Functionalization Mechanism of Reduced Graphene Oxide Flakes with BF3·THF and Its Influence on Interaction with Li+ Ions in Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Molecular Analysis
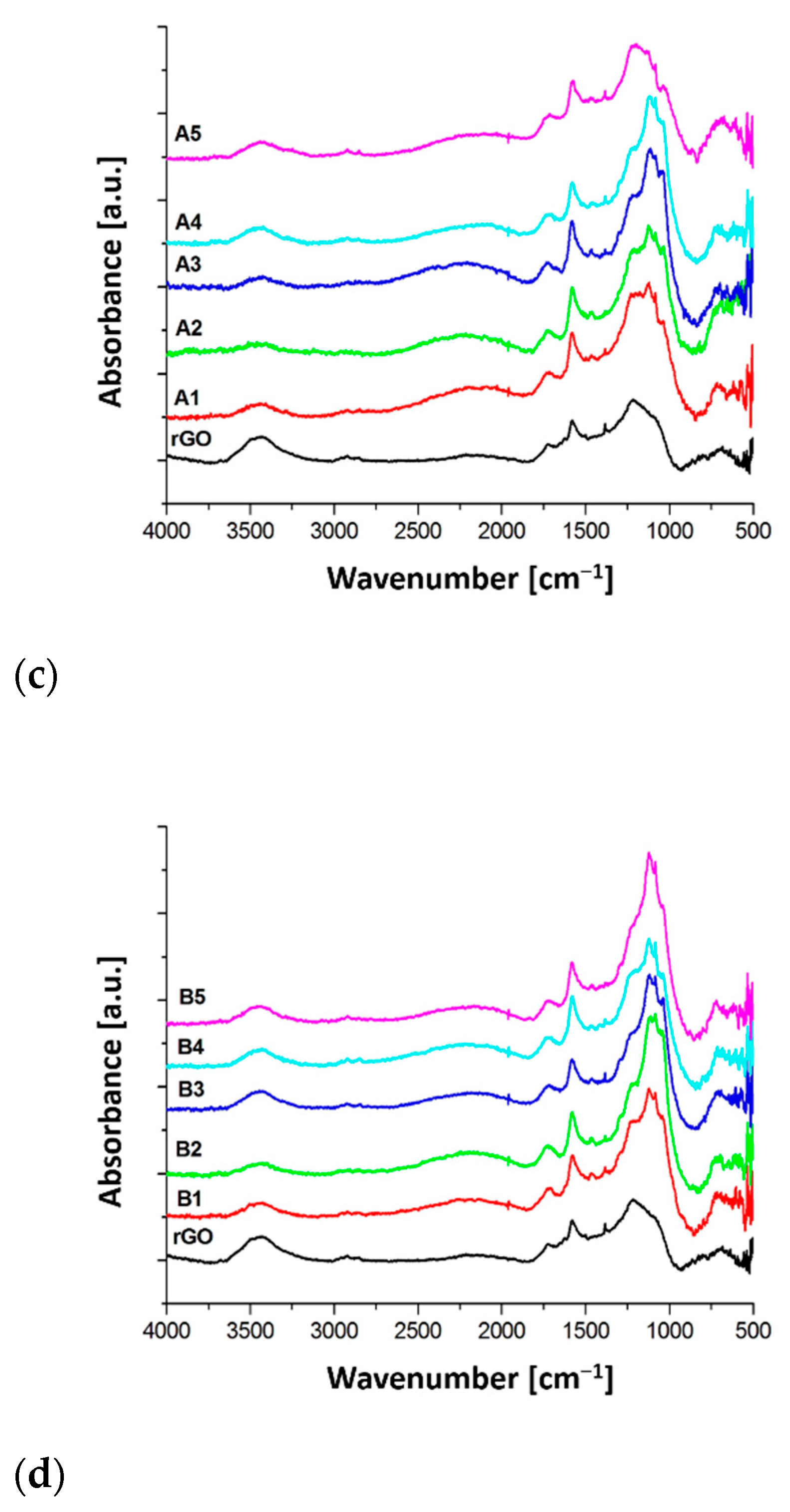
3.2. FTIR
3.3. Raman Spectroscopy
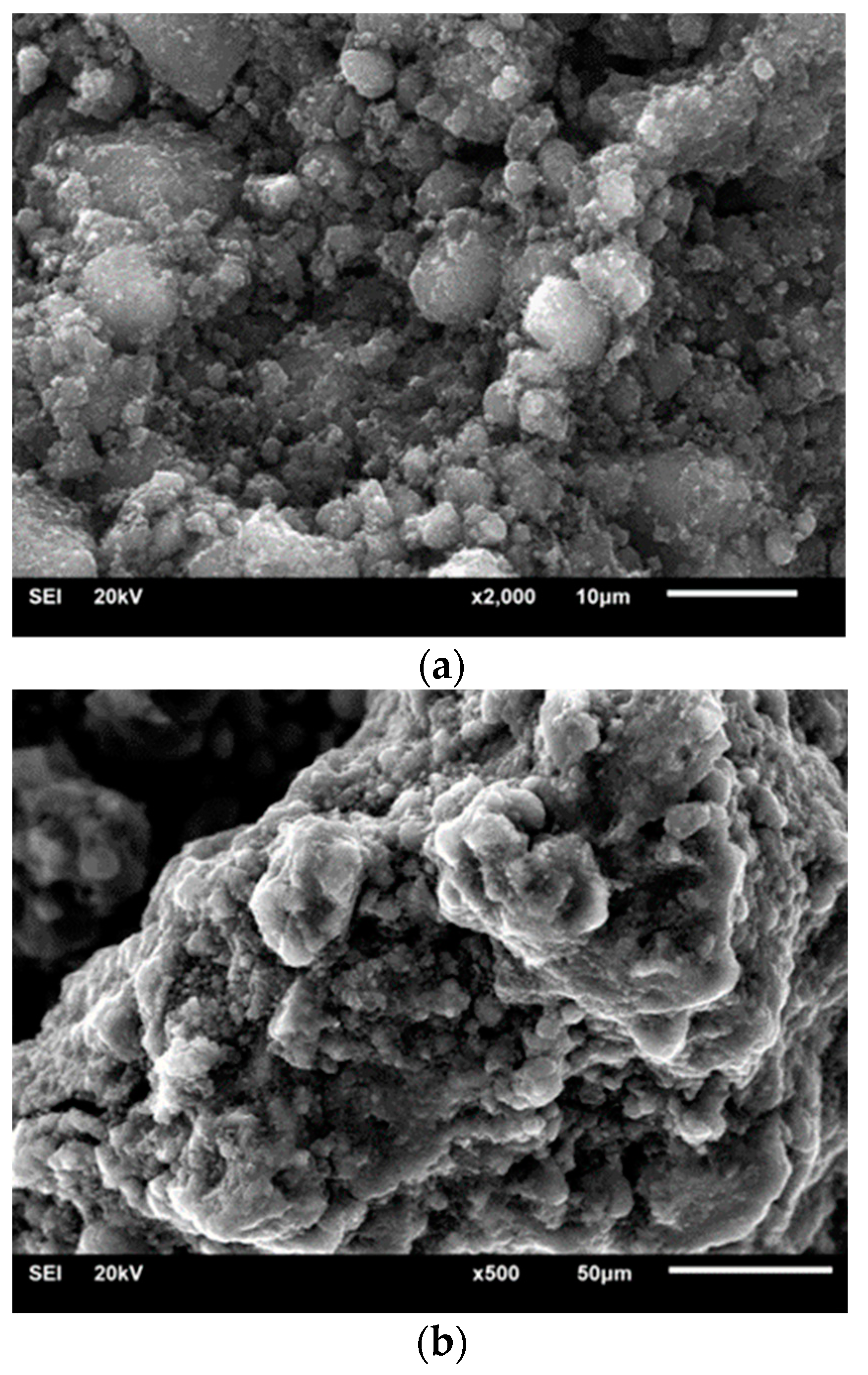
3.4. SEM
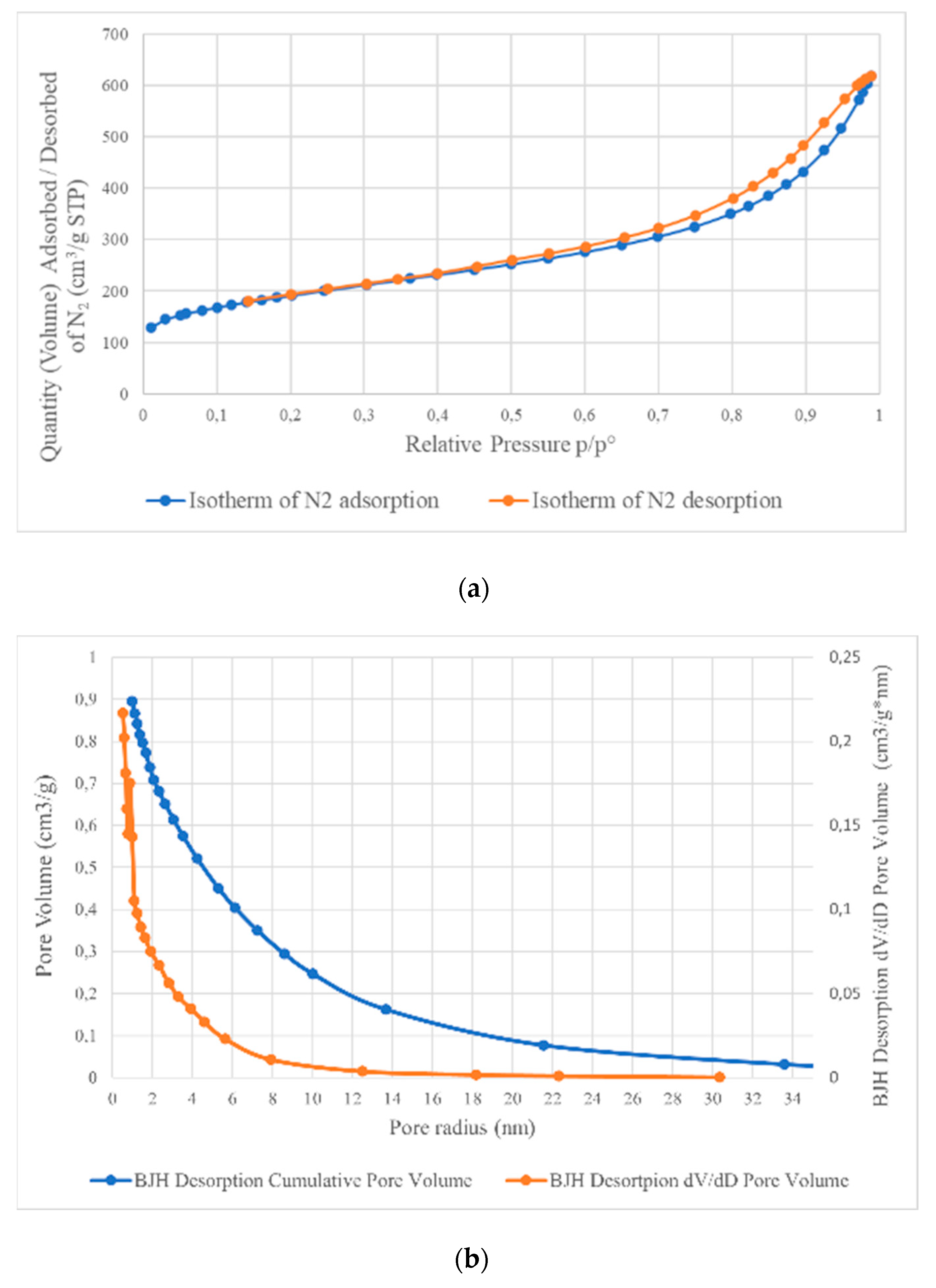
3.5. Analysis of Specific Surface Area (BET)
3.6. Determination of Electrochemical Properties by Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L. Lithium-ion battery recycling processes: Research towards a sustainable course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Damien, D.; Sudeep, P.M.; Narayanan, T.N.; Anantharaman, M.R.; Ajayan, P.M.; Shaijumon, M.M. Fluorinated graphene based electrodes for high performance primary lithium batteries. RSC Adv. 2013, 3, 25702. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, L.; Wang, Y.; Wang, Y.; Shi, Y. Fluorinated graphene as a cathode material for high performance primary lithium ion batteries. New Carbon Mater. 2015, 30, 79–85. [Google Scholar] [CrossRef]
- Bai, A.; Wang, L.; Li, J.; He, X.; Wang, J.; Wang, J. Composite of graphite/phosphorus as anode for lithium-ion batteries. J. Power Source 2015, 289, 100–104. [Google Scholar] [CrossRef]
- Wu, C.-H.; Pu, N.-W.; Liu, Y.-M.; Chen, C.-Y.; Peng, Y.-Y.; Cheng, T.-Y.; Lin, M.-H.; Ger, M.-D. Improving rate capability of lithium-ion batteries using holey graphene as the anode material. J. Taiwan Inst. Chem. Eng. 2017, 80, 511–517. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M. Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, D.H.; Park, C.; Kim, D.-W. Nanocrystalline silicon embedded in an alloy matrix as an anode material for high energy density lithium-ion batteries. J. Power Source 2018, 395, 328–335. [Google Scholar] [CrossRef]
