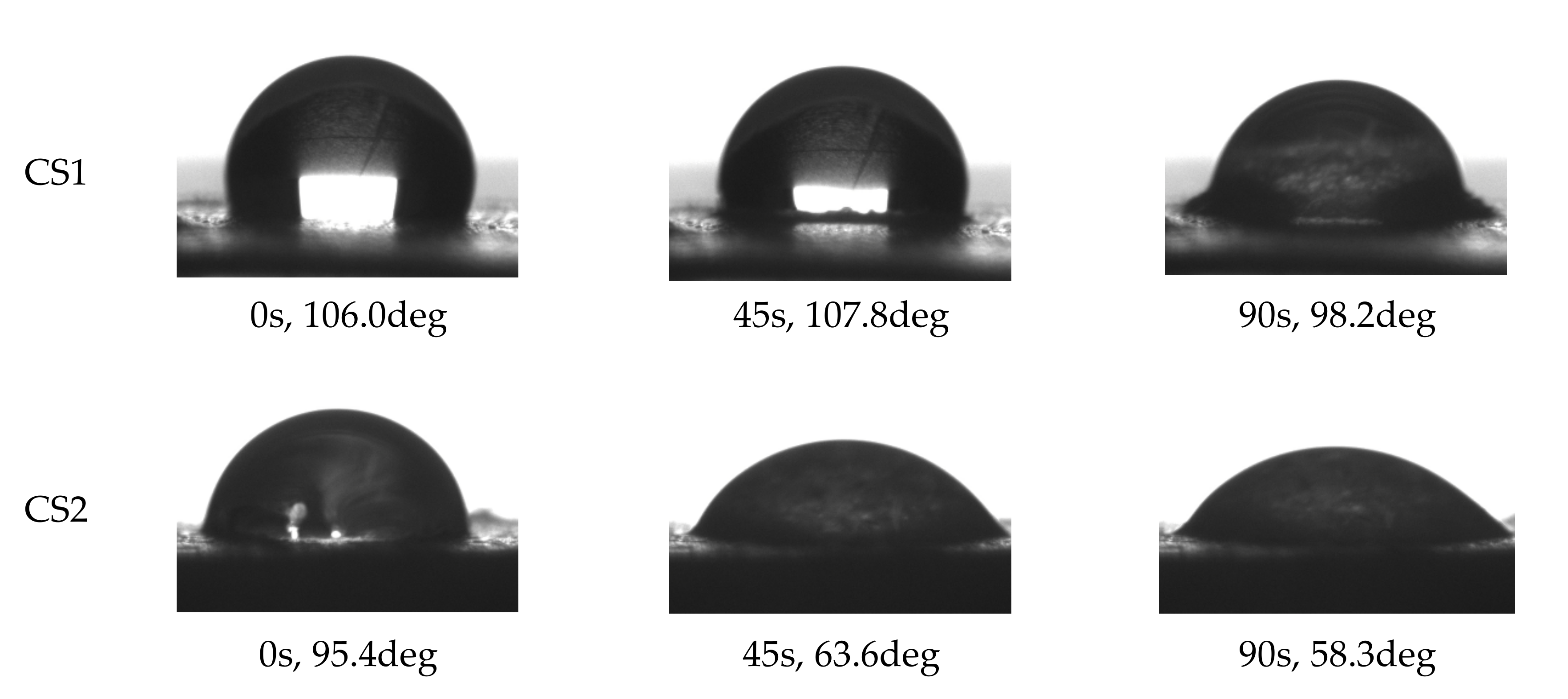

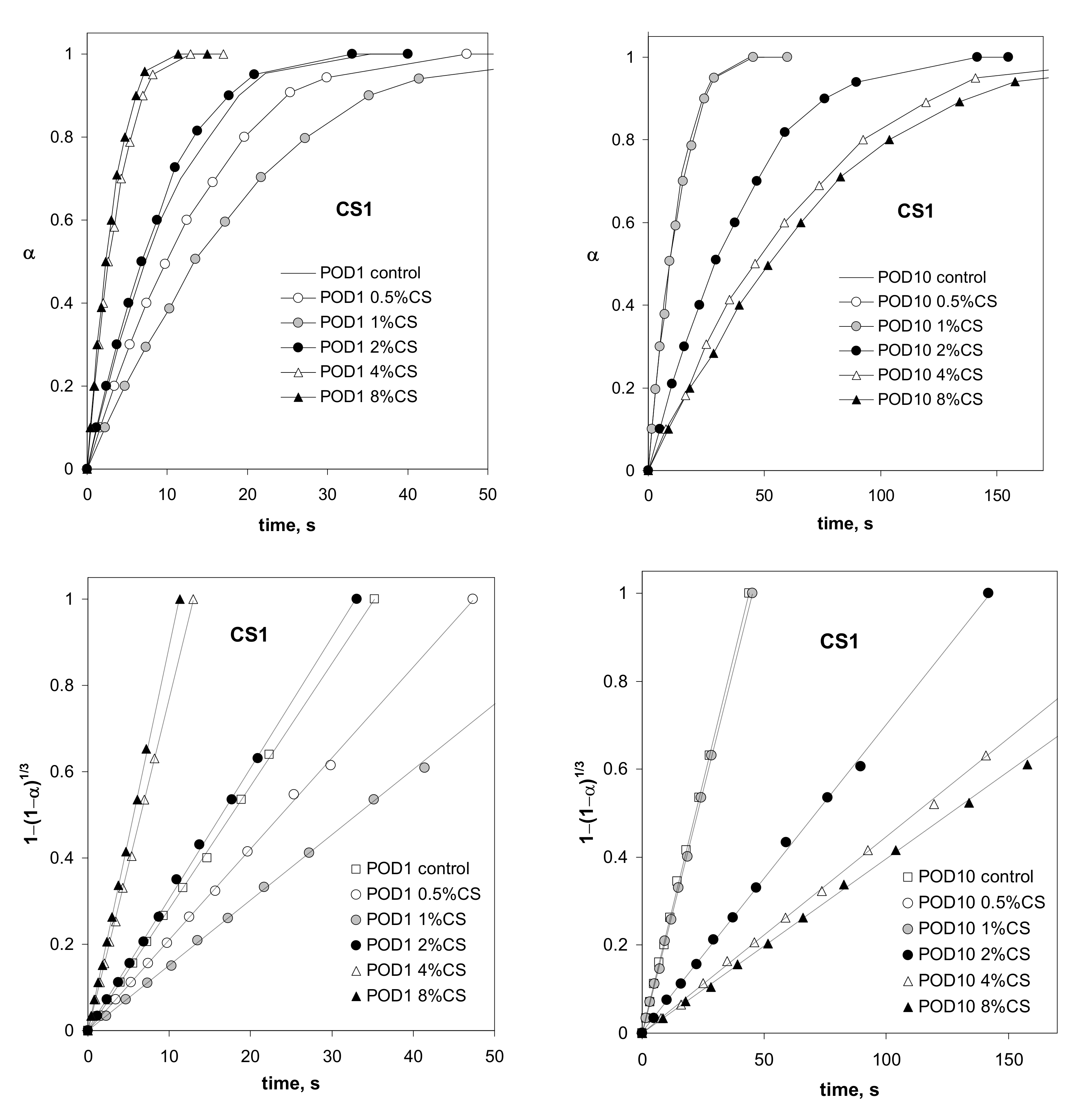
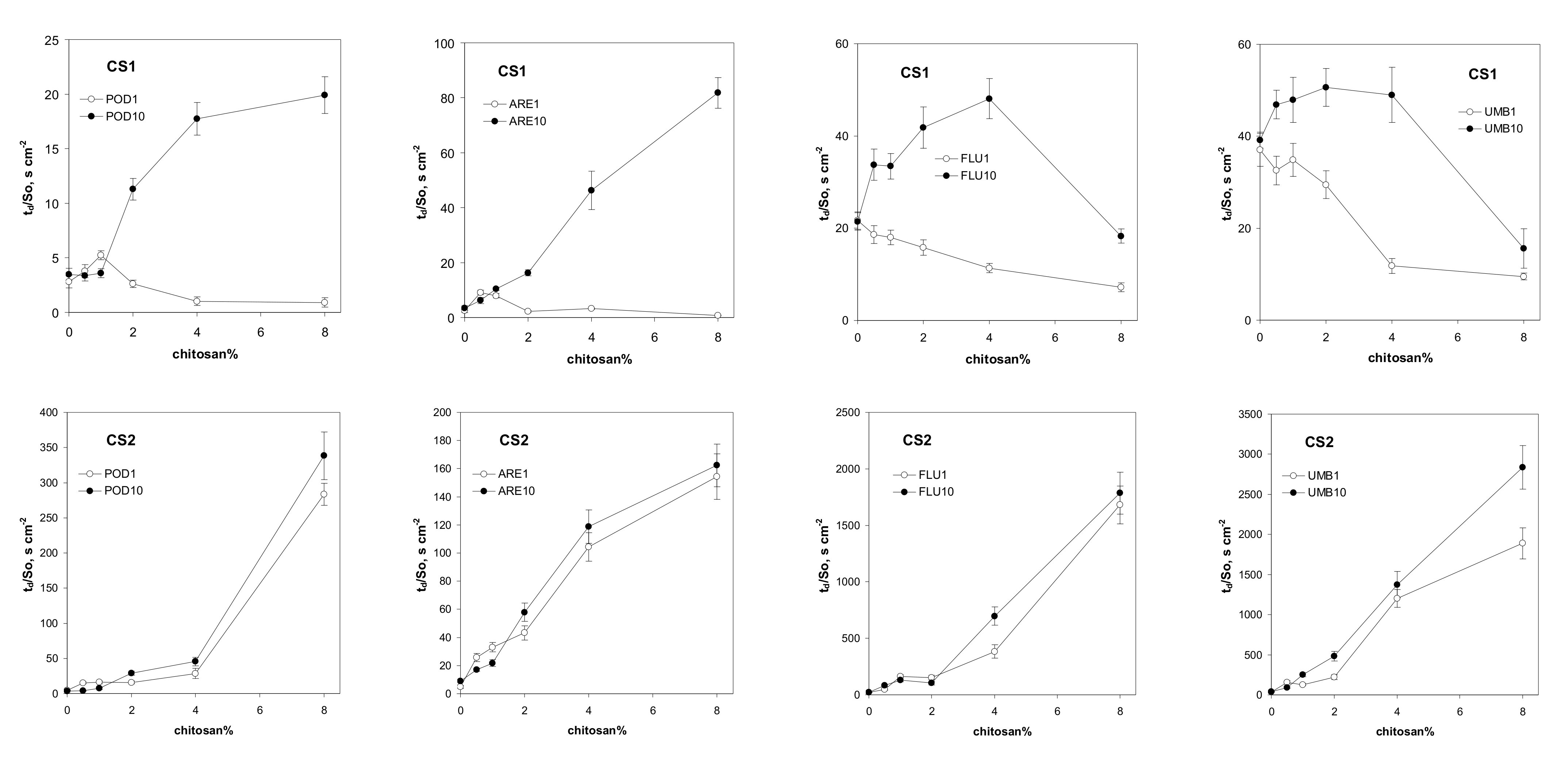
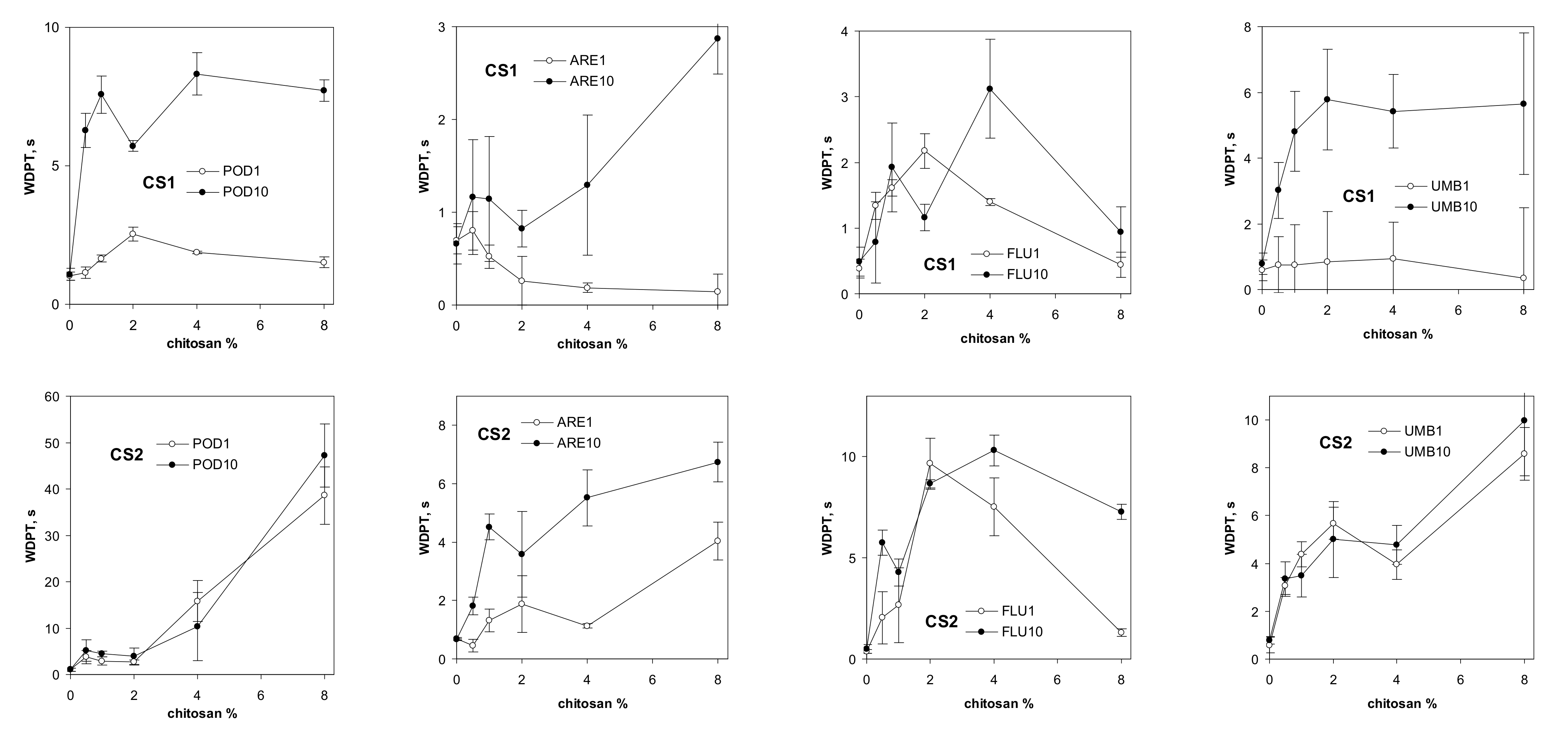
| Abbreviation | POD | ARE | FLU | UMB |
|---|---|---|---|---|
| Soil type | Podzol | Arenosol | Fluvisol | Umbrisol |
| Locality | Trawniki | Strzyzewice | Dorohucza | Prusy |
| Longitude E | 22°58′41″ | 22°26′6″ | 22°59′38″ | 21°41′59″ |
| Latitude N | 51°9′14″ | 51°2′9″ | 51°9′43″ | 50°49′25″ |
| pH | 4.1 | 5.5 | 6.5 | 7.7 |
