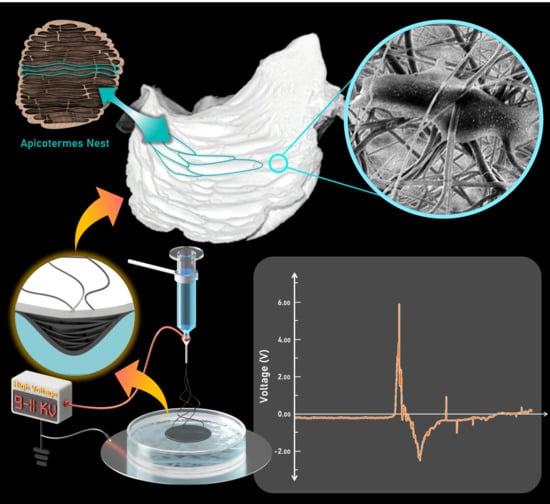
Fabrication of Piezoelectric Electrospun Termite Nest-like 3D Scaffolds for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
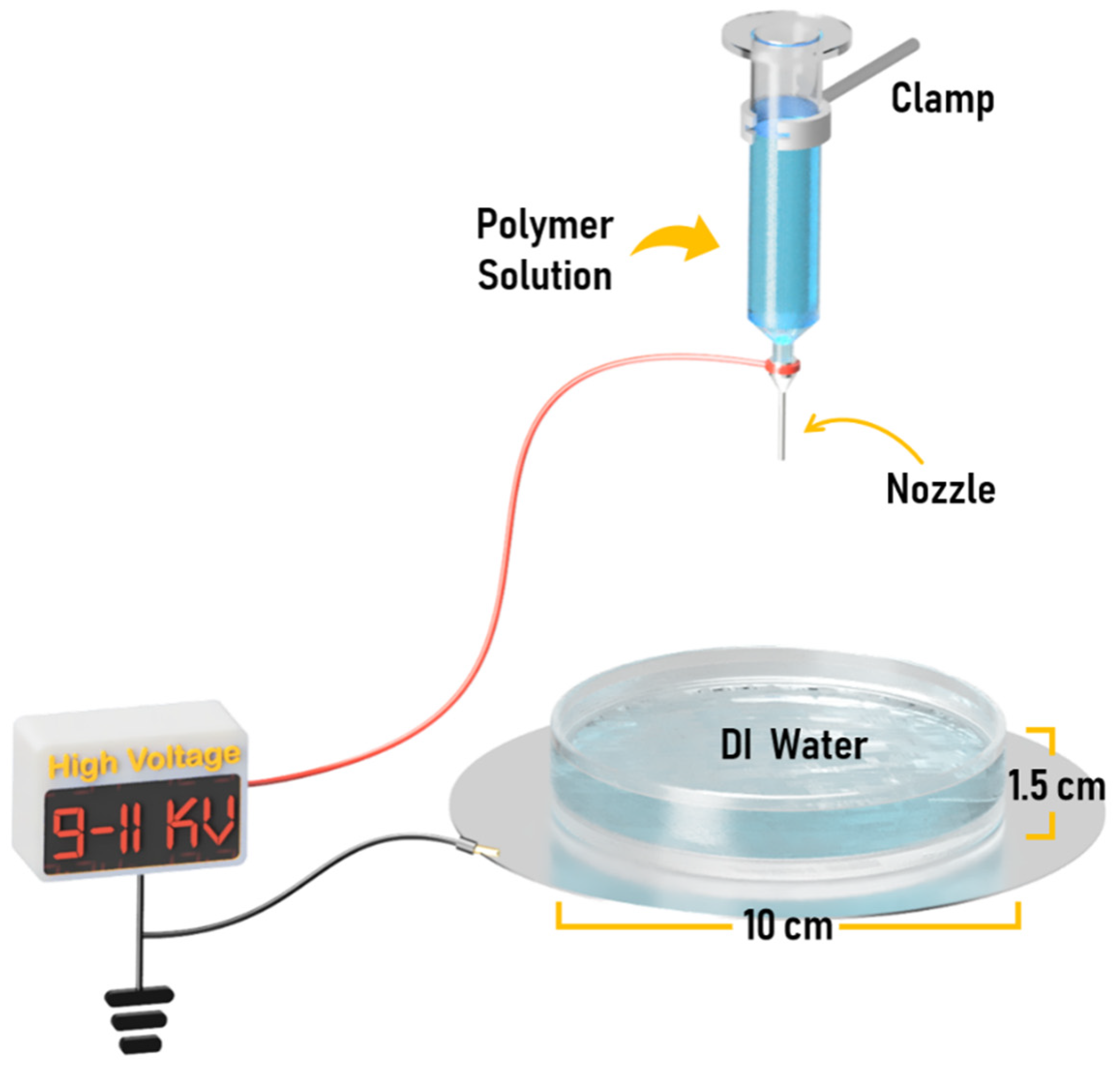
2.2. Electrospinning Process
2.3. Scanning Electron Microscopy
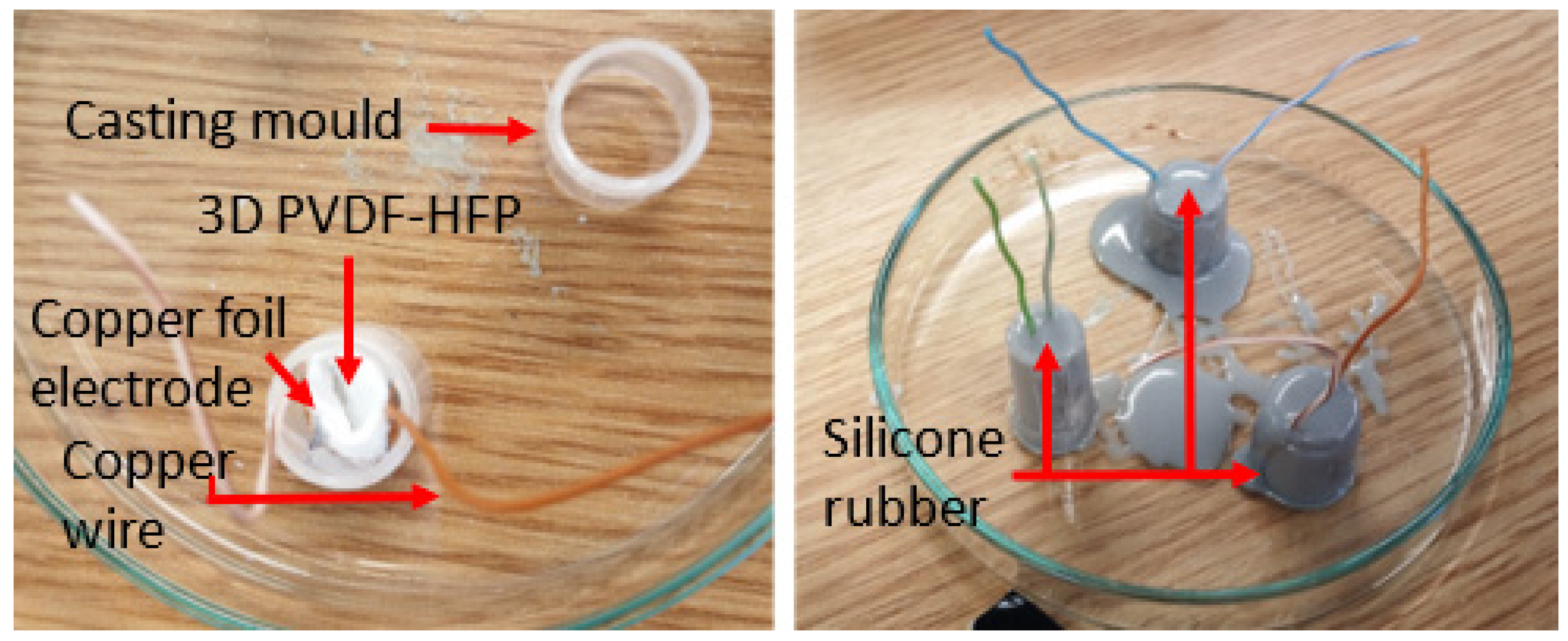
2.4. Generator Assembly for Piezoelectric Response Testing of the 3D Nanofiber Scaffolds
2.5. Surface Modification
2.6. In Vitro Cell Culture
3. Results and Discussion
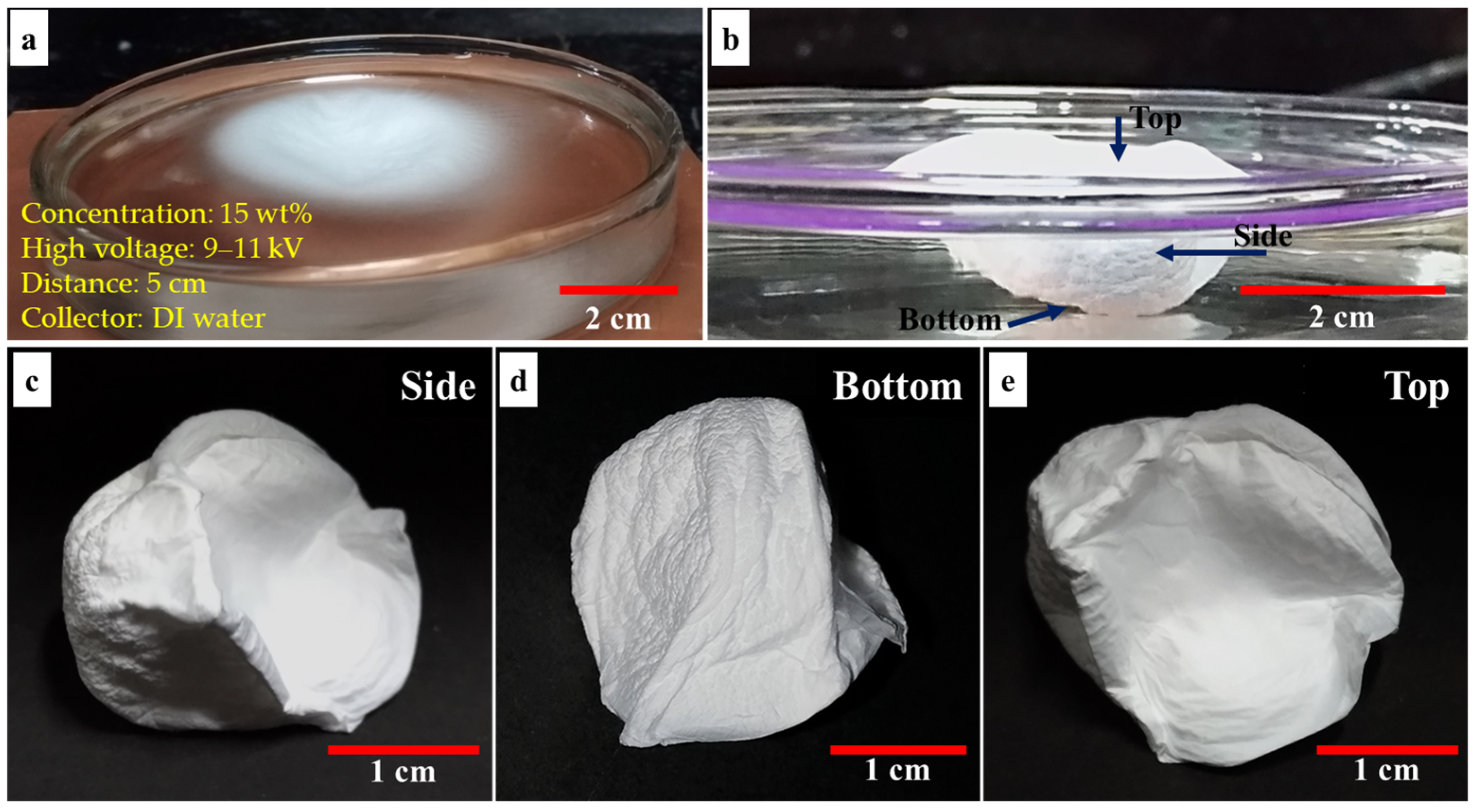
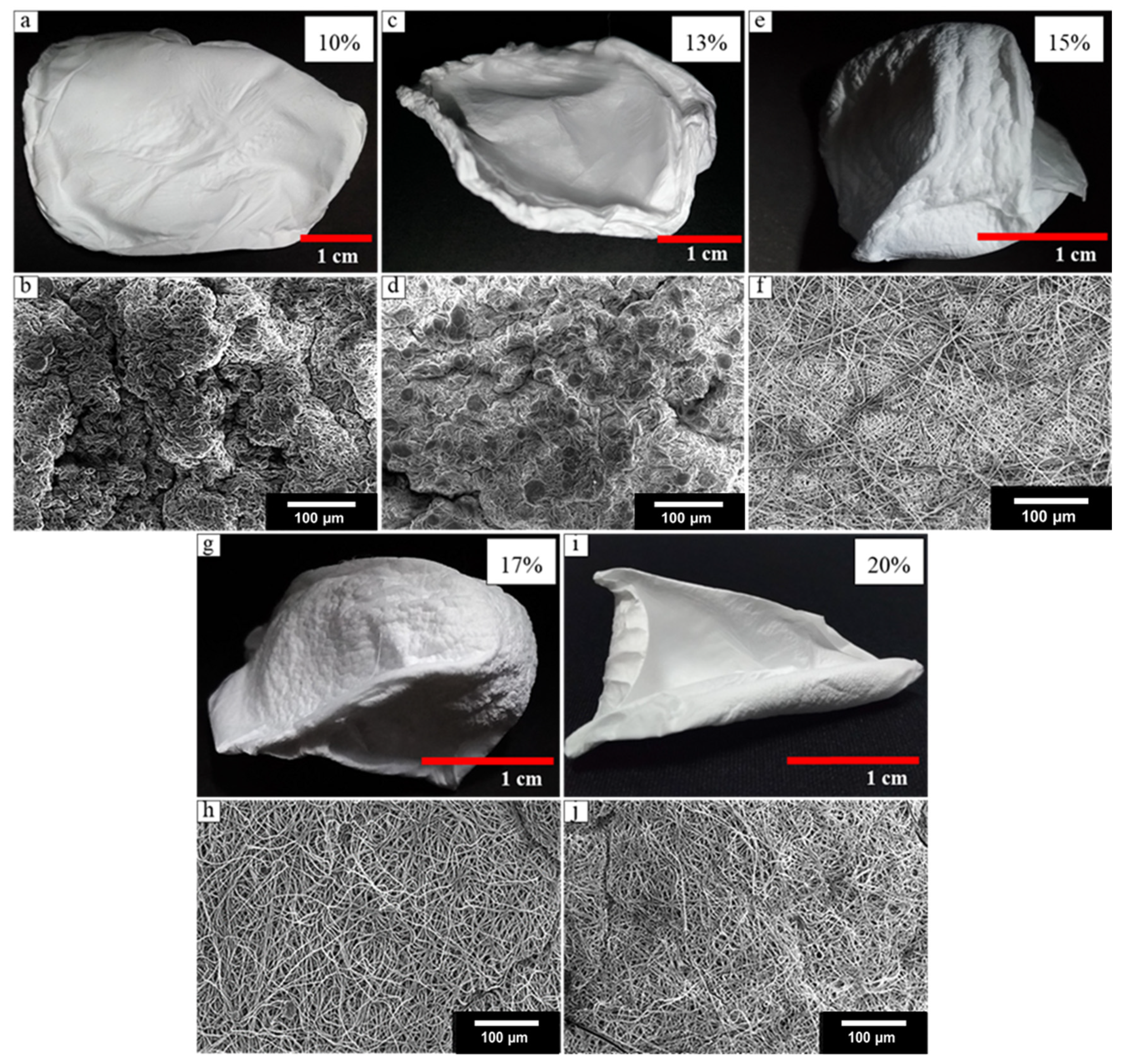
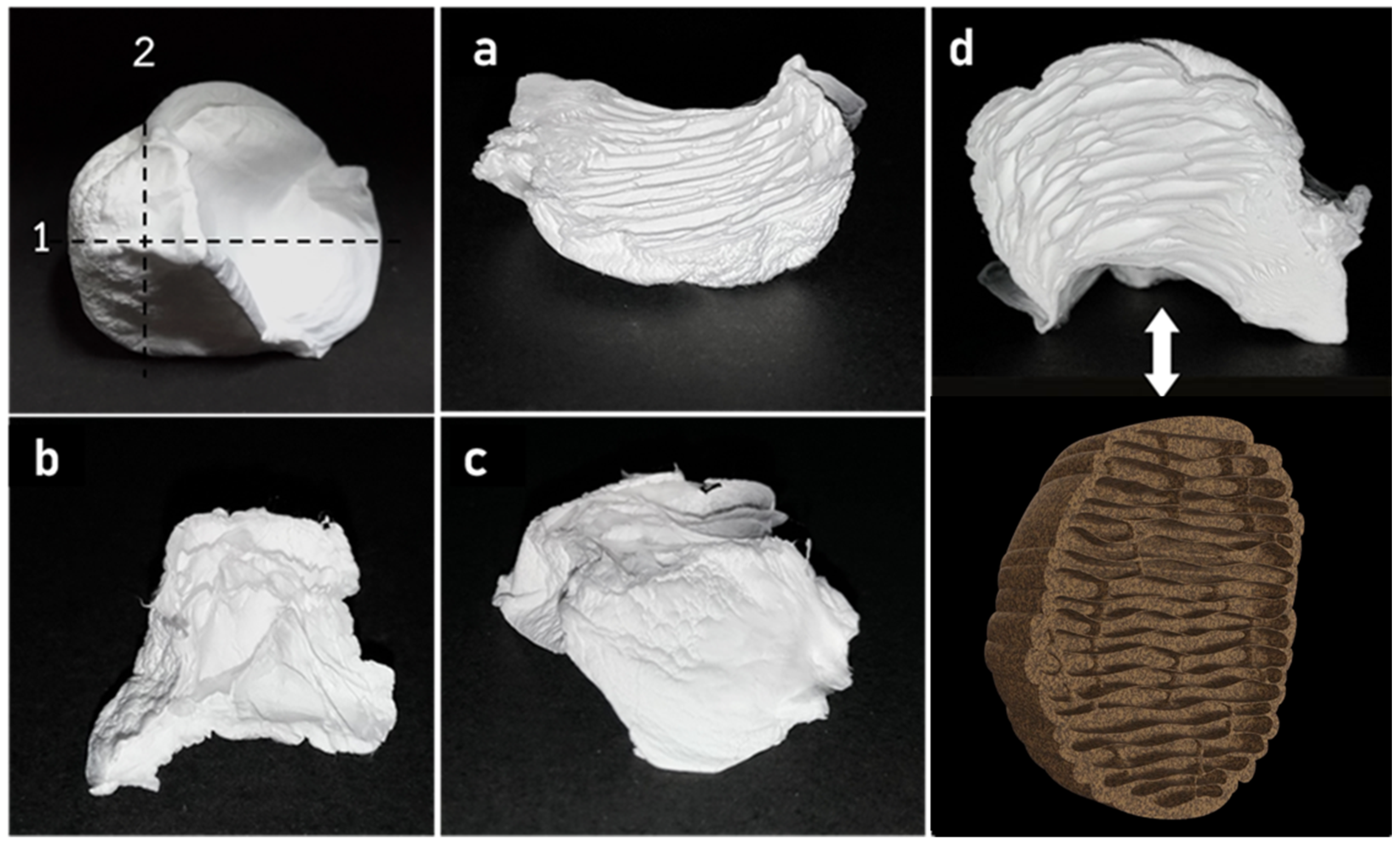
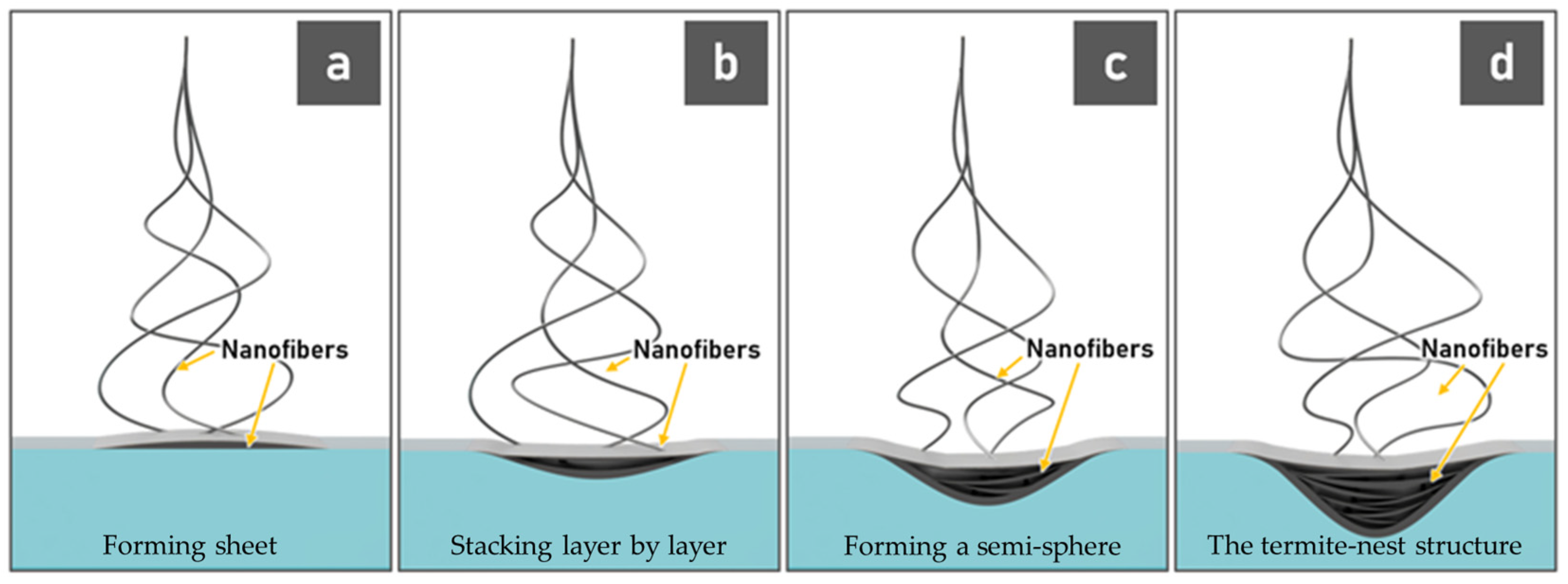
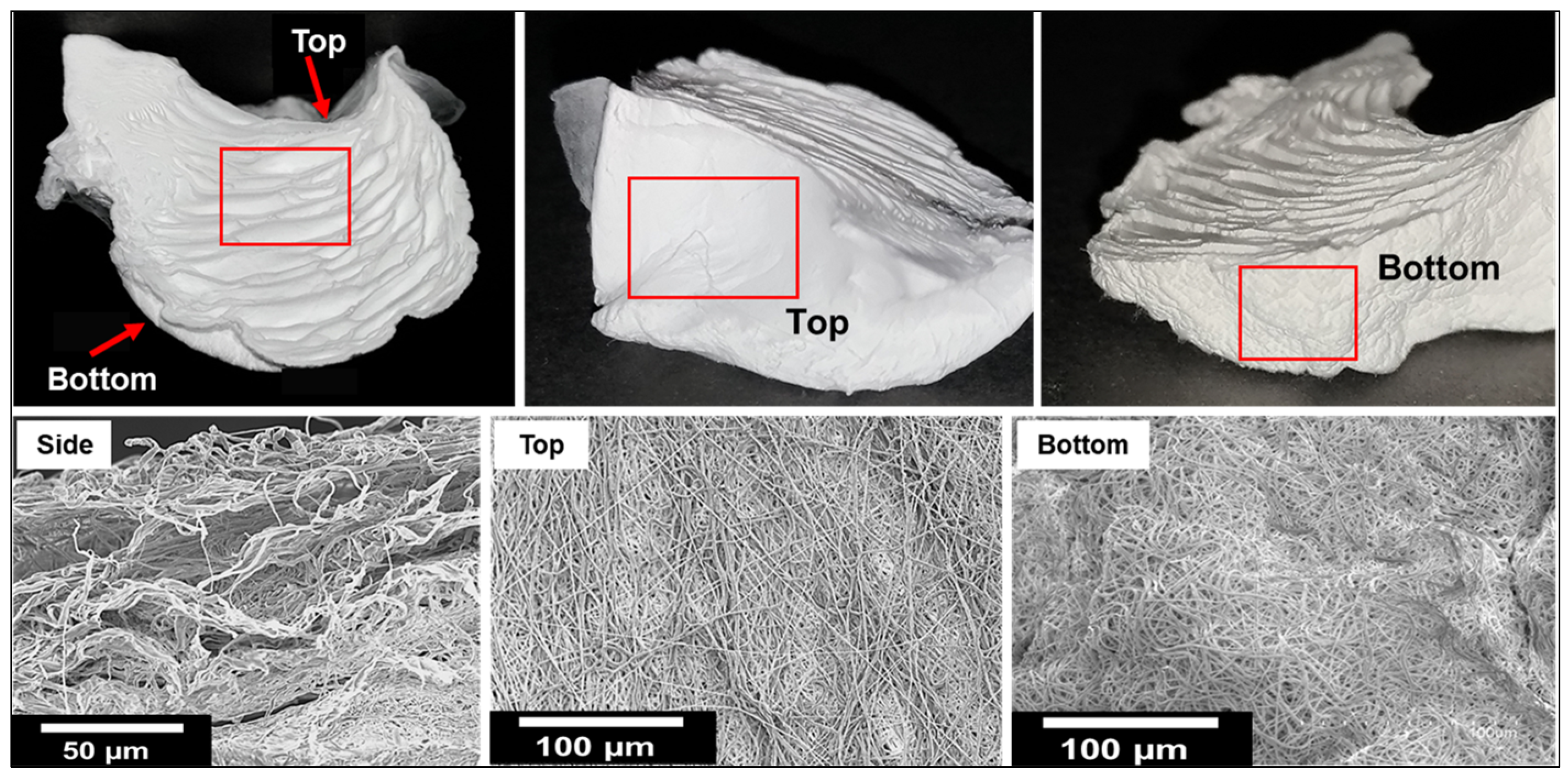
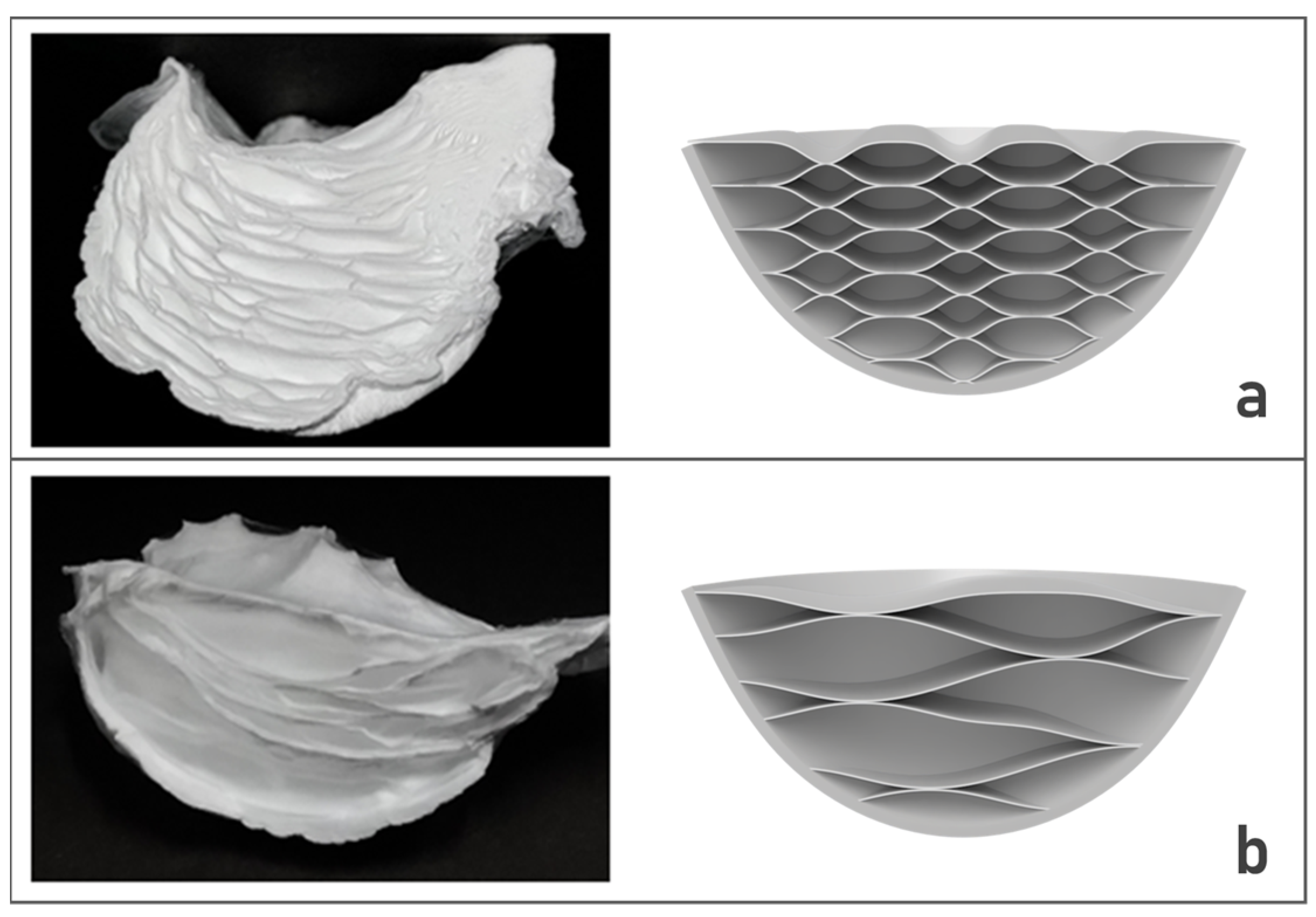
3.1. Fabrication of 3D Nanofiber Scaffolds
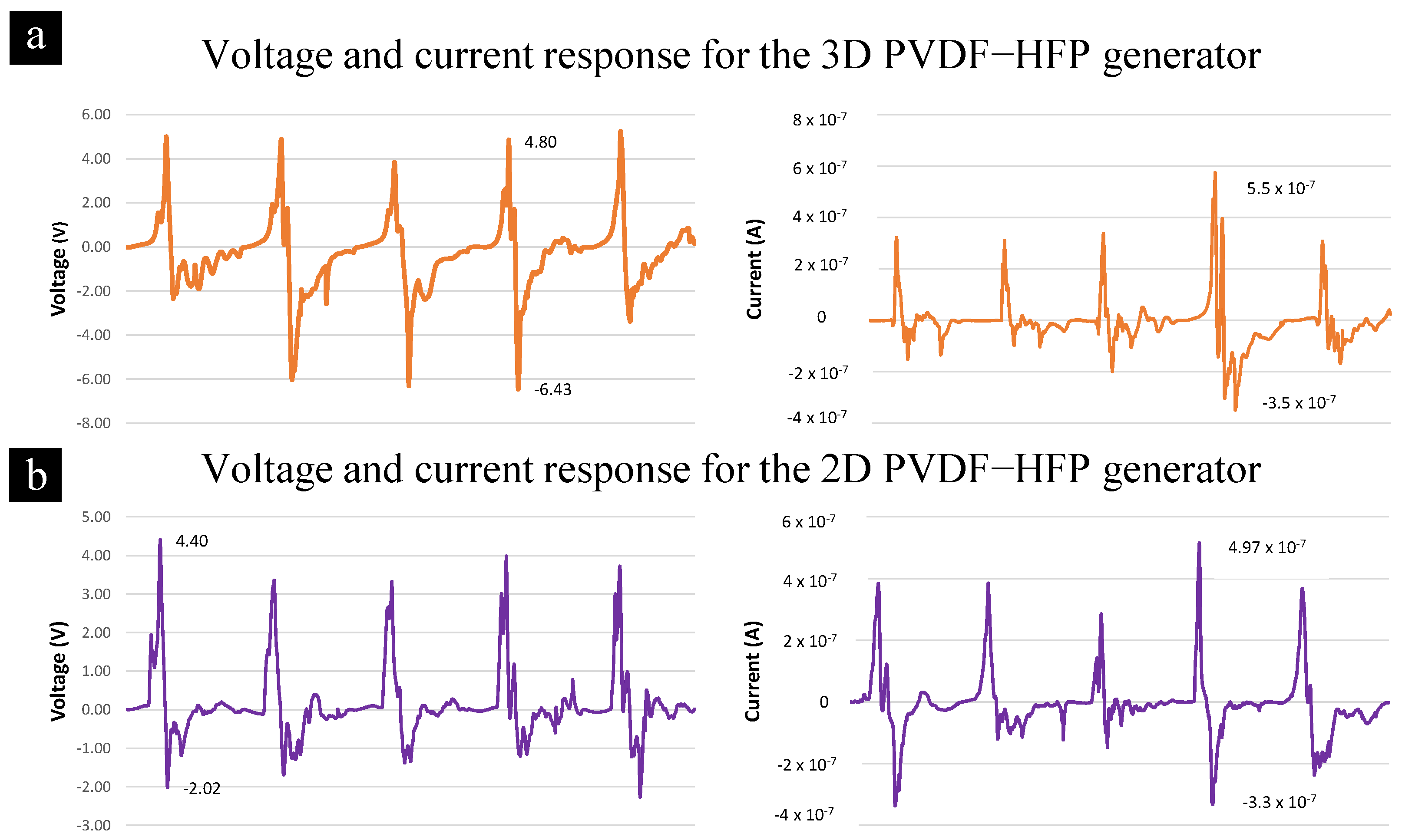
3.2. Piezoelectricity of 3D Nanofibers
3.3. Cell Morphology and Attachment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, H.; Tucker, N. Electrospinning predictions using artificial neural networks. Polymers 2015, 58, 22–29. [Google Scholar] [CrossRef][Green Version]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Radacsi, N.; Giapis, K.P.; Ovari, G.; Szabó-Révész, P.; Ambrus, R. Electrospun nanofiber-based