- Carn, F.; Morcrette, M.; Desport, B.; Backov, R. Lithium-ion battery electrode prepared by confining carbon nanotubes/V 2O5 nanoribbons suspension in model air-liquid foams. Solid State Sci. 2013, 17, 134–139. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, S.; Xiao, Z.; Wang, H.; Ma, X. Efficient conductive networks constructed from ultra-low concentration carbon nanotube suspension for Li ion battery cathodes. Carbon N. Y. 2018, 132, 323–328. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Tlili, I.; Tian, Z.; Bakouri, M.; Safaei, M.R.; Goodarzi, M. Thermal evaluation of graphene nanoplatelets nanofluid in a fast-responding HP with the potential use in solar systems in smart cities. Appl. Sci. 2019, 9, 2101. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.; Wang, Y.; Ren, J. Novel sponge-like N-doped graphene film as high-efficiency electrode for Li-ion battery. Appl. Surf. Sci. 2019, 485, 529–535. [Google Scholar] [CrossRef]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, L.; Zhou, S.; Zhao, D.; Wu, L. Facile Synthesis of Hierarchically Ordered Porous Carbon via in Situ Self-Assembly of Colloidal Polymer and Silica Spheres and Its Use as a Catalyst Support. Chem. Mater. 2010, 22, 3433–3440. [Google Scholar] [CrossRef]
- Liu, S.; Liu, C.; Guo, J.; Yan, W. Microstructure and Superior Electrochemical Activity of Cu 3 P/Reduced Graphene Oxide Composite for an Anode in Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2390–A2397. [Google Scholar] [CrossRef]
- Yang, W.; Gao, H.; Zhao, Y.; Bi, K.; Li, X. Facile preparation of nitrogen-doped graphene sponge as a highly efficient oil absorption material. Mater. Lett. 2016, 178, 95–99. [Google Scholar] [CrossRef]
- Xie, X.; Wang, S.; Kretschmer, K.; Wang, G. Two-dimensional layered compound based anode materials for lithium-ion batteries and sodium-ion batteries. J. Colloid Interface Sci. 2017, 499, 17–32. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Gao, Y.; Cao, C.; Han, J.; Feng, Y.; Feng, W. Free-standing fluorine and nitrogen co-doped graphene paper as a high-performance electrode for flexible sodium-ion batteries. Carbon N. Y. 2017, 116, 338–346. [Google Scholar] [CrossRef]
- Ao, Z.; Jiang, Q.; Li, S.; Liu, H.; Peeters, F.M.; Li, S.; Wang, G. Enhancement of the Stability of Fluorine Atoms on Defective Graphene and at Graphene/Fluorographene Interface. ACS Appl. Mater. Interfaces 2015, 7, 19659–19665. [Google Scholar] [CrossRef]
