Morphology and Properties of Poly(2,6-dimethyl-1,4-phenylene oxide)/Polyamide 11 Hybrid Nanocomposites: Effect of Silica Surface Modification
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Processing
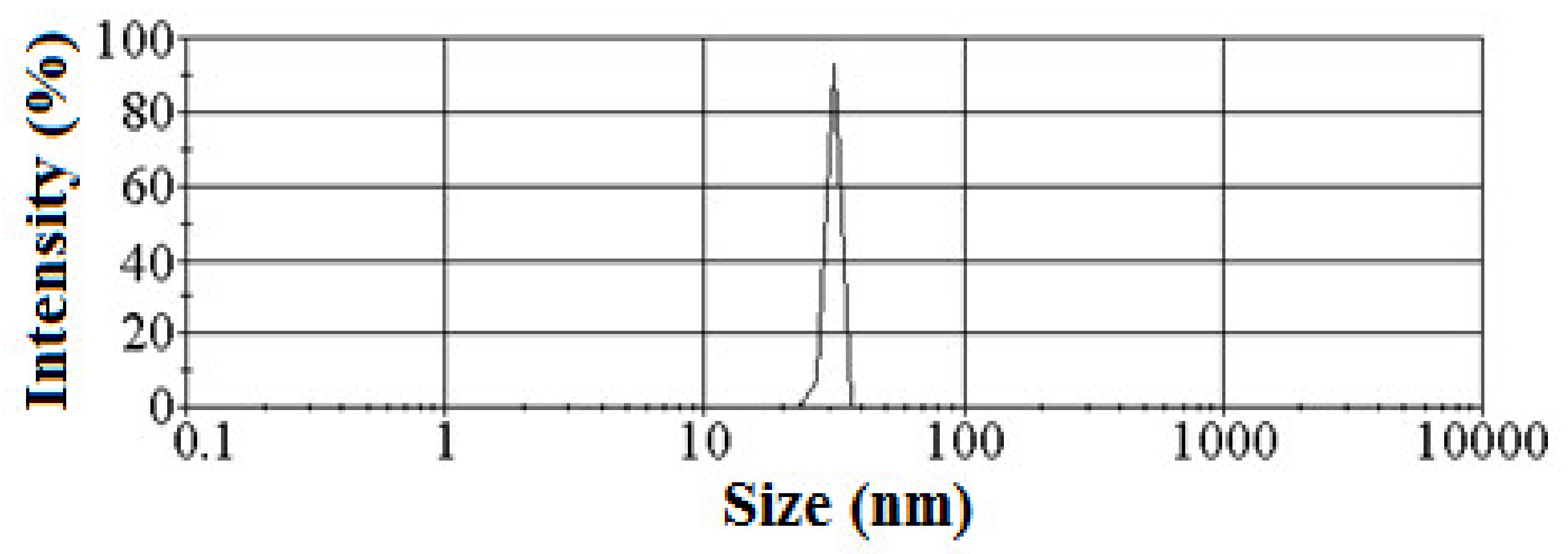
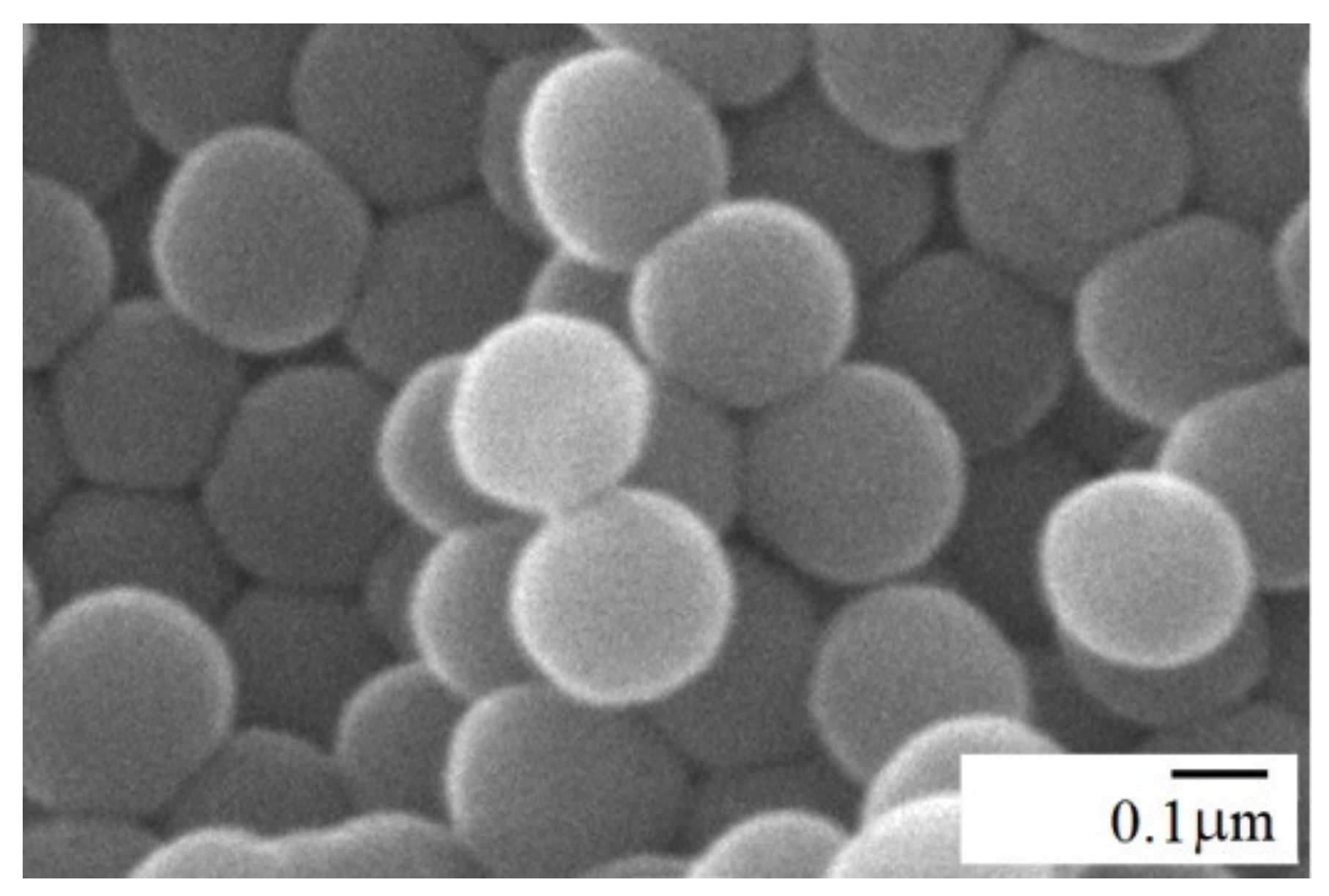
2.2. Silica Preparation and Characterization
2.3. Composites Preparation
2.4. Methods
3. Results and Discussion
3.1. Graft Copolymer Formation
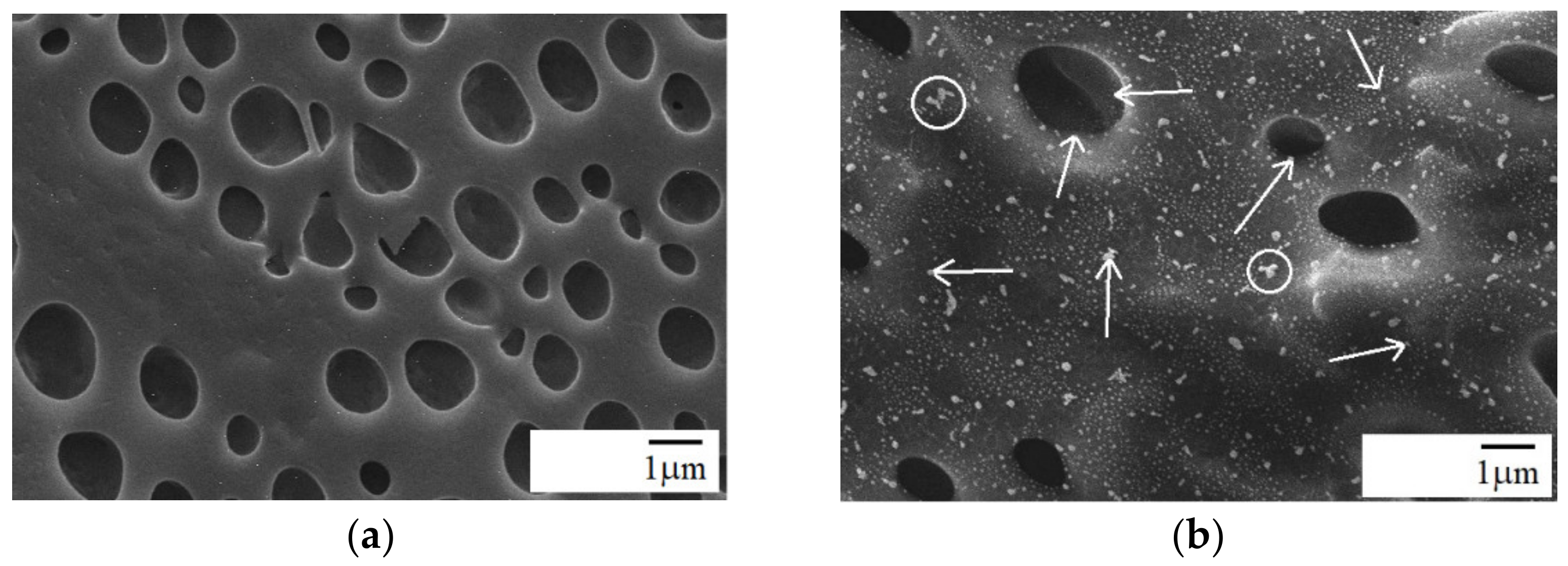
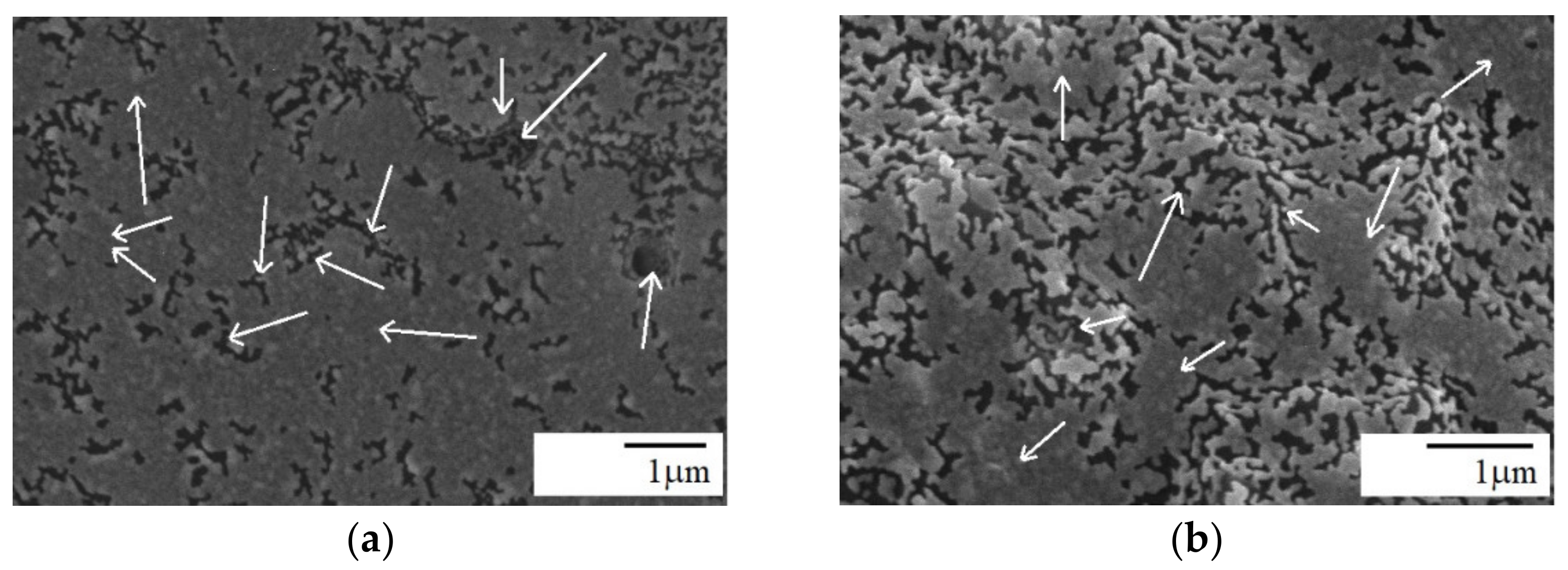
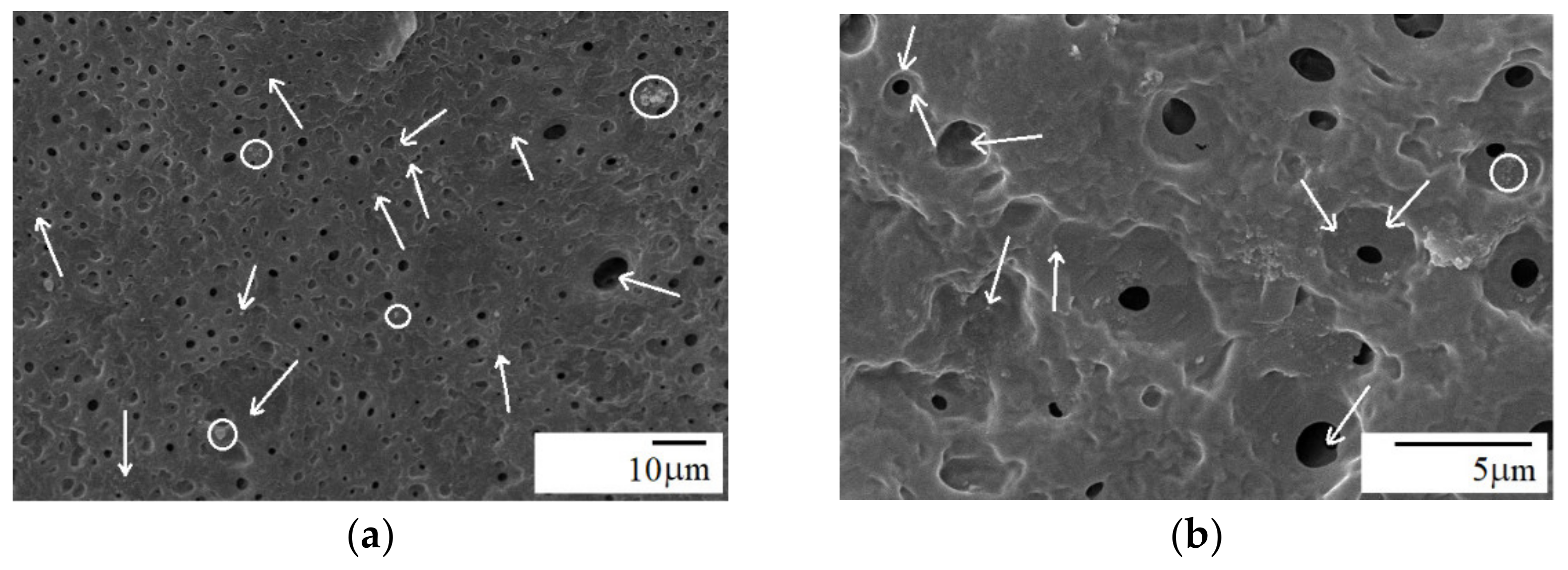
3.2. Morphology
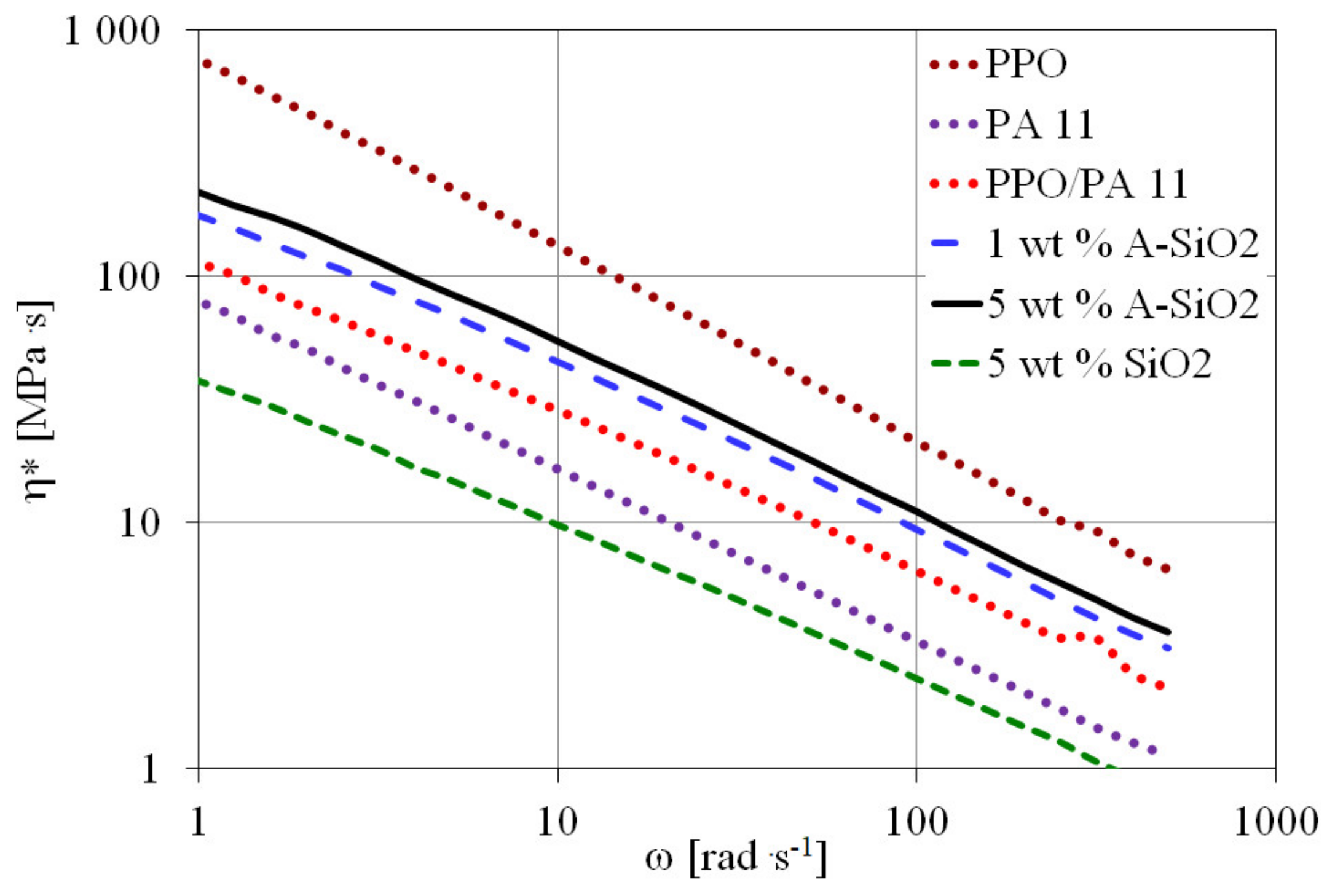
Dynamic Viscosity
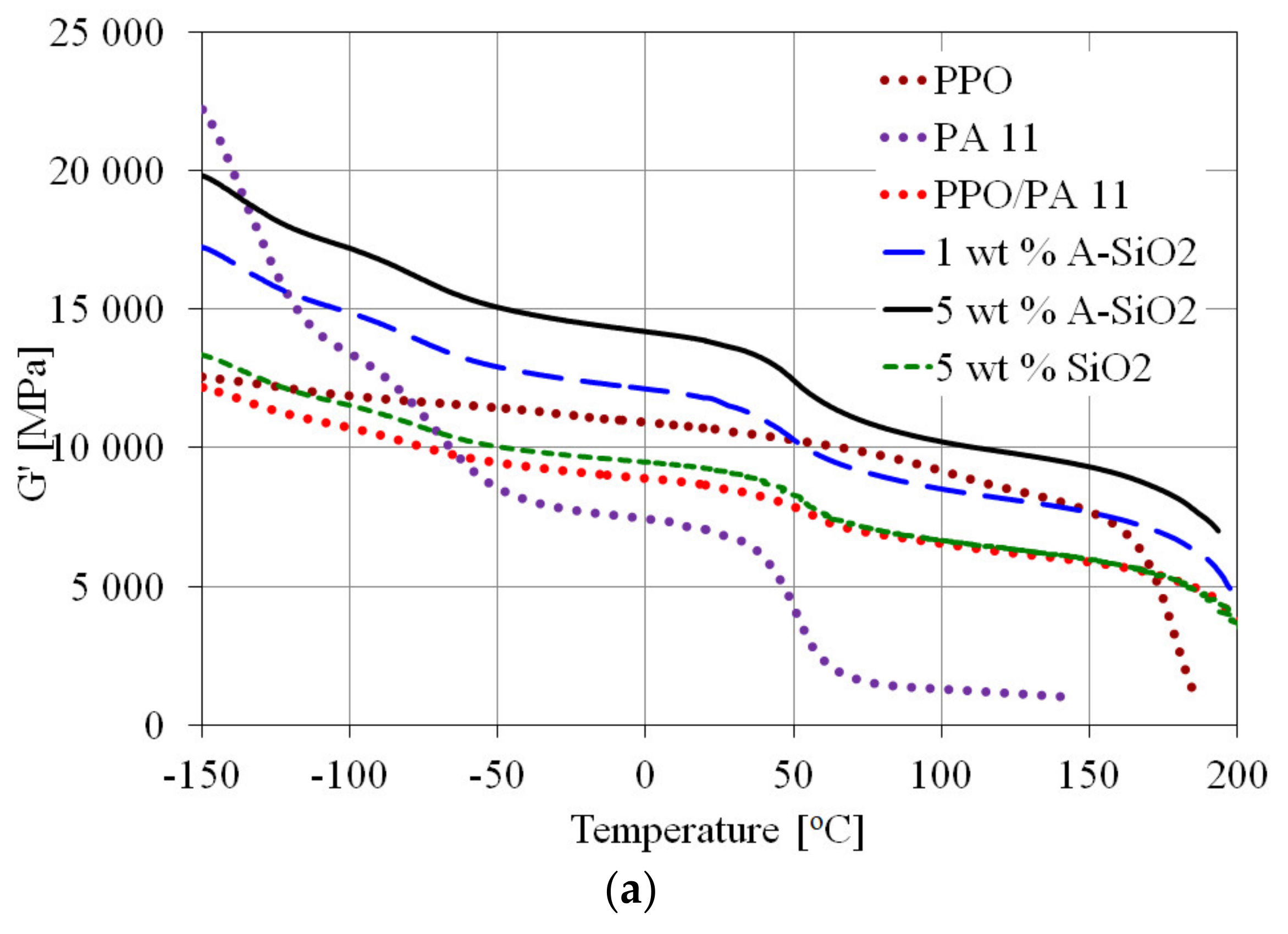
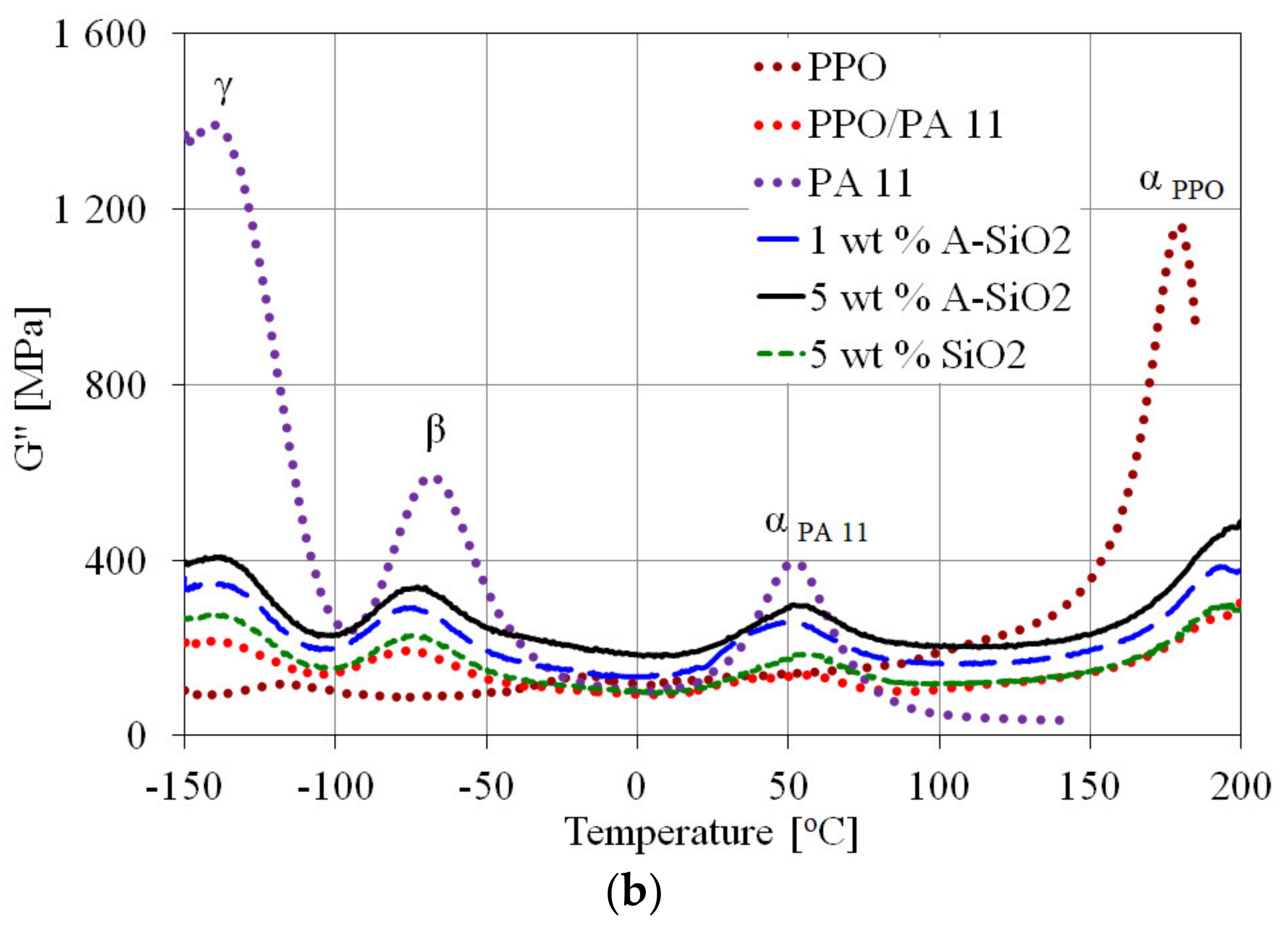
3.3. Dynamic Mechanical Analysis
3.4. Mechanical Properties
| Sample | Tensile Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (MPa) | Flexural Modulus (MPa) | Flexural Strength (MPa) | Impact Strength (kJ/m2) |
|---|---|---|---|---|---|---|
| PPO | 2515 ± 49 | 70 ± 1 | 10 ± 1 | 2250 ± 30 | 58 ± 1 | 6 ± 0.6 |
| PA11 | 1240 ± 55 | 42 ± 1 | 299 ± 9 | 1180 ± 35 | 45 ± 1 | 15 ± 0.6 |
| PPO/PA11 | 2192 ± 33 | 54 ± 1 | 7 ± 0.3 | 2080 ± 24 | 75 ± 1 | 5 ± 0.5 |
| 1 wt.% A-SiO2 | 4843 ± 53 | 60 ± 1 | 7 ± 0.2 | 2165 ± 28 | 86 ± 1 | 7 ± 0.3 |
| 5 wt.% A-SiO2 | 4998 ± 48 | 64 ± 1 | 7 ± 0.3 | 2322 ± 20 | 90 ± 1 | 6 ± 0.4 |
| 5 wt.% SiO2 | 2408 ± 48 | 56 ± 1 | 5 ± 0.2 | 2239 ± 55 | 77 ± 3 | 3 ± 0.3 |
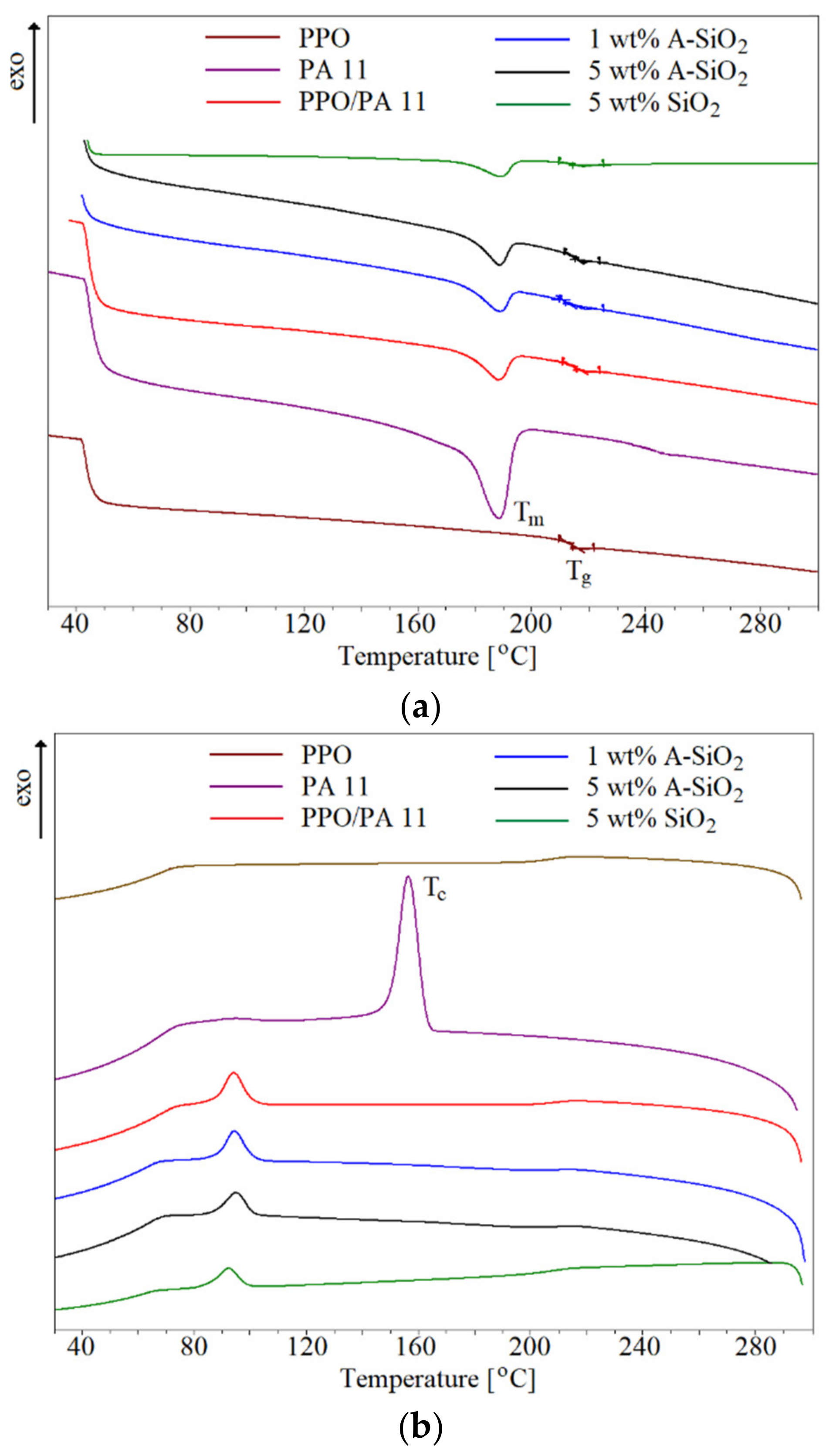
3.5. Thermal Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Yang, G. An unusual morphology and crystallization behavior in situ formed polyphenylene oxide/polyamide 6 blends. J. Mater. Sci. 2010, 45, 987–992. [Google Scholar] [CrossRef]
