The Influence of Silica Nanoparticles on the Thermal and Mechanical Properties of Crosslinked Hybrid Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Composites
2.3. Characterization Methods
3. Results
3.1. ATR/FTIR Analysis
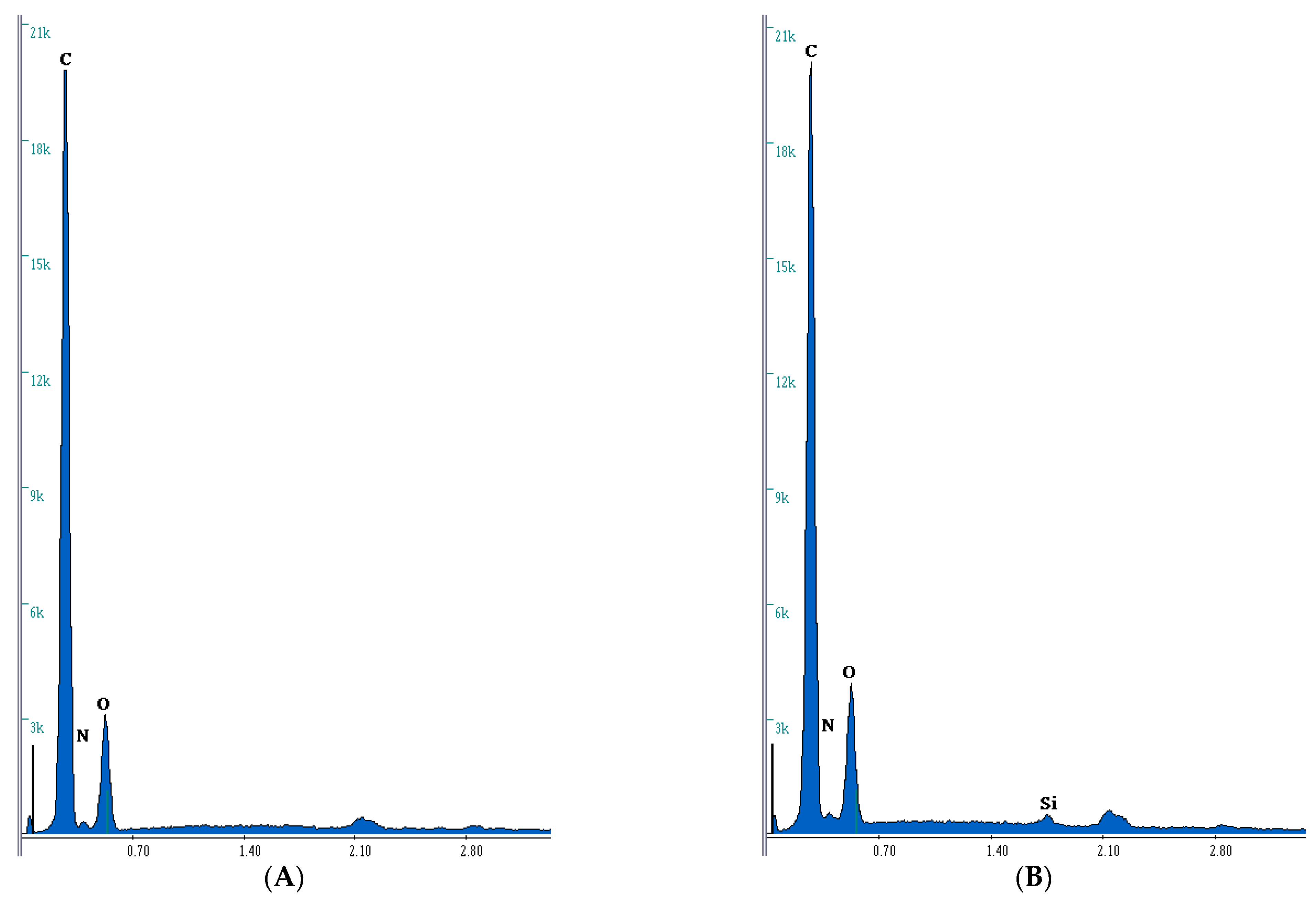
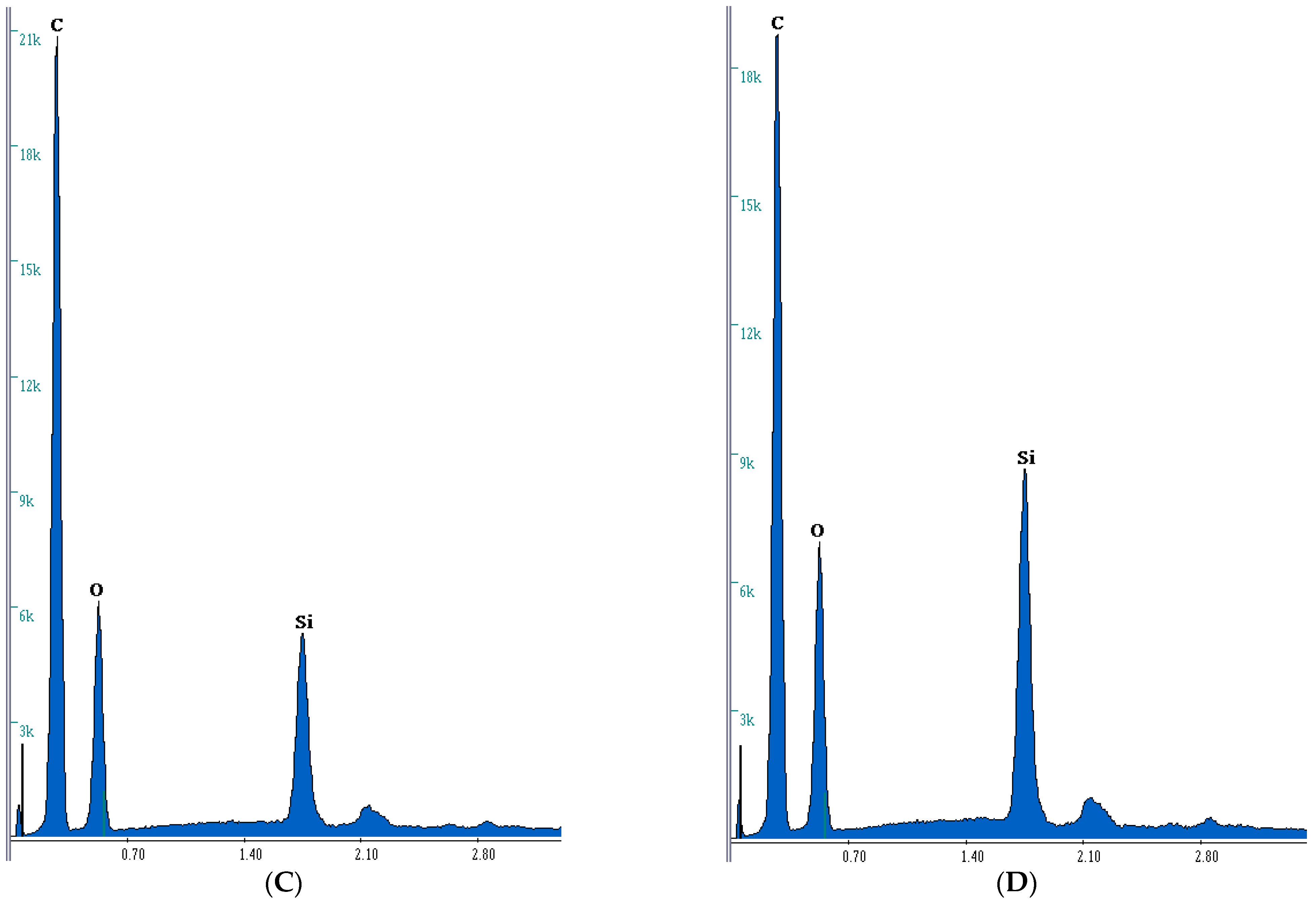
3.2. Microanalysis Study
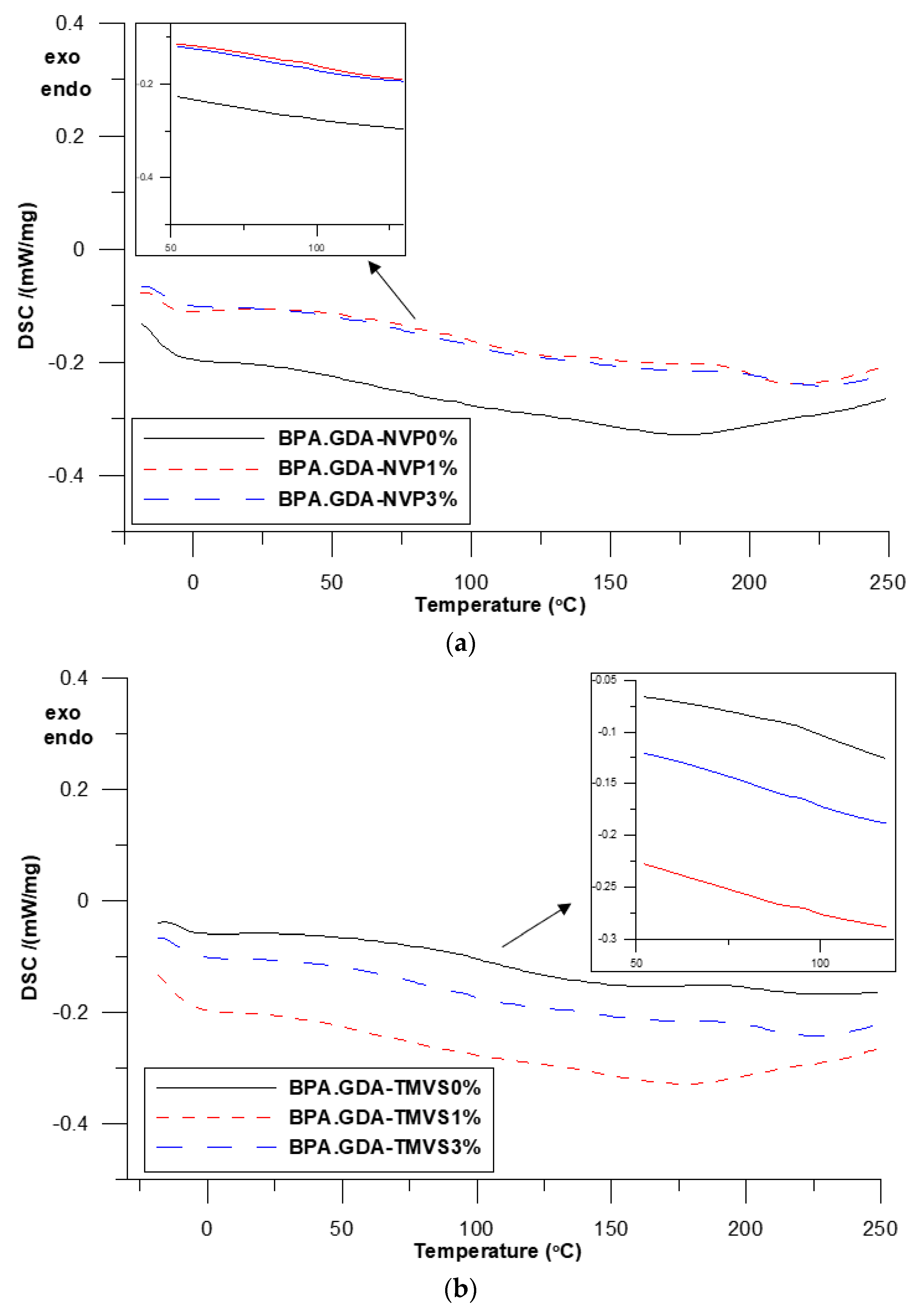
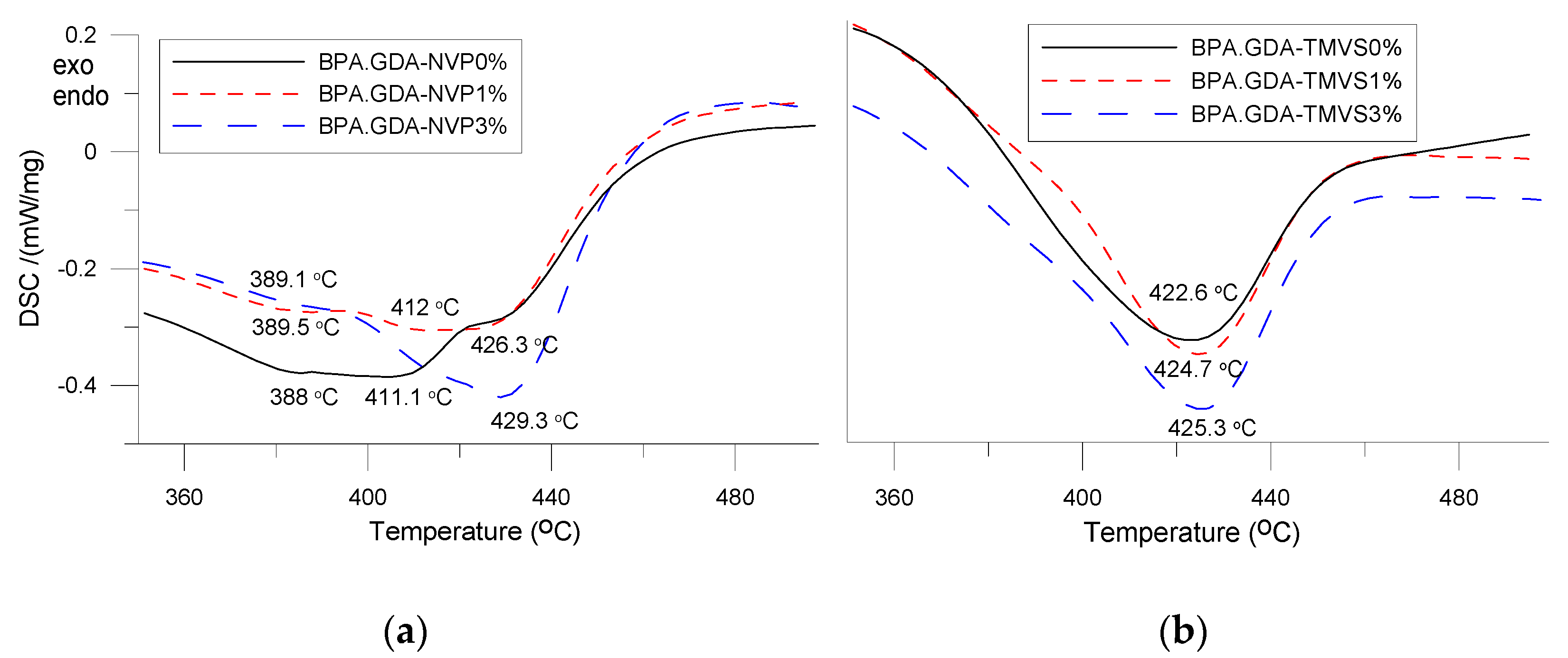
3.3. DSC Analysis
3.4. Mechanical Properties
3.4.1. Hardness Tests
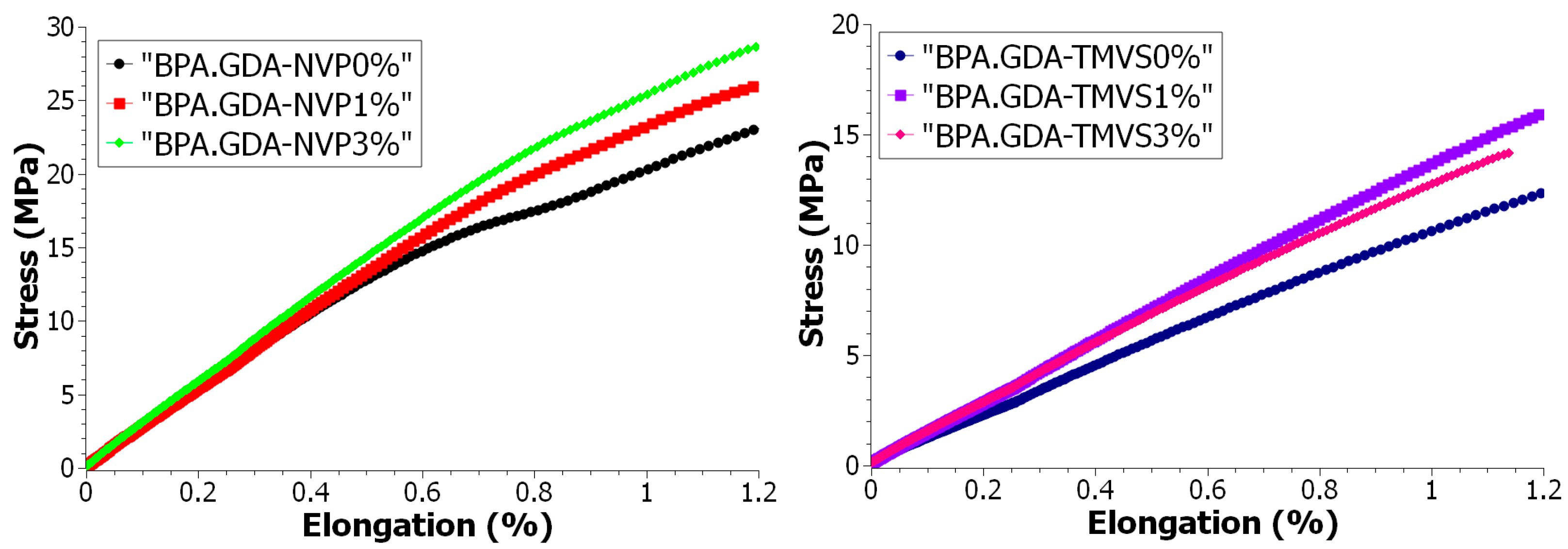
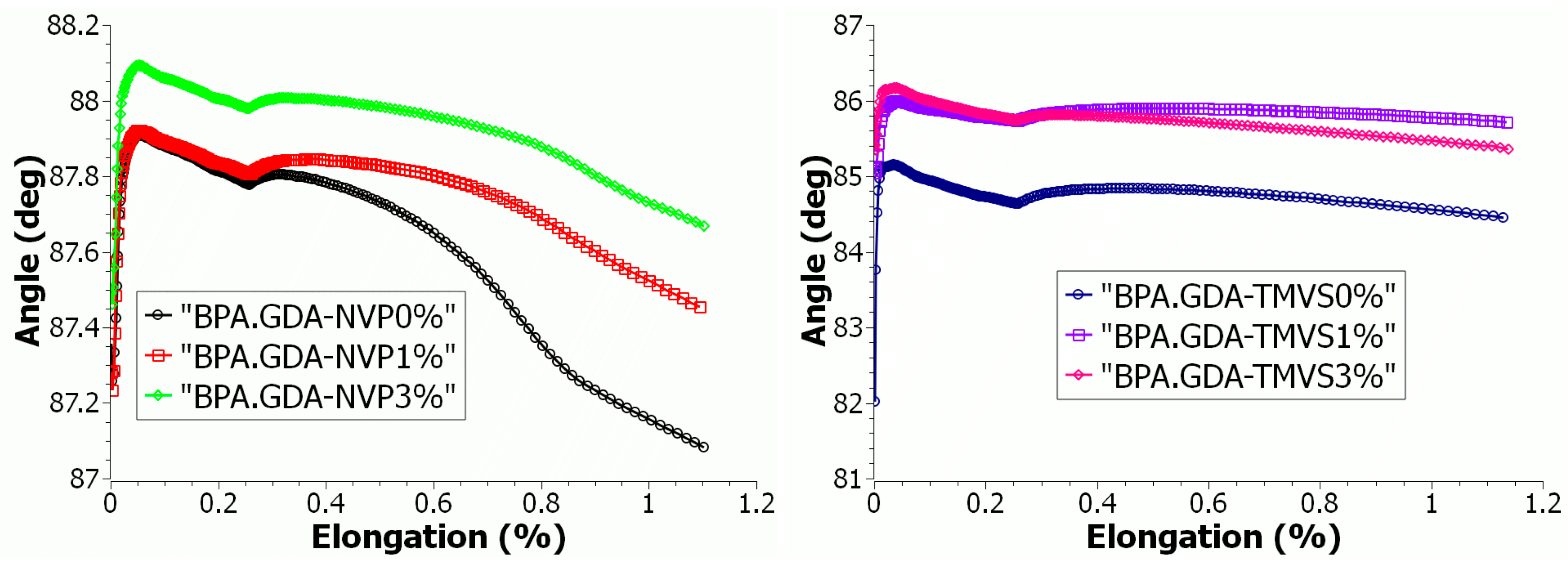
3.4.2. Mechanical Strength Tests during Uniaxial Tensile
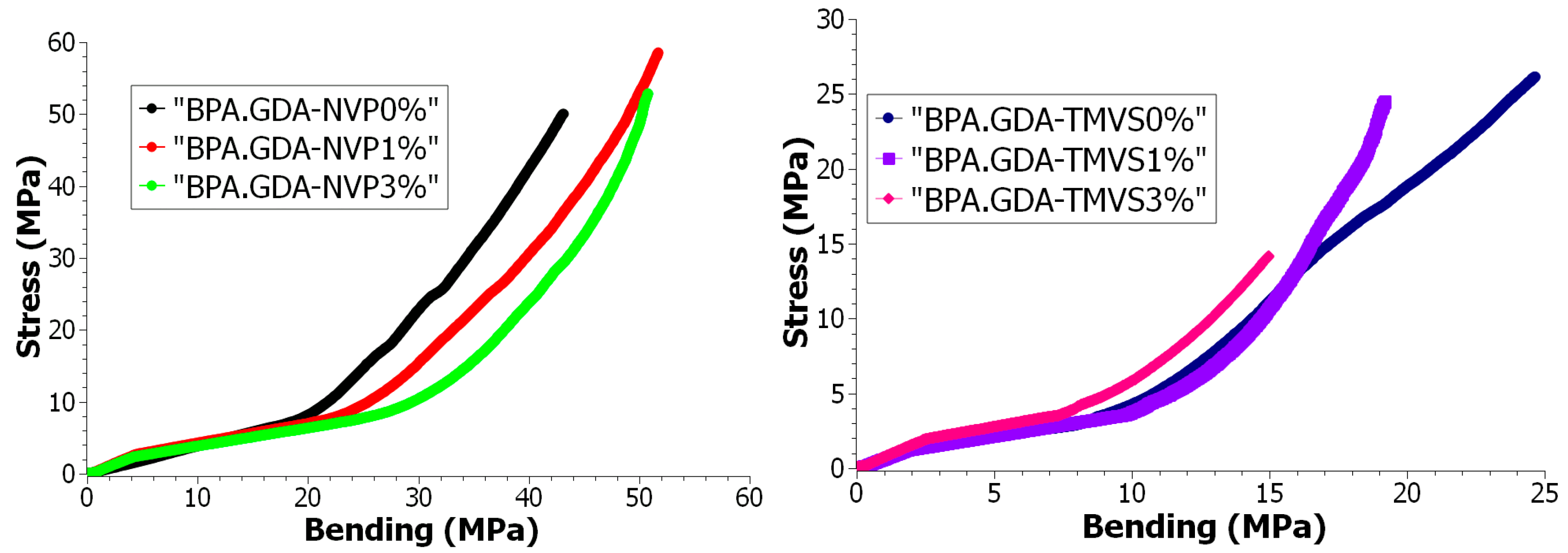
3.4.3. Mechanical Durability Tests during Bending
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, R.G. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar]
- Chujo, Y. Organic-inorganic hybrid materials. Curr. Opin. Solid State Mater. Sci. 1996, 1, 806–811. [Google Scholar] [CrossRef]
- Tiwari, I.; Mahanwar, P.A. Polyacrylate/silica hybrid materials: A step towards multifunctional properties. J. Dispers. Sci. Technol. 2019, 40, 925–957. [Google Scholar] [CrossRef]
- Gomez-Romero, P. Hybrid Organic–Inorganic Materials—In Search of Synergic Activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Agudelo, N.A.; Perez, L.D.; Lopez, B.L. A novel method for the synthesis of polystyrene-graft-silica particles using random copolymers based on styrene and triethoxyvinylsilane. Appl. Surf. Sci. 2011, 257, 8581–8586. [Google Scholar] [CrossRef]