- Tahara, K.; Iwasaki, T.; Furuyama, S.; Matsutani, A.; Hatano, M. Asymmetric transport property of fluorinated graphene. Appl. Phys. Lett. 2013, 103, 143106. [Google Scholar] [CrossRef]
- Poh, H.L.; Sofer, Z.; Klímová, K.; Pumera, M. Fluorographenes via thermal exfoliation of graphite oxide in SF 6, SF 4 and MoF 6 atmospheres. J. Mater. Chem. C 2014, 2, 5198–5207. [Google Scholar] [CrossRef]
- Bon, S.B.; Valentini, L.; Verdejo, R.; Garcia Fierro, J.L.; Peponi, L.; Lopez-Manchado, M.A.; Kenny, J.M. Plasma Fluorination of Chemically Derived Graphene Sheets and Subsequent Modification With Butylamine. Chem. Mater. 2009, 21, 3433–3438. [Google Scholar] [CrossRef]
- Gong, P.; Wang, Z.; Li, Z.; Mi, Y.; Sun, J.; Niu, L.; Wang, H.; Wang, J.; Yang, S. Photochemical synthesis of fluorinated graphene via a simultaneous fluorination and reduction route. RSC Adv. 2013, 3, 6327–6330. [Google Scholar] [CrossRef]
- Bruna, M.; Massessi, B.; Cassiago, C.; Battiato, A.; Vittone, E.; Speranza, G.; Borini, S. Synthesis and properties of monolayer graphene oxyfluoride. J. Mater. Chem. 2011, 21, 18730–18737. [Google Scholar] [CrossRef]
- Zhai, J.; Lei, Z.; Rooney, D.; Sun, K. Top-down synthesis of iron fluoride/reduced graphene nanocomposite for high performance lithium-ion battery. Electrochim. Acta 2019, 313, 497–504. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Contreras-Cid, A.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Flores, M.; Fuentealba, P.; Neira-Carrillo, A.; Verdejo, R.; López-Manchado, M.A. Synthesis of fluorinated graphene oxide by using an easy one-pot deoxyfluorination reaction. J. Colloid Interface Sci. 2018, 524, 219–226. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Li, Z.; Gong, P.; Liu, X.; Zhang, L.; Ren, J.; Wang, H.; Yang, S. Synthesis of fluorinated graphene with tunable degree of fluorination. Carbon N. Y. 2012, 50, 5403–5410. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Long, P.; Gao, Y.; Qin, C.; Cao, C.; Feng, Y.; Feng, W. Hydrothermal preparation of fluorinated graphene hydrogel for high-performance supercapacitors. J. Power Source 2016, 312, 146–155. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and Nitrogen Co-Doped Graphene for Metal-Free Catalytic Oxidation Reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef]