niflumic acid capsules with superior physicochemical properties. J. Pharm. Biomed. Anal. 2019, 166, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, P.; Mei, N.; Chen, X.; Shao, Z.; Pan, L.; Wu, C. Silk fibroin modified porous poly(ε-caprolactone) scaffold for human fibroblast culture in vitro. J. Mater. Sci. Mater. Electron. 2004, 15, 671–677. [Google Scholar] [CrossRef]
- Qin, X.; Wu, D. Effect of different solvents on poly(caprolactone) (PCL) electrospun nonwoven membranes. J. Therm. Anal. Calorim. 2012, 107, 1007–1013. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Mironov, V.; Stampfl, J.; Liska, R. Engineering 3D cell-culture matrices: Multiphoton processing technologies for biological and tissue engineering applications. Expert Rev. Med. Devices 2012, 9, 613–633. [Google Scholar] [CrossRef]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef]
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif. Organs 2006, 30, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Tuning the properties of PVDF or PVDF-HFP fibrous materials decorated with ZnO nanoparticles by applying electrospinning alone or in conjunction with electrospraying. Fibers Polym. 2017, 18, 649–657. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Superhydrophobic PVDF and PVDF-HFP nanofibrous mats with antibacterial and anti-biofouling properties. Appl. Surf. Sci. 2016, 363, 363–371. [Google Scholar] [CrossRef]
- Laroche, G.; Marois, Y.; Guidoin, R.; King, M.W.; Martin, L.; How, T.; Douville, Y. Polyvinylidene fluoride (PVDF) as a biomaterial: From polymeric raw material to monofilament vascular suture. J. Biomed. Mater. Res. 1995, 29, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Dommer, M.; Chang, C.; Lin, L. Piezoelectric nanofibers for energy scavenging applications. Nano Energy 2012, 1, 356–371. [Google Scholar] [CrossRef]
- Najjar, R.; Luo, Y.; Jao, D.; Brennan, D.; Xue, Y.; Beachley, V.; Hu, X.; Xue, W. Biocompatible silk/polymer energy harvesters using stretched poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) nanofibers. Polymers 2017, 9, 479. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; Al-Maadeed, M.A.A. Stretchable electrospun PVDF-HFP/Co-ZnO nanofibers as piezoelectric nanogenerators. Sci. Rep. 2018, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.S.; Subramania, A.; Jung, Y.S.; Kim, K.J. High-performance quasi-solid-state dye-sensitized solar cell based on an electrospun PVdF-HFP membrane electrolyte. Langmuir 2008, 24, 9816–9819. [Google Scholar] [CrossRef]