- Chong, A.S.M.; Zhao, X.S.; Kustedjo, A.T.; Qiao, S.Z. Functionalization of large-pore mesoporous silicas with organosilanes by direct synthesis. Micropor. Mesopor. Mat. 2004, 72, 33–42. [Google Scholar] [CrossRef]
- Donescu, D.; Serban, S.; Petcu, C.; Nistor, C.L.; Ghiurea, M.; Corobea, M.C. Polymer-silica hybrids obtained by microemulsion polymerization. Colloid Polym. Sci. 2007, 285, 1455–1462. [Google Scholar] [CrossRef]
- Donescu, D.; Somoghi, R.; Spataru, C.I.; Manaila-Maximean, D.; Panaitescu, D.M.; Vasile, E.; Nistor, C.L. Hybrid polymeric latexes containing magnetite. Colloid Polym. Sci. 2013, 291, 2345–2358. [Google Scholar] [CrossRef]
- Kickelbick, G. Introduction to hybrid materials. In Hybrid Materials: Synthesis, Characterization and Applications; Kickelbick, G., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 1–48. [Google Scholar]
- Gharzouli, N.; Doufnoune, R.; Riahi, F.; Bouchareb, S. Effects of nanosilica filler surface modification and compatibilization on the mechanical, thermal and microstructure of PP/EPR blends. J. Adhes. Sci. Technol. 2019, 33, 445–467. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, A.; Celli, J.; Njuguna, Y.; Habibi, R.K. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Chung, D.D.L. Composite materials. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-VCH: New York, NY, USA, 2011; pp. 1–39. [Google Scholar]