- Jitender, P.S.; Praveen, K. Optical and Luminescence Study of PVP/PPO Membranes. Int. J. New. Hor. Phys. 2015, 2, 95–97. [Google Scholar] [CrossRef]
- De Bie, V.G.; Anderson, P.D.; van Breemen, L.C.A. The effect of an adhesive interaction on predicting the scratch response of PS/PPO blends. Polymer 2019, 172, 91–99. [Google Scholar] [CrossRef]
- Kerdou, I.; Cailloux, J.; Martinez, J.C.; Santana, O.; Maspoch, M.L.; Puiggali, J.; Franco, L. Biphasic polylactide/polyamide 6,10 blends: Influence of composition on polyamide structure and polyester crystallization. Polymer 2020, 202, 122676. [Google Scholar] [CrossRef]
- Gao, F. Advances in Polymer Nanocomposites. Types and Applications; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Coppola, B.; Scarfato, P.; Incarnato, L.; Di, M.L. Morphology development and mechanical properties variation during cold-drawing of polyethylene-clay nanocomposites fibers. Polymers 2017, 9, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, J.; Xie, H.; Lai, X.; Li, H.; Zeng, X. Significant improvement of tribological performances of polyamide 46/polyphenylene oxide alloy by functionalized zirconium phosphate. Tribol. Int. 2018, 128, 204–213. [Google Scholar] [CrossRef]
- Elias, L.; Fenouillot, F.; Majeste, J.C.; Cassagnau, P. Morphology and rheology of immiscible polymer blends filled with silica nanoparticles. Polymer 2007, 48, 6029–6040. [Google Scholar] [CrossRef]
- Ray, S.S.; Pouliot, S.; Bousmina, M.; Utracki, L.A. Role of organically modified layered silicate as an active interfacial modifier in immiscible polystyrene/polypropylene blends. Polymer 2004, 45, 8403–8413. [Google Scholar]