- Miao, R.; Liu, B.; Zhu, Z.; Liu, Y.; Li, J.; Wang, X.; Li, Q. PVDF-HFP-based porous polymer electrolyte membranes for lithiumion batteries. J. Power Sources 2008, 184, 420–426. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of nanofibers and their applications for energy devices. J. Nanomater. 2015, 2015, 140716. [Google Scholar] [CrossRef]
- Mandal, D.; Yoon, S.; Kim, K.J. Origin of Piezoelectricity in an Electrospun Poly(vinylidene fluoride-trifluoroethylene) Nanofiber Web-Based Nanogenerator and Nano-Pressure Sensor. Macromol. Rapid Commun. 2011, 32, 831–837. [Google Scholar] [CrossRef]
- Vong, M.; Speirs, E.; Klomkliang, C.; Akinwumi, I.; Nuansing, W.; Radacsi, N. Controlled three-dimensional polystyrene micro- and nanostructures fabricated by three-dimensional electrospinning. RSC Adv. 2018, 8, 15501–15512. [Google Scholar] [CrossRef]
- Radacsi, N.; Nuansing, W. Chapter 7 Fabrication of 3D and 4D polymer micro- and nanostructures based on electrospinning. In 3D and 4D Printing of Polymer Nanocomposite Materials: Processes; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 191–229. [Google Scholar]