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Mallakpour, S.; Naghdi, M. Polymer/SiO2 nanocomposites: Production and applications. Prog. Mater. Sci. 2018, 97, 409–447. [Google Scholar] [CrossRef]
- Mammeri, F.; Le Bourhis, E.; Rozes, L.; Sanchez, C. Mechanical properties of hybrid organic–inorganic materials. J. Mater. Chem. 2005, 15, 3789–3811. [Google Scholar] [CrossRef]
- Balguri, P.K.; Samuel, D.G.H.; Thumu, U. A review on mechanical properties of epoxy nanocomposites. Mater. Today Proc. 2021, 44, 346–355. [Google Scholar] [CrossRef]
- Fahmi, A.; Pietsch, T.; Mendoza, C.; Cheval, N. Functional hybrid materials. Mater. Today 2009, 12, 44–50. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites—Processing, properties and applications: A review. Compos. B Eng. 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Parashar, V.; Kukreja, P.; Narula, A.K. Curing and Thermal Properties of Epoxy Resin Containing Nanosilica Particles. Asian J. Chem. 2020, 22, 3973–3980. [Google Scholar]
- Ghaemy, M.; Bazzar, M.; Mighani, H. Effect of nanosilica on the kinetics of cure reaction and thermal degradation of epoxy resin. Chin. J. Polym. Sci. 2011, 29, 141–148. [Google Scholar] [CrossRef]