- Lee, S.H.; Kontopoulou, M.; Park, C.B. Effect of nanosilica on the co-continuous morphology of polypropylene/polyolefin elastomer blends. Polymer 2010, 51, 1147–1155. [Google Scholar] [CrossRef]
- Jeziorska, R.; Szadkowska, A.; Studzinski, M.; Zubrowska, M. The use of modified silica to control the morphology of polyamide 11 and poly(phenylene oxide) blends. Polimery 2021, 66, 399–410. [Google Scholar] [CrossRef]
- Haneef, I.N.H.M.; Shaffiar, N.M.; Shaharuddin, S.I.S.; Hamid, A.M.A.; Sabri, M.F.M.; Afifi, A.M. Effect of HNT on mechanical and thermal properties of poly(lactic acid)/polypropylene carbonate blends. Polimery 2021, 66, 459–465. [Google Scholar] [CrossRef]
- Zhou, H.J.; Rong, M.Z.; Zhang, M.Q.; Friedrich, K. Effects of reactive compatibilization on the performance of nano-silica filled polypropylene composites. J. Mater. Sci. 2006, 41, 5767–5770. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Jin, R. Toughening of poly(2,6-dimethyl-1,4-phenylene oxide)/nylon 6 alloys with functionalized elastomers via reactive compatibilization: Morphology, mechanical properties, and rheology. Eur. Polym. J. 2004, 40, 1223–1232. [Google Scholar] [CrossRef]
- Jeziorska, R.; Wielgosz, Z.; Szadkowska, A.; Studzinski, M.; Komornicki, R.; Stasinski, J.; Kania-Szarek, A. Structure, thermal characterization and mechanical properties of poly(phenylene oxide) and high impact polystyrene blend. Polimery 2016, 61, 710–718. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Wang, S.; Ji, J.L. Effect of POSS on morphology and properties of poly(2,6-dimethyl-1,4-phenylene oxide)/polyamide 6 blends. Eur. Polym. J. 2009, 45, 2202–2210. [Google Scholar] [CrossRef]