- Chen, H.; Peng, Y.; Wu, S.; Tan, L.P. Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair. Materials 2016, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Simonet, M.; Schneider, O.D.; Neuenschwander, P.; Stark, W.J. Ultraporous 3D polymer meshes by low-temperature electrospinning: Use of ice crystals as a removable void template. Polym. Eng. Sci. 2007, 47, 2020–2026. [Google Scholar] [CrossRef]
- Kim, M.S.; Son, J.; Lee, H.; Hwang, H.; Choi, C.H.; Kim, G. Highly porous 3D nanofibrous scaffolds processed with an electrospinning/laser process. Curr. Appl. Phys. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.; Zhang, H.; Li, M.; Duvail, J.-L.; Jiang, X.; Yin, H. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Kim, G.; Son, J.; Park, S.; Kim, W. Hybrid Process for Fabricating 3D Hierarchical Scaffolds Combining Rapid Prototyping and Electrospinning. Macromol. Rapid Commun. 2008, 29, 1577–1581. [Google Scholar] [CrossRef]
- Kim, G.; Kim, W. Highly porous 3D nanofiber scaffold using an electrospinning technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 104–110. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Oh, H.; Jeong, Y.H.; Cho, D.-W. Fabrication of a three-dimensional nanofibrous scaffold with lattice pores using direct-write electrospinning. Mater. Lett. 2013, 93, 397–400. [Google Scholar] [CrossRef]