- Pascual-Sanchez, V.; Barrientos-Ramirez, S.; Martin-Martinez, J.M. Comparative Study of the Effect of Addition of Silica and Silicate Nanofillers on the Properties of Epoxy Coatings. Compos. Interfaces 2010, 17, 513–532. [Google Scholar] [CrossRef]
- Kumar, S.R.; Patnaik, A.; Meena, A.; Godara, M. Effect of adding nanosilica particulate filler on the wear behavior of dental composite. Polym. Compos. 2018, 39, E332–E341. [Google Scholar]
- Kumar, S.R.; Patnaik, A.; Bhat, I.K. Wear behavior of light-cured dental composite reinforced with silane-treated nanosilica filler. Polym. Adv. Technol. 2018, 29, 1394–1403. [Google Scholar] [CrossRef]
- Podkoscielna, B. New photoluminescent copolymers of naphthalene-2,7-diol dimethacrylate and N-vinyl-2-pyrrolidone. J. Therm. Anal. Calorim. 2014, 116, 785–793. [Google Scholar] [CrossRef]
- Benadda, M.; Ferrahi, M.I.; Belbachir, M. Synthesis of Poly(N-vinyl-2- pyrrolidone-co-methyl methacrylate) by Maghnite-H+ a Non-toxic Catalyst. Bull. Chem. React. Eng. Catal. 2014, 9, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Podkoscielna, B.; Sobiesiak, M. Synthesis and characterization of organic–inorganic hybrid microspheres. Adsorption 2016, 22, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Podkoscielna, B.; Fila, K.; Gil, M.; Nowak, J. Synthesis and characterization of new hybrid microspheres with amide functionalization. Adsorp. Sci. Technol. 2017, 35, 422–431. [Google Scholar] [CrossRef]