- Lambert, J.B.; Mazzola, E.P.; Ridge, C.D. Nuclear Magnetic Resonance Spectroscopy: An Introduction to Principles, Applications, and Experimental Methods; Wiley: Hoboken, NJ, USA, 2003; Volume 41. [Google Scholar]
- Chen, L.; Lei, J.; Wang, F.; Wang, G.; Feng, H. Facile synthesis of graphene sheets from fluorinated graphite. RSC Adv. 2015, 5, 40148–40153. [Google Scholar] [CrossRef]
- Oh, T.; Choi, C.K.; Lee, K.M. Investigation of a-C:F films as hydrogenated diamond-like carbon and low-k materials. Thin Solid Film 2005, 475, 109–112. [Google Scholar] [CrossRef]
- Mihály, J.; Sterkel, S.; Ortner, H.M.; Kocsis, L.; Hajba, L.; Furdyga, É.; Minka, J. FTIR and FT-Raman spectroscopic study on polymer based high pressure digestion vessels. Croat. Chem. Acta 2006, 79, 497–501. [Google Scholar]
- Qiang, L.; Zhang, B.; Gao, K.; Gong, Z.; Zhang, J. Hydrophobic, mechanical, and tribological properties of fluorine incorporated hydrogenated fullerene-like carbon films. Friction 2013, 1, 350–358. [Google Scholar] [CrossRef]
- Huang, K.P.; Lin, P.; Shih, H.C. Structures and properties of fluorinated amorphous carbon films. J. Appl. Phys. 2004, 96, 354–360. [Google Scholar] [CrossRef]
- Buc, D.; Bello, I.; Caplovicova, M.; Mikula, M.; Kovac, J.; Hotovy, I.; Chong, Y.M.; Siu, G.G. Analysis of magnetron sputtered boron oxide films. Thin Solid Films 2007, 515, 8723–8727. [Google Scholar] [CrossRef]
- Jacox, M.E.; Irikura, K.K.; Thompson, W.E. The reaction of BF3 with H2O: Infrared spectrum of BF2OH trapped in solid neon. J. Chem. Phys. 2000, 113, 5705–5715. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Stalla, D.; Tekeei, A.; Suppes, G.; Jalisatgi, S.; Lee, M.; Hawthorne, F.; Robertson, J.D.; Firlej, L.; et al. Infrared study of boron-carbon chemical bonds in boron-doped activated carbon. Carbon N. Y. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Beams, R.; Gustavo Cançado, L.; Novotny, L. Raman characterization of defects and dopants in graphene. J. Phys. Condens. Matter 2015, 27, 083002. [Google Scholar] [CrossRef]