- Xu, T.; Miszuk, J.M.; Zhao, Y.; Sun, H.; Fong, H. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv. Healthc. Mater. 2015, 4, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xu, H.; Jiang, Q.; Yang, Y. Novel 3D electrospun scaffolds with fibers oriented randomly and evenly in three dimensions to closely mimic the unique architectures of extracellular matrices in soft tissues: Fabrication and mechanism study. Langmuir 2013, 29, 2311–2318. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, S.M.; Kang, H.Y.; Min, H.-J.; Lee, R.; Ikram, M.; Subhan, F.; Jin, S.W.; Jeong, Y.H.; Kwak, J.-Y.; et al. Bioactive fish collagen/polycaprolactone composite nanofibrous scaffolds fabricated by electrospinning for 3D cell culture. J. Biotechnol. 2015, 205, 47–58. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Latifi, M.; Amani-Tehran, M.; Teo, W.E.; Ramakrishna, S. A note on the 3D structural design of electrospun nanofibers. J. Eng. Fibers Fabr. 2012, 7, 155892501200700204. [Google Scholar] [CrossRef]
- Kostakova, E.K.; Seps, M.; Pokorny, P.; Lukáš, D. Study of polycaprolactone wet electrospinning process. Express Polym. Lett. 2014, 8, 554–564. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Fang, J.; Yao, G.; Zhao, H.; Dodson, M.; Wang, X. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. J. Biomed. Mater. Res. A 2010, 94, 499–508. [Google Scholar]
- Correia, D.M.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Carabineiro, S.; Ribelles, J.L.G.; Lanceros-Méndez, S. Influence of oxygen plasma treatment parameters on poly(vinylidene fluoride) electrospun fiber mats wettability. Prog. Org. Coat. 2015, 85, 151–158. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Characterization of the Surface Biocompatibility of the Electrospun PCL-Collagen Nanofibers Using Fibroblasts. Biomacromolecules 2005, 6, 2583–2589. [Google Scholar] [CrossRef]
- Perna, A.; Theraulaz, G. When social behaviour is moulded in clay: On growth and form of social insect nests. J. Exp. Biol. 2017, 220, 83–91. [Google Scholar] [CrossRef]

| Resistive Load (MΩ) | 1.5 | 3.0 | 4.5 | 6.0 | 7.5 | 9.0 |
|---|---|---|---|---|---|---|
| VPP (V) | 2.86 | 4.79 | 4.32 | 4.22 | 4.36 | 5.15 |
| VRMS (mV) | 288 | 533 | 479 | 529 | 520 | 605 |
| IPP (µA) | 1.85 | 1.54 | 0.93 | 0.67 | 0.56 | 0.55 |
| IRMS (nA) | 191.1 | 172.6 | 105.3 | 85.6 | 68.0 | 65.8 |