- Podkoscielna, B. The highly crosslinked dimethacrylic/divinylbenzene copolymers: Characterization and thermal studies. J. Therm. Anal. Calorim. 2011, 104, 725–730. [Google Scholar] [CrossRef]
- Sobiesiak, M.; Podkoscielna, B.; Sevastyanova, O. Thermal degradation behavior of lignin-modified porous styrene-divinylbenzene and styrene-bisphenol A glycerolate diacrylate copolymer microspheres. J. Anal. Appl. Pyrolysis 2017, 123, 364–375. [Google Scholar] [CrossRef]
- Driemel, A.; Har-Peled, S.; Wenk, C. Approximating the Fréchet distance for realistic curves in near linear time. Discret. Comput. Geom. 2012, 48, 94–127. [Google Scholar] [CrossRef] [Green Version]
- Alt, H.; Godau, M. Measuring the resemblance of polygonal curves. In Proceedings of the 8th Annual Symposium on Computational Geometry, Berlin, Germany, 10–12 June 1992; pp. 102–109. [Google Scholar]


| No. | Composite | BPA.GDA | NVP | TMVS | NANOSiO2 | IQ |
|---|---|---|---|---|---|---|
| g | wt.% | |||||
| 1 | BPA.GDA-NVP0% | 8.4 | 3.6 | 0 | 0 | 1 |
| 2 | BPA.GDA-NVP1% | 8.4 | 3.6 | 0 | 1 | 1 |
| 3 | BPA.GDA-NVP3% | 8.4 | 3.6 | 0 | 3 | 1.5 |
| 4 | BPA.GDA-TMVS0% | 8.4 | 0 | 3.6 | 0 | 1 |
| 5 | BPA.GDA-TMVS1% | 8.4 | 0 | 3.6 | 1 | 1 |