- Li, Y.J.; Shimizu, H. Novel morphologies of poly(phenylene oxide) (PPO)/polyamide 6 (PA6) blend nanocomposites. Polymer 2004, 45, 7381–7388. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, Z.; Cheng, J.; Fang, Z. Influence of carbon nanotubes with different functional groups on the morphology and properties of PPO/PA6 blends. J. Appl. Polym. Sci. 2010, 116, 1322–1328. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Cramez, M.C.; Garcia, C.B.; Kearns, M.P.; Maziers, E. Effect of the processing conditions on the microstructure and properties of rotational molded polyamide 11. J. Appl. Polym. Sci 2008, 108, 939–946. [Google Scholar] [CrossRef]
- Chiang, C.R.; Chang, F.C. Polymer blends of polyamide-6 (PA6) and poly(phenylene ether) (PPE) compatibilized by a multifunctional epoxy coupler. J. Polym. Sci. B Polym. Phys. 1998, 36, 1805–1819. [Google Scholar] [CrossRef]
- Chiang, C.R.; Chang, F.C. Polymer blends of polyamide-6 and poly(phenylene oxide) compatibilized by styrene-co-glycidyl methacrylate. J. Polym. Sci. 1996, 61, 2411–2421. [Google Scholar] [CrossRef]
- Chiang, C.R.; Chang, F.C. Polymer blends of polyamide-6 (PA6) and poly(phenylene oxide) (PPO) compatibilized by styrene-maleic anhydride (SMA) copolymer. Polymer 1997, 38, 4807–4817. [Google Scholar] [CrossRef]
- Martino, L.; Basilissi, L.; Farina, H.; Ortenzi, M.A.; Zini, E.; Di Silvestro, G.; Scandola, M. Bio-based polyamide 11: Synthesis, rheology and solid-state properties of star structures. Eur. Polym. J. 2014, 59, 69–77. [Google Scholar] [CrossRef]
- Bourmaud, A.; Le Duigou, A.; Gourier, C.; Baley, C. Influence of processing temperature on mechanical performance of unidirectional polyamide 11–flax fibre composites. Ind. Crops. Prod. 2016, 84, 151–165. [Google Scholar] [CrossRef]