- Nanda, S.S.; Kim, M.J.; Yeom, K.S.; An, S.S.A.; Ju, H.; Yi, D.K. Raman spectrum of graphene with its versatile future perspectives. TrAC Trends Anal. Chem. 2016, 80, 125–131. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar]
- Wall, M. The Raman Spectroscopy of Graphene and the Determination of Layer Thickness; Thermo Fisher Scientific: Madison, WI, USA, 2011. [Google Scholar]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. In New Developments in Photon and Materials Research; Nova Science Publishers: New York, NY, USA, 2013; pp. 403–418. [Google Scholar]
- Grodecki, K. Spektroskopia ramanowska grafenu. Electron. Mater. 2013, 41, 47–53. [Google Scholar]
- Loryuenyong, V.; Totepvimarn, K.; Eimburanapravat, P.; Boonchompoo, W.; Buasri, A. Preparation and characterization of reduced graphene oxide sheets via water-based exfoliation and reduction methods. Adv. Mater. Sci. Eng. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- An S., J.; Li, J.; Daniel, C.; Mohany, D.; Nagpure, S.; Wood D., L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Ueda, M.; Ohe, M.; Kim J., H.; Yonezawa, S.; Takashima, M. Effects of surface fluorination on the electrochemical properties and thermal stability of LiFePO4 cathode for lithium-ion batteries. J. Fluor. Chem. 2013, 149, 88–94. [Google Scholar] [CrossRef]




| Functionalization Time (h) | ||||||
|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | 120 | ||
| BF3·THF concentration (%) | 1.5 | A1 | A2 | A3 | A4 | A5 |
| 3 | B1 | B2 | B3 | B4 | B5 | |
| Sample Name | Sample Description |
|---|---|
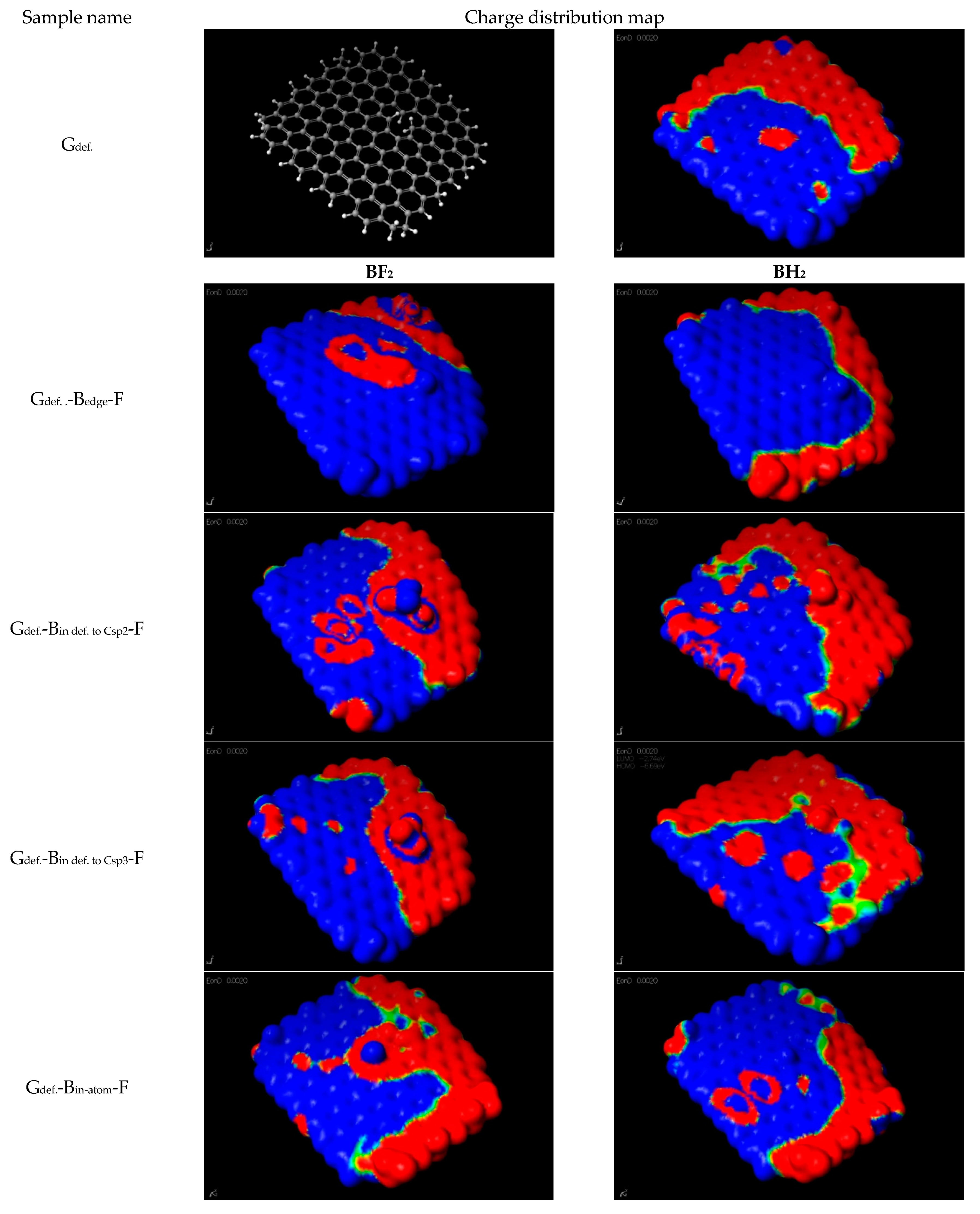
| Gdef. | A model graphene flake made of 149 C atoms and 46 hydrogen atoms. Additionally, the model has a structural defect in the form of a vacancy of one C atom. |
| Gdef.-Bedge-F | As a result of reaction with a hydrogen substitution reaction (on the edge of the flake) with BF3·THF, a Cgraphene-BF2 group is formed |