| 6 | BPA.GDA-TMVS3% | 8.4 | 0 | 3.6 | 3 | 1.5 |
| No. | Composite | C | Si | O | N |
|---|---|---|---|---|---|
| wt.%/at.% | wt.%/at.% | wt.%/at.% | wt.%/at.% | ||
| 1 | BPA.GDA-NVP0% | 72.93/77.82 | -/- | 22.70/18.19 | 4.37/3.99 |
| 3 | BPA.GDA-NVP3% | 69.13/74.43 | 0.37/0.17 | 23.81/19.25 | 6.64/6.13 |
| 4 | BPA.GDA-TMVS0% | 69.88/76.73 | 4.27/2.01 | 25.76/21.23 | -/- |
| 6 | BPA.GDA-TMVS3% | 66.87/74.59 | 6.38/3.04 | 26.67/22.33 | -/- |
| Sample | Composite | Tg | Tmax1 (°C) | Tmax2 | Tmax3 |
|---|---|---|---|---|---|
| A | BPA.GDA-NVP0% | 89.1 | 388 | 411.1 | 426.0 |
| B | BPA.GDA-NVP1% | 91.8 | 389 | - | 426.3 |
| C | BPA.GDA-NVP3% | 98.0 | - | - | 429.3 |
| D | BPA.GDA-TMVS0% | 99.1 | - | - | 422.6 |
| E | BPA.GDA-TMVS1% | 100.6 | - | - | 424.7 |
| F | BPA.GDA-TMVS3% | 107.1 | - | - | 425.3 |
| Sample | Composites | Mean Value, Shore Degrees, D Scale | Diameter µm | High µm |
|---|---|---|---|---|
| A | BPA.GDA-NVP0% | 84.28 | 262.80 | 60 |
| B | BPA.GDA-NVP1% | 92.12 | 283.12 | 74 |
| C | BPA.GDA-NVP3% | 92.18 | 299.34 | 78 |
| D | BPA.GDA-TMVS0% | 82.17 | 489.12 | 60 |
| E | BPA.GDA-TMVS1% | 82.28 | 585.74 | 73 |
| F | BPA.GDA-TMVS3% | 85.65 | 596.56 | 75 |
| Samples | Composites | Young’s Module Et | Tensile Strength σm | Elongation εm | Sample Width b | Sample Thickness h |
|---|---|---|---|---|---|---|
| MPa | MPa | % | mm | mm | ||