- Le Duigou, A.; Bourmaud, A.; Gourier, C.; Baley, C. Multi-scale shear properties of flax fibre reinforced polyamide 11 biocomposites. Composites Part A 2016, 85, 123–129. [Google Scholar] [CrossRef]
- Gourier, C.; Bourmaud, A.; Le Duigou, A.; Baley, C. Influence of PA11 and PP thermoplastic polymers on recycling stability of unidirectional flax fibre reinforced biocomposites. Polym. Degrad. Stab. 2017, 136, 1–9. [Google Scholar] [CrossRef]
- Sahnoune, M.; Taguet, A.; Otazaghine, B.; Kaci, M.; Lopez-Cuesta, J.M. Effects of functionalized halloysite on morphology and properties of polyamide-11/SEBS-g-MA blends. Eur. Polym. J. 2017, 90, 418–430. [Google Scholar] [CrossRef]
- He, X.; Yang, J.; Zhu, L.; Wang, B.; Sun, G.; Lv, P.; Phang, I.Y.; Liu, T. Morphology and melt rheology of nylon 11/clay nanocomposites. J. Appl. Polym. Sci. 2006, 102, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Jeziorska, R.; Abramowicz, A.; Szadkowska, A.; Pasnik, A.; Spasowka, E. Poly(phenylene oxide) and renewable polyamide 11 blends compatibilized by ethylene-n-octene copolymer. J. Renew. Mater. 2018, 6, 772–783. [Google Scholar] [CrossRef]
- Jeziorska, R.; Swierz-Motysia, B.; Zielecka, M.; Szadkowska, A.; Studzinski, M. Structure and mechanical properties of low-density polyethylene/spherical silica nanocomposites prepared by melt mixing: The joint action of silica’s size, functionality, and compatibilizer. J. Appl. Polym. Sci. 2012, 125, 4326–4337. [Google Scholar] [CrossRef]
- Studzinski, M.; Jeziorska, R.; Szadkowska, A.; Zielecka, M. Modified nanosilica-filled polypropylene composites with glycidyl methacrylate grafted ethylene/n-octene copolymer as compatibilizer. Polimery 2014, 59, 625–635. [Google Scholar] [CrossRef]