| Gdef.-Bin def. to Csp2-F | As a result of a hydrogen substitution reaction at carbon Csp2 (in the graphene flake defect) with BF3·THF, a Cgraphene-BF2 group is formed |
| Gdef.-Bin def. to Csp3-F | As a result of a hydrogen substitution reaction at carbon Csp3 (in the graphene flake defect) with BF3·THF, a Cgraphene-BF2 group is formed |
| Gdef.-Bin-atom-F | As a result of a reaction of Csp2 substitution in the graphene with BF3·THF, a -Cgraphene-B-F group is formed. |
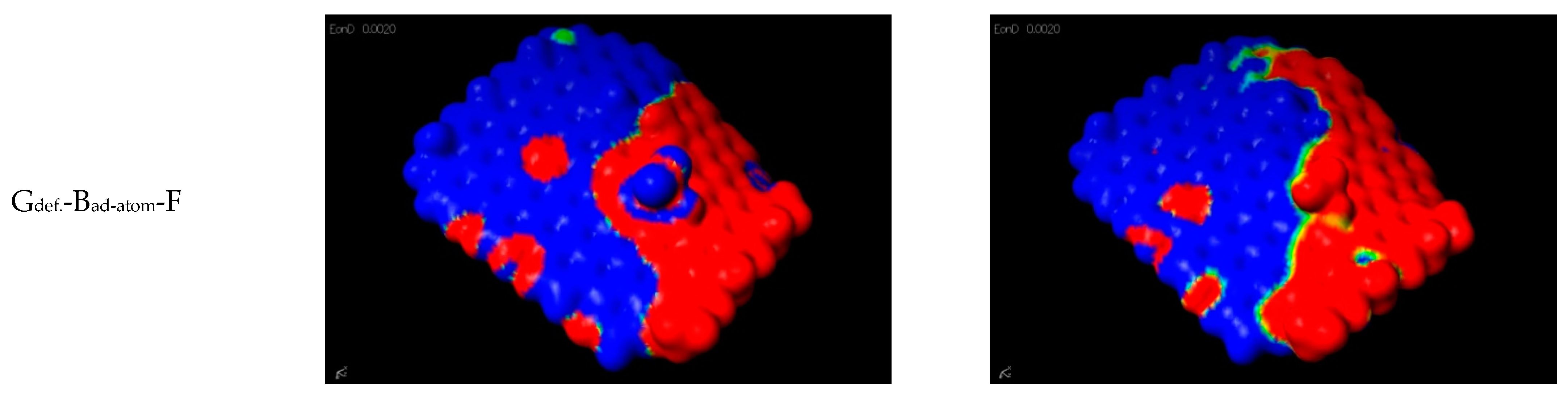
| Gdef.-Bad-atom-F | As a result of a reaction of Csp2 substitution in the graphene with BF3·THF, a Cgraphene-BF2 group is formed. |
| Gdef.-Bedge-H | As a result of a hydrogen substitution reaction (at the edge of a flake) with BF3·THF, a Cgraphene-BH2 is formed. |
| Gdef.-Bin def. to Csp2-H | As a result of a hydrogen substitution reaction at carbon Csp2 (in the graphene flake defect) with BF3·THF, a Cgraphene-BH2 group is formed. |
| Gdef.-Bin def. to Csp3-H | As a result of a hydrogen substitution reaction at carbon Csp3 (in the graphene flake defect) with BF3·THF, a Cgraphene-BH2 group is formed. |
| Gdef.-Bin-atom-H | As a result of a reaction of Csp2 substitution in the graphene with BF3·THF, a -Cgraphene-B-H group is formed. |
| Gdef.-Bad-atom-H | As a result of a reaction of Csp2 substitution in the graphene with BF3·THF, a -Cgraphene-BH2 group is formed. |
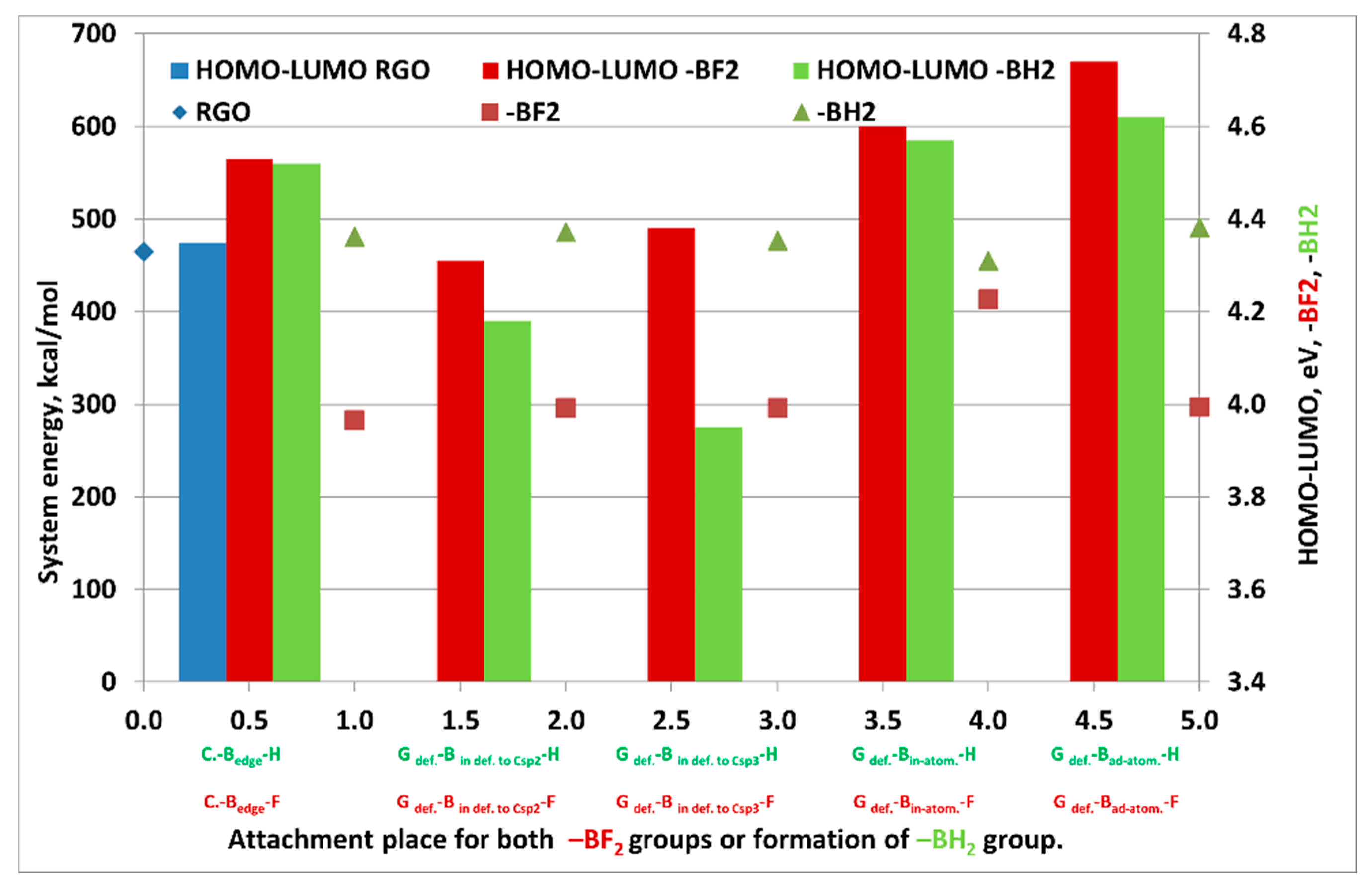
| Sample Name | HOMO, eV | LUMO, eV | ΔE = EHOMO − ELUMO, eV | System Energy, kcal/mol |
|---|---|---|---|---|
| Gdef. | −6.77 | −2.42 | 4.35 | 465.0 |
| Gdef.-Bedge-F | −6.83 | −2.30 | 4.53 | 283.0 |
| Gdef.-Bin def. to Csp2-F | −6.72 | −2.41 | 4.31 | 296.0 |
| Gdef.-Bin def. to Csp3-F | −6.80 | −2.42 | 4.38 | 296.0 |
| Gdef.-Bin-atom-F | −7.00 | −2.40 | 4.6 | 413.0 |
| Gdef.-Bad-atom-F | −6.97 | −2.23 | 4.74 | 297.0 |
| Gdef.-Bedge-H | −6.82 | −2.30 | 4.52 | 481.0 |
| Gdef.-Bin def. to Csp2-H | −6.60 | −2.42 | 4.18 | 486.0 |
| Gdef.-Bin def. to Csp3-H | −6.69 | −2.74 | 3.95 | 477.0 |
| Gdef.-Bin-atom-H | −6.93 | −2.36 | 4.57 | 455.0 |