| A | BPA.GDA-NVP0% | 2542.80 | 50.12 | 3.2 | 10.62 | 2.12 |
| B | BPA.GDA-NVP1% | 2576.15 | 58.53 | 3.5 | 10.09 | 2.06 |
| C | BPA.GDA-NVP3% | 2794.53 | 52.85 | 2.6 | 9.15 | 2.01 |
| D | BPA.GDA-TMVS0% | 1038.56 | 26.11 | 3.8 | 8.00 | 2.92 |
| E | BPA.GDA-TMVS1% | 1322.04 | 24.50 | 2.2 | 8.85 | 1.97 |
| F | BPA.GDA-TMVS3% | 1309.18 | 14.16 | 1.1 | 9.56 | 2.05 |
| Samples | Composites | Young’s Module Ef MPa | Bending Strength σfM MPa | Elongation εfM % | Sample Thickness h mm | Sample Width b mm | Cross Section Area A0 mm2 |
|---|---|---|---|---|---|---|---|
| A | BPA.GDA-NVP0% | 1770.02 | 103.96 | 2.9 | 2.09 | 10.07 | 21.04 |
| B | BPA.GDA-NVP1% | 3787.36 | 110.01 | 3.3 | 2.08 | 8.85 | 18.40 |
| C | BPA.GDA-NVP3% | 4204.99 | 125.83 | 3.6 | 2.00 | 9.91 | 19.82 |
| D | BPA.GDA-TMVS0% | 1514.66 | 42.51 | 3.2 | 2.92 | 10.06 | 29.37 |
| E | BPA.GDA-TMVS1% | 1725.73 | 45.27 | 3.2 | 2.01 | 9.48 | 19.05 |
| F | BPA.GDA-TMVS3% | 2389.02 | 35.84 | 1.4 | 2.06 | 9.86 | 20.31 |
| Compared Curves | Fréchet Distance |
|---|---|
| NVP family | |
| BPA.GDA-NVP0%/BPA.GDA-NVP1% | 2.959537 |
| BPA.GDA-NVP0%/BPA.GDA-NVP3% | 5.536141 |
| BPA.GDA-NVP1%/BPA.GDA-NVP3% | 2.576815 |
| TMVS family | |
| BPA.GDA-TMVS0%/BPA.GDA-TMVS1% | 3.524791 |
| BPA.GDA-TMVS0%/BPA.GDA-TMVS3% | 2.39698 |
| BPA.GDA-TMVS1%/BPA.GDA-TMVS3% | 1.128088 |
| Compared Curves | Fréchet Distance |