| PAVG (nW) | 55.0 | 92.0 | 50.4 | 45.3 | 35.3 | 39.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muenwacha, T.; Weeranantanapan, O.; Chudapongse, N.; Diaz Sanchez, F.J.; Maensiri, S.; Radacsi, N.; Nuansing, W. Fabrication of Piezoelectric Electrospun Termite Nest-like 3D Scaffolds for Tissue Engineering. Materials 2021, 14, 7684. https://doi.org/10.3390/ma14247684
Muenwacha T, Weeranantanapan O, Chudapongse N, Diaz Sanchez FJ, Maensiri S, Radacsi N, Nuansing W. Fabrication of Piezoelectric Electrospun Termite Nest-like 3D Scaffolds for Tissue Engineering. Materials. 2021; 14(24):7684. https://doi.org/10.3390/ma14247684
Chicago/Turabian StyleMuenwacha, Thanapon, Oratai Weeranantanapan, Nuannoi Chudapongse, Francisco Javier Diaz Sanchez, Santi Maensiri, Norbert Radacsi, and Wiwat Nuansing. 2021. "Fabrication of Piezoelectric Electrospun Termite Nest-like 3D Scaffolds for Tissue Engineering" Materials 14, no. 24: 7684. https://doi.org/10.3390/ma14247684
APA StyleMuenwacha, T., Weeranantanapan, O., Chudapongse, N., Diaz Sanchez, F. J., Maensiri, S., Radacsi, N., & Nuansing, W. (2021). Fabrication of Piezoelectric Electrospun Termite Nest-like 3D Scaffolds for Tissue Engineering. Materials, 14(24), 7684. https://doi.org/10.3390/ma14247684