- Zielecka, M.; Bujnowska, E.; Suwala, K.; Wenda, M. Sol-Gel-Derived Silicon-Containing Hybrids. In Recent Applications in Sol-Gel Synthesis, 1st ed.; Chandra, U., Ed.; InTech: Houston, TX, USA, 2017; pp. 1–23. ISBN 9789535132462. [Google Scholar]
- Jeziorska, R.; Wielgosz, Z.; Abramowicz, A.; Szadkowska, A.; Zielecka, M.; Dzierzawski, J.; Komornicki, R.; Jaczewska, T.; Firlik, S. High-Impact Thermoplastic Compositions Containing Poly(phenylene oxide). Polish Patent 224 607, 31 January 2017. [Google Scholar]
- Ricou, P.; Pinel, E.; Juhasz, N. Temperature experiments for improved accuracy in the calculation of polyamide-11 crystallinity by X-ray diffraction. Adv. X-ray Anal. 2005, 48, 170–175. [Google Scholar]
- Hoseini, A.H.A.; Arjmand, M.; Sundararaj, U.; Trifkovic, M. Tunable electrical conductivity of polystyrene/polyamide-6/carbon nanotube blend nanocomposites via control of morphology and nanofiller localization. Eur. Polym. J. 2017, 95, 418–429. [Google Scholar] [CrossRef]
- Zou, H.; Wang, K.; Zhang, Q.; Fu, Q. A change of phase morphology in poly(p-phenylene sulfide)/polyamide 66 blends induced by adding multi-walled carbon nanotubes. Polymer 2006, 47, 7821–7826. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Vassiliou, A.; Pavlidou, E.; Karayannidis, G.P. Compatibilisation effect of PP-g-MA copolymer on iPP/SiO2 nanocomposites prepared by melt mixing. Eur. Polym. J. 2005, 41, 1965–1978. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Pavlidou, E.; Bikiaris, D. Thermal degradation mechanism of HDPE nanocomposites containing fumed silica nanoparticles. Thermochim. Acta 2009, 485, 65–71. [Google Scholar] [CrossRef]