|---|---|
| NVP family | |
| BPA.GDA-NVP0%/BPA.GDA-NVP1% | 9.13941 |
| BPA.GDA-NVP0%/BPA.GDA-NVP3% | 8.486294 |
| BPA.GDA-NVP1%/BPA.GDA-NVP3% | 0.7185488 |
| TMVS family | |
| BPA.GDA-TMVS0%/BPA.GDA-TMVS1% | 5.132055 |
| BPA.GDA-TMVS0%/BPA.GDA-TMVS3% | 9.985838 |
| BPA.GDA-TMVS1%/BPA.GDA-TMVS3% | 5.002452 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klepka, T.; Podkościelna, B.; Czerwiński, D.; Samujło, B. The Influence of Silica Nanoparticles on the Thermal and Mechanical Properties of Crosslinked Hybrid Composites. Materials 2021, 14, 7431. https://doi.org/10.3390/ma14237431
Klepka T, Podkościelna B, Czerwiński D, Samujło B. The Influence of Silica Nanoparticles on the Thermal and Mechanical Properties of Crosslinked Hybrid Composites. Materials. 2021; 14(23):7431. https://doi.org/10.3390/ma14237431
Chicago/Turabian StyleKlepka, Tomasz, Beata Podkościelna, Dariusz Czerwiński, and Bronisław Samujło. 2021. "The Influence of Silica Nanoparticles on the Thermal and Mechanical Properties of Crosslinked Hybrid Composites" Materials 14, no. 23: 7431. https://doi.org/10.3390/ma14237431
APA StyleKlepka, T., Podkościelna, B., Czerwiński, D., & Samujło, B. (2021). The Influence of Silica Nanoparticles on the Thermal and Mechanical Properties of Crosslinked Hybrid Composites. Materials, 14(23), 7431. https://doi.org/10.3390/ma14237431