| Gdef.-Bad-atom-H | −6.85 | −2.23 | 4.62 | 491.0 |
| Sample Name | Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|---|
| 1310–1280 cm−1 (C-F (s) in CF-CH3 and C-F (as) in CF2 | 1126 cm−1 C-F (ss) in CF2 /B-F (s) | 1085 cm−1 (C-F (s) in CF)/B-OH (s) | 1055 cm−1 (C-O-C as)) | 1035 cm−1 (C-F (s) from CF) | 1018 cm−1 (C-B) | |
| A1 | - | 0.21 | 0.06 | - | 0.14 | 0.04 |
| A2 | - | 0.25 | 0.06 | 0.01 | 0.22 | 0.06 |
| A3 | 0.02 | 0.27 | 0.03 | 0.02 | 0.20 | 0.04 |
| A4 | 0.02 | 0.22 | 0.05 | - | 0.19 | 0.05 |
| A5 | 0.01 | 0.12 | 0.05 | 0.01 | 0.14 | 0.03 |
| B1 | 0.02 | 0.09 | 0.05 | 0.01 | 0.10 | 0.02 |
| B2 | 0.04 | 0.08 | 0.05 | 0.01 | 0.14 | 0.03 |
| B3 | 0.03 | 0.15 | 0.07 | - | 0.20 | 0.05 |
| B4 | 0.01 | 0.20 | 0.09 | - | 0.19 | 0.08 |
| B5 | 0.02 | 0.26 | 0.10 | - | 0.21 | 0.12 |
| Parameter | 0 | A1 | A2 | A3 | A4 | A5 | Band |
|---|---|---|---|---|---|---|---|
| Area | 41,684.09 | 58,487.56 | 64,284.79 | 11,1025.87 | 95,620.83 | 101,770.27 | Peak D |
| FWHD | 124.56 | 54.29 | 53.60 | 65.57 | 73.29 | 81.31 | |
| Intensity(A.U) | 326.76 | 872.73 | 1008.73 | 1354.54 | 1048.21 | 1261.63 | |
| Raman shift | 1341.58 | 1339.32 | 1339.28 | 1340.39 | 1340.43 | 1341.41 | |
| Area | 43,444.72 | 40,909.61 | 39,571.78 | 64,445.61 | 37,184.21 | 39,406.77 | Peak G |
| FWHD | 143.15 | 23.04 | 20.52 | 30.89 | 24.81 | 27.14 | |
| Intensity(A.U) | 363.95 | 1387.60 | 1471.78 | 1603.80 | 1185.59 | 1439.51 | |
| Raman shift | 1580.70 | 1566.53 | 1566.06 | 1568.22 | 1567.30 | 1570.73 | |
| Area | 33,384.10 | 122,004.23 | 130,523.80 | 148,816.48 | 101,785.16 | 128,345.60 | Peak 2D |
| FWHD | 203.60 | 127.93 | 135.84 | 114.73 | 123.89 | 146.59 | |
| Intensity(A.U) | 226.35 | 779.66 | 856.84 | 1101.80 | 744.65 | 879.08 | |
| Raman shift | 2698.19 | 2674.78 | 2674.91 | 2675.59 | 2675.32 | 2682.03 | |
| Area | - | 38,543.12 | 41,647.04 | 33,785.90 | 49,792.27 | 58,367.66 | Peak D’ |
| FWHD | - | 94.98 | 72.45 | 69.10 | 67.66 | 71.98 | |
| Intensity(A.U) | - | 426.52 | 610.73 | 586.99 | 689.81 | 822.08 | |
| Raman shift | - | 1604.59 | 1594.56 | 1599.18 | 1598.95 | 1602.38 | |