- Prashantha, K.; Schmitt, H.; Lacrampe, M.F.; Krawczak, P. Mechanical behavior and essential work of fracture of halloysite nanotubes filled polyamide 6 nanocomposites. Compos. Sci. Technol. 2011, 71, 1859–1866. [Google Scholar]
- Sangroniz, L.; Palacios, J.K.; Fernández, M.; Eguiazabal, J.I.; Santamaria, A.; Müller, A.J. Linear and non-linear rheological behavior of polypropylene/polyamide blends modified with a compatibilizer agent and nanosilica and its relationship with the morphology. Eur. Polym. J. 2016, 83, 10–21. [Google Scholar] [CrossRef]
- Zhou, R.J.; Burkhart, T. Polypropylene/SiO2 nanocomposites filled with different nanosilicas: Thermal and mechanical properties, morphology and interphase characterization. J. Mater. Sci. 2011, 46, 1228–1238. [Google Scholar] [CrossRef]
- Jeziorska, R.; Swierz-Motysia, B.; Zielecka, M.; Studzinski, M. Polyamide/spherical nanosilica nanocomposites. Polimery 2009, 54, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Jeziorska, R.; Swierz-Motysia, B.; Szadkowska, A.; Marciniec, B.; Maciejewski, H.; Dutkiewicz, M.; Leszczynska, I. Effect of POSS on morphology, thermal and mechanical properties of polyamide 6. Polimery 2011, 56, 809–816. [Google Scholar] [CrossRef]

| Sample | Gel Content (%) |
|---|---|
| PPO/PA11 | 0 |
| 1 wt.% A-SiO2 | 12 |
| 5 wt.% A-SiO2 | 18 |
| Sample | η* (100 rad s−1) (MPa s) |
|---|---|
| PPO | 21.3 |
| PA11 | 3.3 |
| PPO/PA11 | 6.3 |
| 1 wt.% A-SiO2 | 9.4 |
| 5 wt.% A-SiO2 | 11.0 |
| 5 wt.% SiO2 | 2.3 |
| Sample | Temperature (°C) | Peak Intensity (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|
| αPPO | αPA 11 | β | γ | αPPO | αPA 11 | β | γ | |
| PPO | 179 | - | −18 | −118 | 117 | - | 13.4 | 11.8 |
| PA11 | - | 52 | −69 | −140 | - | 39.7 | 59.4 | 139 |