| PD, (g cm−3) | 2.54 | 2.62 | 2.62 | 2.68 |
| Nitrogen (%) | 0.16 | 0.13 | 0.46 | 0.14 |
| Total organic carbon (%) | 0.65 | 1.55 | 3.04 | 0.9 |
| Sand (0.063–2 mm) (%) | 72.4 | 47.1 | 20.2 | 10.4 |
| Silt (0.002–0.063 mm) (%) | 25.9 | 46.2 | 52.2 | 72.4 |
| Clay (<0.002 mm) (%) | 1.7 | 6.7 | 27.6 | 17.2 |
| N [%] | TOC (%) | PD (g cm−3) | DD | M (kDa) | η (1% Solution) (cP) | x (at pH = 4, µ = 0.01) | θ (deg) | |
|---|---|---|---|---|---|---|---|---|
| CS1 | 7.51 | 41.59 | 1.51 | 0.77 | 699 | 111.0 | 5.75 | 106.0 |
| CS2 | 7.79 | 41.27 | 1.54 | 0.91 | 280 | 12.3 | 2.25 | 95.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczuk, A.; Kercheva, M.; Hristova, M.; Jozefaciuk, G. Impact of Chitosan on Water Stability and Wettability of Soils. Materials 2021, 14, 7724. https://doi.org/10.3390/ma14247724
Adamczuk A, Kercheva M, Hristova M, Jozefaciuk G. Impact of Chitosan on Water Stability and Wettability of Soils. Materials. 2021; 14(24):7724. https://doi.org/10.3390/ma14247724
Chicago/Turabian StyleAdamczuk, Agnieszka, Milena Kercheva, Mariana Hristova, and Grzegorz Jozefaciuk. 2021. "Impact of Chitosan on Water Stability and Wettability of Soils" Materials 14, no. 24: 7724. https://doi.org/10.3390/ma14247724
APA StyleAdamczuk, A., Kercheva, M., Hristova, M., & Jozefaciuk, G. (2021). Impact of Chitosan on Water Stability and Wettability of Soils. Materials, 14(24), 7724. https://doi.org/10.3390/ma14247724