| Area | - | 28,219.95 | 17,986.16 | 18,834.84 | 25,609.52 | 32,190.22 | Peak D+G |
| FWHD | - | 118.16 | 76.25 | 88.23 | 107.32 | 78.18 | |
| Intensity(A.U) | - | 342.23 | 395.32 | 411.94 | 373.54 | 412.70 | |
| Raman shift | - | 2460.62 | 2451.43 | 2458.03 | 2455.01 | 2921.88 | |
| IDA/IGA | 0.96 | 1.43 | 1.62 | 1.72 | 2.57 | 2.58 | |
| IDI/IGI | 0.89 | 0.63 | 0.69 | 0.84 | 0.88 | 0.88 |
| Sample | Specific Surface Area (BET), m2/g | Total Pore Volume (BJH), cm3/g | Average Pore Size (Radius) (BJH), nm |
|---|---|---|---|
| A1 | 654 | 0.895 | 3.49 |
| B1 | 638 | 0.909 | 3.61 |
| A5 | 660 | 0.909 | 3.44 |
| B5 | 586 | 0.885 | 3.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, Ł.; Balik, M.; Warga, T.; Acznik, I.; Lota, K.; Miszczak, S.; Sobczyk-Guzenda, A.; Kyzioł, K.; Zawadzki, P.; Wosiak, A. Functionalization Mechanism of Reduced Graphene Oxide Flakes with BF3·THF and Its Influence on Interaction with Li+ Ions in Lithium-Ion Batteries. Materials 2021, 14, 679. https://doi.org/10.3390/ma14030679
Kaczmarek Ł, Balik M, Warga T, Acznik I, Lota K, Miszczak S, Sobczyk-Guzenda A, Kyzioł K, Zawadzki P, Wosiak A. Functionalization Mechanism of Reduced Graphene Oxide Flakes with BF3·THF and Its Influence on Interaction with Li+ Ions in Lithium-Ion Batteries. Materials. 2021; 14(3):679. https://doi.org/10.3390/ma14030679
Chicago/Turabian StyleKaczmarek, Łukasz, Magdalena Balik, Tomasz Warga, Ilona Acznik, Katarzyna Lota, Sebastian Miszczak, Anna Sobczyk-Guzenda, Karol Kyzioł, Piotr Zawadzki, and Agnieszka Wosiak. 2021. "Functionalization Mechanism of Reduced Graphene Oxide Flakes with BF3·THF and Its Influence on Interaction with Li+ Ions in Lithium-Ion Batteries" Materials 14, no. 3: 679. https://doi.org/10.3390/ma14030679
APA StyleKaczmarek, Ł., Balik, M., Warga, T., Acznik, I., Lota, K., Miszczak, S., Sobczyk-Guzenda, A., Kyzioł, K., Zawadzki, P., & Wosiak, A. (2021). Functionalization Mechanism of Reduced Graphene Oxide Flakes with BF3·THF and Its Influence on Interaction with Li+ Ions in Lithium-Ion Batteries. Materials, 14(3), 679. https://doi.org/10.3390/ma14030679