| PPO/PA11 | 192 | 47 | −77 | −140 | 26.9 | 13.4 | 19.3 | 21.8 |
| 1 wt.% A-SiO2 | 194 | 55 | −74 | −139 | 29.7 | 18.7 | 22.9 | 27.5 |
| 5 wt.% A-SiO2 | 198 | 56 | −72 | −138 | 47.4 | 29.6 | 33.4 | 40.6 |
| 5 wt.% SiO2 | 195 | 58 | −72 | −138 | 29.6 | 18.6 | 22.5 | 27.4 |
| Sample | Tg (C) | Tm (°C) | Tc (°C) | Tm–Tc (°C) | Xc (%) |
|---|---|---|---|---|---|
| PPO | 215 | - | - | - | - |
| PA11 | - | 188 | 157 | 31 | 16.6 |
| PPO/PA11 | 216 | 188 | 94 | 94 | 0.72 |
| 1 wt.% A-SiO2 | 216 | 190 | 96 | 94 | 0.68 |
| 5 wt.% A-SiO2 | 215 | 189 | 95 | 94 | 0.56 |
| 5 wt.% SiO2 | 212 | 189 | 92 | 97 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeziorska, R.; Szadkowska, A.; Studzinski, M. Morphology and Properties of Poly(2,6-dimethyl-1,4-phenylene oxide)/Polyamide 11 Hybrid Nanocomposites: Effect of Silica Surface Modification. Materials 2022, 15, 3421. https://doi.org/10.3390/ma15103421
Jeziorska R, Szadkowska A, Studzinski M. Morphology and Properties of Poly(2,6-dimethyl-1,4-phenylene oxide)/Polyamide 11 Hybrid Nanocomposites: Effect of Silica Surface Modification. Materials. 2022; 15(10):3421. https://doi.org/10.3390/ma15103421
Chicago/Turabian StyleJeziorska, Regina, Agnieszka Szadkowska, and Maciej Studzinski. 2022. "Morphology and Properties of Poly(2,6-dimethyl-1,4-phenylene oxide)/Polyamide 11 Hybrid Nanocomposites: Effect of Silica Surface Modification" Materials 15, no. 10: 3421. https://doi.org/10.3390/ma15103421
APA StyleJeziorska, R., Szadkowska, A., & Studzinski, M. (2022). Morphology and Properties of Poly(2,6-dimethyl-1,4-phenylene oxide)/Polyamide 11 Hybrid Nanocomposites: Effect of Silica Surface Modification. Materials, 15(10), 3421. https://doi.org/10.3390/ma15103421






